Molecular Characterisation of Uterine Endometrial Proteins during Early Stages of Pregnancy in Pigs by MALDI TOF/TOF
Abstract
1. Introduction
2. Results
2.1. Identification of Uterine Endometrial DEPs during the Preimplantation and Peri-Implantation Period
2.2. Network Interaction of Identified DEPs during the Preimplantation and Peri-Implantation Period and Their Involvement in the Biological Pathways and Molecular Processes
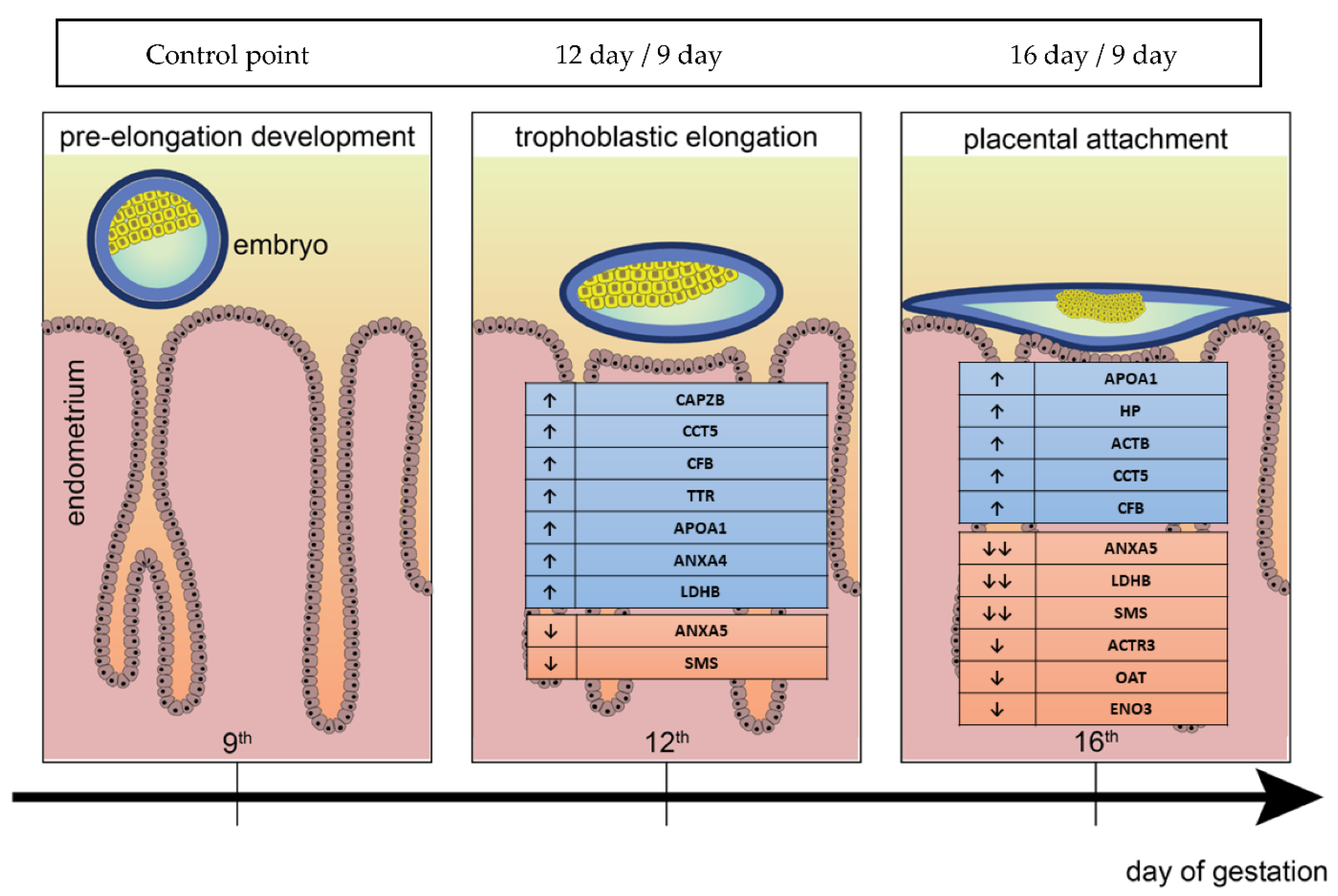
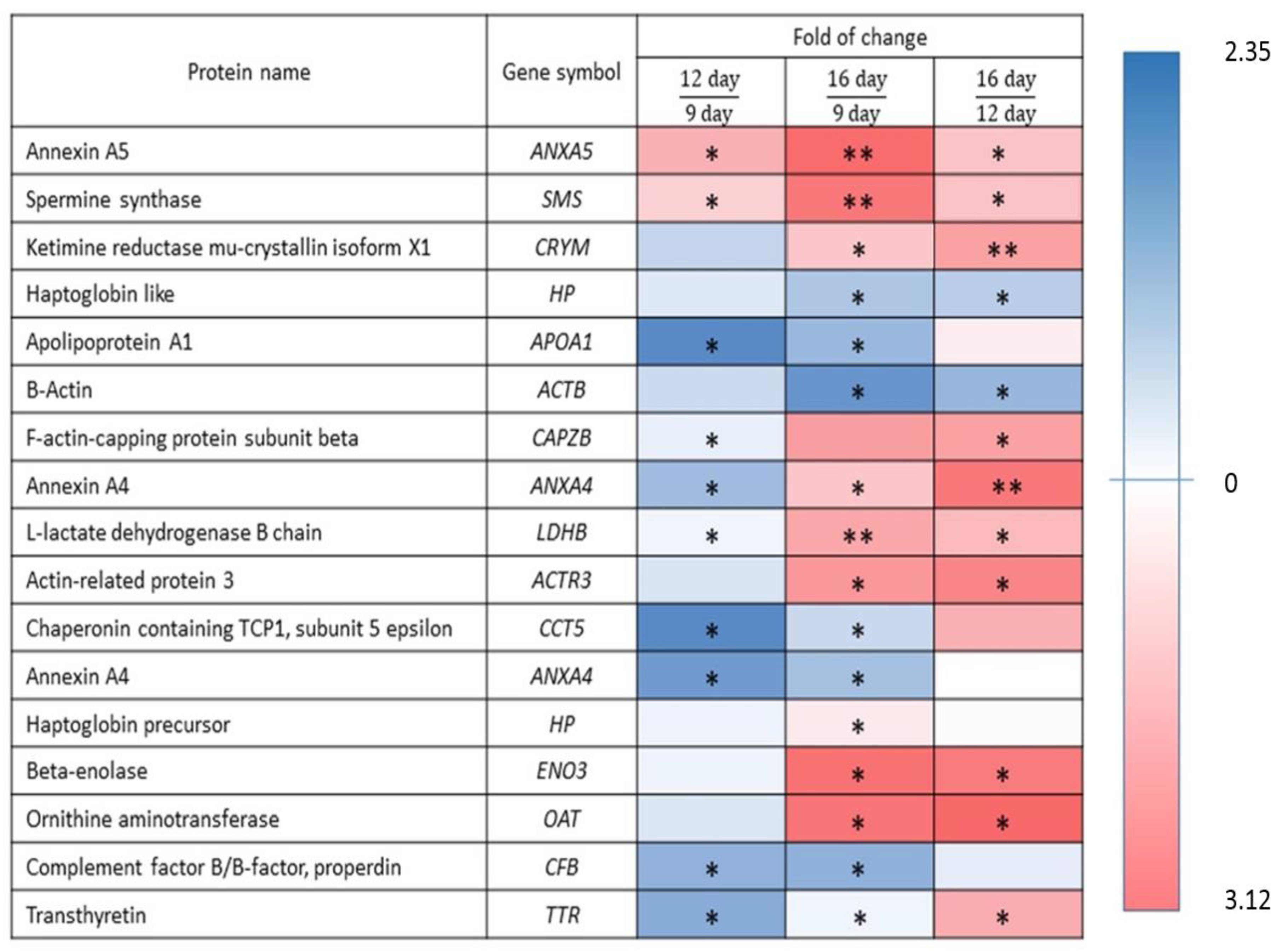
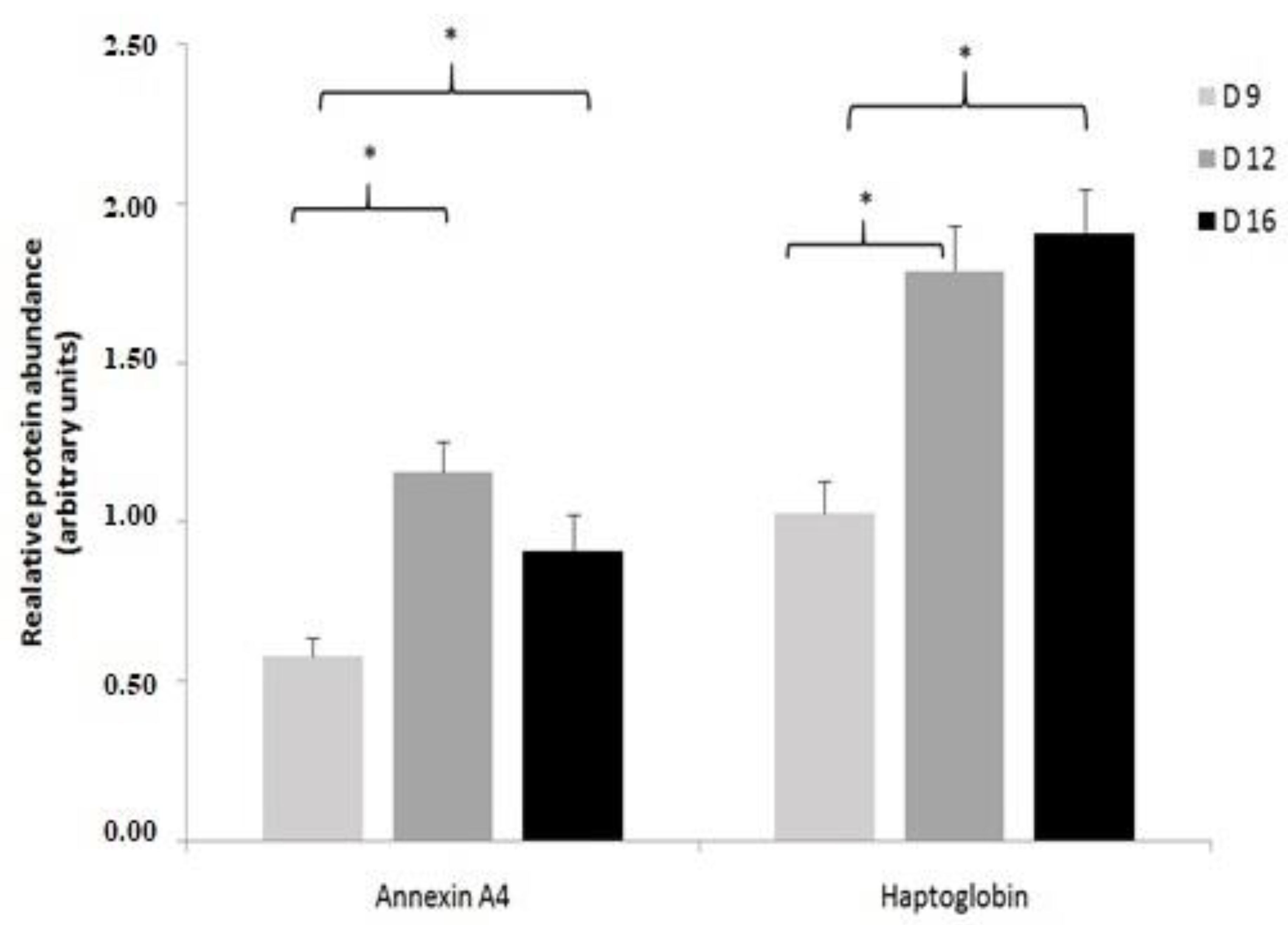
2.3. Validation of Identified Uterine Endometrial DEPs during the Preimplantation and Peri-Implantation Period
3. Discussion
3.1. Molecular Characterisation of Upregulated DEPs during 9D, 12D and 16D of Pregnancy
3.2. Molecular Characterisation of Down-Regulated DEPs during 9D, 12D and 16D of Pregnancy
3.3. Protein Networks Interaction—Molecular Processes
4. Materials and Methods
4.1. Protein Extraction
4.2. Protein Identification
4.3. Validation of Endometrial Proteins Using Western Blotting
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Chae, J.I.; Kim, J.; Lee, S.G.; Jeon, Y.J.; Kim, D.W.; Soh, Y.; Seo, K.S.; Lee, H.K.; Choi, N.J.; Ryu, J.; et al. Proteomic analysis of pregnancy-related proteins from pig uterus endometrium during pregnancy. Proteome Sci. 2011, 9, 41. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, H.; Kong, S.; Wang, S.; Wang, H.; Wang, H.; Armant, D.R. Physiological and molecular determinants of embryo implantation. Mol. Asp. Med. 2013, 34, 939–980. [Google Scholar] [CrossRef] [PubMed]
- Pierzchała, M.; Pierzchała, D.; Ogłuszka, M.; Poławska, E.; Blicharski, T.; Roszczyk, A.; Nawrocka, A.; Urbański, P.; Stepanow, K.; Ciepłoch, A.; et al. Identification of Differentially Expressed Gene Transcripts in Porcine Endometrium during Early Stages of Pregnancy. Life 2020, 10, 68. [Google Scholar] [CrossRef]
- Massimiani, M.; Lacconi, V.; La Civita, F.; Ticconi, C.; Rago, R.; Campagnolo, L. Molecular Signaling Regulating Endometrium–Blastocyst Crosstalk. Int. J. Mol. Sci. 2019, 21, 23. [Google Scholar] [CrossRef]
- Drzewiecka, E.; Kozlowska, W.; Zmijewska, A.; Wydorski, P.; Franczak, A. Electromagnetic Field (EMF) Radiation Alters Estrogen Release from the Pig Myometrium during the Peri-Implantation Period. Int. J. Mol. Sci. 2021, 22, 2920. [Google Scholar] [CrossRef]
- Kaczmarek, M.M.; Najmula, J.; Guzewska, M.M.; Przygrodzka, E. MiRNAs in the Peri-Implantation Period: Contribution to Embryo–Maternal Communication in Pigs. Int. J. Mol. Sci. 2020, 21, 2229. [Google Scholar] [CrossRef]
- Samborski, A.; Graf, A.; Krebs, S.; Kessler, B.; Bauersachs, S. Deep Sequencing of the Porcine Endometrial Transcriptome on Day 14 of Pregnancy1. Biol. Reprod. 2013, 88, 84. [Google Scholar] [CrossRef]
- Vallet, J.L.; Christenson, R.K.; Trout, W.E.; Klemcke, H.G. Conceptus, progesterone, and breed effects on uterine protein secretion in swine. J. Anim. Sci. 1998, 76, 2657–2670. [Google Scholar] [CrossRef] [PubMed]
- Salamonsen, L.A.; Edgell, T.; Rombauts, L.J.; Stephens, A.N.; Robertson, D.M.; Rainczuk, A.; Nie, G.; Hannan, N. Proteomics of the human endometrium and uterine fluid: A pathway to biomarker discovery. Fertil. Steril. 2013, 99, 1086–1092. [Google Scholar] [CrossRef]
- Kim, S.-M.; Kim, J.-S. A Review of Mechanisms of Implantation. Dev. Reprod. 2017, 21, 351–359. [Google Scholar] [CrossRef]
- Ziecik, A.J.; Waclawik, A.; Kaczmarek, M.M.; Blitek, A.; Jalali, B.M.; Andronowska, A. Mechanisms for the Establishment of Pregnancy in the Pig. Reprod. Domest. Anim. 2011, 46, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Geisert, R.D.; Schmitt, R.A.M. Early embryonic survival in the pig: Can it be improved? J. Anim. Sci. 2002, 80, E54–E65. [Google Scholar]
- Chen, T.Y.; Stott, P.G.; Athorn, R.Z.; Bouwman, E.G.; Langendijk, P. Undernutrition during early follicle development has irreversible effects on ovulation rate and embryos. Reprod. Fertil. Dev. 2012, 24, 886. [Google Scholar] [CrossRef]
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.K.; Wessels, J.M.; Kerr, A.; Tayade, C. An Overview of Molecular and Cellular Mechanisms Associated with Porcine Pregnancy Success or Failure. Reprod. Dom. Anim. 2012, 47, 394–401. [Google Scholar] [CrossRef]
