Abstract
Urate homeostasis in humans is a complex and highly heritable process that involves i.e., metabolic urate biosynthesis, renal urate reabsorption, as well as renal and extrarenal urate excretion. Importantly, disturbances in urate excretion are a common cause of hyperuricemia and gout. The majority of urate is eliminated by glomerular filtration in the kidney followed by an, as yet, not fully elucidated interplay of multiple transporters involved in the reabsorption or excretion of urate in the succeeding segments of the nephron. In this context, genome-wide association studies and subsequent functional analyses have identified the ATP-binding cassette (ABC) transporter ABCG2 as an important urate transporter and have highlighted the role of single nucleotide polymorphisms (SNPs) in the pathogenesis of reduced cellular urate efflux, hyperuricemia, and early-onset gout. Recent publications also suggest that ABCG2 is particularly involved in intestinal urate elimination and thus may represent an interesting new target for pharmacotherapeutic intervention in hyperuricemia and gout. In this review, we specifically address the involvement of ABCG2 in renal and extrarenal urate elimination. In addition, we will shed light on newly identified polymorphisms in ABCG2 associated with early-onset gout.
Keywords:
gout; early-onset gout; hyperuricemia; urate; uric acid; ABCG2; BCRP; ABC transporter; single nucleotide polymorphism; SNP 1. Introduction
Gout is the clinical manifestation of hyperuricemia which is triggered by urate precipitation (deposition of monosodium urate crystals) in the synovial fluid of joints and other tissues [1,2]. The disease is primarily associated with severe arthropathy, which manifests mainly in the metatarsophalangeal joints (podagra), but also in other joints of the foot, ankles, knee, wrist, fingers, and elbows [3]. In the pathogenesis of the disease, urate deposits promote inflammatory responses in the synovial membrane (synovitis) and thus arthritis characterized by sudden, severe attacks of pain, swelling, redness, and tenderness in the affected joints. Depending on the course of the disease, the symptoms of gout can occur both as acute episodic flares (gout attacks) and persist chronically and, if left untreated, can lead to irreversible deformations and impaired mobility of the affected joints [3]. In addition, gout nephropathy, a form of chronic tubulointerstitial nephritis, induced by the deposition of urate precipitates in the distal collecting ducts and the medullary interstitium may cause progressive chronic kidney disease [4]. Furthermore, gout and hyperuricemia have been associated with a subset of comorbidities including metabolic syndrome, diabetes, hypertension as well as cardiovascular and cerebrovascular disease [5,6,7,8,9,10,11,12,13]. In most patients, the onset of gout occurs after the age of 60, with the incidence being about three times higher in men than in women [14]. However, a significant proportion of patients develop primary hyperuricemia and gout symptoms before the age of 40, which is defined as the pathotype of early-onset gout [15,16]. In addition to environmental factors, genetic predispositions leading to chronic, yet asymptomatic hyperuricemia in childhood and adolescence are considered to be the main causes for the early onset of the disease. Although not every patient with hyperuricemia necessarily develops gout [17], it is considered to be the major risk factor for the development and progression of the disease. In this review, we will specifically address the pathogenesis and genetic background of early-onset gout and highlight the role of intestinal uric acid transport in this context.
2. Gout and Hyperuricemia
Hyperuricemia represents a prolonged pathophysiological increased serum urate concentration, often defined as >6.0 mg/dL (>360 µmol/L) for females and >7.0 mg/dL (>420 µmol/L) for males [1,18], which is either caused by an increased hepatic biosynthesis or a reduced renal or intestinal excretion of urate [19]. Under physiological conditions, urate is derived from the enzymatic degradation of purine nucleobases/nucleotides, which are involved in a multitude of biochemical processes, such as energy metabolism and the formation of RNA and DNA [20]. In humans, urate is the terminal metabolite of purine catabolism derived from purines that do not enter the salvage pathway for the resynthesis of ATP or GTP [19]. Therefore, secondary hyperuricemia can be induced by an excessive intake of purine-rich food (e.g., red meat, offal, seafood) [21], cellular degradation processes, and high cell turnover in the context of leukemia/lymphoma [22] or anticancer treatment with chemo- or radiation therapy [23], which all increase the availability of free purines. In addition to a diet high in purines, other lifestyle-related behaviors such as excessive intake of fructose [24,25] and alcohol abuse [26,27] can also trigger hyperuricemia, which explains the high prevalence in industrialized countries and the increasing prevalence in developing countries [28]. Aside from the aforementioned environmental factors, also genetic defects in enzymes responsible for the biotransformation of purine bases can favor primary hyperuricemia, as is the case in Lesch–Nyhan or Kelley–Seegmiller syndromes [29]. In line with this notion, the heritability of hyperuricemia is substantial, suggesting important genetic contributions to urate homeostasis [30]. In pharmacotherapy, uricostatic drugs like the xanthinoxidase inhibitor allopurinol can be used to normalize hyperuricemia by preventing the last step in urate biosynthesis. Under this treatment, intermediates of the purine metabolism such as inosine, hypoxanthine, and xanthine accumulate, yet exhibit a better water solubility and a lower tendency to form crystals than urate. Unlike secondary hyperuricemias that are triggered by increased urate biosynthesis, the vast number (>90%) of primary hyperuricemia cases result from a decreased ability of the kidney or intestine to excrete urate [31]. The majority of urate (roughly 70%) is eliminated by the kidney, where it is freely filtered by the glomerulus [32]. Urate homeostasis is primarily influenced by renal proximal tubule cells, which express several transporters that either reabsorb urate (e.g., URAT1 at the apical and GLUT9 at the basolateral membrane) [33,34,35,36] or are involved in urate excretion (e.g., NPT1/4 at the apical and OAT1/3 at the basolateral membrane) [20,35,37,38]. Indeed, uricosuric drugs such as the URAT1 inhibitors benzbromarone as well as probenecid and lesinurad are used in pharmacotherapy to treat hyperuricemia by inhibiting renal reabsorption of urate [39]. In addition to transporters of the salute carrier (SLC) and the organic anion transporter (OAT) protein families, ABC transporters such as ABCG2 and ABCC4 are also involved in urate excretion [32,37]. As the previously mentioned other transporters, ABCG2 was shown to be located in the apical brush border membrane of renal proximal tubule cell [40]. In the intestine, the major site for the remaining 30% of urate excretion, the mechanisms of urate excretion are less well defined [38]. Urate transporters GLUT9 [41] and in particular ABCG2 [42] are highly expressed in intestinal epithelial cells and may thus represent interesting new pharmacological targets for the treatment of hyperuricemia [43,44,45,46,47]. Nonetheless, with regard to the sites of urate excretion (kidney & intestine) and the complex interplay of transporter-mediated excretion and reabsorption of urate in the kidney, the mechanisms of urate homeostasis are still not fully understood. However, single nucleotide polymorphisms (SNPs) in different genes involved in urate transport have been associated with hyperuricemia [48], thereby emphasizing the multicausal complexity of gout pathology [49]. In this article, we aim to focus on ABCG2, which has been identified as an important urate transporter in the intestine and the kidney [40,50,51,52], and discuss its role in renal and extra-renal urate excretion as well as in primary hyperuricemia and early-onset gout.
