Phototoxicities Caused by Continuous Light Exposure Were Not Induced in Retinal Ganglion Cells Transduced by an Optogenetic Gene
Abstract
1. Introduction
2. Results
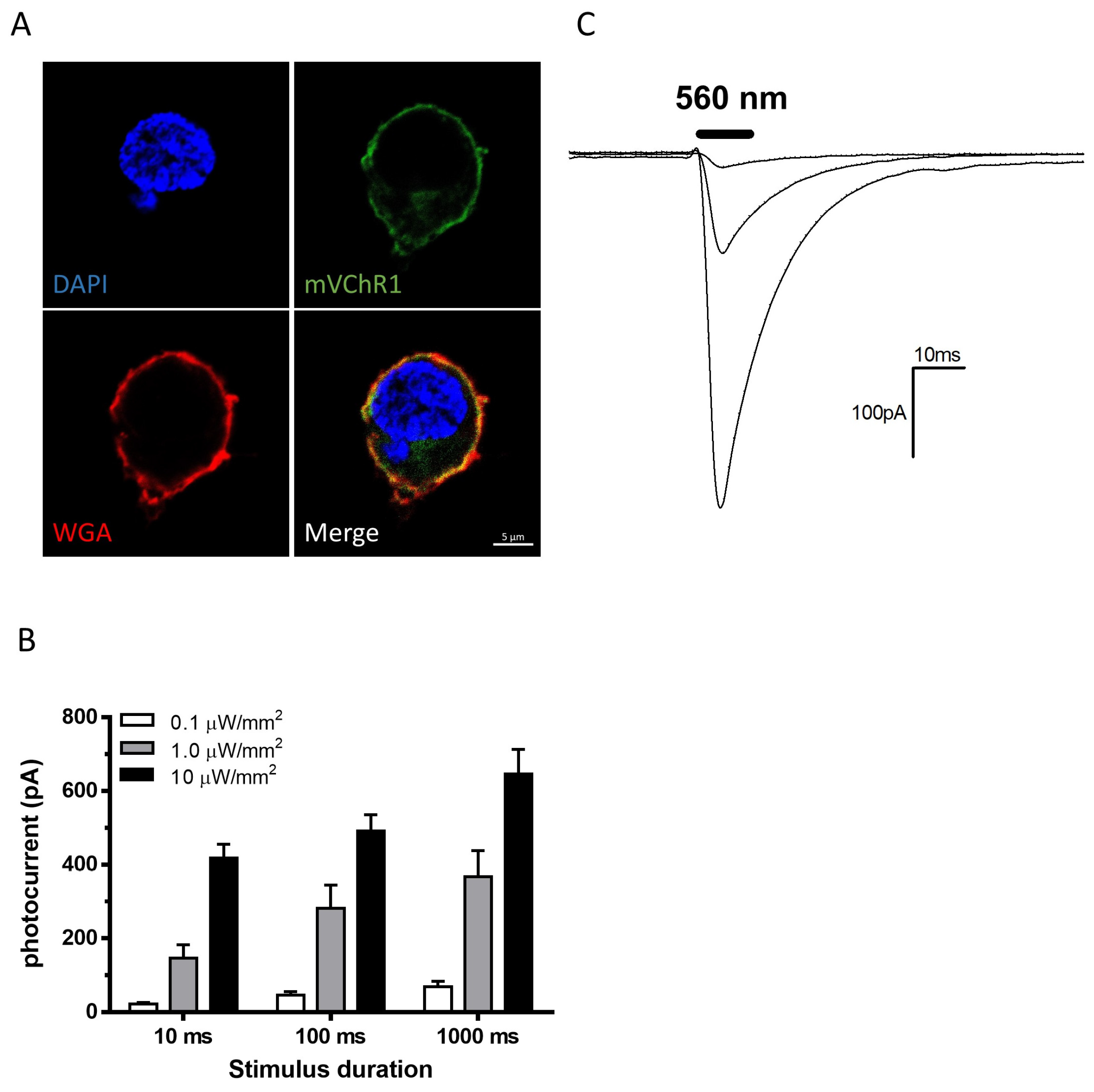
2.1. Patch Clamp Recordings
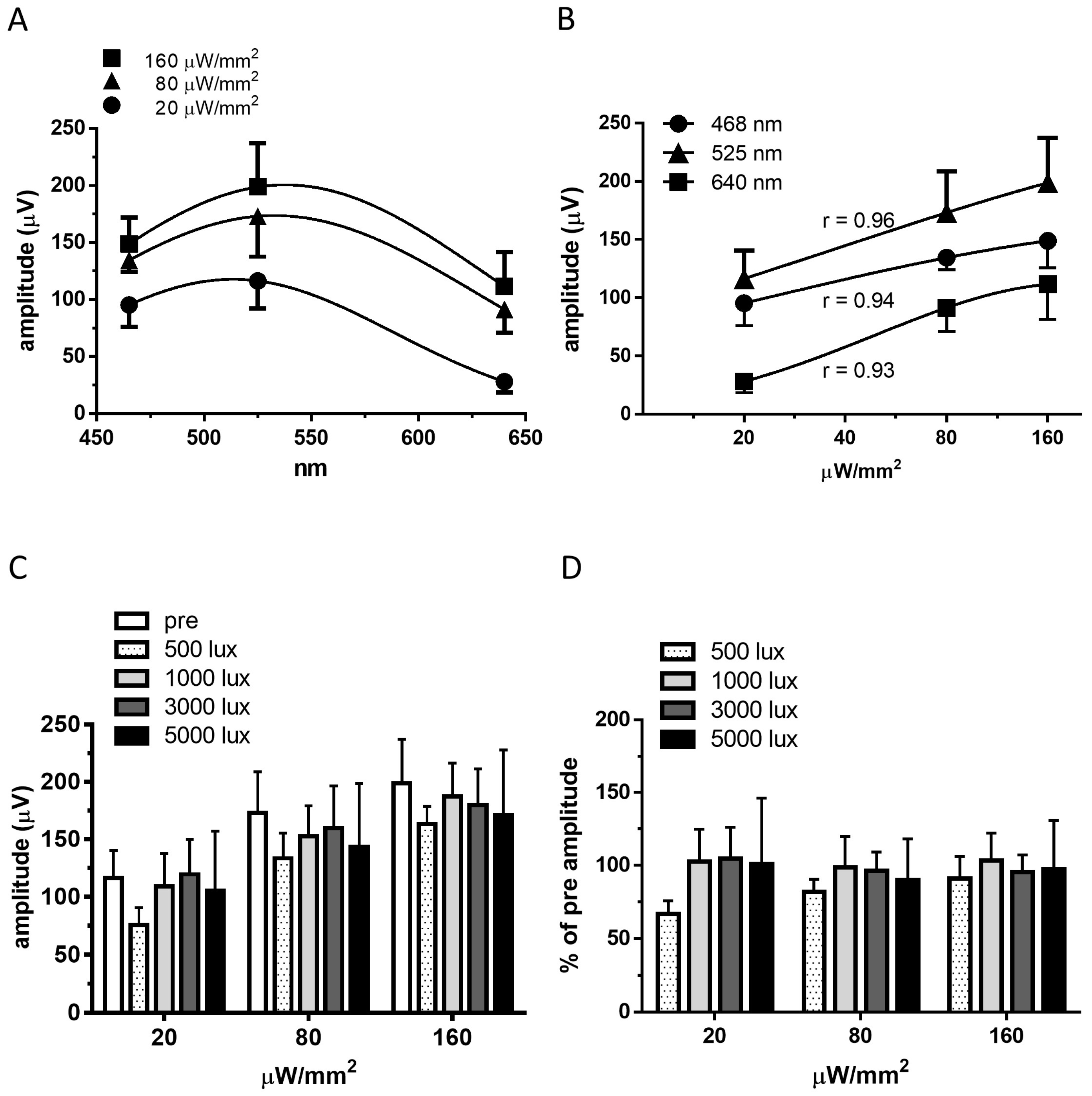
2.2. Recordings of Visually Evoked Potentials
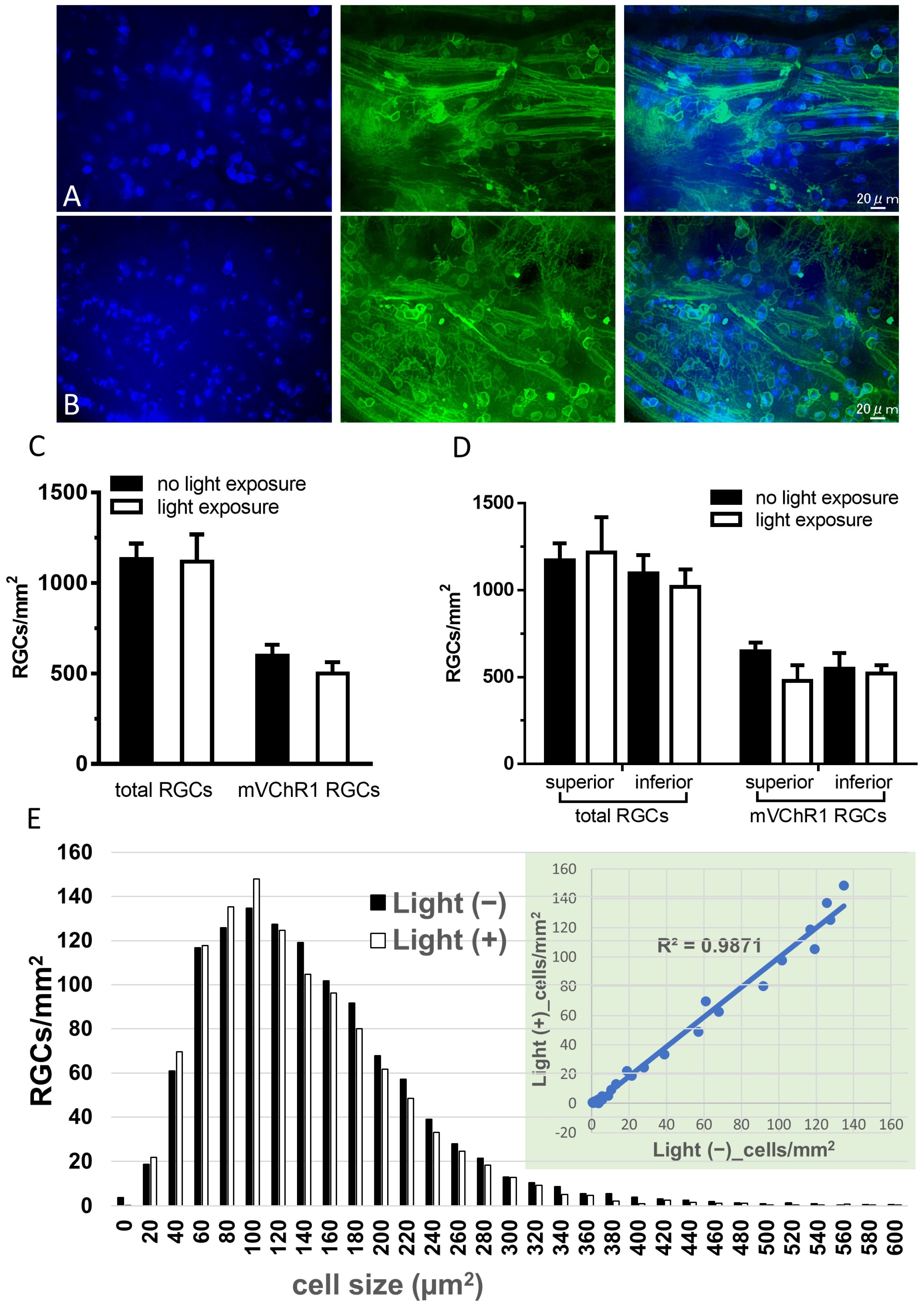
2.3. Number of RGCs
3. Discussion
4. Materials and Methods
4.1. mVChR1 Gene Transduction into HEK293 Cells
4.2. Patch Clamp Recordings
4.3. Animals
4.4. Preparation of the AAV Vector
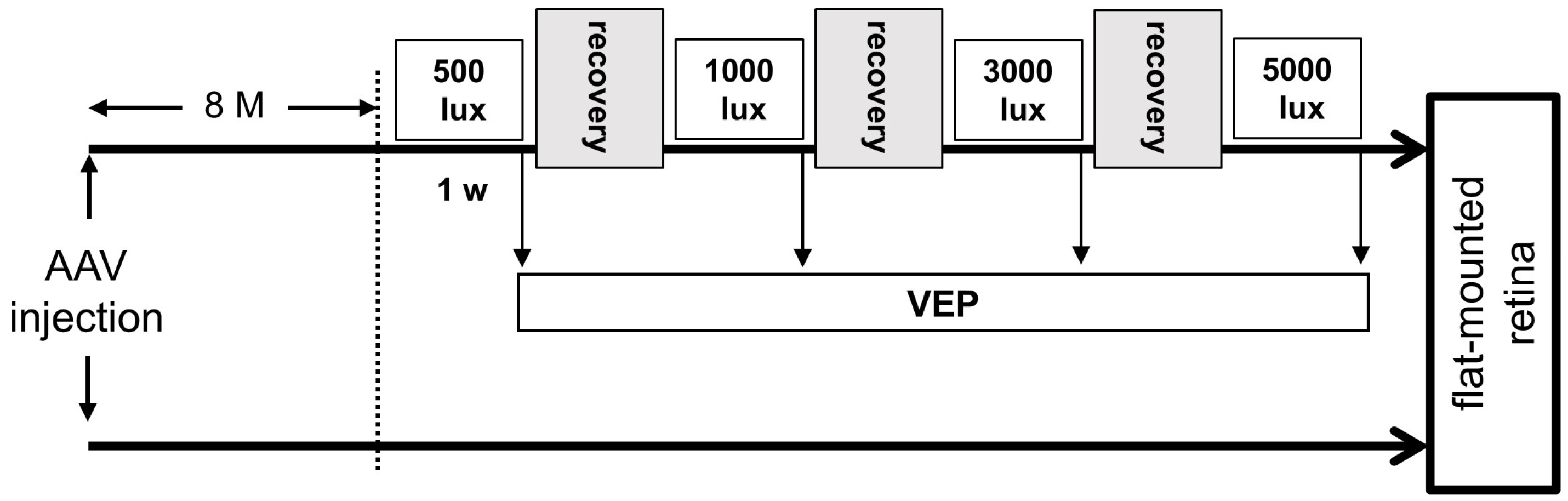
4.5. Intravitreal Injection of AAV
4.6. Light Exposure
4.7. Recording VEPs
4.8. Preparations of Whole Mounted Retina and Retinal Section
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noell, W.K.; Walker, V.S.; Kang, B.S.; Berman, S. Retinal damage by light in rats. Investig. Ophthalmol. 1966, 5, 450–473. [Google Scholar]
- Noell, W.K.; Albrecht, R. Irreversible effects on visible light on the retina: Role of vitamin A. Science 1971, 172, 76–79. [Google Scholar] [CrossRef]
- Humphries, M.M.; Rancourt, D.; Farrar, G.J.; Kenna, P.; Hazel, M.; Bush, R.A.; Sieving, P.A.; Sheils, D.M.; McNally, N.; Creighton, P.; et al. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat. Genet. 1997, 15, 216–219. [Google Scholar] [CrossRef]
- Grimm, C.; Wenzel, A.; Hafezi, F.; Yu, S.; Redmond, T.M.; Remé, C.E. Protection of Rpe65-deficient mice identifies rhodopsin as a mediator of light-induced retinal degeneration. Nat. Genet. 2000, 25, 63–66. [Google Scholar] [CrossRef]