- Sharkey, A.M.; Smith, S.K. The endometrium as a cause of implantation failure. Best Pract. Res. Clin. Obstet. Gynaecol. 2003, 17, 289–307. [Google Scholar] [CrossRef]
- Buca, D.; Bologna, G.; D’Amico, A.; Cugini, S.; Musca, F.; Febbo, M.; D’Arcangelo, D.; Buca, D.; Simeone, P.; Liberati, M.; et al. Extracellular Vesicles in Feto–Maternal Crosstalk and Pregnancy Disorders. Int. J. Mol. Sci. 2020, 21, 2120. [Google Scholar] [CrossRef]
- Duley, L. The Global Impact of Pre-eclampsia and Eclampsia. Semin. Perinatol. 2009, 33, 130–137. [Google Scholar] [CrossRef]
- Kolakowska, J.; Souchelnytskyi, S.; Saini, R.K.R.; Franczak, A. Proteomic analysis of the endometrium during early pregnancy in the domestic pig. Reprod. Fertil. Dev. 2017, 29, 2255–2268. [Google Scholar] [CrossRef]
- Jalali, B.M.; Bogacki, M.; Dietrich, M.; Likszo, P.; Wasielak, M. Proteomic analysis of porcine endometrial tissue during peri-implantation period reveals altered protein abundance. J. Proteom. 2015, 125, 76–88. [Google Scholar] [CrossRef]
- Wu, P.; Berg, C.V.D.; Alfirevic, Z.; O’Brien, S.; Röthlisberger, M.; Baker, P.N.; Kenny, L.C.; Kublickiene, K.; Duvekot, J.J. Early Pregnancy Biomarkers in Pre-Eclampsia: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2015, 16, 23035–23056. [Google Scholar] [CrossRef]
- Tayade, C.; Fang, Y.; Croy, B.A. A review of gene expression in porcine endometrial lymphocytes, endothelium and trophoblast during pregnancy success and failure. J. Reprod. Dev. 2007, 53, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Wang, X.; Johnson, G.A.; Wu, G. Select nutrients and their effects on conceptus development in mammals. Anim. Nutr. 2015, 1, 85–95. [Google Scholar] [CrossRef]
- Riding, G.A.; Jones, A.; Holland, M.K.; Hill, J.R.; Lehnert, S.A. Proteomic analysis of bovine conceptus fluids during early pregnancy. Proteomics 2008, 8, 160–177. [Google Scholar] [CrossRef] [PubMed]
- Kayser, J.-P.R.; Kim, J.G.; Cerny, R.L.; Vallet, J.L. Global characterization of porcine intrauterine proteins during early pregnancy. Reproduction 2006, 131, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Casado-Vela, J.; Rodriguez-Suarez, E.; Iloro, I.; Ametzazurra, A.; Alkorta, N.; García-Velasco, J.A.; Matorras, R.; Prieto, B.; González, S.; Nagore, D.; et al. Comprehensive Proteomic Analysis of Human Endometrial Fluid Aspirate. J. Proteome Res. 2009, 8, 4622–4632. [Google Scholar] [CrossRef] [PubMed]
- Wessels, J.M.; Linton, N.F.; Croy, B.A.; Tayade, C. A review of molecular contrasts between arresting and viable porcine attachment sites. Am. J. Reprod. Immunol. 2007, 58, 470–480. [Google Scholar] [CrossRef]
- Forde, N.; Bazer, F.W.; Spencer, T.E.; Lonergan, P. ‘Conceptualizing’ the Endometrium: Identification of Conceptus-Derived Proteins during Early Pregnancy in Cattle1. Biol. Reprod. 2015, 92, 156. [Google Scholar] [CrossRef]
- Forde, N.; McGettigan, P.; Mehta, J.P.; O’Hara, L.; Mamo, S.; Bazer, F.W.; Spencer, T.E.; Lonergan, P. Proteomic analysis of uterine fluid during the pre-implantation period of pregnancy in cattle. Reproduction 2014, 147, 575–587. [Google Scholar] [CrossRef]
- Brooks, K.; Burns, G.W.; Moraes, J.G.; Spencer, T.E. Analysis of the Uterine Epithelial and Conceptus Transcriptome and Luminal Fluid Proteome during the Peri-Implantation Period of Pregnancy in Sheep. Biol. Reprod. 2016, 95, 88. [Google Scholar] [CrossRef]
- Smits, K.; Willems, S.; Van Steendam, K.; Van De Velde, M.; De Lange, V.; Ververs, C.; Roels, K.; Govaere, J.; Van Nieuwerburgh, F.; Peelman, L.; et al. Proteins involved in embryo-maternal interaction around the signalling of maternal recognition of pregnancy in the horse. Sci. Rep. 2018, 8, 5249. [Google Scholar] [CrossRef]
- Verma, P.; Nair, R.R.; Singh, S.; Rajender, S.; Khanna, A.; Jha, R.K.; Singh, K. High Level of APOA1 in Blood and Maternal Fetal Interface Is Associated with Early Miscarriage. Reprod. Sci. 2019, 26, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Brosens, J.J.; Hodgetts, A.; Feroze-Zaidi, F.; Sherwin, J.R.; Fusi, L.; Salker, M.S.; Higham, J.; Rose, G.L.; Kajihara, T.; Young, S.L.; et al. Proteomic analysis of endometrium from fertile and infertile patients suggests a role for apolipoprotein A-I in embryo implantation failure and endometriosis. Mol. Hum. Reprod. 2010, 16, 273–285. [Google Scholar] [CrossRef]