3. ABCG2 and Its Function in Renal Urate Elimination
ABCG2 (also known as BCRP) is a multi-drug efflux pump that has been described to contribute to transport processes in many different tissues and cell types. It belongs to the ABC (ATP-binding cassette) transporter superfamily [53] and has the ability to transport a variety of substrates across the membrane [54,55]. ABCG2 is highly expressed in the placental syncytiotrophoblasts [56], but can also be found at the entry and exit point of the human body including endothelial cells of the cerebral blood-brain barrier [57] and canalicular membrane of the liver [58] as well as polar epithelial cells of the intestine [42] and the kidney [50,59]. Based on its function and localization, ABCG2 is thought to act as a “gatekeeper”, preventing endo- or exotoxins and xenobiotics from crossing biological barriers and entering sensitive tissues [60]. Although these functions of ABCG2 are thought to serve to maintain the healthy state of the organism, they also appear to be responsible for ABCG2-related interference with pharmacotherapeutic interventions to treat certain diseases. In this regard, overexpression of ABCG2 has been associated with multidrug resistance to chemotherapy [61,62], which is associated with poor prognosis in the treatment of certain cancers [63,64]. With regard to its protein structure, ABCG2 consists of an ATP-hydrolyzing nucleotide-binding domain, which is located in the cytoplasm and provides energy for the transport process, and a transmembrane domain, which is responsible for the binding of substrates and their transport across the membrane (Figure 1). Moreover, ABCG2 is a so-called “half-transporter” that needs to homodimerize to form a functional transporter [60]. Recently, several high-resolution 3D structures of the ABCG2 protein bound to different substrates and inhibitors have been solved [65,66,67,68], which help to understand the molecular mechanisms of substrate selection, substrate binding, and substrate transport of ABCG2 [69,70]. In addition to its role as an efflux pump with broad specificity, ABCG2 has been proposed to be involved in renal and intestinal urate excretion [40,50,51,52,71]. The function of ABCG2 as a urate transporter was inferred from genome-wide association analyses and subsequent functional studies, which specifically demonstrated a strong association of a missense SNP in the ABCG2 gene (rs2231142; Q141K) with hyperuricemia [72,73,74], an SNP that could be causally related to at least 10% of all gout cases [50]. The Q141K polymorphism has been associated with a reduced ABCG2 surface expression and decreases cellular urate efflux to approximately half of wild-type ABCG2 levels [50,52,75,76,77]. In structural predictions derived from homology models [78] as well as structural cryo-EM data [65], Q141 was shown to be located in the nucleotide-binding domain of the transporter and to form a hydrogen bond to N158 of an α-helix within the nucleotide-binding domain adjacent to transmembrane helix 1 of ABCG2. This connection might be responsible to convey conformational changes induced by ATP binding or ATP hydrolysis from the nucleotide-binding domain to the substrate transporting transmembrane domain, thereby potentially explaining the partial loss of function of the Q141K-mutated transporter. However, also misfolding, reduced protein stability, and reduced membrane expression due to increased proteasomal degradation of Q141K-mutated ABCG2 [75,79] are also discussed as causes of the urate excretion deficit. Recent findings suggest that Q141K- and M71V-related dysfunction is due to aberrant trafficking of ABCG2 to the plasma membrane due to quality control mechanisms in the endoplasmic reticulum rather than reduced ABCG2 transport activity [80]. Moreover, the c.C421 > A mutation that leads to the Q141K polymorphism promotes microRNA-mediated suppression of ABCG2 translation, so that cell type-specific processing of the ABCG2 3`UTR along with cell type-specific microRNA expression profiles may have a profound impact on functional ABCG2 bioavailability in individuals carrying the Q141K polymorphism [76]. In the kidney of humans and mice, ABCG2 was shown to be expressed in the apical membrane of the brush border of proximal tubule epithelial cells [40,50], although in the analyses of The Human Protein Atlas consortium ABCG2 could not be detected at the protein level in human kidney biopsies [81]. Nonetheless, also findings from other groups indicate relevant renal ABCG2 expression [59]. The renal expression pattern of ABCG2 partially resembles the expression pattern of urate reabsorbing transporter URAT1, thereby indicating a functional interplay of both transporters in renal urate handling [40]. However, in measurements of renal urate excretion after a purine challenge (oral administration of inosine, which is rapidly metabolized to urate), human subjects carrying the ABCG2 transport function impairing Q141K polymorphism showed no significant differences in urate excretion and a fraction of filtered urate load (FEUA defined as the ratio between the renal clearance of uric acid to the renal clearance of creatinine), although their serum urate levels were significantly elevated [40]. In the same study, renal urate excretion was also investigated in an orthologous Q140K knock-in mouse model. Here, only the male animals displayed elevated serum urate levels and had, in contrast to humans, at least a significantly reduced fraction of filtered urate load but again no change in urinary urate excretion [40]. Interestingly, these sex-related phenotypes were consistent with the increased prevalence of gout in human males [28]. However, the results of both human and mouse experiments suggest that the hyperuricemia induced by the ABCG2 Q141K polymorphism is not caused by a significant effect on renal urate excretion, but is likely to be triggered by different mechanism [40]. These findings, which are in line with the ABCG2 expression data from The Human Protein Atlas consortium [81], also raise the fundamental question of whether ABCG2 is in fact of any significance for renal urate excretion. In this regard, conflicting results regarding the involvement of ABCG2 in renal urate elimination have been obtained in experiments with ABCG2 knockout mice [51,71]. In both studies, serum urate concentrations of ABCG2 knockout animals were elevated compared to their wildtype littermates. However, while one study did not observe significant differences in renal urate elimination [71], the other study found a significant reduction of about 30% [51]. Nevertheless, these two animal studies, as well as the translational study by Hoque and colleagues, indicate that ABCG2 primarily affects extrarenal regulation of urate homeostasis, which is further discussed below. It should be mentioned that in the kidney, ABCG2 is only one of many renal transporters that are able to excrete urate [37] so that ABCG2 loss of function may be compensated by other transporters. In conclusion, the previously assumed relevance of ABCG2 for renal urate elimination has been questioned by recent studies and therefore further future studies are needed to definitively elucidate this issue.
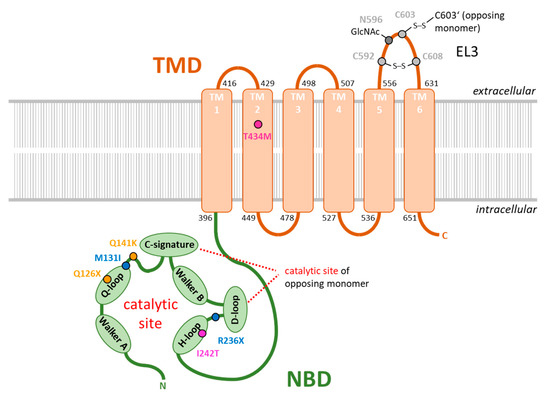
Figure 1.
Polymorphisms in ABCG2 protein sequence associated with pediatric-onset hyperuricemia and early-onset gout. Schematic overview of the ABCG2 domain structure consisting of a nucleotide-binding domain (light green, NBD) and a transmembrane domain (light brown, TMD) modified from [69]. Single membrane-spanning α-helices (TM1–6) were structured according to the information of published protein sequences (NCBI accession number: NP_001335914.1). The catalytic site for ATP hydrolysis is formed by the sequence motifs Walker A, Q-loop, Walker B, and H-loop of one monomer, and the c-signature and D-loop from the other monomer. Cysteine bridge forming residues and N-acetylation sites within extracellular loop 3 (EL3) are marked in grey. SNPs involved in pediatric-onset hyperuricemia and early-onset of gout published in recent seminal publications are highlighted in different colors (yellow, dark blue, and purple).