- Organisciak, D.T.; Wang, H.M.; Li, Z.Y.; Tso, M.O. The protective effect of ascorbate in retinal light damage of rats. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1580–1588. [Google Scholar]
- Tomita, H.; Kotake, Y.; Anderson, R.E. Mechanism of protection from light-induced retinal degeneration by the synthetic antioxidant phenyl-N-tert-butylnitrone. Investig. Ophthalmol. Vis. Sci. 2005, 46, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Li, F.; Elliott, M.H.; Dittmar, M.; Anderson, R.E. Protective effect of TEMPOL derivatives against light-induced retinal damage in rats. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1900–1905. [Google Scholar] [CrossRef] [PubMed]
- Bi, A.; Cui, J.; Ma, Y.P.; Olshevskaya, E.; Pu, M.; Dizhoor, A.M.; Pan, Z.H. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron 2006, 50, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Sugano, E.; Yawo, H.; Ishizuka, T.; Isago, H.; Narikawa, S.; Kügler, S.; Tamai, M. Restoration of visual response in aged dystrophic RCS rats using AAV-mediated channelopsin-2 gene transfer. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3821–3826. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Sugano, E.; Fukazawa, Y.; Isago, H.; Sugiyama, Y.; Hiroi, T.; Ishizuka, T.; Mushiake, H.; Kato, M.; Hirabayashi, M.; et al. Visual properties of transgenic rats harboring the channelrhodopsin-2 gene regulated by the thy-1.2 promoter. PLoS ONE 2009, 4, e7679. [Google Scholar] [CrossRef]
- Tomita, H.; Sugano, E.; Isago, H.; Hiroi, T.; Wang, Z.; Ohta, E.; Tamai, M. Channelrhodopsin-2 gene transduced into retinal ganglion cells restores functional vision in genetically blind rats. Exp. Eye Res. 2010, 90, 429–436. [Google Scholar] [CrossRef]
