Hybrid Methacrylated Gelatin and Hyaluronic Acid Hydrogel Scaffolds. Preparation and Systematic Characterization for Prospective Tissue Engineering Applications
Abstract
:1. Introduction
2. Results and Discussion
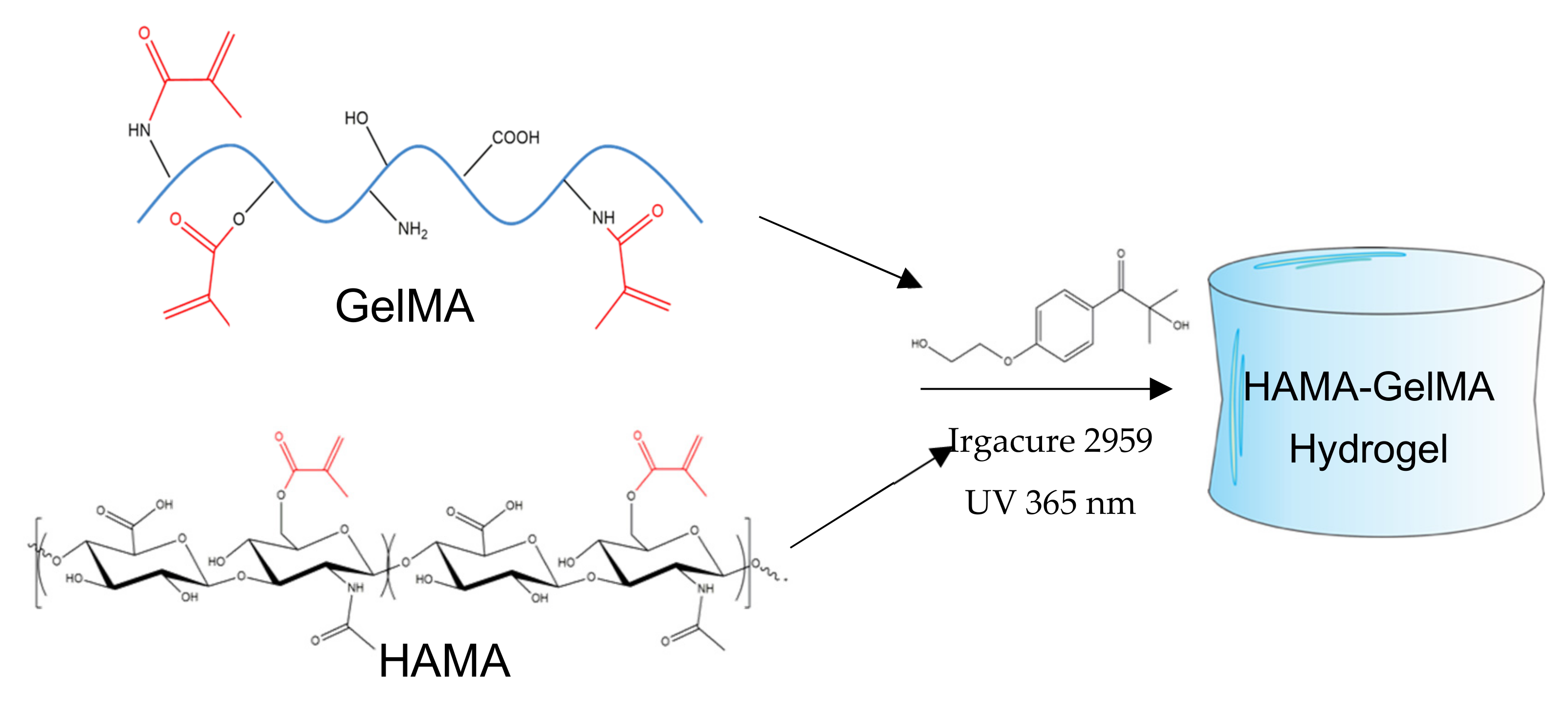
2.1. Synthesis and Preparation of HAMA-GelMA Hydrogels
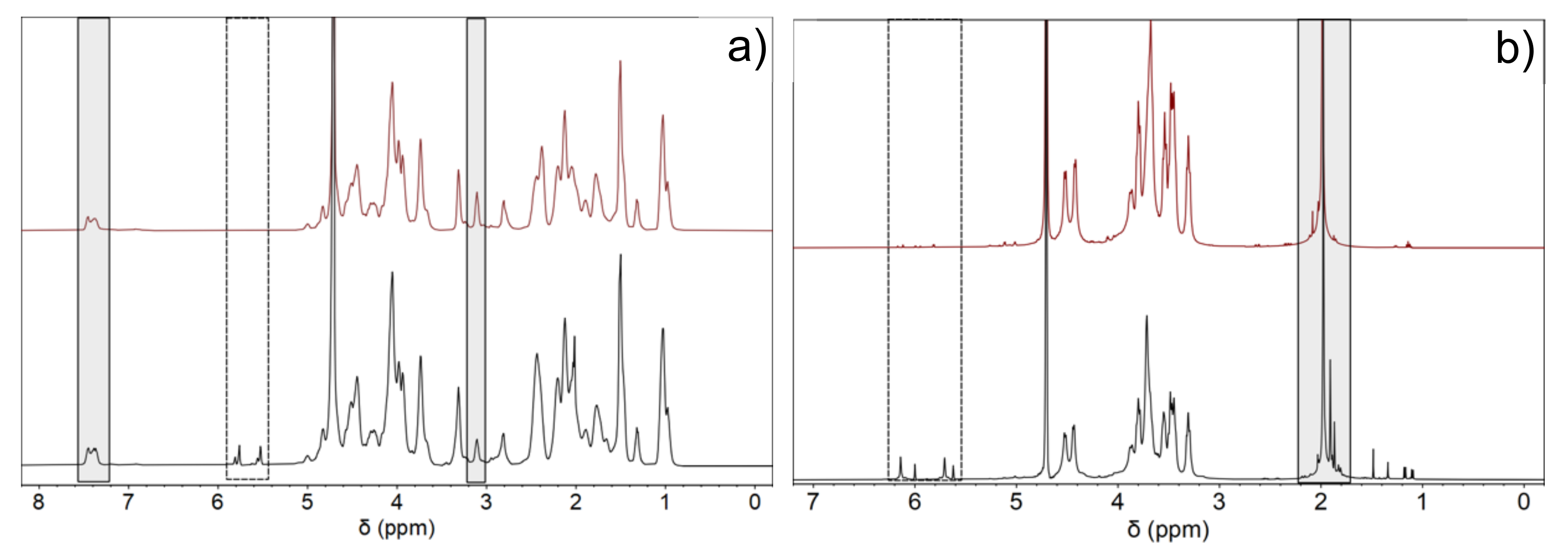
2.2. Physico-Chemical Characterization
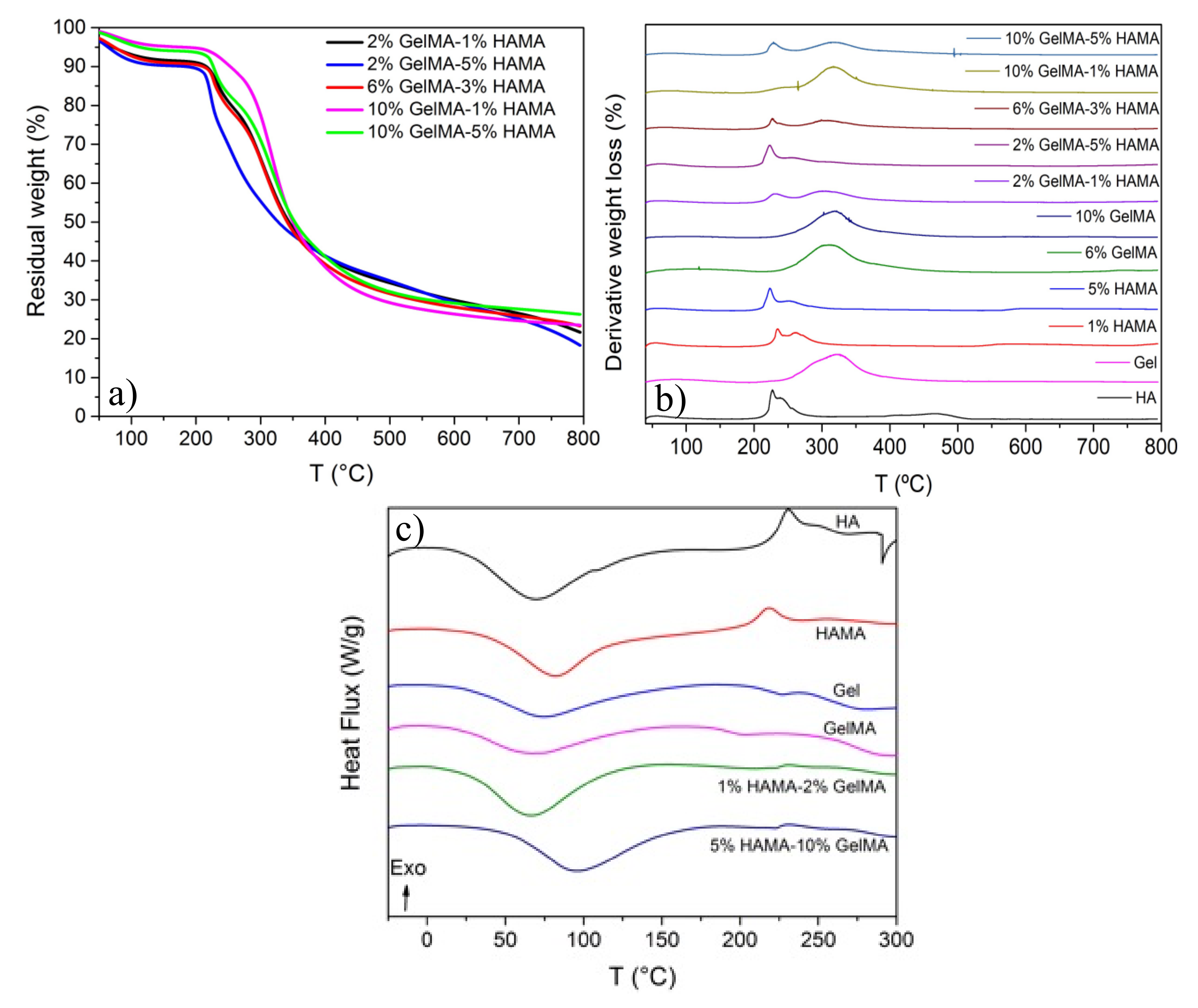
2.3. Thermal Characterization
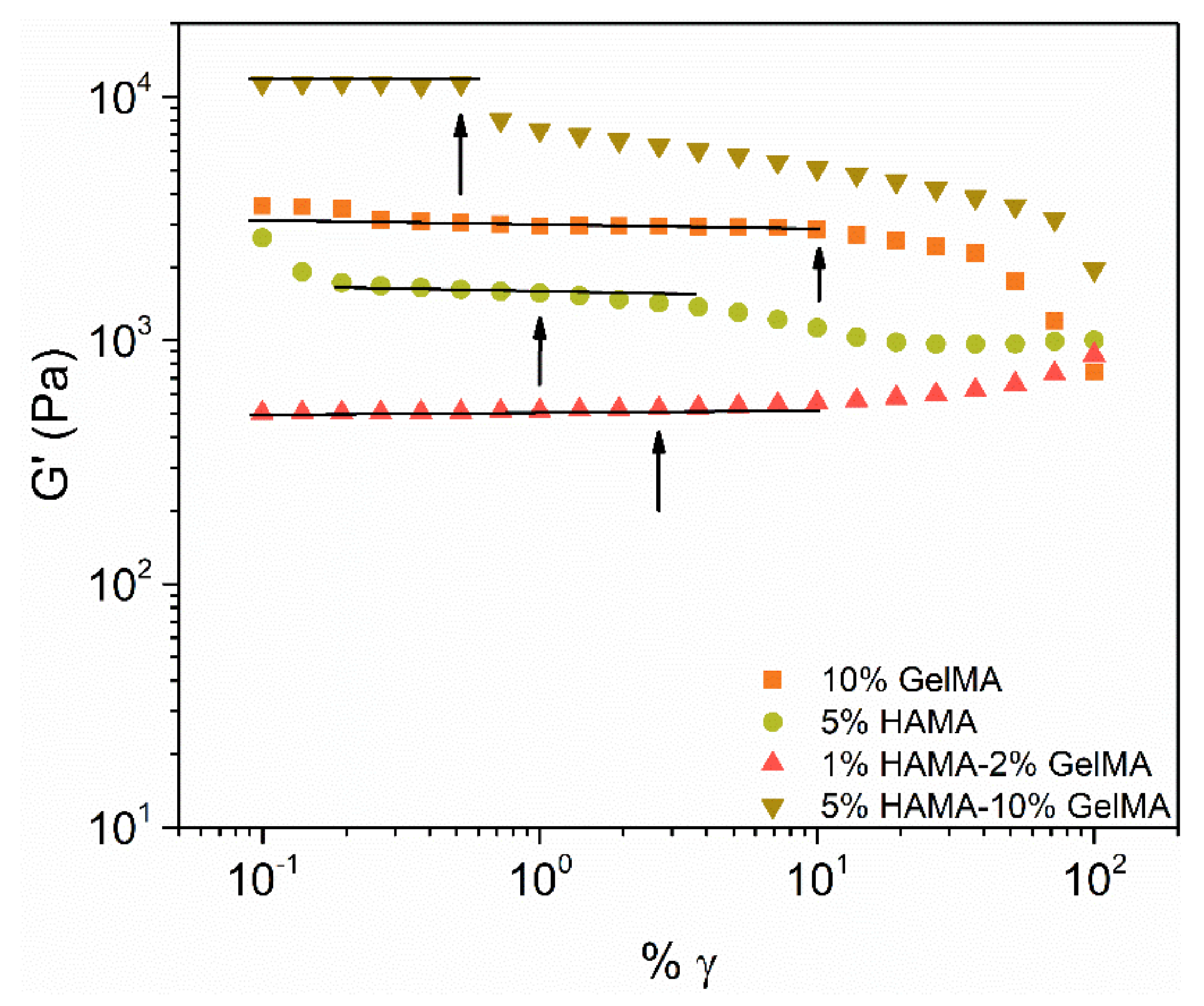
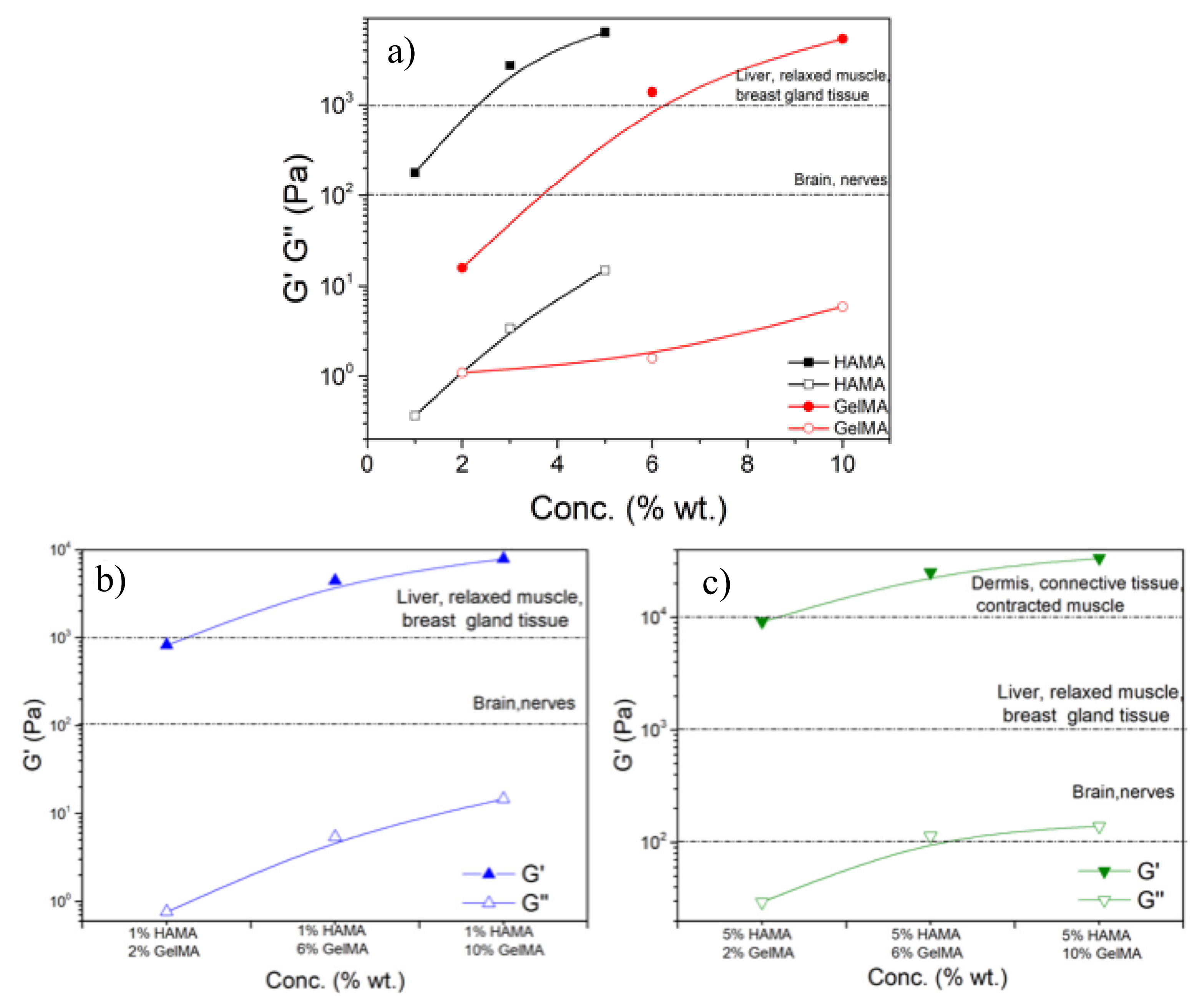
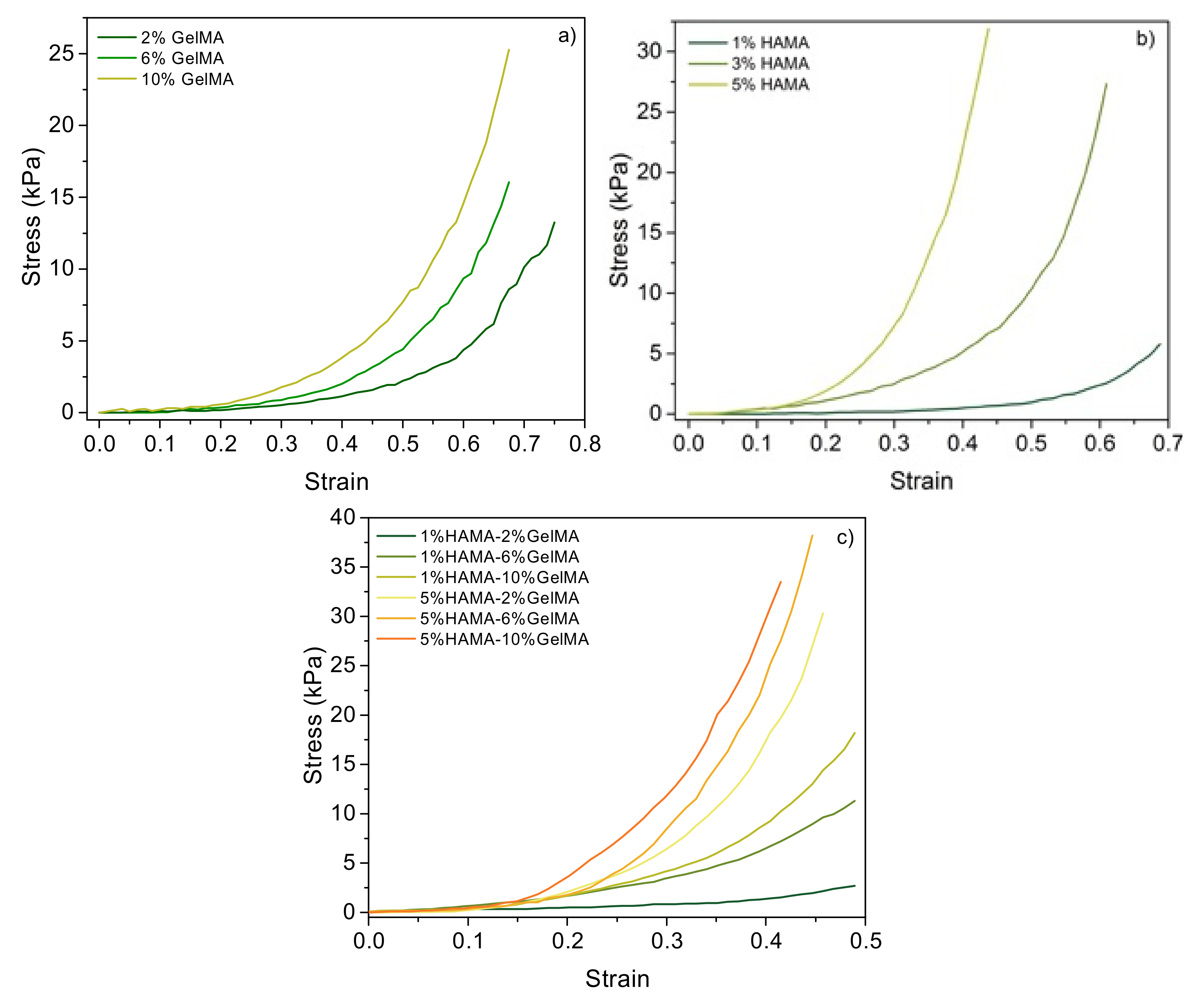
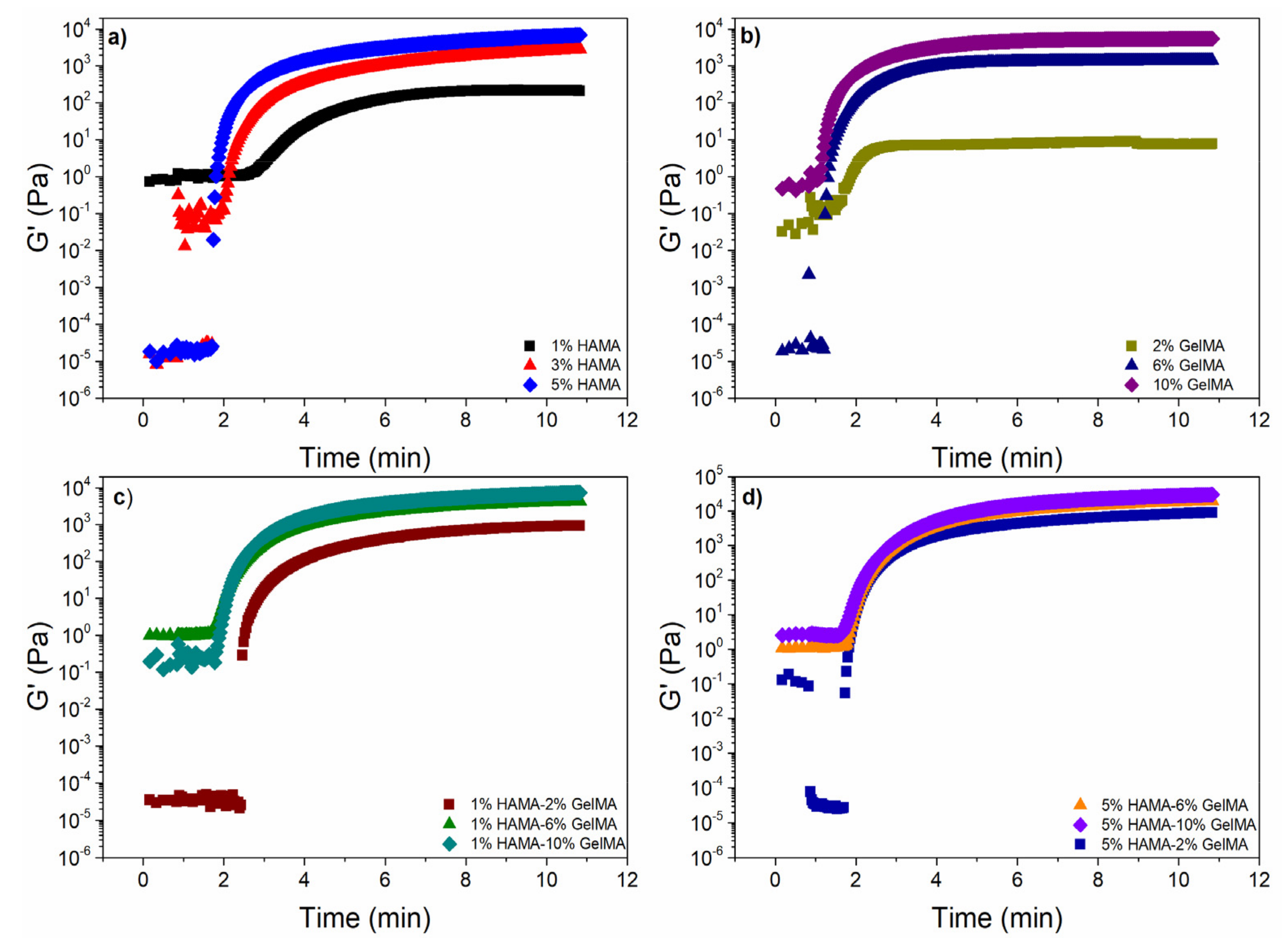
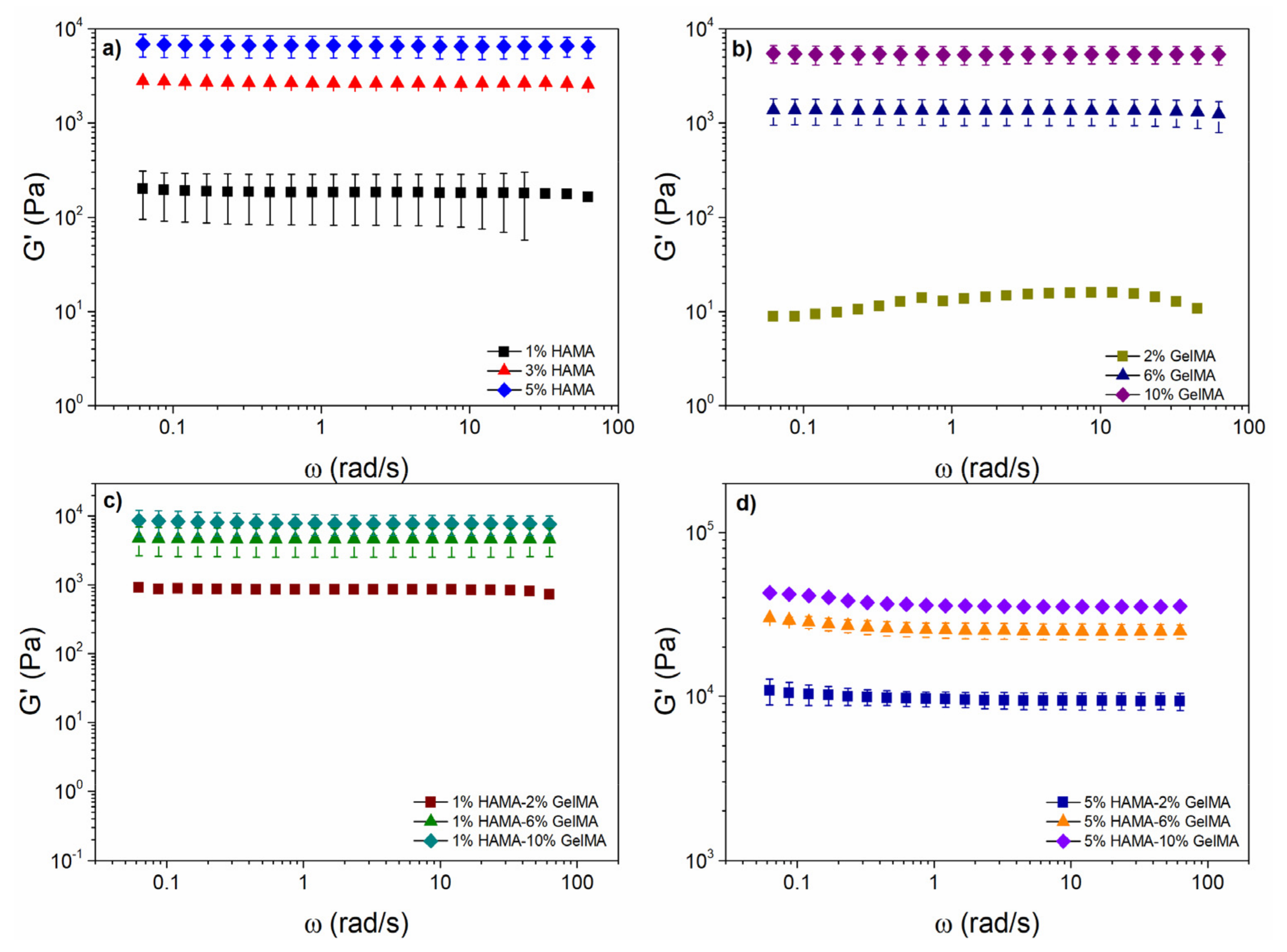
2.4. Rheological Properties of the Scaffolds
2.5. Structure and Morphology of the Scaffolds
2.6. Swelling Degree
2.7. In Vitro Biodegradation
3. Materials and Methods
3.1. Materials
3.2. Gelatin and Hyaluronic Acid Methacrylation
3.3. Hydrogel Preparation
3.4. Gel Fraction
3.5. Apparent Density
3.6. Fourier-Transform Infrared Spectroscopy
3.7. Raman Imaging
3.8. Thermal Analysis
3.9. Oscillatory Rheological Measurements
3.10. Mechanical Compression Tests
3.11. Field-Emission Scanning Electron Microscopy
3.12. Mercury Immersion Porosimetry
3.13. Swelling
3.14. In Vitro Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Sample | Concentration (w/v%) | |
|---|---|---|
| HAMA | 1 | 1.92 |
| 3 | 1.00 | |
| 5 | 0.88 | |
| GelMA | 2 | 0.94 |
| 6 | 0.52 | |
| 10 | 0.35 | |
| 1% HAMA/X% GelMA | 2 | 1.69 |
| 6 | 0.93 | |
| 10 | 1.00 | |
| 5% HAMA/X% GelMA | 2 | 0.91 |
| 6 | 0.86 | |
| 10 | 0.77 |
References
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef]
- Gu, L.; Li, T.; Song, X.; Yang, X.; Li, S.; Chen, L.; Liu, P.; Gong, X.; Chen, C.; Sun, L. Preparation and characterization of methacrylated gelatin/bacterial cellulose composite hydrogels for cartilage tissue engineering. Regen. Biomater. 2020, 7, 195–202. [Google Scholar] [CrossRef]
- Motealleh, A.; Kehr, N.S. Nanocomposite Hydrogels and Their Applications in Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 938. [Google Scholar] [CrossRef]
- Zhang, F.; He, C.; Cao, L.; Feng, W.; Wang, H.; Mo, X.; Wang, J. Fabrication of gelatin-hyaluronic acid hybrid scaffolds with tunable porous structures for soft tissue engineering. Int. J. Biol. Macromol. 2011, 48, 474–481. [Google Scholar] [CrossRef]
- Vanderhooft, J.L.; Alcoutlabi, M.; Magda, J.J.; Prestwich, G.D. Rheological properties of cross-linked hyaluronan-gelatin hydrogels for tissue engineering. Macromol. Biosci. 2009, 9, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dave, P.N.; Gor, A. Natural Polysaccharide-Based Hydrogels and Nanomaterials. In Handbook of Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 36–66. ISBN 9780128133514. [Google Scholar]
