Role of Receptor Protein Tyrosine Phosphatase β/ζ in Neuron–Microglia Communication in a Cellular Model of Parkinson’s Disease
Abstract
:1. Introduction
2. Results
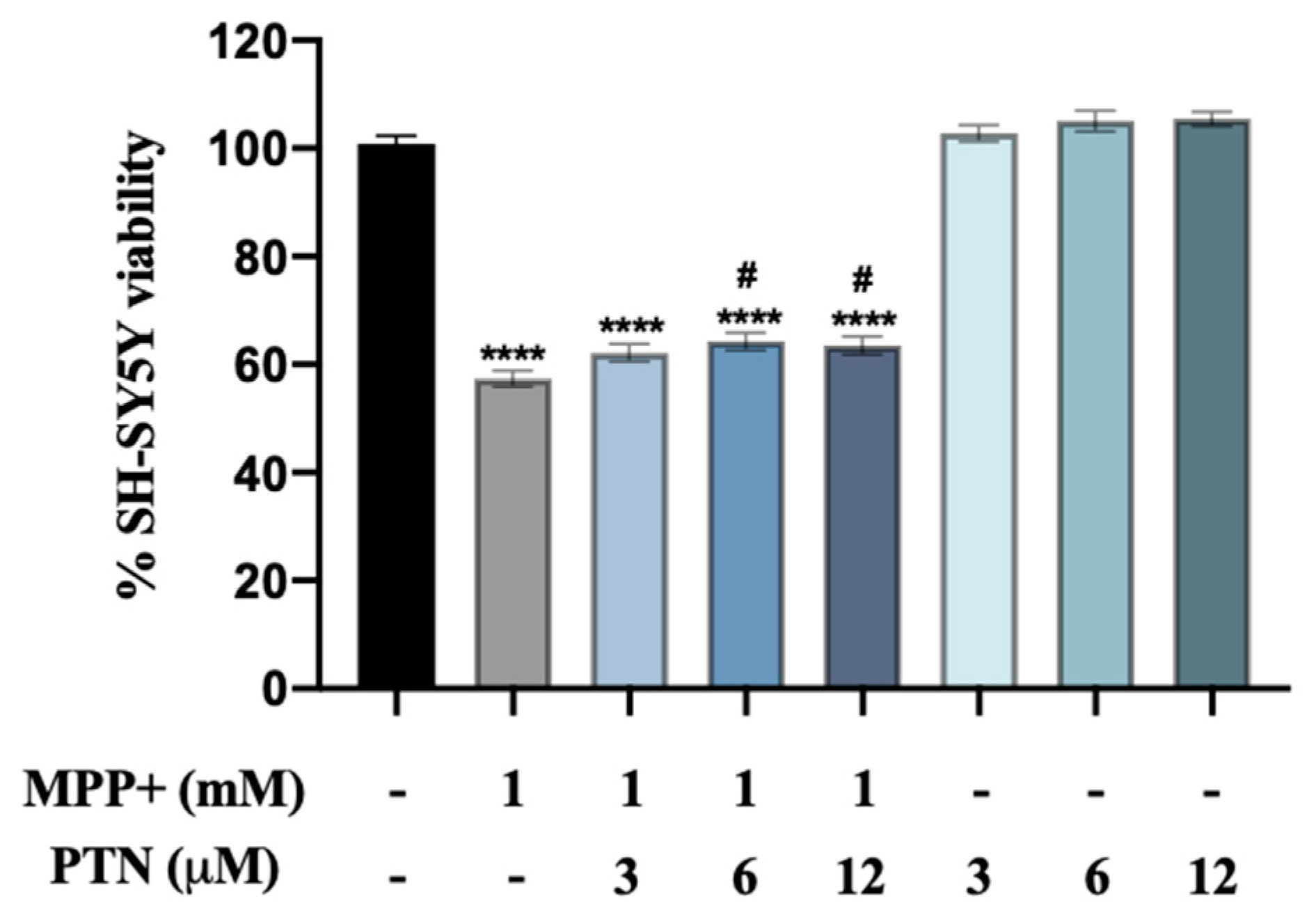
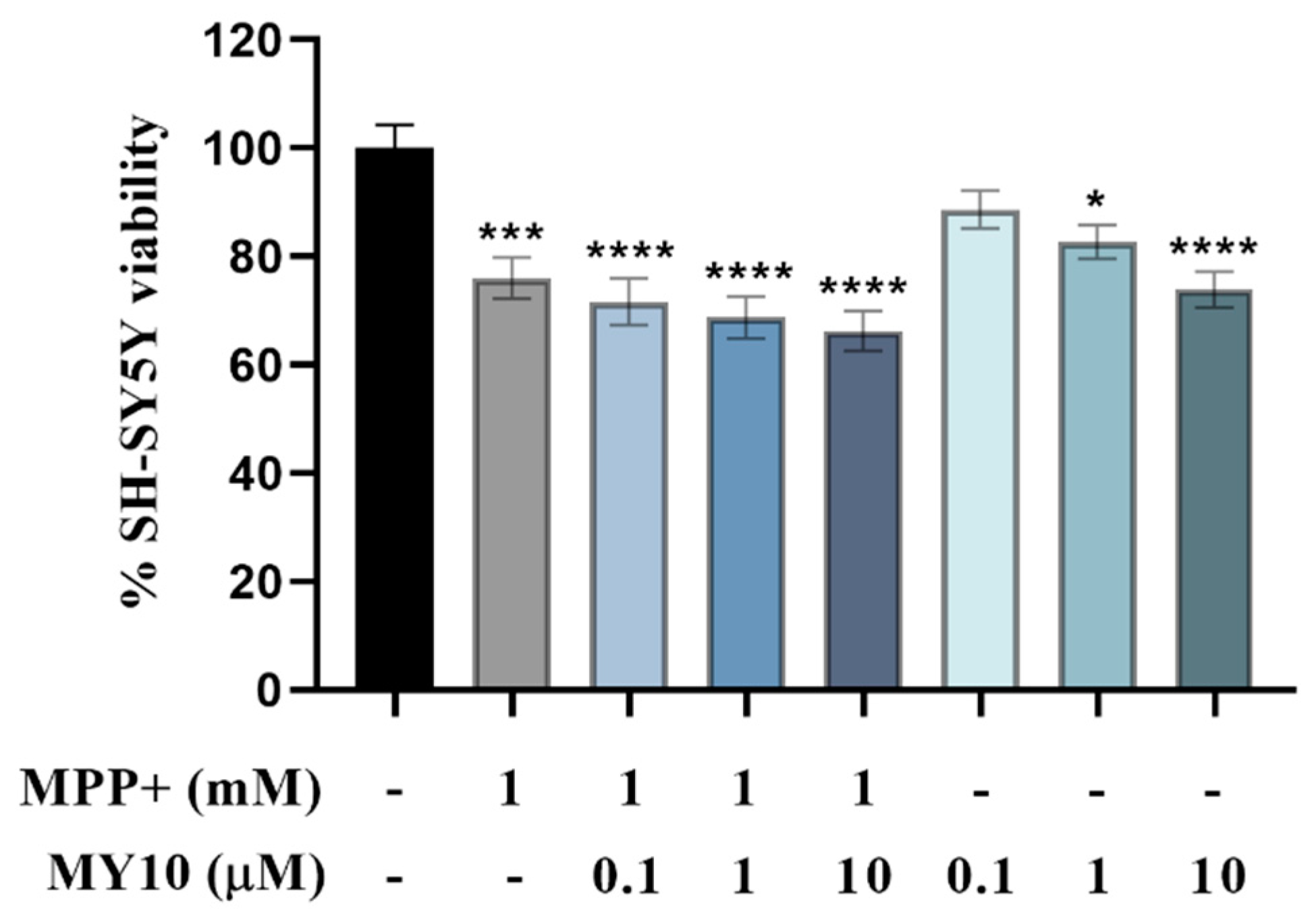
2.1. PTN but Not MY10 Partially Protects against MPP+ SH-SY5Y Cell Toxicity
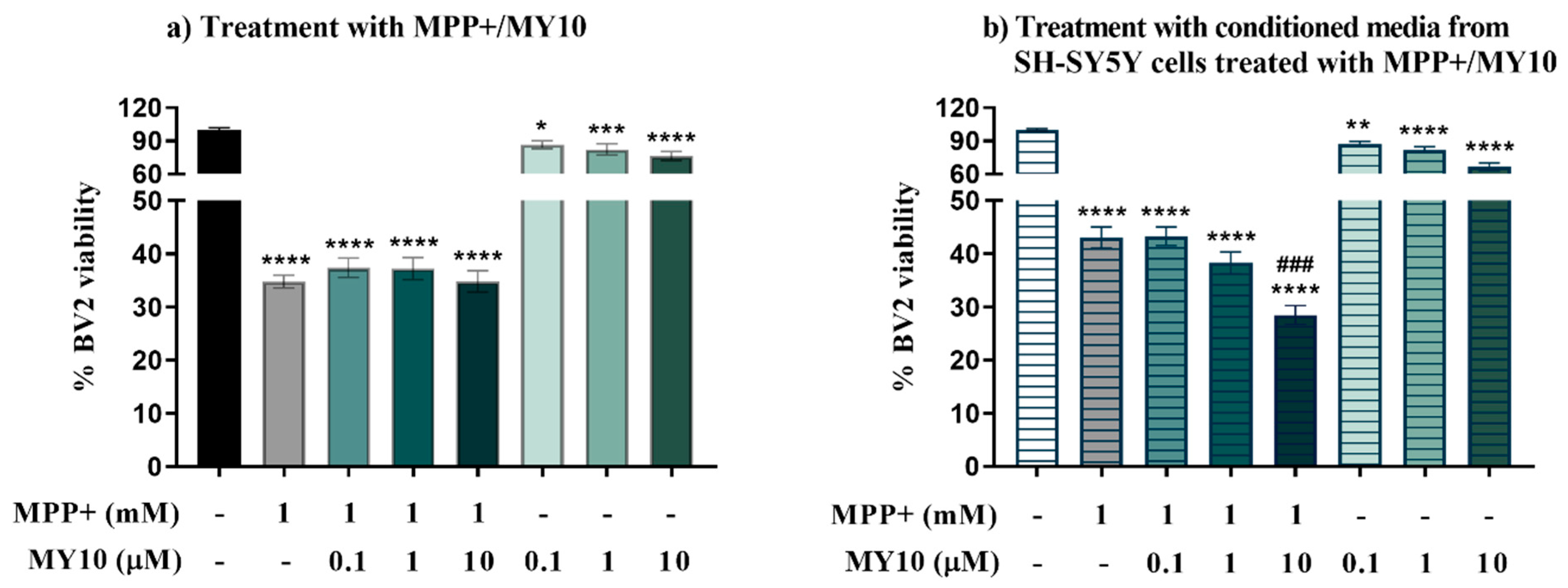
2.2. Conditioned Media from MPP+-Injured SH-SY5Y Cells Treated with the Rptpβ/Ζ Inhibitor MY10, Aggravates the MPP+-Induced Loss of Microglial Viability
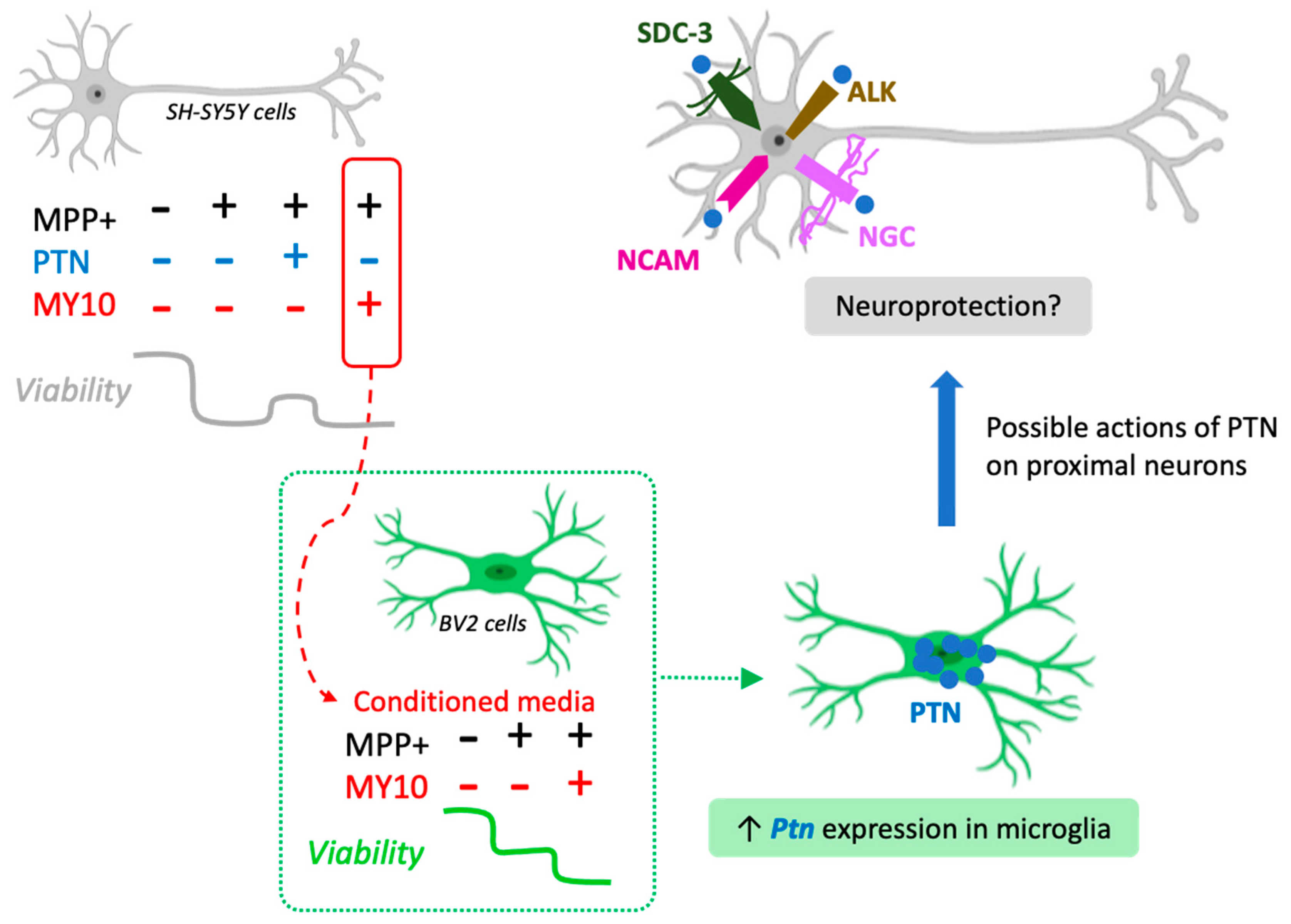
2.3. Upregulated Ptn Expression in Microglia Injured by Media Of MPP+-Treated SH-SY5Y Cells
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Treatments
4.1.1. Neuroblastoma SH-SY5Y Cells
4.1.2. BV2 Microglial Cell Cultures
4.2. Cell Viability–MTT Assay
4.3. RNA Extraction and cDNA Synthesis
4.4. Real-Time Quantitative-PCR Analysis
5. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hauser, D.N.; Hastings, T.G. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease and monogenic parkinsonism. Neurobiol. Dis. 2013, 51, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, O.A.; Malagelada, C.; Greene, L.A. Cell death pathways in Parkinson’s disease: Proximal triggers, distal effectors, and final steps. Apoptosis 2009, 14, 478–500. [Google Scholar] [CrossRef] [PubMed]