- Sugano, E.; Isago, H.; Wang, Z.; Murayama, N.; Tamai, M.; Tomita, H. Immune responses to adeno-associated virus type 2 encoding channelrhodopsin-2 in a genetically blind rat model for gene therapy. Gene Ther. 2011, 18, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Sugano, E.; Tabata, K.; Takahashi, M.; Nishiyama, F.; Shimizu, H.; Sato, M.; Tamai, M.; Tomita, H. Local and systemic responses following intravitreous injection of AAV2-encoded modified Volvox channelrhodopsin-1 in a genetically blind rat model. Gene Ther. 2016, 23, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Sugano, E.; Murayama, N.; Ozaki, T.; Nishiyama, F.; Tabata, K.; Takahashi, M.; Saito, T.; Tamai, M. Restoration of the majority of the visual spectrum by using modified Volvox channelrhodopsin-1. Mol. Ther. 2014, 22, 1434–1440. [Google Scholar] [CrossRef]
- Klapoetke, N.C.; Murata, Y.; Kim, S.S.; Pulver, S.R.; Birdsey-Benson, A.; Cho, Y.K.; Morimoto, T.K.; Chuong, A.S.; Carpenter, E.J.; Tian, Z.; et al. Independent optical excitation of distinct neural populations. Nat. Methods 2014, 11, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Sahel, J.A.; Boulanger-Scemama, E.; Pagot, C.; Arleo, A.; Galluppi, F.; Martel, J.N.; Esposti, S.D.; Delaux, A.; de Saint Aubert, J.B.; de Montleau, C.; et al. Partial recovery of visual function in a blind patient after optogenetic therapy. Nat. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- García-Ayuso, D.; Salinas-Navarro, M.; Nadal-Nicolás, F.M.; Ortín-Martínez, A.