- Nieto-Suárez, M.; López-Quintela, M.A.; Lazzari, M. Preparation and characterization of crosslinked chitosan/gelatin scaffolds by ice segregation induced self-assembly. Carbohydr. Polym. 2016, 141, 175–183. [Google Scholar] [CrossRef] [PubMed]
- McKee, T.J.; Perlman, G.; Morris, M.; Komarova, S.V. Extracellular matrix composition of connective tissues: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Novak, U.; Kaye, A.H. Extracellular matrix and the brain: Components and function. J. Clin. Neurosci. 2000, 7, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Rose, J.B.; Pacelli, S.; El Haj, A.J.; Dua, H.S.; Hopkinson, A.; White, L.J.; Rose, F.R.A.J. Gelatin-based materials in ocular tissue engineering. Materials 2014, 7, 3106–3135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camci-Unal, G.; Cuttica, D.; Annabi, N.; Demarchi, D.; Khademhosseini, A. Synthesis and characterization of hybrid hyaluronic acid-gelatin hydrogels. Biomacromolecules 2013, 14, 1085–1092. [Google Scholar] [CrossRef]
- Chandra, P.K.; Atala, A.A. Use of matrix and seeding with cells for vasculature of organs. In Encyclopedia of Tissue Engineering and Regenerative Medicine; Academic Press: New York, NY, USA, 2019; Volume 1–3, pp. 425–446. ISBN 9780128136997. [Google Scholar]
- Loessner, D.; Meinert, C.; Kaemmerer, E.; Martine, L.C.; Yue, K.; Levett, P.A.; Klein, T.J.; Melchels, F.P.W.; Khademhosseini, A.; Hutmacher, D.W. Functionalization, preparation and use of cell-laden gelatin methacryloyl-based hydrogels as modular tissue culture platforms. Nat. Protoc. 2016, 11, 727–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eke, G.; Mangir, N.; Hasirci, N.; MacNeil, S.; Hasirci, V. Development of a UV crosslinked biodegradable hydrogel containing adipose derived stem cells to promote vascularization for skin wounds and tissue engineering. Biomaterials 2017, 129, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Van, A.I.; Bulcke, D.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Masters, K.S.; Shah, D.N.; Leinwand, L.A.; Anseth, K.S. Crosslinked hyaluronan scaffolds as a biologically active carrier for valvular interstitial cells. Biomaterials 2005, 26, 2517–2525. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [Green Version]
- Benton, J.A.; Deforest, C.A.; Vivekanandan, V.; Anseth, K.S. Photocrosslinking of gelatin macromers to synthesize porous hydrogels that promote valvular interstitial cell function. Tissue Eng. Part A 2009, 15, 3221–3230. [Google Scholar] [CrossRef]