4. Relevance of ABCG2 in Extrarenal Urate Elimination
As earlier studies indicated that ABCG2 is also expressed in the liver [58] and intestine [42] and that ABCG2 may function as a urate transporter [50,52], it was reasonable to speculate that ABCG2 plays a role in extrarenal urate excretion possibly via the bile or intestine [82]. However, accurate non-invasive measurements of intestinal/biliary urate secretion are not possible in humans because the secreted urate is largely metabolized by the bacterial flora in the intestine. Therefore, the role of ABCG2 in extrarenal urate secretion was revealed in animal experiments using the in situ intestinal “closed-loop” perfusion method [51,71]. Since most vertebrates, including rodents used for animal studies, express the enzyme uricase which converts uric acid to allantoin and which has been lost during human evolution [83], a direct relation of results from animal experiments studying urate metabolism and urate transport to the human organism is inadequate. To account for that, rodents used in both studies were treated with the uricase inhibitor oxonate, a pharmacological intervention that increased the serum urate levels in mice and rats to the magnitude of serum urate levels in humans [51,71]. By administration of radioactive labeled uric acid, Hosomi and colleagues demonstrated that, in addition to the substantial fraction of renal urate elimination [51], there is direct urate excretion via the intestine (mainly in the ileum) and only minor urate excretion via the bile [51,71]. These findings, therefore, suggest that the intestine is the main site of extrarenal urate excretion. In the intestine of mice, ABCG2 expression is mainly located at the villi brush border of epithelial cells of the ileum and the jejunum [40]. Interestingly, in this study, the observed overall expression levels of the protein in the intestine are much higher than in the kidney. In rats, ABCG2 expression in the gut was found to further increase in response to increased blood urate concentrations after oxonate treatment [84]. To study the contribution of ABCG2 in intestinal urate excretion (and renal urate excretion which was already discussed above), oxonate-treated ABCG2 knockout mice were used [51,71] and showed a reduction in intestinal urate elimination by roughly 40–50%. These findings also suggest that other yet unknown transporters besides ABCG2 are involved in intestinal urate secretion [51,71]. A similar severe loss in intestinal urate elimination was also observed in the aforementioned study by Hogue and colleagues in an orthologous Q140K knock-in mouse model but in absence of the uricase inhibitor oxonate [40]. Consistent with these data, Q140K knock-in in mice resulted in a marked reduction in intestinal ABCG2 expression. In contrast, only subtle changes in urate elimination and ABCG2 expression were observed in the kidney in the same knock-in mouse model. These results are further supported by the observation of other authors, which indicate that the ABCG2 Q141K polymorphism and fractional renal clearance both contribute significantly but independently to the risk of hyperuricemia in humans [73]. In addition, impaired intestinal urate excretion induced by the orthologous murine Q140K mutation or complete ABCG2 knockout may explain hyperuricemia despite unaltered renal urate excretion in the respective mouse models [71,85,86] as well as in human individuals carrying the Q141K polymorphism [40]. However, due to the rise in serum urate levels (sUA) caused by the lack of intestinal urate secretion, an indirect increase in the fraction of filtered urate load (FEUA) could be expected in patients with ABCG2 dysfunction although this was not observed in the previously mentioned study [40]. In addition, ABCG2-mediated intestinal urate elimination appears to play an important role in compensating for the loss of renal urate elimination in chronic kidney disease [35]. Taken together, recent publications indicate that the major site of action of the ABCG2 transporter is regulating urate homeostasis in the intestine.
5. ABCG2 Polymorphisms in Pediatric-Onset Hyperuricemia and Early-Onset Gout
Hyperuricemia and gout pathology has often been shown to be related to genetic predisposition [30] and to be affected by SNPs in many of the genes encoding urate transporters [87,88]. Among these, especially SNPs of ABCG2 have been highly associated with pediatric-onset hyperuricemia and early-onset gout [89,90,91,92,93]. These polymorphisms are summarized and organized in Table 1 according to the standard nomenclature rules for molecular diagnostics [94]. Furthermore, the localization of these polymorphisms on the protein sequence of ABCG2 is shown in Figure 1. It should be noted that there is a subset of other function-impairing SNPs in ABCG2 [95,96], but most of them have not yet been associated with pediatric hyperuricemia or early-onset gout. One of the best-studied variations of the ABCG2 amino acid sequence is the previously discussed Q141K polymorphism, which also gives rise to other important clinical phenotypes, such as in the pharmacokinetics and tissue distribution of drugs transported by ABCG2 [97]. The polymorphism is highly associated with early-onset hyperuricemia, gout, and hyperuricemia-associated comorbidities, which cause a high mortality rate in hemodialysis patients [98]. Although the F489L polymorphism has not been as well studied in the context of disease, it shows a similar inhibitory effect on the ABCG2 transport function as the Q141K mutation. As with the Q141K mutation, ABCG2 carriers with the F489L mutation show reduced expression and reduced ABCG2 transport capacity [75]. Inhibition of proteasomal degradation could partially restore the transport function of both ABCG2 variants. In contrast to the Q141K polymorphism, which causes amino acid sequence alterations in the nucleotide-binding domain, the F489L polymorphism is localized in the transmembrane domain. This shows that the impairment of ABCG2 function can be caused by changes in amino acid structure in different domains of the transporter. Polymorphisms in the transmembrane domain have often been associated with decreased surface expression of the ABCG2 transporter and impaired substrate transport abilities [99]. However, there is not much literature to support their clinical impact in both late and early-onset hyperuricemia and gout. The clinical importance of a certain polymorphism on the development of hyperuricemia and gout usually is related to its minor allele frequency in humans and its functional impact on the protein of interest. Due to genetic drift caused by spatial separation of populations, certain polymorphisms have accumulated in different ethnicities. For example, the frequency of V12M polymorphism is high in Mexican Indians but low in Caucasian and Middle Eastern populations [97]. In contrast, the Q141K and Q126X polymorphisms are enriched in Japanese populations, whereas in Caucasians, Q141K is not as common and Q126X is virtually absent [97]. Our understanding of the genetic variations in the ABCG2 sequence associated with hyperuricemia and gout is still incomplete, as evidenced by the recent discovery of less common polymorphisms previously unrecognized or not studied in the context of hyperuricemia and gout [89,90,95,100]. Two of these newly identified rare polymorphisms have been recently described in a case report of a 12-year-old Czech girl of Roma ethnicity with chronic asymptomatic pediatric-onset of hyperuricemia [89]. In this regard, several rare diseases have been found to occur primarily or exclusively in individuals of Roma ethnicity, and many of the mutations underlying these diseases have been recently discovered, such as for Charcot-Marie tooth disease