- Harms, A.S.; Ferreira, S.A.; Romero-Ramos, M. Periphery and brain, innate and adaptive immunity in Parkinson’s disease. Acta Neuropathol. 2021, 141, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Nicola, D.; Perry, V.H. Microglial Dynamics and Role in the Healthy and Diseased Brain. Neuroscientist 2015, 21, 169–184. [Google Scholar] [CrossRef] [Green Version]
- Troncoso-Escudero, P.; Parra, A.; Nassif, M.; Vidal, R.L. Outside in: Unraveling the Role of Neuroinflammation in the Progression of Parkinson’s Disease. Front. Neurol. 2018, 9. [Google Scholar] [CrossRef]
- Reale, M.; Iarlori, C.; Thomas, A.; Gambi, D.; Perfetti, B.; Di Nicola, M.; Onofrj, M. Peripheral cytokines profile in Parkinson’s disease. Brain Behav. Immun. 2009, 23, 55–63. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Y.; Cao, Z.; Liu, Q.; Cheng, Y. Cerebrospinal fluid inflammatory cytokine aberrations in Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis: A systematic review and meta-analysis. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Gerhard, A.; Pavese, N.; Hotton, G.; Turkheimer, F.; Es, M.; Hammers, A.; Eggert, K.; Oertel, W.; Banati, R.B.; Brooks, D.J. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol. Dis. 2006, 21, 404–412. [Google Scholar] [CrossRef]
- Szepesi, Z.; Manouchehrian, O.; Bachiller, S.; Deierborg, T. Bidirectional Microglia–Neuron Communication in Health and Disease. Front. Cell. Neurosci. 2018, 12. [Google Scholar] [CrossRef]