- Prakash Parthiban, S.; Rana, D.; Jabbari, E.; Benkirane-Jessel, N.; Ramalingam, M. Covalently immobilized VEGF-mimicking peptide with gelatin methacrylate enhances microvascularization of endothelial cells. Acta Biomater. 2017, 51, 330–340. [Google Scholar] [CrossRef]
- Visser, J.; Gawlitta, D.; Benders, K.E.M.; Toma, S.M.H.; Pouran, B.; van Weeren, P.R.; Dhert, W.J.A.; Malda, J. Endochondral bone formation in gelatin methacrylamide hydrogel with embedded cartilage-derived matrix particles. Biomaterials 2015, 37, 174–182. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic acid: Molecular mechanisms and therapeutic trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.M.; Zhang, C.L.; Hou, S.P.; Yu, X.; Cui, F.Z.; Xu, Q.Y.; Sheng, S.L.; Cui, H.; Li, H.D. Hyaluronic acid hydrogel as Nogo-66 receptor antibody delivery system for the repairing of injured rat brain: In vitro. J. Control. Release 2005, 102, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Bencherif, S.A.; Srinivasan, A.; Horkay, F.; Hollinger, J.O.; Matyjaszewski, K.; Washburn, N.R. Influence of the degree of methacrylation on hyaluronic acid hydrogels properties. Biomaterials 2008, 29, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Slevin Oligosaccharides of hyaluronan induce angiogenesis through distinct CD44 and RHAMM-mediated signalling pathways involving Cdc2 and γ-adducin. Int. J. Oncol. 2009, 35, 761–773. [CrossRef] [Green Version]
- Braun, M. Cellular adhesion molecules on vascular smooth muscle cells. Cardiovasc. Res. 1999, 41, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermatoendocrinology 2012, 4, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Almeida, L.D.F.; Babo, P.S.; Silva, C.R.; Rodrigues, M.T.; Hebling, J.; Reis, R.L.; Gomes, M.E. Hyaluronic acid hydrogels incorporating platelet lysate enhance human pulp cell proliferation and differentiation. J. Mater. Sci. Mater. Med. 2018, 29, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hjortnaes, J.; Camci-Unal, G.; Hutcheson, J.D.; Jung, S.M.; Schoen, F.J.; Kluin, J.; Aikawa, E.; Khademhosseini, A. Directing valvular interstitial cell myofibroblast-like differentiation in a hybrid hydrogel platform. Adv. Healthc. Mater. 2015, 4, 121–130. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Chen, Y.C. Regeneration of neurite-like cells from induced pluripotent stem cells in self-assembled hyaluronic acid-gelatin microhydrogel. J. Taiwan Inst. Chem. Eng. 2016, 67, 74–87. [Google Scholar] [CrossRef]