- Jain, C.V. Molecular Regulation of Trophoblast Survival during Placentation and Pathologies of Placental Insufficiency. 2016. Available online: https://digitalcommons.wayne.edu/oa_dissertations/1642 (accessed on 15 December 2015).
- Thulasiraman, V.; Yang, C.; Frydman, J. In vivo newly translated polypeptides are sequestered in a protected folding environment. EMBO J. 1999, 18, 85–95. [Google Scholar] [CrossRef]
- Yam, A.Y.; Xia, Y.; Lin, H.-T.J.; Burlingame, A.; Gerstein, M.; Frydman, J. Defining the TRiC/CCT interactome links chaperonin function to stabilization of newly made proteins with complex topologies. Nat. Struct. Mol. Biol. 2008, 15, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Regal, J.F.; Gilbert, J.S.; Burwick, R.M. The complement system and adverse pregnancy outcomes. Mol. Immunol. 2015, 67, 56–70. [Google Scholar] [CrossRef]
- Noris, M.; Remuzzi, G. Overview of Complement Activation and Regulation. Semin. Nephrol. 2013, 33, 479–492. [Google Scholar] [CrossRef]
- Moza Jalali, B.; Likszo, P.; Skarzynski, D.J. Proteomic and network analysis of pregnancy induced changes in the porcine endometrium on Day 12 of gestation. Mol. Reprod. Dev. 2016, 83, 827–841. [Google Scholar] [CrossRef] [PubMed]
- Hasty, L.A.; Brockman, W.W.; Lambris, J.D.; Lyttle, C.R. Hormonal Regulation of Complement Factor B in Human Endometrium. Am. J. Reprod. Immunol. 1993, 30, 63–67. [Google Scholar] [CrossRef]
- Vaiman, M.; Chardon, P.; Tothschild, M. Porcine major histocompatibility complex. Rev. Sci. Tech. 1998, 17, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Buske, B.; Brunsch, C.; Zeller, K.; Reinecke, P.; Brockmann, G. Analysis of properdin (BF) genotypes associated with litter size in a commercial pig cross population. J. Anim. Breed. Genet. 2005, 122, 259–263. [Google Scholar] [CrossRef]
- Kao, L.C.; Tulac, S.; Lobo, S.; Imani, B.; Yang, J.P.; Germeyer, A.; Osteen, K.; Taylor, R.N.; Lessey, B.A.; Giudice, L.C. Global gene profiling in human endometrium during the window of implantation. Endocrinology 2002, 143, 2119–2138. [Google Scholar] [CrossRef] [PubMed]
- Mirkin, S.; Arslan, M.; Churikov, D.; Corica, A.; Diaz, J.; Williams, S.; Bocca, S.; Oehninger, S. In search of candidate genes critically expressed in the human endometrium during the window of implantation. Hum. Reprod. 2005, 20, 2104–2117. [Google Scholar] [CrossRef]
- Ponnampalam, A.P.; Rogers, P.A. Cyclic changes and hormonal regulation of annexin IV mRNA and protein in human endometrium. Mol. Hum. Reprod. 2006, 12, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Greening, D.W.; Nguyen, H.P.; Evans, J.; Simpson, R.J.; Salamonsen, L. Modulating the endometrial epithelial proteome and secretome in preparation for pregnancy: The role of ovarian steroid and pregnancy hormones. J. Proteom. 2016, 144, 99–112. [Google Scholar] [CrossRef]
- Talbi, S.; Hamilton, A.E.; Vo, K.C.; Tulac, S.; Overgaard, M.T.; Dosiou, C.; Le Shay, N.; Nezhat, C.N.; Kempson, R.; Lessey, B.A.; et al. Molecular Phenotyping of Human Endometrium Distinguishes Menstrual Cycle Phases and Underlying Biological Processes in Normo-Ovulatory Women. Endocrinology 2006, 147, 1097–1121. [Google Scholar] [CrossRef]
- Riesewijk, A.; Martín, J.; Van Os, R.; Horcajadas, J.A.; Polman, J.; Pellicer, A.; Mosselman, S.; Simón, C. Gene expression profiling of human endometrial receptivity on days LH+2 versus LH+7 by microarray technology. Mol. Hum. Reprod. 2003, 9, 253–264. [Google Scholar] [CrossRef]
- Ponnampalam, A.P.; Weston, G.C.; Trajstman, A.C.; Susil, B.; Rogers, P.A. Molecular classification of human endometrial cycle stages by transcriptional profiling. Mol. Hum. Reprod. 2004, 10, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. Changes in the protein content of mouse uterine flushings during normal pregnancy and delayed implantation, and after ovariectomy and oestradiol administration. Reproduction 1977, 50, 29–36. [Google Scholar] [CrossRef]