- Bartels, T.; De Schepper, S.; Hong, S. Microglia modulate neurodegeneration in Alzheimer’s and Parkinson’s diseases. Science 2020, 370, 66–69. [Google Scholar] [CrossRef]
- Nasrolahi, A.; Mahmoudi, J.; Akbarzadeh, A.; Karimipour, M.; Sadigh-Eteghad, S.; Salehi, R.; Farhoudi, M. Neurotrophic factors hold promise for the future of Parkinson’s disease treatment: Is there a light at the end of the tunnel? Rev. Neurosci. 2018, 29, 475–489. [Google Scholar] [CrossRef]
- Pöyhönen, S.; Er, S.; Domanskyi, A.; Airavaara, M. Effects of Neurotrophic Factors in Glial Cells in the Central Nervous System: Expression and Properties in Neurodegeneration and Injury. Front. Physiol. 2019, 10, 486. [Google Scholar] [CrossRef]
- Herradón, G.; Pérez-García, C. Targeting midkine and pleiotrophin signalling pathways in addiction and neurodegenerative disorders: Recent progress and perspectives. Br. J. Pharmacol. 2014, 171, 837–848. [Google Scholar] [CrossRef] [Green Version]
- Herradon, G.; Ramos-Alvarez, M.P.; Gramage, E. Connecting Metainflammation and Neuroinflammation Through the PTN-MK-RPTPβ/ζ Axis: Relevance in Therapeutic Development. Front. Pharmacol. 2019, 10, 377. [Google Scholar] [CrossRef]