- Ingber, D.E. Cellular mechanotransduction: Putting all the pieces together again. FASEB J. 2006, 20, 811–827. [Google Scholar] [CrossRef]
- Arya, A.D.; Hallur, P.M.; Karkisaval, A.G.; Gudipati, A.; Rajendiran, S.; Dhavale, V.; Ramachandran, B.; Jayaprakash, A.; Gundiah, N.; Chaubey, A. Gelatin Methacrylate Hydrogels as Biomimetic Three-Dimensional Matrixes for Modeling Breast Cancer Invasion and Chemoresponse in Vitro. ACS Appl. Mater. Interfaces 2016, 8, 22005–22017. [Google Scholar] [CrossRef]
- Poldervaart, M.T.; Goversen, B.; De Ruijter, M.; Abbadessa, A.; Melchels, F.P.W.; Öner, F.C.; Dhert, W.J.A.; Vermonden, T.; Alblas, J. 3D bioprinting of methacrylated hyaluronic acid (MeHA) hydrogel with intrinsic osteogenicity. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levett, P.A.; Melchels, F.P.W.; Schrobback, K.; Hutmacher, D.W.; Malda, J.; Klein, T.J. A biomimetic extracellular matrix for cartilage tissue engineering centered on photocurable gelatin, hyaluronic acid and chondroitin sulfate. Acta Biomater. 2014, 10, 214–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razzak, M.A.; Kim, M.; Kim, H.J.; Park, Y.C.; Chung, D. Deciphering the interactions of fish gelatine and hyaluronic acid in aqueous solutions. Int. J. Biol. Macromol. 2017, 102, 885–892. [Google Scholar] [CrossRef]
- De Oliveira, S.A.; da Silva, B.C.; Riegel-Vidotti, I.C.; Urbano, A.; de Sousa Faria-Tischer, P.C.; Tischer, C.A. Production and characterization of bacterial cellulose membranes with hyaluronic acid from chicken comb. Int. J. Biol. Macromol. 2017, 97, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Farshi Azhar, F.; Olad, A.; Mirmohseni, A. Development of novel hybrid nanocomposites based on natural biodegradable polymer-montmorillonite/polyaniline: Preparation and characterization. Polym. Bull. 2014, 71, 1591–1610. [Google Scholar] [CrossRef]
- Pawlak, A.; Mucha, M. Thermogravimetric and FTIR studies of chitosan blends. Thermochim. Acta 2003, 396, 153–166. [Google Scholar] [CrossRef]
- Smitha, B.; Sridhar, S.; Khan, A.A. Chitosan-sodium alginate polyion complexes as fuel cell membranes. Eur. Polym. J. 2005, 41, 1859–1866. [Google Scholar] [CrossRef]
- Florczyk, S.J.; Wang, K.; Jana, S.; Wood, D.L.; Sytsma, S.K.; Sham, J.G.; Kievit, F.M.; Zhang, M. Porous chitosan-hyaluronic acid scaffolds as a mimic of glioblastoma microenvironment ECM. Biomaterials 2013, 34, 10143–10150. [Google Scholar] [CrossRef] [Green Version]
- Pieróg, M.; Gierszewska-Drużyńska, M.; Ostrowska-Czubenko, J. Effect of Ionic Crosslinking Agents on Swelling Behaviour of Chitosan Hydrogel Membranes. Prog. Chem. Appl. Chitin Deriv. 2009, XIV, 75–82. [Google Scholar]