- Beier, H.M.; Beier-Hellwig, K. Specific secretory protein of the female genital tract. Eur. J. Endocrinol. 1973, 74, S404–S425. [Google Scholar] [CrossRef] [PubMed]
- Mullur, R.; Liu, Y.-Y.; Brent, G.A. Thyroid Hormone Regulation of Metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Diao, H.; Xiao, S.; Cui, J.; Chun, J.; Xu, Y.; Ye, X. Progesterone receptor-mediated up-regulation of transthyretin in preimplantation mouse uterus. Fertil. Steril. 2010, 93, 2750–2753. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, C.; Zhang, Z. Transthyretin and Normal Human Pregnancy: Mini Review. Crit. Rev. Eukaryot. Gene Expr. 2016, 26, 273–277. [Google Scholar] [CrossRef]
- Flamini, M.I.; Fu, X.; Sanchez, A.M.; Giretti, M.S.; Garibaldi, S.; Goglia, L.; Pisaneschi, S.; Tosi, V.; Genazzani, A.R.; Simoncini, T. Effects of raloxifene on breast cancer cell migration and invasion through the actin cytoskeleton. J. Cell. Mol. Med. 2009, 13, 2396–2407. [Google Scholar] [CrossRef]
- Bunnell, T.M.; Burbach, B.J.; Shimizu, Y.; Ervasti, J.M. β-Actin specifically controls cell growth, migration, and the G-actin pool. Mol. Biol. Cell 2011, 22, 4047–4058. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.J.; Terry, V.; Shorey, C.D.; Murphy, C.R. Alterations in distribution of actin binding proteins in uterine stromal cells during decidualization in the rat. Cell Biol. Int. 1998, 22, 237–243. [Google Scholar] [CrossRef]
- Breuiller-Fouche, M.; Germain, G. Gene and protein expression in the myometrium in pregnancy and labor. Reproduction 2006, 131, 837–850. [Google Scholar] [CrossRef]
- Greguła-Kania, M.; Kosior-Korzecka, U.; Patkowski, K.; Juszczuk-Kubiak, E.; Plewik, M.; Gruszecki, T.M. Acute-phase proteins, cortisol and haematological parameters in ewes during the periparturient period. Reprod. Domest. Anim. 2020, 55, 393–400. [Google Scholar] [CrossRef]
- Dobryszycka, W. Biological functions of haptoglobin--new pieces to an old puzzle. Eur. J. Clin. Chem. Clin. Biochem. 1997, 35, 647–654. [Google Scholar]
- Hoffman, L.H.; Winfrey, V.P.; Blaeuer, G.L.; Olson, G.E. A haptoglobin-like glycoprotein is produced by implantation-stage rabbit endometrium1. Biol. Reprod. 1996, 55, 176–184. [Google Scholar] [CrossRef][Green Version]
- Herrler, A.; von Rango, U.; Beier, H.M. Embryo-maternal signalling: How the embryo starts talking to its mother to accomplish implantation. Reprod. Biomed. Online 2003, 6, 244–256. [Google Scholar] [CrossRef]
- El Ghmati, S.M.; Van Hoeyveld, E.M.; Van Strijp, J.G.; Ceuppens, J.L.; Stevens, E.A. Identification of haptoglobin as an alternative ligand for CD11b/CD18. J. Immunol. 1996, 156, 2542–2552. [Google Scholar]
- Ewoldsen, M.A.; Ostlie, N.S.; Warner, C.M. Killing of mouse blastocyst stage embryos by cytotoxic T lymphocytes directed to major histocompatibility complex antigens. J. Immunol. 1987, 138, 2764–2770. [Google Scholar]
- Bottini, N.; Gimelfarb, A.; Gloria-Bottini, F.; La Torre, M.; Lucarelli, P.; Lucarini, N. Haptoglobin genotype and natural fertility in humans. Fertil. Steril. 1999, 72, 293–296. [Google Scholar] [CrossRef]
- Tait, J.F.; Sakata, M.; McMullen, B.A.; Miao, C.H.; Funakoshi, T.; Hendrickson, L.E.; Fujikawa, K. Placental anticoagulant proteins: Isolation and comparative characterization of four members of the lipocortin family. Biochemistry 1988, 27, 6268–6276. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Suda, M.; Nakao, H.; Nagoya, T.; Saino, Y.; Arai, K.; Mizoguchi, T.; Sato, F.; Yoshizaki, H.; Hirata, M.; et al. Structure and Expression of cDNA for an Inhibitor of Blood Coagulation Isolated from Human Placenta: A New Lipocortin-Like Protein. J. Biochem. 1987, 102, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, U.; Abel, K.J.; Bohn, H.; Lobermann, H.; Lottspeich, F.; Kupper, H. Characterization of cDNA encoding human placental anticoagulant protein (PP4): Homology with the lipocortin family. Proc. Natl. Acad. Sci. USA 1988, 85, 3708–3712. [Google Scholar] [CrossRef] [PubMed]