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Villegas-Pérez, M.P. Sectorial loss of retinal ganglion cells in inherited photoreceptor degeneration is due to RGC death. Br. J. Ophthalmol. 2014, 98, 396–401. [Google Scholar] [CrossRef][Green Version]
- Ranchon, I.; Chen, S.; Alvarez, K.; Anderson, R.E. Systemic administration of phenyl-N-tert-butylnitrone protects the retina from light damage. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1375–1379. [Google Scholar] [PubMed]
- Ishizawa, Y.; Sharp, R.; Liebman, P.A.; Eckenhoff, R.G. Halothane binding to a G protein coupled receptor in retinal membranes by photoaffinity labeling. Biochemistry 2000, 39, 8497–8502. [Google Scholar] [CrossRef]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-Timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef]
- Maeda, A.; Maeda, T.; Golczak, M.; Chou, S.; Desai, A.; Hoppel, C.L.; Matsuyama, S.; Palczewski, K. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J. Biol. Chem. 2009, 284, 15173–15183. [Google Scholar] [CrossRef]
- Kim, S.R.; Jang, Y.P.; Jockusch, S.; Fishkin, N.E.; Turro, N.J.; Sparrow, J.R. The all-trans-retinal dimer series of lipofuscin pigments in retinal pigment epithelial cells in a recessive Stargardt disease model. Proc. Natl Acad. Sci. USA 2007, 104, 19273–19278. [Google Scholar] [CrossRef]
- Mata, N.L.; Weng, J.; Travis, G.H. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc. Natl Acad. Sci. USA 2000, 97, 7154–7159. [Google Scholar] [CrossRef]