- Macosko, C.W. Rheology: Principles, Measurements, and Applications; VCH: New York, NY, USA, 1994; ISBN 9780471185758. [Google Scholar]
- Cui, Y.; Tan, M.; Zhu, A.; Guo, M. Strain hardening and highly resilient hydrogels crosslinked by chain-extended reactive pseudopolyrotaxane. RSC Adv. 2014, 4, 56791–56797. [Google Scholar] [CrossRef]
- Shah, J.V.; Janmey, P.A. Strain hardening of fibrin gels and plasma clots. Rheol. Acta 1997, 36, 262–268. [Google Scholar] [CrossRef]
- Magami, S.M.; Williams, R.L. Gelation studies on acrylic acid-based hydrogels via in situ photo-crosslinking and rheology. J. Appl. Polym. Sci. 2018, 135, 46691. [Google Scholar] [CrossRef]
- Alcantara, M.R.; Vanin, J. Rheological properties of lyotropic liquid crystals. Colloids Surf. A Physicochem. Eng. Asp. 1995, 97, 151–156. [Google Scholar] [CrossRef]
- Soltero, J.F.A.; Bautista, F.; Pecina, E.; Puig, J.E.; Manero, O.; Proverbio, Z.; Schulz, P.C. Rheological behavior in the didodecyldimethylammonium bromide/water system. Colloid Polym. Sci. 2000, 278, 37–47. [Google Scholar] [CrossRef]
- Liao, H.; Zhang, H.; Chen, W. Differential physical, rheological, and biological properties of rapid in situ gelable hydrogels composed of oxidized alginate and gelatin derived from marine or porcine sources. J. Mater. Sci. Mater. Med. 2009, 20, 1263–1271. [Google Scholar] [CrossRef]
- Hassan, N.; Soltero, A.; Pozzo, D.; Messina, P.V.; Ruso, J.M. Bioinspired templates for the synthesis of silica nanostructures. Soft Matter 2012, 8, 9553. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Shiroud Heidari, B.; Ruan, R.; De-Juan-Pardo, E.M.; Zheng, M.; Doyle, B. Biofabrication and Signaling Strategies for Tendon/Ligament Interfacial Tissue Engineering. ACS Biomater. Sci. Eng. 2021, 7, 383–399. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, S.; Chen, X. Injectable hydrogels for tendon and ligament tissue engineering. J. Tissue Eng. Regen. Med. 2020, 14, 1333–1348. [Google Scholar] [CrossRef]
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Rezaei Kolahchi, A.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 2017, 62, 42–63. [Google Scholar] [CrossRef]
- Romn, J.; Cabãas, M.V.; Pẽa, J.; Vallet-Regí, M. Control of the pore architecture in three-dimensional hydroxyapatite- reinforced hydrogel scaffolds. Sci. Technol. Adv. Mater. 2011, 12, 45003. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Chang, Y.H. Differentiation of induced pluripotent stem cells toward neurons in hydrogel biomaterials. Colloids Surf. B Biointerfaces 2013, 102, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.C.; García-Carvajal, Z.Y.; Jobbágy, M.; Rubio, F.; Yuste, L.; Rojo, F.; Ferrer, M.L.; Del Monte, F. Poly(vinyl alcohol) scaffolds with tailored morphologies for drug delivery and controlled release. Adv. Funct. Mater. 2007, 17, 3505–3513. [Google Scholar] [CrossRef] [Green Version]