- Masuda, J.; Takayama, E.; Satoh, A.; Ida, M.; Shinohara, T.; Kojima-Aikawa, K.; Ohsuzu, F.; Nakanishi, K.; Kuroda, K.; Murakami, M.; et al. Levels of annexin IV and V in the plasma of pregnant and postpartum women. Thromb. Haemost. 2004, 91, 1129–1136. [Google Scholar] [CrossRef]
- Degrelle, S.A.; Blomberg, L.A.; Garrett, W.M.; Li, R.W.; Talbot, N.C. Comparative proteomic and regulatory network analyses of the elongating pig conceptus. Proteomics 2009, 9, 2678–2694. [Google Scholar] [CrossRef]
- Geisert, R.D.; Brookbank, J.W.; Roberts, R.M.; Bazer, F.W. Establishment of Pregnancy in the Pig: II. Cellular Remodeling of the Porcine Blastocyst during Elongation on Day 12 of Pregnancy12. Biol. Reprod. 1982, 27, 941–955. [Google Scholar] [CrossRef]
- Mosser, G.; Ravanat, C.; Freyssinet, J.-M.; Brisson, A. Sub-domain structure of lipid-bound annexin-V resolved by electron image analysis. J. Mol. Biol. 1991, 217, 241–245. [Google Scholar] [CrossRef]
- Krikun, G.; Lockwood, C.J.; Wu, X.X.; Zhou, X.D.; Guller, S.; Calandri, C.; Guha, A.; Nemerson, Y.; Rand, J.H. The expression of the placental anticoagulant protein, annexin V, by villous trophoblasts: Immunolocalization and in vitro regulation. Placenta 1994, 15, 601–612. [Google Scholar] [CrossRef]
- Wang, X.; Campos, B.; Kaetzel, M.A.; Dedman, J.R. Annexin V is critical in the maintenance of murine placental integrity. Am. J. Obstet. Gynecol. 1999, 180, 1008–1016. [Google Scholar] [CrossRef]
- Rand, J.H. The pathogenic role of annexin-V in the antiphospholipid syndrome. Curr. Rheumatol. Rep. 2000, 2, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Shu, F.; Sugimura, M.; Kanayama, N.; Kobayashi, H.; Kobayashi, T.; Terao, T. Immunohistochemical study of annexin V expression in placentae of preeclampsia. Gynecol. Obstet. Investig. 2000, 49, 17–23. [Google Scholar] [CrossRef]
- Gardner, D.K. Lactate production by the mammalian blastocyst: Manipulating the microenvironment for uterine implantation and invasion? Bioessays 2015, 37, 364–371. [Google Scholar] [CrossRef]
- Zuo, R.-J.; Gu, X.-W.; Qi, Q.; Wang, T.-S.; Zhao, X.-Y.; Liu, J.-L.; Yang, Z.-M. Warburg-like Glycolysis and Lactate Shuttle in Mouse Decidua during Early Pregnancy. J. Biol. Chem. 2015, 290, 21280–21291. [Google Scholar] [CrossRef]
- Xiao, S.; Li, R.; El Zowalaty, A.E.; Diao, H.; Zhao, F.; Choi, Y.; Ye, X. Acidification of uterine epithelium during embryo implantation in mice. Biol. Reprod. 2017, 96, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, S.; Brinster, R. Lactate dehydrogenase isozymes in the early mouse embryo. Exp. Cell Res. 1967, 46, 89–92. [Google Scholar] [CrossRef]
- Song, N.; Liu, H.; Ma, X.; Zhang, S. Placental Growth Factor Promotes Metastases of Ovarian Cancer through MiR-543-Regulated MMP7. Cell. Physiol. Biochem. 2015, 37, 1104–1112. [Google Scholar] [CrossRef]
- Brand, A.; Singer, K.; Koehl, G.E.; Kolitzus, M.; Schoenhammer, G.; Thiel, A.; Matos, C.; Bruss, C.; Klobuch, S.; Peter, K.; et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016, 24, 657–671. [Google Scholar] [CrossRef]
- Hunt, T.K.; Aslam, R.S.; Beckert, S.; Wagner, S.; Ghani, Q.P.; Hussain, M.Z.; Roy, S.; Sen, C.K. Forum original research communication. Antioxid. Redox Signal. 2007, 9, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Porporato, P.E.; Payen, V.L.; De Saedeleer, C.J.; Préat, V.; Thissen, J.-P.; Feron, O.; Sonveaux, P. Lactate stimulates angiogenesis and accelerates the healing of superficial and ischemic wounds in mice. Angiogenesis 2012, 15, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Hoffmann, P.; Völkl, S.; Meidenbauer, N.; Ammer, J.; Edinger, M.; Gottfried, E.; Schwarz, S.; Rothe, G.; Hoves, S.; et al. Inhibitory effect of tumor cell–derived lactic acid on human T cells. Blood 2007, 109, 3812–3819. [Google Scholar] [CrossRef]
- Vauti, F.; Prochnow, B.R.; Freese, E.; Ramasamy, S.K.; Ruiz, P.; Arnold, H.-H. Arp3 is required during preimplantation development of the mouse embryo. FEBS Lett. 2007, 581, 5691–5697. [Google Scholar] [CrossRef]
- Sun, S.C.; Wang, Q.L.; Gao, W.W.; Xu, Y.N.; Liu, H.L.; Cui, X.S.; Kim, N.H. Actin nucleator Arp2/3 complex is essential for mouse preimplantation embryo development. Reprod. Fertil. Dev. 2013, 25, 617–623. [Google Scholar] [CrossRef]