- González-Castillo, C.; Ortuño-Sahagún, D.; Guzmán-Brambila, C.; Pallàs, M.; Rojas-Mayorquí n, A.E. Pleiotrophin as a central nervous system neuromodulator, evidences from the hippocampus. Front. Cell. Neurosci. 2015, 8, 8–443. [Google Scholar] [CrossRef] [Green Version]
- Maeda, N.; Hamanaka, H.; Shintani, T.; Nishiwaki, T.; Noda, M. Multiple receptor-like protein tyrosine phosphatases in the form of chondroitin sulfate proteoglycan. FEBS Lett. 1994, 354, 67–70. [Google Scholar] [CrossRef] [Green Version]
- Herradon, G.; Ezquerra, L. Blocking Receptor Protein Tyrosine Phosphatase β/ζ: A Potential Therapeutic Strategy for Parkinsons Disease. Curr. Med. Chem. 2009, 16, 3322–3329. [Google Scholar] [CrossRef]
- Perez-Pinera, P.; Zhang, W.; Chang, Y.; Vega, J.A.; Deuel, T.F. Anaplastic lymphoma kinase is activated through the pleiotrophin/receptor protein-tyrosine phosphatase β/ζ signaling pathway: An alternative mechanism of receptor tyrosine kinase activation. J. Biol. Chem. 2007, 282, 28683–28690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pariser, H.; Ezquerra, L.; Herradon, G.; Perez-Pinera, P.; Deuel, T.F. Fyn is a downstream target of the pleiotrophin/receptor protein tyrosine phosphatase β/ζ-signaling pathway: Regulation of tyrosine phosphorylation of Fyn by pleiotrophin. Biochem. Biophys. Res. Commun. 