- Salerno, A.; Zeppetelli, S.; Di Maio, E.; Iannace, S.; Netti, P.A. Architecture and properties of bi-modal porous scaffolds for bone regeneration prepared via supercritical CO 2 foaming and porogen leaching combined process. J. Supercrit. Fluids 2012, 67, 114–122. [Google Scholar] [CrossRef]
- Silva, M.M.C.G.; Cyster, L.A.; Barry, J.J.A.; Yang, X.B.; Oreffo, R.O.C.; Grant, D.M.; Scotchford, C.A.; Howdle, S.M.; Shakesheff, K.M.; Rose, F.R.A.J. The effect of anisotropic architecture on cell and tissue infiltration into tissue engineering scaffolds. Biomaterials 2006, 27, 5909–5917. [Google Scholar] [CrossRef]
- Ikeda, T.; Ikeda, K.; Yamamoto, K.; Ishizaki, H.; Yoshizawa, Y.; Yanagiguchi, K.; Yamada, S.; Hayashi, Y. Fabrication and characteristics of chitosan sponge as a tissue engineering scaffold. Biomed Res. Int. 2014, 2014, 786892. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Goimil, L.; Jaeger, P.; Ardao, I.; Gómez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C.; García-González, C.A. Preparation and stability of dexamethasone-loaded polymeric scaffolds for bone regeneration processed by compressed CO2 foaming. J. CO2 Util. 2018, 24, 89–98. [Google Scholar] [CrossRef]
- Alizadeh, M.; Abbasi, F.; Khoshfetrat, A.B.; Ghaleh, H. Microstructure and characteristic properties of gelatin/chitosan scaffold prepared by a combined freeze-drying/leaching method. Mater. Sci. Eng. C 2013, 33, 3958–3967. [Google Scholar] [CrossRef]
- Sivashanmugam, A.; Arun Kumar, R.; Vishnu Priya, M.; Nair, S.V.; Jayakumar, R. An overview of injectable polymeric hydrogels for tissue engineering. Eur. Polym. J. 2015, 72, 543–565. [Google Scholar] [CrossRef]
- Galeska, I.; Kim, T.K.; Patil, S.D.; Bhardwaj, U.; Chattopadhyay, D.; Papadimitrakopoulos, F.; Burgess, D.J. Controlled release of dexamethasone from PLGA microspheres embedded within polyacid-containing PVA hydrogels. AAPS J. 2005, 7, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Möller, L.; Krause, A.; Dahlmann, J.; Gruh, I.; Kirschning, A.; Dräger, G. Preparation and evaluation of hydrogel-composites from methacrylated hyaluronic acid, alginate, and gelatin for tissue engineering. Int. J. Artif. Organs 2011, 34, 93–102. [Google Scholar] [CrossRef]