types 4D and 4G [101,102], the congenital cataract facial dysmorphism neuropathy [103], the Gitelman syndrome [104], and the Galactokinase deficiency [105]. In the afore-mentioned case of the 12-year old girl, DNA sequencing analysis of the ABCG2 gene revealed the presence of heterozygously expressed missense (c.393G > T, p.M131I) and nonsense (c.706C > T, p.R236X) mutations (Figure 1, blue residues) causing the pediatric-onset of hyperuricemia observed in the girl`s ancestry and the early-onset of gout especially in male individuals of the maternal line of inheritance. In the study, the functional consequences of the mutations were investigated in comparative in vitro experiments. Due to the in-frame stop codon induced by the R236X mutation, the ABCG2 protein sequence was truncated to about 1/3 of the full-length protein, with the mutant protein lacking a functional transmembrane domain. Therefore, no plasma membrane localization and no urate transport activity of the mutant protein could be observed. In contrast, the M131I mutation was translated to a full-length protein with no impairments in N-glycosylation at residue N596 and normal membrane localization. However, the urate transport capabilities of the M131I mutant were reduced to <15% of wildtype levels [89]. M131 itself was found to be a highly conserved residue that is localized close to the Q-loop within the nucleotide-binding domain of ABCG2 (Figure 1). The conserved glutamine Q126 in the center of the Q-loop is responsible for the coordination of the magnesium ion associated with ATP in the catalytic center of the protein [68]. M131I may thus alter the spatial orientation of the Q-loop or sterically hinder the coordination of Mg-ATP, thereby drastically reducing ABCG2′s ATP hydrolysis capabilities necessary for providing the energy for substrate transport across the membrane. Another newly identified polymorphism associated with pediatric hyperuricemia and early-onset gout is I242T, which was found in the lineage of another young European girl and was analyzed in a similar way [93]. Like the aforementioned M131I mutant, the I242T mutant ABCG2 variant showed no impairment in glycosylation and membrane localization, although its urate transport abilities were drastically reduced. This effect could be coincidentally related to the close localization of the mutants at the conserved H243 within the H-loop or also called histidine switch of the catalytic center of ABCG2 (Figure 1). The H-loop is responsible for coordinating the γ-phosphate of ATP, which is responsible for ATP hydrolysis. For further research, I242T and M131I may represent interesting new candidates to study the consequences of ABCG2 loss-of-function without disrupting ABCG2 membrane localization and protein-protein interactions. These representative case reports also show that depending on the severity of the disruption of the urate transportability of ABCG2, homo- or heterozygosity of the dysfunctional polymorphisms and further genetic predispositions in other genes involved in urate homeostasis [106], hyperuricemia can already occur in childhood (pediatric-onset), which increases the risk for the development of early-onset gout. This allows the risk allele of a particular polymorphism to be identified and considered for clinical diagnosis. Interestingly, compared to patients with late-onset gout, patients with early-onset gout also show clinical symptoms that indicate a more severe disease pattern. This includes a prolonged disease duration, a different localization of the first occurring arthritis (with a lower incidence of typical metatarsophalangeal manifestations and a higher incidence of ankle- or mid-foot involvement in early-onset gout), a higher flare frequency (gout attacks), and an increased overall number of involved joints [15,16]. In terms of gout-associated comorbidities, late-onset gout patients are more likely to suffer from chronic kidney disease, metabolic syndrome, and cardiovascular disease, a phenomenon probably related to the age difference between the two patient groups [16]. However, these comorbidities occur at a younger age in patients with early-onset gout. In contrast, a recent study showed that patients diagnosed with gout at age 40 or younger may be at increased risk for cardiovascular disease and recurrent gout compared to those diagnosed later in life [107]. In this study, of 427 adult patients diagnosed with gout at a New England multispecialty group practice, 327 who were aged 40 years or younger at diagnosis were more likely to have cardiovascular risk factors. For example, these younger patients had a significantly higher body mass index than gout patients over 40 years of age, and a substantial proportion of the younger patients also suffered from hypertension or hyperlipidemia. Moreover, early-onset gout patients were less likely to achieve a serum uric acid level below 6.0 mg/dL after therapeutic intervention as compared to late-onset gout patients. Therefore, clinical screening for hyperuricemia in genetically predisposed families and prompt urate-lowering therapy in pediatric, adolescent, or young adult patients with still asymptomatic chronic hyperuricemia could help delay the onset of gout and the development of hyperuricemia-related comorbidities [108,109,110]. With regard to the treatment of cardiovascular comorbidities in hyperuricemia patients, it should be noted that blood pressure-lowering drugs such as the AT1 receptor blocker telmisartan have been shown to inhibit the transport activity of ABCG2 [75] thereby potentially exacerbating hyperuricemia in patients with a corresponding genetic predisposition. In view of the emerging role of ABCG2 and its importance for intestinal excretion of uric acid, it may in principle represent a novel pharmacotherapeutic target to lower uric acid levels [43,44,45,46,47]. As speculation, this may be accomplished by modifying ABCG2 expression and function in intestinal epithelial cells. For example, in patients expressing mutant forms of ABCG2, this opens up the possibility of developing small molecule drugs with high pre-systemic elimination to target the function, cellular handling, or expression of ABCG2 predominantly in intestinal epithelial cells, thereby locally normalizing the impaired intestinal uric acid excretion in these individuals without interfering with the function of ABCG2 in other tissues (e.g., extrusion of xenobiotics). This area of research, therefore, shows great potential for the development of targeted pharmacotherapies for specific populations of genetically predisposed individuals with early-onset gout and thus warrants innovative research in the near future.

Table 1.
Polymorphisms in ABCG2 protein sequence associated with pediatric-onset hyperuricemia and early-onset gout.
6. Conclusions
Gout is a major health care burden in developed countries, where it affects about 1% to 2% of the adult population and is the most common cause of inflammatory arthritis in men. In addition to obesity and hyperuricemia, lifestyle changes that have developed in industrialized countries in recent decades, such as a diet rich in red meat and fructose, physical inactivity, and increased alcohol consumption, may play a role in the shift toward a younger age of manifestation of gout in the population and require early intervention. As there is evidence that early onset of hyperuricemia and gout is associated not only with a severe clinical course of gouty arthritis, but also with other comorbidities, such as hypertension, metabolic syndrome, and cardiovascular complications, early detection of hyperuricemia in younger patients with genetic predisposition and early uric acid-lowering therapy should be considered to reduce morbidity and mortality in these patients.