- Boycott, B.B.; Wässle, H. The morphological types of ganglion cells of the domestic cat’s retina. J. Physiol. 1974, 240, 397–419. [Google Scholar] [CrossRef]
- Citron, M.C.; Emerson, R.C.; Levick, W.R. Nonlinear measurement and classification of receptive fields in cat retinal ganglion cells. Ann. Biomed. Eng. 1988, 16, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Dacey, D.M. Morphology of a small-field bistratified ganglion cell type in the macaque and human retina. Vis. Neurosci. 1993, 10, 1081–1098. [Google Scholar] [CrossRef] [PubMed]
- Peichl, L. Alpha ganglion cells in mammalian retinae: Common properties, species differences, and some comments on other ganglion cells. Vis. Neurosci. 1991, 7, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Enroth-Cugell, C.; Robson, J.G. The contrast sensitivity of retinal ganglion cells of the cat. J. Physiol. 1966, 187, 517–552. [Google Scholar] [CrossRef]
- Huxlin, K.R.; Goodchild, A.K. Retinal ganglion cells in the albino rat: Revised morphological classification. J. Comp. Neurol. 1997, 385, 309–323. [Google Scholar] [CrossRef]
- Sun, W.; Li, N.; He, S. Large-Scale morophological survey of rat retinal ganglion cells. Vis. Neurosci. 2002, 19, 483–493. [Google Scholar] [CrossRef]
- Santos, A.; Humayun, M.S.; de Juan, E., Jr.; Greenburg, R.J.; Marsh, M.J.; Klock, I.B.; Milam, A.H. Preservation of the inner retina in retinitis pigmentosa. A morphometric analysis. Arch. Ophthalmol. 1997, 115, 511–515. [Google Scholar] [CrossRef]