- Daza Agudelo, J.I.; Badano, J.M.; Rintoul, I. Kinetics and thermodynamics of swelling and dissolution of PVA gels obtained by freeze-thaw technique. Mater. Chem. Phys. 2018, 216, 14–21. [Google Scholar] [CrossRef]
- Siangsanoh, C.; Ummartyotin, S.; Sathirakul, K.; Rojanapanthu, P.; Treesuppharat, W. Fabrication and characterization of triple-responsive composite hydrogel for targeted and controlled drug delivery system. J. Mol. Liq. 2018, 256, 90–99. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Li, X.; Wen, P.; Zhang, Y.; Long, Y.; Wang, X.; Guo, Y.; Xing, F.; Gao, J. Preparation of aligned porous gelatin scaffolds by unidirectional freeze-drying method. Acta Biomater. 2010, 6, 1167–1177. [Google Scholar] [CrossRef]
- Claaßen, C.; Claaßen, M.H.; Truffault, V.; Sewald, L.; Tovar, G.E.M.; Borchers, K.; Southan, A. Quantification of Substitution of Gelatin Methacryloyl: Best Practice and Current Pitfalls. Biomacromolecules 2018, 19, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Smeds, K.A.; Pfister-Serres, A.; Hatchell, D.L.; Grinstaff, M.W. Synthesis of a novel polysaccharide hydrogel. J. Macromol. Sci. Pure Appl. Chem. 1999, 36, 981–989. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Siegwart, D.J.; Srinivasan, A.; Horkay, F.; Hollinger, J.O.; Washburn, N.R.; Matyjaszewski, K. Nanostructured hybrid hydrogels prepared by a combination of atom transfer radical polymerization and free radical polymerization. Biomaterials 2009, 30, 5270–5278. [Google Scholar] [CrossRef] [Green Version]
- Ho, S.T.; Hutmacher, D.W. A comparison of micro CT with other techniques used in the characterization of scaffolds. Biomaterials 2006, 27, 1362–1376. [Google Scholar] [CrossRef] [PubMed]



| Samples | Gel Fraction (%) | E (kPa) | Density (g/mL) | εMIP (%) | MIP—Mean Pore Diameter (μm) |
|---|---|---|---|---|---|
| 1% HAMA | 100 ± 1 | 1.0 ± 0.2 | 0.065 ± 0.004 | 94 ± 2 | 89 ± 12 |
| 3% HAMA | 100 ± 1 | 6.6 ± 0.9 | 0.072 ± 0.006 | 75 ± 3 | 54 ± 10 |
| 5% HAMA | 100 ± 1 | 12.4 ± 2.1 | 0.084 ± 0.006 | 65 ± 1 | 32 ± 6 |
| 2% GelMA | 70 ± 6 | 0.8 ± 0.1 | 0.072 ± 0.005 | 89 ± 2 | 26 ± 4 |
| 6% GelMA | 79 ± 3 | 2.1 ± 0.3 | 0.105 ± 0.006 | 77 ± 3 | 17 ± 3 |
| 10% GelMA | 79 ± 1 | 3.1 ± 0.5 | 0.157 ± 0.008 | 67 ± 4 | 11 ± 2 |
| 2% GelMA-1% HAMA | 71 ± 5 | 2.0 ± 0.2 | 0.070 ± 0.002 | 91 ± 1 | 31 ± 4 |
| 2% GelMA-5% HAMA | 80 ± 2 | 12.8 ± 1.9 | 0.079 ± 0.003 | 76 ± 2 | 19 ± 3 |
| 6% GelMA-1% HAMA | 83 ± 3 | 5.6 ± 1.1 | 0.101 ± 0.006 | 81± 4 | 22 ± 5 |
| 6% GelMA-5% HAMA | 87 ± 2 | 9.0 ± 1.2 | 0.103 ± 0.004 | 74 ± 4 | 20 ± 2 |