Author Contributions
R.E. and R.A.B. wrote the manuscript. Both authors contributed to the article and approved the submitted version. Both authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the European Regional Development Fund of the European Commission (W21029490) to R.A.B and the Publication Fund of the Martin-Luther-University Halle/Wittenberg (VAT DE 811353703).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that the review article was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Bardin, T.; Richette, P. Definition of hyperuricemia and gouty conditions. Curr. Opin. Rheumatol. 2014, 26, 186–191. [Google Scholar] [CrossRef]
- Masseoud, D.; Rott, K.; Liu-Bryan, R.; Agudelo, C. Overview of Hyperuricaemia and Gout. Curr. Pharm. Des. 2005, 11, 4117–4124. [Google Scholar] [CrossRef] [PubMed]
- Grassi, W.; De Angelis, R. Clinical features of gout. Reumatismo 2012, 63, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Kc, M.; Leslie, S.W. Uric Acid Nephrolithiasis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Lanaspa, M.A.; Andres-Hernando, A.; Kuwabara, M. Uric acid and hypertension. Hypertens. Res. 2020, 43, 832–834. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M.; Niwa, K.; Hisatome, I.; Nakagawa, T.; Roncal-Jimenez, C.A.; Andres-Hernando, A.; Bjornstad, P.; Jensen, T.; Sato, Y.; Milagres, T.; et al. Asymptomatic Hyperuricemia Without Comorbidities Predicts Cardiometabolic Diseases: Five-Year Japanese Cohort Study. Hypertension 2017, 69, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, H.; Tian, W.; Shi, L.; Chen, L.; Li, J.; Zhao, S.; Qi, G. Association between serum uric acid levels and coronary artery disease in different age and gender: A cross-sectional study. Aging Clin. Exp. Res. 2019, 31, 1783–1790. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Hsu, H.-L.; Huang, Y.-C.; Lee, M.; Huang, W.-Y.; Huang, Y.-C.; Lee, T.-H.; Lee, J.-D. Gouty Arthritis in Acute Cerebrovascular Disease. Cerebrovasc. Dis. 2009, 28, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Thottam, G.E.; Krasnokutsky, S.; Pillinger, M.H. Gout and Metabolic Syndrome: A Tangled Web. Curr. Rheumatol. Rep. 2017, 19, 60. [Google Scholar] [CrossRef]
- Ejaz, A.A.; Nakagawa, T.; Kanbay, M.; Kuwabara, M.; Kumar, A.; Arroyo, F.E.G.; Roncal-Jimenez, C.; Sasai, F.; Kang, D.-H.; Jensen, T.; et al. Hyperuricemia in Kidney Disease: A Major Risk Factor for Cardiovascular Events, Vascular Calcification, and Renal Damage. Semin. Nephrol. 2020, 40, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lozada, L.G.; Rodriguez-Iturbe, B.; Kelley, E.E.; Nakagawa, T.; Madero, M.; Feig, D.I.; Borghi, C.; Piani, F.; Cara-Fuentes, G.; Bjornstad, P.; et al. Uric Acid and Hypertension: An Update With Recommendations. Am. J. Hypertens. 2020, 33, 583–594. [Google Scholar] [CrossRef]
- Johnson, R.J.; Bakris, G.L.; Borghi, C.; Chonchol, M.B.; Feldman, D.; Lanaspa, M.A.; Merriman, T.R.; Moe, O.W.; Mount, D.B.; Lozada, L.G.S.; et al. Hyperuricemia, Acute and Chronic Kidney Disease, Hypertension, and Cardiovascular Disease: Report of a Scientific Workshop Organized by the National Kidney Foundation. Am. J. Kidney Dis. 2018, 71, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ge, J.-Y.; Zhang, Y.-Y.; Wang, F.-F.; Ji, Y.; Li, H.-Y. The relationship between elevated serum uric acid and arterial stiffness in a healthy population. Vascular 2020, 28, 494–501. [Google Scholar] [CrossRef]
- Soriano, L.C.; Rothenbacher, D.; Choi, H.K.; Rodríguez, L.A.G. Contemporary epidemiology of gout in the UK general population. Arthritis Res. Ther. 2011, 13, R39. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Fang, W.; Zeng, X.; Zhang, Y.; Ma, Y.; Sheng, F.; Zhang, X. Clinical characteristics of early- and late-onset gout: A cross-sectional observational study from a Chinese gout clinic. Medicine 2016, 95, e5425. [Google Scholar] [CrossRef]
- Pascart, T.; Norberciak, L.; Ea, H.; Guggenbuhl, P.; Lioté, F. Patients With Early-Onset Gout and Development of Earlier Severe Joint Involvement and Metabolic Comorbid Conditions: Results From a Cross-Sectional Epidemiologic Survey. Arthritis Rheum. 2019, 71, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.C. Gout—An update of aetiology, genetics, co-morbidities and management. Maturitas 2018, 118, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Sui, X.; Church, T.S.; Meriwether, R.A.; Lobelo, F.; Blair, S.N. Uric acid and the development of metabolic syndrome in women and men. Metabolism 2008, 57, 845–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estiverne, C.; Mandal, A.K.; Mount, D.B. Molecular Pathophysiology of Uric Acid Homeostasis. Semin. Nephrol. 2020, 40, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.K.; Atkinson, K.; Karlson, E.W.; Willett, W.; Curhan, G. Purine-Rich Foods, Dairy and Protein Intake, and the Risk of Gout in Men. N. Engl. J. Med. 2004, 350, 1093–1103. [Google Scholar] [CrossRef] [Green Version]
- Pui, C.-H.; Mahmoud, H.H.; Wiley, J.M.; Woods, G.M.; Leverger, G.; Camitta, B.; Hastings, C.; Blaney, S.M.; Relling, M.V.; Reaman, G.H. Recombinant Urate Oxidase for the Prophylaxis or Treatment of Hyperuricemia in Patients with Leukemia or Lymphoma. J. Clin. Oncol. 2001, 19, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.S.; Kaplow, R. Tumor Lysis Syndrome: Implications for Oncology Nursing Practice. Semin. Oncol. Nurs. 2021, 37, 151136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, L.; Zhang, Y.; Zeng, C. Recent advances in fructose intake and risk of hyperuricemia. Biomed. Pharmacother. 2020, 131, 110795. [Google Scholar] [CrossRef]
- Sayehmiri, K.; Ahmadi, I.; Anvari, E. Fructose Feeding and Hyperuricemia: A Systematic Review and Meta-Analysis. Clin. Nutr. Res. 2020, 9, 122–133. [Google Scholar] [CrossRef]
- Hernández-Rubio, A.; Sanvisens, A.; Bolao, F.; Pérez-Mañá, C.; García-Marchena, N.; Fernández-Prendes, C.; Muñoz, A.; Muga, R. Association of hyperuricemia and gamma glutamyl transferase as a marker of metabolic risk in alcohol use disorder. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Choi, H.K.; McCormick, N.; Lu, N.; Rai, S.K.; Yokose, C.; Zhang, Y. Population Impact Attributable to Modifiable Risk Factors for Hyperuricemia. Arthritis Rheumatol. 2020, 72, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Dehlin, M.; Jacobsson, L.; Roddy, E. Global epidemiology of gout: Prevalence, incidence, treatment patterns and risk factors. Nat. Rev. Rheumatol. 2020, 16, 380–390. [Google Scholar] [CrossRef]