2005, 332, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Panicker, N.; Saminathan, H.; Jin, H.; Neal, M.; Harischandra, D.S.; Gordon, R.; Kanthasamy, K.; Lawana, V.; Sarkar, S.; Luo, J.; et al. Fyn kinase regulates microglial neuroinflammatory responses in cell culture and animal models of parkinson’s disease. J. Neurosci. 2015, 35, 10058–10077. [Google Scholar] [CrossRef] [PubMed]
- Pastor, M.; Fernández-Calle, R.; Di Geronimo, B.; Vicente-Rodríguez, M.; Zapico, J.M.; Gramage, E.; Coderch, C.; Pérez-García, C.; Lasek, A.W.; Puchades-Carrasco, L.; et al. Development of inhibitors of receptor protein tyrosine phosphatase β/ζ (PTPRZ1) as candidates for CNS disorders. Eur. J. Med. Chem. 2018, 144, 318–329. [Google Scholar] [CrossRef]
- Fernández-Calle, R.; Vicente-Rodríguez, M.; Gramage, E.; Pita, J.; Pérez-García, C.; Ferrer-Alcón, M.; Uribarri, M.; Ramos, M.P.; Herradón, G. Pleiotrophin regulates microglia-mediated neuroinflammation. J. Neuroinflamm. 2017, 14, 46. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Calle, R.; Galán-Llario, M.; Gramage, E.; Zapatería, B.; Vicente-Rodríguez, M.; Zapico, J.M.; de Pascual-Teresa, B.; Ramos, A.; Ramos-Álvarez, M.P.; Uribarri, M.; et al. Role of RPTPβ/ζ in neuroinflammation and microglia-neuron communication. Sci. Rep. 2020, 10, 20259. [Google Scholar] [CrossRef]
- Kim, C.; Park, S. IGF-1 protects SH-SY5Y cells against MPP+-induced apoptosis via PI3K/PDK-1/Akt pathway. Endocr. Connect. 2018, 7, 443–455. [Google Scholar] [CrossRef] [Green Version]