| 10% GelMA-1% HAMA | 91 ± 6 | 9.5 ± 1.7 | 0.149 ± 0.009 | 68 ± 5 | 13 ± 3 |
| 10% GelMA-5% HAMA | 95 ± 4 | 18.3 ± 2.4 | 0.192 ± 0.013 | 54 ± 6 | 8 ± 2 |
| Scaffolds | k | n |
|---|---|---|
| 1% HAMA | 0.80 ± 0.09 | 0.22 ± 0.07 |
| 3% HAMA | 0.74 ± 0.06 | 0.23 ± 0.05 |
| 5% HAMA | 0.72 ± 0.03 | 0.29 ± 0.02 |
| 2% GelMA | 0.70 ± 0.07 | 0.28 ± 0.05 |
| 6% GelMA | 0.65 ± 0.05 | 0.30 ± 0.04 |
| 10% GelMA | 0.51 ± 0.01 | 0.31 ± 0.01 |
| 2% GelMA-1% HAMA | 0.69 ± 0.11 | 0.29 ± 0.08 |
| 2% GelMA-5% HAMA | 0.62 ± 0.07 | 0.35 ± 0.06 |
| 6% GelMA-1% HAMA | 0.70 ± 0.10 | 0.25 ± 0.08 |
| 6% GelMA-5% HAMA | 0.66 ± 0.04 | 0.29 ± 0.05 |
| 10% GelMA-1% HAMA | 0.77 ± 0.08 | 0.25 ± 0.09 |
| 10% GelMA-5% HAMA | 0.67 ± 0.02 | 0.35 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velasco-Rodriguez, B.; Diaz-Vidal, T.; Rosales-Rivera, L.C.; García-González, C.A.; Alvarez-Lorenzo, C.; Al-Modlej, A.; Domínguez-Arca, V.; Prieto, G.; Barbosa, S.; Soltero Martínez, J.F.A.; et al. Hybrid Methacrylated Gelatin and Hyaluronic Acid Hydrogel Scaffolds. Preparation and Systematic Characterization for Prospective Tissue Engineering Applications. Int. J. Mol. Sci. 2021, 22, 6758. https://doi.org/10.3390/ijms22136758
Velasco-Rodriguez B, Diaz-Vidal T, Rosales-Rivera LC, García-González CA, Alvarez-Lorenzo C, Al-Modlej A, Domínguez-Arca V, Prieto G, Barbosa S, Soltero Martínez JFA, et al. Hybrid Methacrylated Gelatin and Hyaluronic Acid Hydrogel Scaffolds. Preparation and Systematic Characterization for Prospective Tissue Engineering Applications. International Journal of Molecular Sciences. 2021; 22(13):6758. https://doi.org/10.3390/ijms22136758
Chicago/Turabian StyleVelasco-Rodriguez, B., T. Diaz-Vidal, L. C. Rosales-Rivera, C. A. García-González, C. Alvarez-Lorenzo, A. Al-Modlej, V. Domínguez-Arca, G. Prieto, S. Barbosa, J. F. A. Soltero Martínez, and et al. 2021. "Hybrid Methacrylated Gelatin and Hyaluronic Acid Hydrogel Scaffolds. Preparation and Systematic Characterization for Prospective Tissue Engineering Applications" International Journal of Molecular Sciences 22, no. 13: 6758. https://doi.org/10.3390/ijms22136758
APA StyleVelasco-Rodriguez, B., Diaz-Vidal, T., Rosales-Rivera, L. C., García-González, C. A., Alvarez-Lorenzo, C., Al-Modlej, A., Domínguez-Arca, V., Prieto, G., Barbosa, S., Soltero Martínez, J. F. A., & Taboada, P. (2021). Hybrid Methacrylated Gelatin and Hyaluronic Acid Hydrogel Scaffolds. Preparation and Systematic Characterization for Prospective Tissue Engineering Applications. International Journal of Molecular Sciences, 22(13), 6758. https://doi.org/10.3390/ijms22136758