- Li, Y.H.; Xu, Y.N.; Lin, Z.L.; Kwon, J.; Cui, X.S.; Kim, N.H. Inhibition of the Arp2/3 complex impairs early embryo development of porcine parthenotes. Anim. Cells Syst. 2016, 20, 253–259. [Google Scholar] [CrossRef]
- Erikson, D.W.; Burghardt, R.C.; Bayless, K.J.; Johnson, G.A. Secreted phosphoprotein 1 (SPP1, osteopontin) binds to integrin alpha v beta 6 on porcine trophectoderm cells and integrin alpha v beta 3 on uterine luminal epithelial cells, and promotes trophectoderm cell adhesion and migration. Biol. Reprod. 2009, 81, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Chan, M.M.; Iritani, B.M. Hem-1: Putting the “WAVE” into actin polymerization during an immune response. FEBS Lett. 2010, 584, 4923–4932. [Google Scholar] [CrossRef]
- Rehman, K.S.; Yin, S.; Mayhew, B.A.; Word, R.A.; Rainey, W.E. Human myometrial adaptation to pregnancy: cDNA microarray gene expression profiling of myometrium from non-pregnant and pregnant women. Mol. Hum. Reprod. 2003, 9, 681–700. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ying, W.; Dunlap, K.A.; Lin, G.; Satterfield, M.C.; Burghardt, R.C.; Wu, G.; Bazer, F.W. Arginine Decarboxylase and Agmatinase: An Alternative Pathway for De Novo Biosynthesis of Polyamines for Development of Mammalian Conceptuses1. Biol. Reprod. 2014, 90, 84. [Google Scholar] [CrossRef] [PubMed]
- Lenis, Y.Y.; Johnson, G.A.; Wang, X.; Tang, W.W.; Dunlap, K.A.; Satterfield, M.C.; Wu, G.; Hansen, T.R.; Bazer, F.W. Functional roles of ornithine decarboxylase and arginine decarboxylase during the peri-implantation period of pregnancy in sheep. J. Anim. Sci. Biotechnol. 2018, 9, 10. [Google Scholar] [CrossRef]
- Tajima, M.; Harada, T.; Ishikawa, T.; Iwahara, Y.; Kubota, T. Augmentation of arginase Ⅱ expression in the human endometrial epithelium in the secretory phase. J. Med. Dent. Sci. 2012, 59, 75–82. [Google Scholar]
- Majumder, S.; Sinha, S.; Siamwala, J.H.; Muley, A.; Seerapu, H.R.; Kolluru, G.K.; Veeriah, V.; Nagarajan, S.; Sridhara, S.R.; Priya, M.K.; et al. A comparative study of NONOate based NO donors: Spermine NONOate is the best suited NO donor for angiogenesis. Nitric Oxide 2014, 36, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Bidarimath, M.; Tayade, C. Pregnancy and spontaneous fetal loss: A pig perspective. Mol. Reprod. Dev. 2017, 84, 856–869. [Google Scholar] [CrossRef]
- Chen, F.; Wang, T.; Feng, C.; Lin, G.; Zhu, Y.; Wu, G.; Johnson, G.; Wang, J. Proteome Differences in Placenta and Endometrium between Normal and Intrauterine Growth Restricted Pig Fetuses. PLoS ONE 2015, 10, e0142396. [Google Scholar] [CrossRef]
- DeSouza, L.; Diehl, G.; Yang, E.C.; Guo, J.; Rodrigues, M.J.; Romaschin, A.D.; Colgan, T.J.; Siu, K.M. Proteomic analysis of the proliferative and secretory phases of the human endometrium: Protein identification and differential protein expression. Proteomics 2005, 5, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Scotchie, J.G.; Fritz, M.A.; Ms, M.M.; Lessey, B.A.; Young, S.L. Proteomic Analysis of the Luteal Endometrial Secretome. Reprod. Sci. 2009, 16, 883–893. [Google Scholar] [CrossRef]
- Arredouani, M.; Matthijs, P.; Van Hoeyveld, E.; Kasran, A.; Baumann, H.; Ceuppens, J.L.; Stevens, E. Haptoglobin directly affects T cells and suppresses T helper cell type 2 cytokine release. Immunology 2003, 108, 144–151. [Google Scholar] [CrossRef]
- Hallen, A.; Cooper, A.J.L.; Jamie, J.F.; Haynes, P.A.; Willows, R.D. Mammalian forebrain ketimine reductase identified as μ-crystallin; potential regulation by thyroid hormones. J. Neurochem. 2011, 118, 379–387. [Google Scholar] [CrossRef]
- Glinoer, D. The regulation of thyroid function in pregnancy: Pathways of endocrine adaptation from physiology to pathology. Endocr. Rev. 1997, 18, 404–433. [Google Scholar] [CrossRef] [PubMed]
- Brent, G.A. The debate over thyroid-function screening in pregnancy. N. Engl. J. Med. 2012, 366, 562–563. [Google Scholar] [CrossRef]
- Bai, S.W.; Herrera-Abreu, M.T.; Rohn, J.L.; Racine, V.; Tajadura, V.; Suryavanshi, N.; Bechtel, S.; Wiemann, S.; Baum, B.; Ridley, A.J. Identification and characterization of a set of conserved and new regulators of cytoskeletal organization, cell morphology and migration. BMC Biol. 2011, 9, 54. [Google Scholar] [CrossRef]