- Nanagiri, A.; Shabbir, N. Lesch Nyhan Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Krishnan, E.; Lessov-Schlaggar, C.N.; Krasnow, R.E.; Swan, G.E. Nature Versus Nurture in Gout: A Twin Study. Am. J. Med. 2012, 125, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J.; Langlois, V.; Noone, D. Hyperuricemia and Hypertension: Links and Risks. Integr. Blood Press. Control 2019, 12, 43–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodward, O.M. ABCG2: The molecular mechanisms of urate secretion and gout. Am. J. Physiol. Physiol. 2015, 309, F485–F488. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Kimura, H.; Chairoungdua, A.; Shigeta, Y.; Jutabha, P.; Cha, S.H.; Hosoyamada, M.; Takeda, M.; Sekine, T.; Igarashi, T.; et al. Molecular identification of a renal urate–anion exchanger that regulates blood urate levels. Nat. Cell Biol. 2002, 417, 447–452. [Google Scholar] [CrossRef]
- Vitart, V.; Rudan, I.; Hayward, C.; Gray, N.K.; Floyd, J.; Palmer, C.N.A.; Knott, S.A.; Kolcic, I.; Polasek, O.; Graessler, J.; et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat. Genet. 2008, 40, 437–442. [Google Scholar] [CrossRef]
- Nigam, S.K.; Bhatnagar, V. The systems biology of uric acid transporters: The role of remote sensing and signaling. Curr. Opin. Nephrol. Hypertens. 2018, 27, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Dalbeth, N.; Gosling, A.L.; Gaffo, A.; Abhishek, A. Gout. Lancet 2021, 397, 1843–1855. [Google Scholar] [CrossRef]
- Bobulescu, I.A.; Moe, O.W. Renal Transport of Uric Acid: Evolving Concepts and Uncertainties. Adv. Chronic Kidney Dis. 2012, 19, 358–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- So, A.; Thorens, B. Uric acid transport and disease. J. Clin. Investig. 2010, 120, 1791–1799. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, V.F.; Kos, I.A.; Vargas-Santos, A.B.; Pinheiro, G.D.R.C.; Paiva, E.D.S. Benzbromarone in the treatment of gout. Adv. Rheumatol. 2019, 59, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoque, K.M.; Dixon, E.E.; Lewis, R.M.; Allan, J.; Gamble, G.D.; Phipps-Green, A.J.; Kuhns, V.L.H.; Horne, A.M.; Stamp, L.K.; Merriman, T.R.; et al. The ABCG2 Q141K hyperuricemia and gout associated variant illuminates the physiology of human urate excretion. Nat. Commun. 2020, 11, 2767. [Google Scholar] [CrossRef]
- DeBosch, B.J.; Kluth, O.; Fujiwara, H.; Schurmann, A.; Moley, K.H. Early-onset metabolic syndrome in mice lacking the intestinal uric acid transporter SLC2A9. Nat. Commun. 2014, 5, 4642. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, H.; Hruz, P.; Zimmermann, C.; Beglinger, C.; Drewe, J. Distribution of breast cancer resistance protein (BCRP/ABCG2) mRNA expression along the human GI tract. Biochem. Pharmacol. 2005, 70, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lin, H.; Niu, Y.; Liu, Y.; Wang, Z.; Song, L.; Gao, L.; Li, L. Mangiferin promotes intestinal elimination of uric acid by modulating intestinal transporters. Eur. J. Pharmacol. 2020, 888, 173490. [Google Scholar] [CrossRef]
- Chen, M.; Ye, C.; Zhu, J.; Zhang, P.; Jiang, Y.; Lu, X.; Wu, H. Bergenin as a Novel Urate-Lowering Therapeutic Strategy for Hyperuricemia. Front. Cell Dev. Biol. 2020, 8, 703. [Google Scholar] [CrossRef]
- Chen, X.; Ge, H.-Z.; Lei, S.-S.; Jiang, Z.-T.; Su, J.; He, X.; Zheng, X.; Wang, H.-Y.; Yu, Q.-X.; Li, B.; et al. Dendrobium officinalis six nostrum ameliorates urate under-excretion and protects renal dysfunction in lipid emulsion-induced hyperuricemic rats. Biomed. Pharmacother. 2020, 132, 110765. [Google Scholar] [CrossRef] [PubMed]
- Ristic, B.; Sikder, M.O.F.; Bhutia, Y.D.; Ganapathy, V. Pharmacologic inducers of the uric acid exporter ABCG2 as potential drugs for treatment of gouty arthritis. Asian J. Pharm. Sci. 2020, 15, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-H.; Chang, Y.-P.; Li, T.; Han, F.; Li, C.-J.; Li, X.-Y.; Xue, M.; Cheng, Y.; Meng, Z.-Y.; Han, Z.; et al. Empagliflozin Attenuates Hyperuricemia by Upregulation of ABCG2 via AMPK/AKT/CREB Signaling Pathway in Type 2 Diabetic Mice. Int. J. Biol. Sci. 2020, 16, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, A.; Köttgen, A.; Yang, Q.; Hwang, S.-J.; Kao, W.L.; Rivadeneira, F.; Boerwinkle, E.; Levy, D.; Hofman, A.; Astor, B.C.; et al. Association of three genetic loci with uric acid concentration and risk of gout: A genome-wide association study. Lancet 2008, 372, 1953–1961. [Google Scholar] [CrossRef] [Green Version]
- Richette, P.; Bardin, T. Gout. Lancet 2010, 375, 318–328. [Google Scholar] [CrossRef]
- Woodward, O.M.; Köttgen, M.; Coresh, J.; Boerwinkle, E.; Guggino, W.B. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc. Natl. Acad. Sci. USA 2009, 106, 10338–10342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosomi, A.; Nakanishi, T.; Fujita, T.; Tamai, I. Extra-Renal Elimination of Uric Acid via Intestinal Efflux Transporter BCRP/ABCG2. PLoS ONE 2012, 7, e30456. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, H.; Takada, T.; Ichida, K.; Nakamura, T.; Nakayama, A.; Ikebuchi, Y.; Ito, K.; Kusanagi, Y.; Chiba, T.; Tadokoro, S.; et al. Common Defects of ABCG2, a High-Capacity Urate Exporter, Cause Gout: A Function-Based Genetic Analysis in a Japanese Population. Sci. Transl. Med. 2009, 1, 5ra11. [Google Scholar] [CrossRef]
- Dean, M.; Hamon, Y.; Chimini, G. The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res. 2001, 42, 1007–1017. [Google Scholar] [CrossRef]
- Mo, W.; Zhang, J.-T. Human ABCG2: Structure, function, and its role in multidrug resistance. Int. J. Biochem. Mol. Boil. 2011, 3, 1–27. [Google Scholar]
- Polgar, O.; Robey, R.W.; Bates, S.E. ABCG2: Structure, function and role in drug response. Expert Opin. Drug Metab. Toxicol. 2007, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q. BCRP/ABCG2 in the placenta: Expression, function and regulation. Pharm. Res. 2008, 25, 1244–1255. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Mojsilovic-Petrovic, J.; Andrade, M.F.; Zhang, H.; Ball, M.; Stanimirovic, D.B. Expression and functional characterization of ABCG2 in brain endothelial cells and vessels. FASEB J. 2003, 17, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Maliepaard, M.; Scheffer, G.L.; Faneyte, I.F.; Van Gastelen, M.A.; Pijnenborg, A.C.; Schinkel, A.H.; Van De Vijver, M.J.; Scheper, R.J.; Schellens, J.H. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001, 61, 3458–3464. [Google Scholar] [PubMed]
- Huls, M.; Brown, C.; Windass, A.; Sayer, R.; Heuvel, J.V.D.; Heemskerk, S.; Russel, F.; Masereeuw, R.; Huls, M.; Brown, C.; et al. The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int. 2008, 73, 220–225. [Google Scholar] [CrossRef] [Green Version]