- Stone, J.L.; Barlow, W.E.; Humayun, M.S.; de Juan, E., Jr.; Milam, A.H. Morphometric analysis of macular photoreceptors and ganglion cells in retinas with retinitis pigmentosa. Arch. Ophthalmol. 1992, 110, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Isago, H.; Sugano, E.; Wang, Z.; Murayama, N.; Koyanagi, E.; Tamai, M.; Tomita, H. Age-Dependent differences in recovered visual responses in Royal College of Surgeons rats transduced with the Channelrhodopsin-2 gene. J. Mol. Neurosci. 2012, 46, 393–400. [Google Scholar] [CrossRef]
- Lin, B.; Peng, E.B. Retinal ganglion cells are resistant to photoreceptor loss in retinal degeneration. PLoS ONE 2013, 8, e68084. [Google Scholar] [CrossRef] [PubMed]
- Pérez de Sevilla Müller, L.; Sargoy, A.; Rodriguez, A.R.; Brecha, N.C. Melanopsin ganglion cells are the most resistant retinal ganglion cell type to axonal injury in the rat retina. PLoS ONE 2014, 9, e93274. [Google Scholar] [CrossRef]
- Sun, W.; Li, N.; He, S. Large-Scale morphological survey of mouse retinal ganglion cells. J. Comp. Neurol. 2002, 451, 115–126. [Google Scholar] [CrossRef]
- Graham, F.L.; van der Eb, A.J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 1973, 52, 456–467. [Google Scholar] [CrossRef]
- Watanabe, Y.; Sugano, E.; Tabata, K.; Ozaki, T.; Saito, T.; Tamai, M.; Tomita, H. Kinetic profiles of photocurrents in cells expressing two types of channelrhodopsin genes. Biochem. Biophys. Res. Commun. 2018, 496, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sugiyama, Y.; Hikima, T.; Sugano, E.; Tomita, H.; Takahashi, T.; Ishizuka, T.; Yawo, H. Molecular determinants differentiating photocurrent properties of two channelrhodopsins from chlamydomonas. J. Biol. Chem. 2009, 284, 5685–5696. [Google Scholar] [CrossRef]