- Matorras, R.; Quevedo, S.; Corral, B.; Prieto, B.; Exposito, A.; Mendoza, R.; Rabanal, A.; Diaz-Nuñez, M.; Ferrando, M.; Elortza, F.; et al. Proteomic pattern of implantative human endometrial fluid in in vitro fertilization cycles. Arch. Gynecol. Obste. 2018, 297, 1577–1586. [Google Scholar] [CrossRef]
- Mullen, M.P.; Elia, G.; Hilliard, M.; Parr, M.H.; Diskin, M.G.; Evans, A.C.; Crowe, M.A. Proteomic characterization of histotroph during the preimplantation phase of the estrous cycle in cattle. J. Proteome Res. 2012, 11, 3004–3018. [Google Scholar] [CrossRef]
- Koch, J.M.; Ramadoss, J.; Magness, R.R. Proteomic Profile of Uterine Luminal Fluid from Early Pregnant Ewes. J. Proteome Res. 2010, 9, 3878–3885. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Gordon, S. The role of scavenger receptors in pathogen recognition and innate immunity. Immunobiology 2004, 209, 39–49. [Google Scholar] [CrossRef]
- Areschoug, T.; Gordon, S. Scavenger receptors: Role in innate immunity and microbial pathogenesis. Cell. Microbiol. 2009, 11, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Clagett-Dame, M.; DeLuca, H.F. The role of vitamin A in mammalian reproduction and embryonic development. Annu. Rev. Nutr. 2002, 22, 347–381. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Ye, Q.; Zeng, X.; Yang, G.; Ye, C.; Chen, M.; Yu, H.; Wang, Y.; Wang, G.; Huang, S.; et al. CBS and MAT2A improve methionine mediated DNA synthesis through SAMTOR/mTORC1/S6K1/CAD pathway during embryo implantation. Cell Prolif. 2021, 54, e12950. [Google Scholar] [CrossRef]
- Li, X.X.; Lee, K.B.; Lee, J.H.; Kim, K.J.; Kim, E.Y.; Han, K.W.; Kim, M.K. Glutathione and cysteine enhance porcine preimplantation embryo development in vitro after intracytoplasmic sperm injection. Theriogenology 2014, 81, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.-W.; Wang, Y.-P.; Chen, I.-C.; Pan, H.-M.; Wu, G.-J. Notch 1 signaling pathway effect on implantation competency. Fertil. Steril. 2011, 96, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Chen, C.-D.; Abraham, C.R. Cell-type dependent modulation of Notch signaling by the amyloid precursor protein. J. Neurochem. 2010, 113, 262–274. [Google Scholar] [CrossRef]
- Dratwa-Chałupnik, A.; Lepczyński, A.; Ożgo, M.; Herosimczyk, A.; Michałek, K.; Niemcewicz, M.; Pałyszka, A.; Skrzypczak, W. Analysis of the efficiency of post-electrphoretic protein staining using colloidal Coomassie Blue G-250. Acta Scient. Polon. Zootech. 2017, 14, 67–76. [Google Scholar]
- Pink, M.; Verma, N.; Rettenmeier, A.W.; Schmitz-Spanke, S. CBB staining protocol with higher sensitivity and mass spectrometric compatibility. Electrophoresis 2010, 31, 593–598. [Google Scholar] [CrossRef]
- Bindea, G.; Galon, J.; Mlecnik, B. CluePedia Cytoscape plugin: Pathway insights using integrated experimental and in silico data. Bioinformatics 2013, 29, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
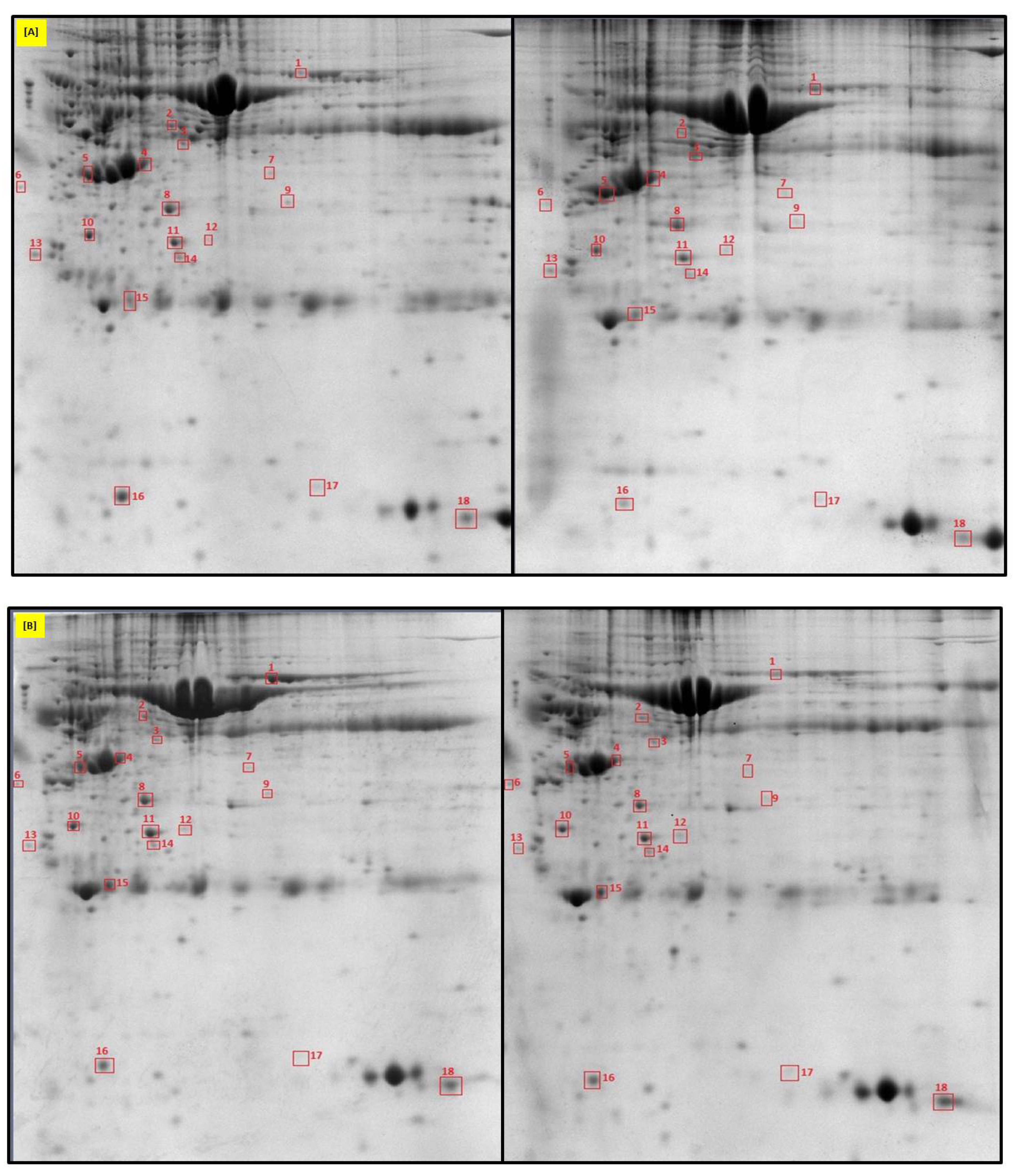
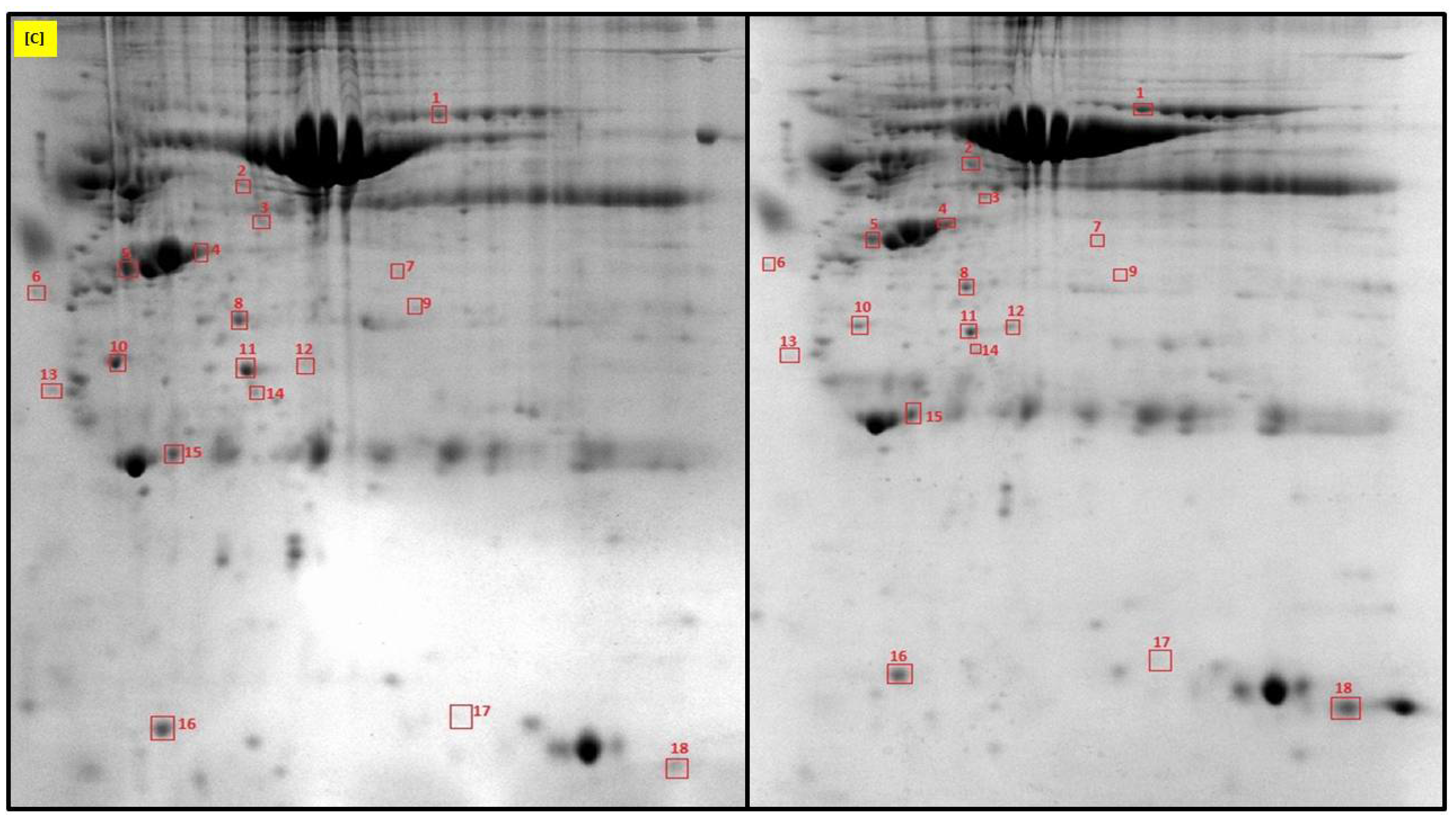
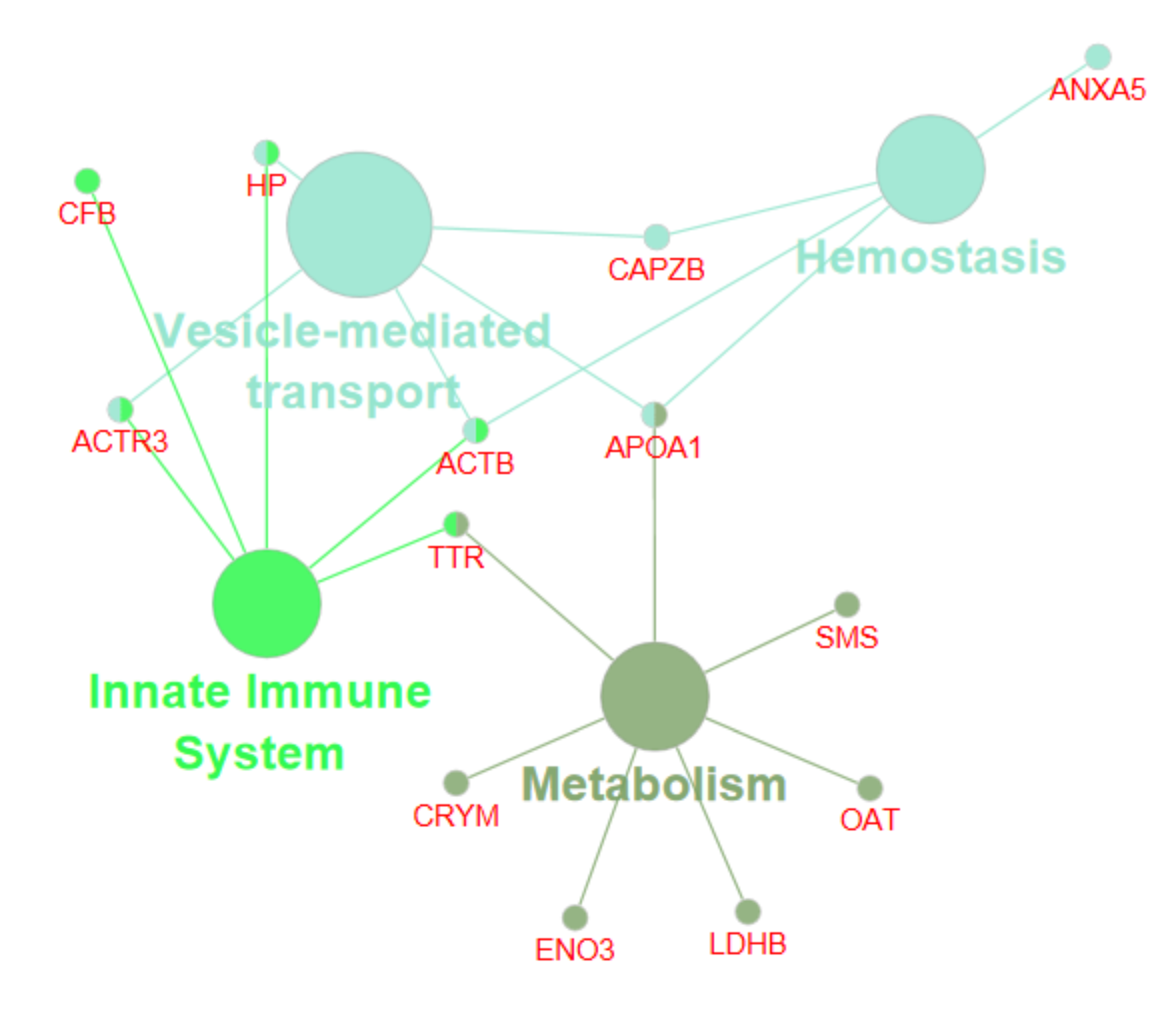

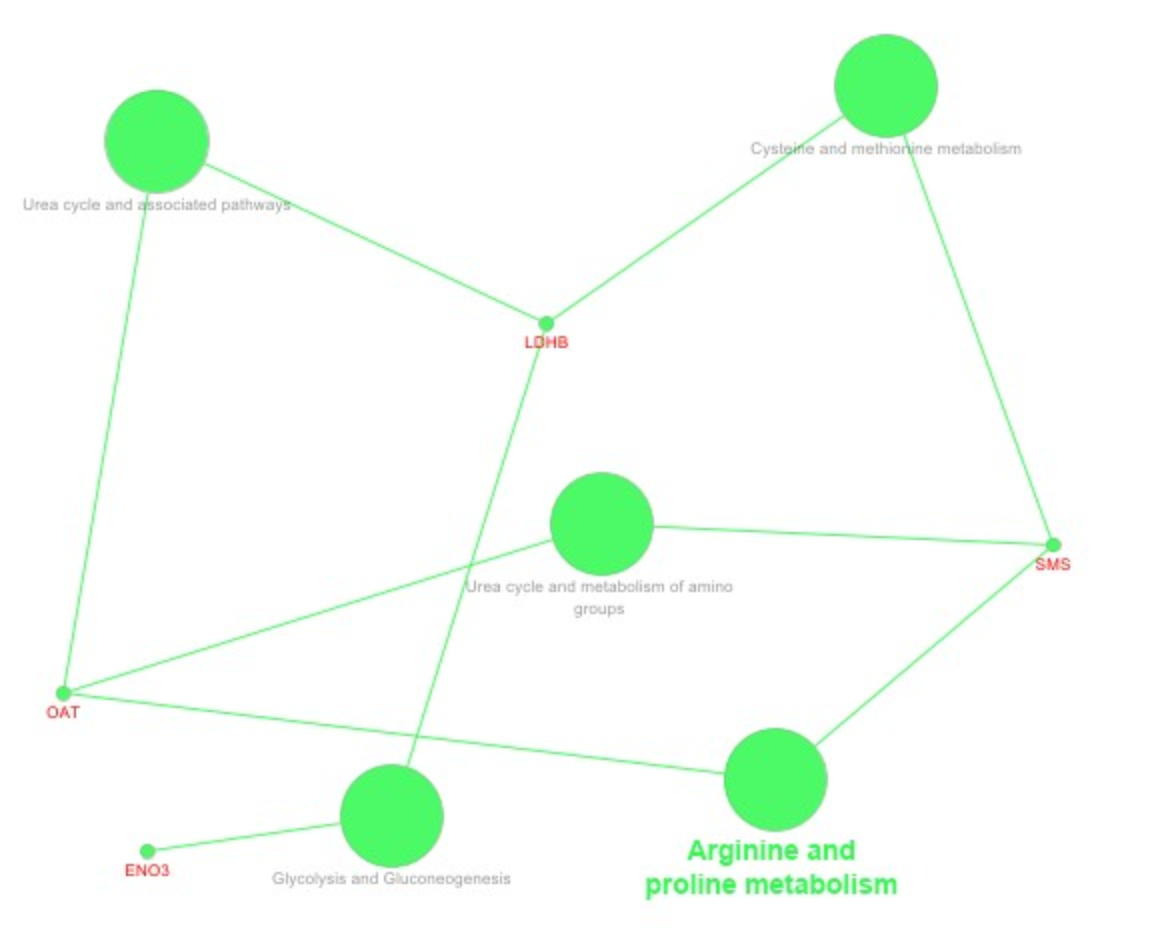
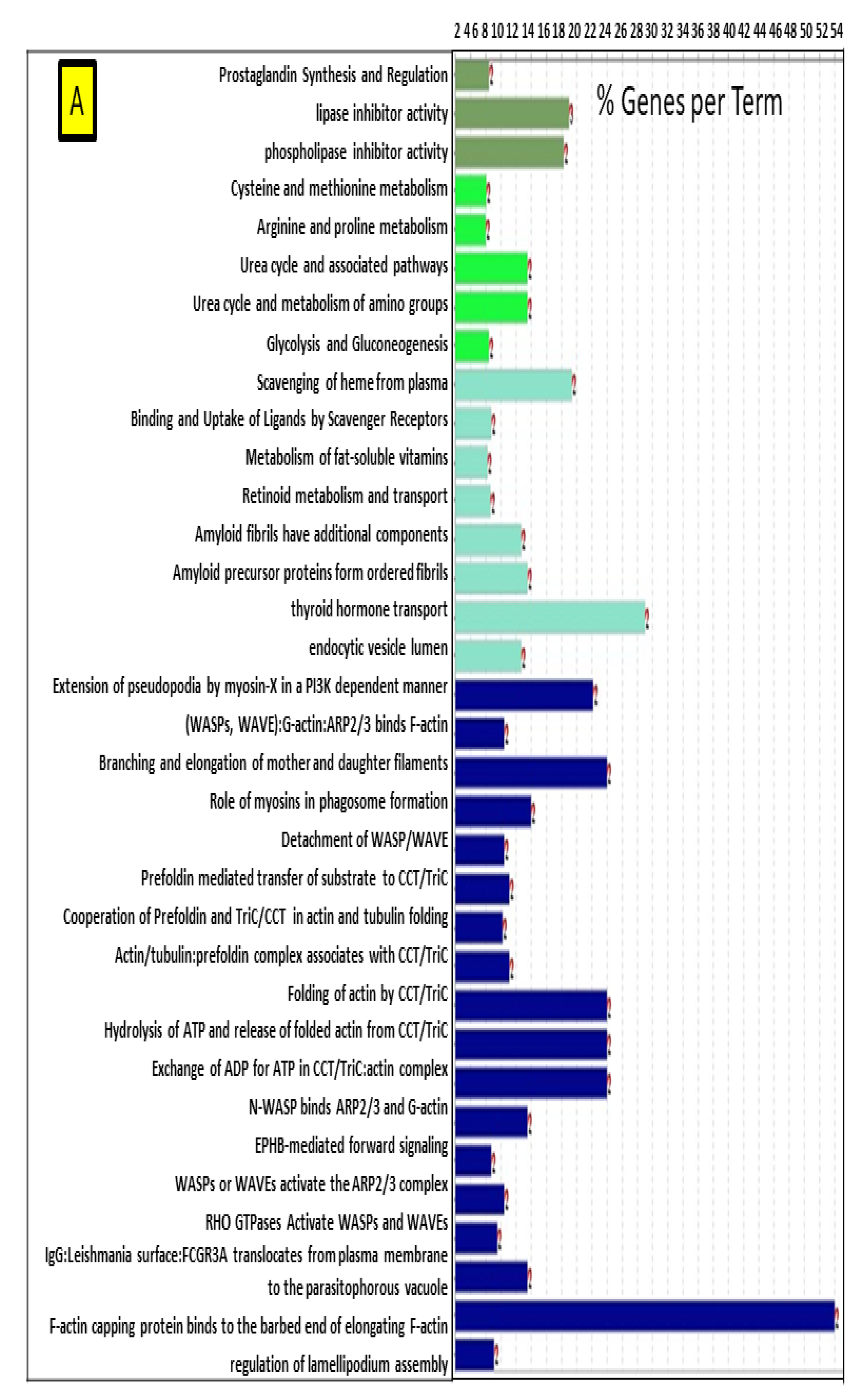
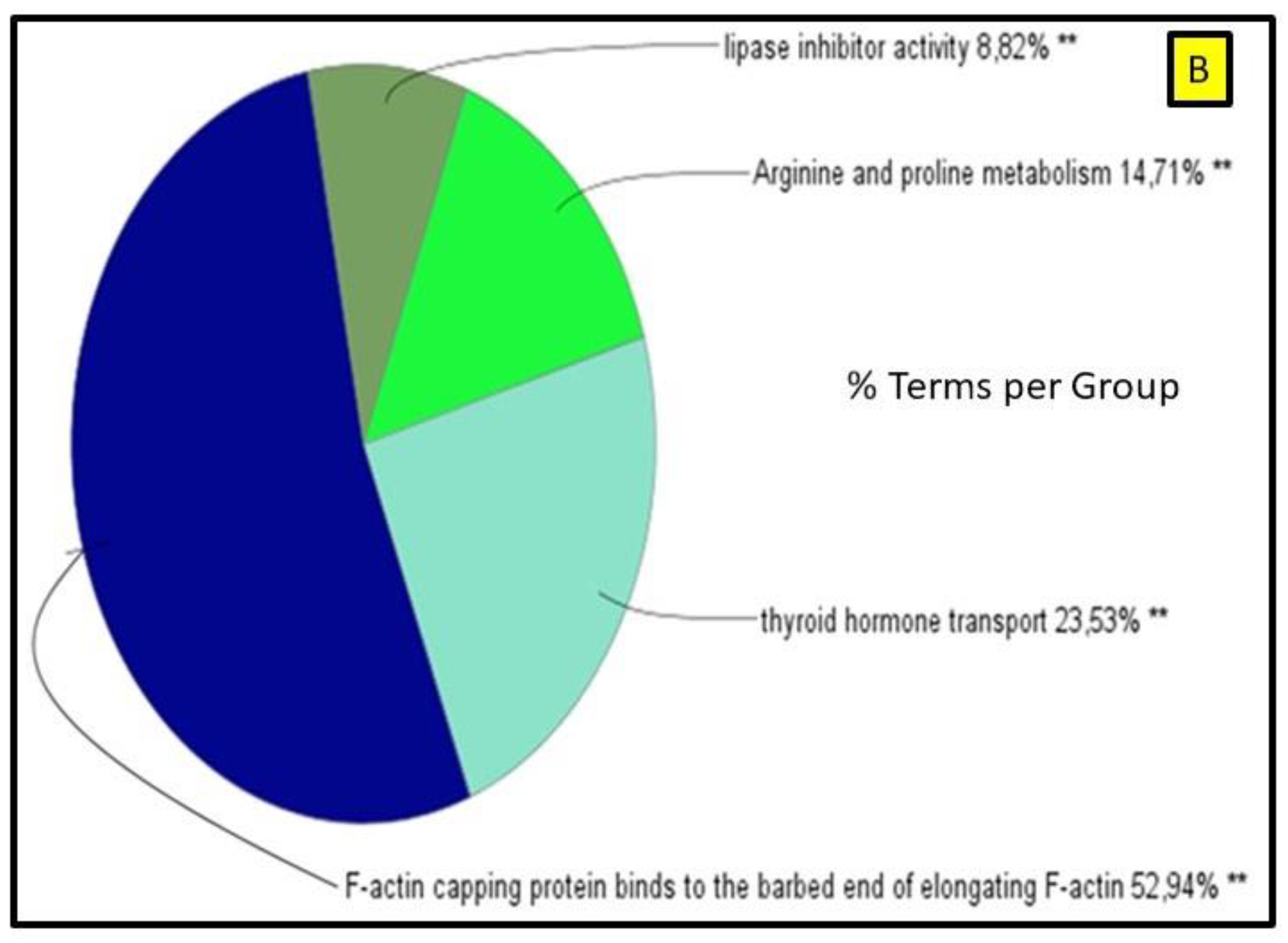

| Protein Name | Gene Symbol | Protein Spot Order Number on 2D Gel | Molecular Weight (Da) | Theoretical pH | Seq Coverage % | Score | Protein Function | NCBI Identification Code | NCBI Ref. Seq. Number |
|---|---|---|---|---|---|---|---|---|---|
| Annexin A5 | ANXA5 | 1 | 36.148 | 4.94 | 37% | 88 | Blood coagulation hemostasis | gi|335293906 | XP_003129266.2 |
| Spermine synthase (PABs-domain containing protein) | SMS | 2 | 41.239 | 4.87 | 41% | 83 | Polyamine biosynthesis | gi|350595577 | XP_020935312.1 |
| Ketimine reductase mu crystalline isoform X1 | CRYM | 3 | 33.508 | 5.16 | 14 | 54 | Transcription co-repressor activity | gi|335284508 | XP_003124605.2 |
| Haptoglobin like | HP (L) | 12 | 38.481 | 6.51 | 11 | 50 | Acute phase response, defense response | gi|350596912 | ACD93463.1 |