- Sarkadi, B.; Ozvegy-Laczka, C.; Német, K.; Váradi, A. ABCG2—A transporter for all seasons. FEBS Lett. 2004, 567, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.; Ross, D.D. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 2003, 22, 7340–7358. [Google Scholar] [CrossRef] [Green Version]
- Robey, R.W.; Polgar, O.; Deeken, J.; To, K.; Bates, S.E. ABCG2: Determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 2007, 26, 39–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.E.; Singh, R.R.; Cho-Vega, J.H.; Drakos, E.; Davuluri, Y.; Khokhar, F.A.; Fayad, L.; Medeiros, L.J.; Vega, F. Sonic hedgehog signaling proteins and ATP-binding cassette G2 are aberrantly expressed in diffuse large B-Cell lymphoma. Mod. Pathol. 2009, 22, 1312–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damiani, D.; Tiribelli, M.; Calistri, E.; Geromin, A.; Chiarvesio, A.; Michelutti, A.; Cavallin, M.; Fanin, R. The prognostic value of P-glycoprotein (ABCB) and breast cancer resistance protein (ABCG2) in adults with de novo acute myeloid leukemia with normal karyotype. Haematologica 2006, 91, 825–828. [Google Scholar] [PubMed]
- Taylor, N.M.I.; Manolaridis, I.; Jackson, S.M.; Kowal, J.; Stahlberg, H.; Locher, K.P. Structure of the human multidrug transporter ABCG2. Nat. Cell Biol. 2017, 546, 504–509. [Google Scholar] [CrossRef]
- Kowal, J.; Ni, D.; Jackson, S.M.; Manolaridis, I.; Stahlberg, H.; Locher, K.P. Structural Basis of Drug Recognition by the Multidrug Transporter ABCG2. J. Mol. Biol. 2021, 433, 166980. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.M.; Manolaridis, I.; Kowal, J.; Zechner, M.; Taylor, N.M.I.; Bause, M.; Bauer, S.; Bartholomaeus, R.; Bernhardt, G.; Koenig, B.; et al. Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat. Struct. Mol. Biol. 2018, 25, 333–340. [Google Scholar] [CrossRef]
- Manolaridis, I.; Jackson, S.M.; Taylor, N.M.I.; Kowal, J.; Stahlberg, H.; Locher, K.P. Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states. Nat. Cell Biol. 2018, 563, 426–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckenstaler, R.; Benndorf, R.A. 3D structure of the transporter ABCG2-What’s new? Br. J. Pharmacol. 2020, 177, 1485–1496. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, P.; Horsey, A.J.; Cox, M.H.; Kerr, I.D. ABCG2: Does resolving its structure elucidate the mechanism? Biochem. Soc. Trans. 2018, 46, 1485–1494. [Google Scholar] [CrossRef]
- Ichida, K.; Matsuo, H.; Takada, T.; Nakayama, A.; Murakami, K.; Shimizu, T.; Yamanashi, Y.; Kasuga, H.; Nakashima, H.; Nakamura, T.; et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat. Commun. 2012, 3, 764. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Guo, S.; Yang, Y.; Wu, J.; Guan, M.; Zou, H.; Jin, L.; Wang, J. Association between ABCG2 Q141K polymorphism and gout risk affected by ethnicity and gender: A systematic review and meta-analysis. Int. J. Rheum. Dis. 2015, 18, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Kannangara, D.; Phipps-Green, A.J.; Dalbeth, N.; Stamp, L.K.; Williams, K.M.; Graham, G.G.; Day, R.O.; Merriman, T.R. Hyperuricaemia: Contributions of urate transporter ABCG2 and the fractional renal clearance of urate. Ann. Rheum. Dis. 2015, 75, 1363–1366. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Miao, L.; Qin, L.; Xiang, Y.; Zhang, X.; Peng, H.; Mailamuguli; Sun, Y.; Yao, H. A meta-analysis of the associations between the Q141K and Q126X ABCG2 gene variants and gout risk. Int. J. Clin. Exp. Pathol. 2015, 8, 9812–9823. [Google Scholar] [PubMed]
- Deppe, S.; Ripperger, A.; Weiss, J.; Ergün, S.; Benndorf, R.A. Impact of genetic variability in the ABCG2 gene on ABCG2 expression, function, and interaction with AT1 receptor antagonist telmisartan. Biochem. Biophys. Res. Commun. 2014, 443, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Ripperger, A.; Benndorf, R.A. The C421A (Q141K) polymorphism enhances the 3′-untranslated region (3′-UTR)-dependent regulation of ATP-binding cassette transporter ABCG2. Biochem. Pharmacol. 2016, 104, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Woodward, O.M.; Tukaye, D.N.; Cui, J.; Greenwell, P.; Constantoulakis, L.M.; Parker, B.S.; Rao, A.; Köttgen, M.; Maloney, P.C.; Guggino, W.B. Gout-causing Q141K mutation in ABCG2 leads to instability of the nucleotide-binding domain and can be corrected with small molecules. Proc. Natl. Acad. Sci. USA 2013, 110, 5223–5228. [Google Scholar] [CrossRef] [Green Version]
- László, L.; Sarkadi, B.; Hegedűs, T. Jump into a New Fold—A Homology Based Model for the ABCG2/BCRP Multidrug Transporter. PLoS ONE 2016, 11, e0164426. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, T.; Wakabayashi, K.; Tamura, A.; Nakagawa, H.; Morishima, Y.; Osawa, Y.; Ishikawa, T. Major SNP (Q141K) Variant of Human ABC Transporter ABCG2 Undergoes Lysosomal and Proteasomal Degradations. Pharm. Res. 2008, 26, 469–479. [Google Scholar] [CrossRef] [Green Version]
- Bartos, Z.; Homolya, L. Identification of Specific Trafficking Defects of Naturally Occurring Variants of the Human ABCG2 Transporter. Front. Cell Dev. Biol. 2021, 9, 615729. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Nakayama, A.; Matsuo, H.; Takada, T.; Ichida, K.; Nakamura, T.; Ikebuchi, Y.; Ito, K.; Hosoya, T.; Kanai, Y.; Suzuki, H.; et al. ABCG2 is a High-Capacity Urate Transporter and its Genetic Impairment Increases Serum Uric Acid Levels in Humans. Nucleosides Nucleotides Nucleic Acids 2011, 30, 1091–1097. [Google Scholar] [CrossRef]
- Varela-Echavarría, A.; Oca-Luna, R.M.; Barrera-Saldaña, H.A. Uricase protein sequences: Conserved during vertebrate evolution but absent in humans. FASEB J. 1988, 2, 3092–3096. [Google Scholar] [CrossRef]
- Morimoto, C.; Tamura, Y.; Asakawa, S.; Kuribayashi-Okuma, E.; Nemoto, Y.; Li, J.; Murase, T.; Nakamura, T.; Hosoyamada, M.; Uchida, S.; et al. ABCG2 expression and uric acid metabolism of the intestine in hyperuricemia model rat. Nucleosides Nucleotides Nucleic Acids 2020, 39, 744–759. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Takada, T.; Nakayama, A.; Shimizu, T.; Sakiyama, M.; Shimizu, S.; Chiba, T.; Nakashima, H.; Nakamura, T.; Takada, Y.; et al. ABCG2 Dysfunction Increases the Risk of Renal Overload Hyperuricemia. Nucleosides Nucleotides Nucleic Acids 2014, 33, 266–274. [Google Scholar] [CrossRef]
- Matsuo, H.; Nakayama, A.; Sakiyama, M.; Chiba, T.; Shimizu, S.; Kawamura, Y.; Nakashima, H.; Nakamura, T.; Takada, Y.; Oikawa, Y.; et al. ABCG2 dysfunction causes hyperuricemia due to both renal urate underexcretion and renal urate overload. Sci. Rep. 2014, 4, 3755. [Google Scholar] [CrossRef] [PubMed]