- Sugano, E.; Tomita, H.; Ishiguro, S.; Abe, T.; Tamai, M. Establishment of effective methods for transducing genes into iris pigment epithelial cells by using adeno-associated virus type 2. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabata, K.; Sugano, E.; Hatakeyama, A.; Watanabe, Y.; Suzuki, T.; Ozaki, T.; Fukuda, T.; Tomita, H. Phototoxicities Caused by Continuous Light Exposure Were Not Induced in Retinal Ganglion Cells Transduced by an Optogenetic Gene. Int. J. Mol. Sci. 2021, 22, 6732. https://doi.org/10.3390/ijms22136732
Tabata K, Sugano E, Hatakeyama A, Watanabe Y, Suzuki T, Ozaki T, Fukuda T, Tomita H. Phototoxicities Caused by Continuous Light Exposure Were Not Induced in Retinal Ganglion Cells Transduced by an Optogenetic Gene. International Journal of Molecular Sciences. 2021; 22(13):6732. https://doi.org/10.3390/ijms22136732
Chicago/Turabian StyleTabata, Kitako, Eriko Sugano, Akito Hatakeyama, Yoshito Watanabe, Tomoya Suzuki, Taku Ozaki, Tomokazu Fukuda, and Hiroshi Tomita. 2021. "Phototoxicities Caused by Continuous Light Exposure Were Not Induced in Retinal Ganglion Cells Transduced by an Optogenetic Gene" International Journal of Molecular Sciences 22, no. 13: 6732. https://doi.org/10.3390/ijms22136732
APA StyleTabata, K., Sugano, E., Hatakeyama, A., Watanabe, Y., Suzuki, T., Ozaki, T., Fukuda, T., & Tomita, H. (2021). Phototoxicities Caused by Continuous Light Exposure Were Not Induced in Retinal Ganglion Cells Transduced by an Optogenetic Gene. International Journal of Molecular Sciences, 22(13), 6732. https://doi.org/10.3390/ijms22136732