| Apolipoprotein A1 | APOA1 | 4 | 30.325 | 5.48 | 33 | 47 | Cholesterol metabolism, transport | gi|164359 | AAA30992.1 |
| B Actin | ACTB | 5 | 41.737 | 5.29 | 34 | 61 | Cytoskeleton organization | gi|45269029 | AAS55927.1 |
| F actin capping protein subunit beta | CAPZB | 10 | 33.017 | 5.53 | 25 | 83 | Isomerase, apoptotic cell clearance | gi|147899312 | NP_001090923.1 |
| Annexin A4 | ANXA4 | 9 | 35.829 | 5.7 | 52 | 120 | Promotes membrane fusion | gi|264681432 | NP_001161111.1 |
| L-Lactate dehydrogenase B chain | LDHB | 8 | 36.612 | 5.57 | 32 | 82 | Glycolysis, carbohydrate metabolism and oxido reduction | gi|164518958 | NP_001106758.1 |
| Actin related protein 3 | ACTR3 | 7 | 47.425 | 5.61 | 19 | 40 | Maintenance of cell polarity, asymmetric cell division | gi|197251946 | NP_001127815.1 |
| Chaperonin containing TCP1, subunit 5 epsilon | CCT5 | 6 | 59.641 | 5.57 | 22 | 64 | Cochaperone, protein folding | gi|523580068 | NP_001265708.1 |
| Annexin A41 | ANXA41 | 11 | 35.829 | 5.7 | 31 | 68 | Promotes membrane fusion | gi|264681432 | NP_001161111.1 |
| Haptoglobin precursor | HP (P) | 16 | 13.843 | 6 | 23 | 56 | Acute phase response, defense response | gi|47522826 | NP_999165.1 |
| Beta enolase | ENO3 | 15 | 47.130 | 8.05 | 12 | 79 | Regulation of blood coagulation | gi|113205498 | NP_001037992.1 |
| Ornithine oxo-acid aminotransferase | OAT | 14 | 48.477 | 6.44 | 18 | 41 | Catalytic, transaminase activity | gi|297591979 | NP_001172070.1 |
| Complement factor B/B factor, properdin | CFB | 13 | 85.865 | 7.45 | 13 | 46 | Innate immunity, complement activity | gi|156120152 | NP_001095294.1 |
| Transthyretin | TTR | 17 | 16.081 | 6.29 | 12 | 22 | Transport, extracellular matrix organization | gi|975233 | CAA61120.1 |
| Target Protein | UniProtKB Accession Number | Gene Symbol | Molecular Weight | Dilution | Host Species | Conjugate | Company | Code Number |
|---|---|---|---|---|---|---|---|---|
| Primary antibody | ||||||||
| GAPDH | P00355 | GAPDH | 36 kDa | 1:10,000 | rabbit | polyclonal | Abcam plc (Cambridge, UK) | ab190304 |
| haptoglobin | Q61646 | HP | 39 kDa | 1:500 | rabbit | polyclonal | Abcam plc | ab231976 |
| annexinA4 | P08132 | ANXA4 | 36 kDa | 1:500 | rabbit | polyclonal | Abcam plc | Ab232805 |
| Secondary antibody | ||||||||
| Peroxidase AffiniPure Goat Anti-Rabbit IgG (H+L) | 1:10,000 | goat | polyclonal, horseradish peroxidase (HRP) conjugates | Jackson ImmunoResearch Europe Ltd. (Ely, UK) | 111-035-003 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierzchała, D.; Liput, K.; Korwin-Kossakowska, A.; Ogłuszka, M.; Poławska, E.; Nawrocka, A.; Urbański, P.; Ciepłoch, A.; Juszczuk-Kubiak, E.; Lepczyński, A.; et al. Molecular Characterisation of Uterine Endometrial Proteins during Early Stages of Pregnancy in Pigs by MALDI TOF/TOF. Int. J. Mol. Sci. 2021, 22, 6720. https://doi.org/10.3390/ijms22136720
Pierzchała D, Liput K, Korwin-Kossakowska A, Ogłuszka M, Poławska E, Nawrocka A, Urbański P, Ciepłoch A, Juszczuk-Kubiak E, Lepczyński A, et al. Molecular Characterisation of Uterine Endometrial Proteins during Early Stages of Pregnancy in Pigs by MALDI TOF/TOF. International Journal of Molecular Sciences. 2021; 22(13):6720. https://doi.org/10.3390/ijms22136720
Chicago/Turabian StylePierzchała, Dorota, Kamila Liput, Agnieszka Korwin-Kossakowska, Magdalena Ogłuszka, Ewa Poławska, Agata Nawrocka, Paweł Urbański, Aleksandra Ciepłoch, Edyta Juszczuk-Kubiak, Adam Lepczyński, and et al. 2021. "Molecular Characterisation of Uterine Endometrial Proteins during Early Stages of Pregnancy in Pigs by MALDI TOF/TOF" International Journal of Molecular Sciences 22, no. 13: 6720. https://doi.org/10.3390/ijms22136720
APA StylePierzchała, D., Liput, K., Korwin-Kossakowska, A., Ogłuszka, M., Poławska, E., Nawrocka, A., Urbański, P., Ciepłoch, A., Juszczuk-Kubiak, E., Lepczyński, A., Ślaska, B., Kowal, K., te Pas, M. F. W., Śmiech, M., Leszczyński, P., Taniguchi, H., Fraser, L., Sobiech, P., Sachajko, M., ... Pierzchała, M. (2021). Molecular Characterisation of Uterine Endometrial Proteins during Early Stages of Pregnancy in Pigs by MALDI TOF/TOF. International Journal of Molecular Sciences, 22(13), 6720. https://doi.org/10.3390/ijms22136720