- Tin, A.; Marten, J.; Halperin Kuhns, V.L.; Li, Y.; Wuttke, M.; Kirsten, H.; Sieber, K.B.; Qiu, C.; Gorski, M.; Yu, Z.; et al. Target genes, variants, tissues and transcriptional pathways influencing human serum urate levels. Nat. Genet. 2019, 51, 1459–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukkunaprasit, T.; Rattanasiri, S.; Turongkaravee, S.; Suvannang, N.; Ingsathit, A.; Attia, J.; Thakkinstian, A. The association between genetic polymorphisms in ABCG2 and SLC2A9 and urate: An updated systematic review and meta-analysis. BMC Med. Genet. 2020, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, Y.; Pavelcová, K.; Bohatá, J.; Ješina, P.; Kubota, Y.; Suzuki, H.; Takada, T.; Stiburkova, B. Identification of Two Dysfunctional Variants in the ABCG2 Urate Transporter Associated with Pediatric-Onset of Familial Hyperuricemia and Early-onset Gout. Int. J. Mol. Sci. 2021, 22, 1935. [Google Scholar] [CrossRef] [PubMed]
- Stiburkova, B.; Pavelcova, K.; Pavlikova, M.; Ješina, P.; Pavelka, K. The impact of dysfunctional variants of ABCG2 on hyperuricemia and gout in pediatric-onset patients. Arthritis Res. 2019, 21, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, F.; Narang, R.K.; Phipps-Green, A.; Gamble, G.G.; Tausche, A.-K.; So, A.; Riches, P.; Andres, M.; Perez-Ruiz, F.; Doherty, M.; et al. Systematic genetic analysis of early-onset gout: ABCG2 is the only associated locus. Rheumatology 2020, 59, 2544–2549. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Ichida, K.; Takada, T.; Nakayama, A.; Nakashima, H.; Nakamura, T.; Kawamura, Y.; Takada, Y.; Yamamoto, K.; Inoue, H.; et al. Common dysfunctional variants in ABCG2 are a major cause of early-onset gout. Sci. Rep. 2013, 3, srep02014. [Google Scholar] [CrossRef] [Green Version]
- Toyoda, Y.; Pavelcová, K.; Klein, M.; Suzuki, H.; Takada, T.; Stiburkova, B. Familial early-onset hyperuricemia and gout associated with a newly identified dysfunctional variant in urate transporter ABCG2. Arthritis Res. 2019, 21, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Ogino, S.; Gulley, M.L.; Dunnen, J.T.D.; Wilson, R.B. Standard Mutation Nomenclature in Molecular Diagnostics: Practical and Educational Challenges. J. Mol. Diagn. 2007, 9, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Homolya, L. Medically Important Alterations in Transport Function and Trafficking of ABCG2. Int. J. Mol. Sci. 2021, 22, 2786. [Google Scholar] [CrossRef] [PubMed]
- Zámbó, B.; Mózner, O.; Bartos, Z.; Török, G.; Várady, G.; Telbisz, Á.; Homolya, L.; Orbán, T.I.; Sarkadi, B. Cellular expression and function of naturally occurring variants of the human ABCG2 multidrug transporter. Cell. Mol. Life Sci. 2020, 77, 365–378. [Google Scholar] [CrossRef] [Green Version]
- Heyes, N.; Kapoor, P.; Kerr, I.D. Polymorphisms of the Multidrug Pump ABCG2: A Systematic Review of Their Effect on Protein Expression, Function, and Drug Pharmacokinetics. Drug Metab. Dispos. 2018, 46, 1886–1899. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, A.; Ichida, K.; Ohkido, I.; Yokoyama, K.; Matsuo, H.; Ohashi, Y.; Takada, T.; Nakayama, A.; Suzuki, H.; Shinomiya, N.; et al. Dysfunctional ABCG2 gene polymorphisms are associated with serum uric acid levels and all-cause mortality in hemodialysis patients. Hum. Cell 2020, 33, 559–568. [Google Scholar] [CrossRef] [Green Version]
- Sjöstedt, N.; Heuvel, J.J.; Koenderink, J.B.; Kidron, H. Transmembrane Domain Single-Nucleotide Polymorphisms Impair Expression and Transport Activity of ABC Transporter ABCG2. Pharm. Res. 2017, 34, 1626–1636. [Google Scholar] [CrossRef] [Green Version]
- Toyoda, Y.; Mančíková, A.; Krylov, V.; Morimoto, K.; Pavelcová, K.; Bohatá, J.; Pavelka, K.; Pavlikova, M.; Suzuki, H.; Matsuo, H.; et al. Functional Characterization of Clinically-Relevant Rare Variants in ABCG2 Identified in a Gout and Hyperuricemia Cohort. Cells 2019, 8, 363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Liu, G.; Liu, Z.; Gong-Lu, L.; Wu, Z. Identification and functional characterization of two missense mutations in NDRG1 associated with Charcot-Marie-Tooth disease type 4D. Hum. Mutat. 2017, 38, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Gabrikova, D.; Mistrik, M.; Bernasovska, J.; Bozikova, A.; Behulova, R.; Tothova, I.; Macekova, S. Founder mutations in NDRG1 and HK1 genes are common causes of inherited neuropathies among Roma/Gypsies in Slovakia. J. Appl. Genet. 2013, 54, 455–460. [Google Scholar] [CrossRef]
- Lassuthova, P.; Sišková, D.; Haberlová, J.; Sakmaryová, I.; Filouš, A.; Seeman, P. Congenital cataract, facial dysmorphism and demyelinating neuropathy (CCFDN) in 10 Czech Gypsy children—Frequent and underestimated cause of disability among Czech Gypsies. Orphanet J. Rare. Dis. 2014, 9, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil-Peña, H.; Coto, E.; Santos, F.; Espino, M.; Cea Crespo, J.M.; Chantzopoulos, G.; Komianou, F.; Gómez, J.; Alonso, B.; Iglesias, S.; et al. A new SLC12A3 founder mutation (p.Val647Met) in Gitelman’s syndrome patients of Roma ancestry. Nefrologia 2017, 37, 423–428. [Google Scholar] [CrossRef]
- Schulpis, K.H.; Thodi, G.; Iakovou, K.; Chatzidaki, M.; Dotsikas, Y.; Molou, E.; Triantafylli, O.; Loukas, Y.L. Clinical evaluation and mutational analysis of GALK and GALE genes in patients with galactosemia in Greece: One novel mutation and two rare cases. J. Pediatr. Endocrinol. Metab. 2017, 30, 775–779. [Google Scholar] [CrossRef]
- Butler, F.; Alghubayshi, A.; Roman, Y. The Epidemiology and Genetics of Hyperuricemia and Gout across Major Racial Groups: A Literature Review and Population Genetics Secondary Database Analysis. J. Pers. Med. 2021, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Piranavan, P.; Sundaresan, D.; Yood, R. Clinical Characteristics of Early-Onset Gout in Outpatient Setting. ACR Open Rheumatol. 2019, 1, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Singer, R.F.; Walters, G. Uric acid lowering therapies for preventing or delaying the progression of chronic kidney disease. Cochrane Database Syst. Rev. 2011, 10, 10. [Google Scholar] [CrossRef]
- Ying, H.; Yuan, H.; Tang, X.; Guo, W.; Jiang, R.; Jiang, C. Impact of Serum Uric Acid Lowering and Contemporary Uric Acid-Lowering Therapies on Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 641062. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.J.; Anoopkumar, K.; Krishnan, V. Asymptomatic hyperuricemia: Is it time to intervene? Clin. Rheumatol. 2017, 36, 2637–2644. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).