- Marchionini, D.M.; Lehrmann, E.; Chu, Y.; He, B.; Sortwell, C.E.; Becker, K.G.; Freed, W.J.; Kordower, J.H.; Collier, T.J. Role of heparin binding growth factors in nigrostriatal dopamine system development and Parkinson’s disease. Brain Res. 2007, 1147, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Calle, R.; Vicente-Rodríguez, M.; Gramage, E.; de la Torre-Ortiz, C.; Pérez-García, C.; Ramos, M.P.; Herradón, G. Endogenous pleiotrophin and midkine regulate LPS-induced glial responses. Neurosci. Lett. 2018, 662, 213–218. [Google Scholar] [CrossRef]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lull, M.E.; Block, M.L. Microglial Activation and Chronic Neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dheen, S.T.; Kaur, C.; Ling, E.-A. Microglial activation and its implications in the brain diseases. Curr. Med. Chem. 2007, 14, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Morales, L.; Pérez-García, C.; Salas, E.; González-Martín, C.; Castillo, C.; Polanco, M.J.; Herradón, G.; Alguacil, L.F. Behavioral and in vitro evaluation of tetrodotoxin tolerability for therapeutic applications. Toxicon 2008, 51, 1530–1534. [Google Scholar] [CrossRef]
- Ezquerra, L.; Herradón, G.; Nguyen, T.; Vogt, T.F.; Bronson, R.; Silos-Santiago, I.; Deuel, T.F. Pleiotrophin is a major regulator of the catecholamine biosynthesis pathway in mouse aorta. Biochem. Biophys. Res. Commun. 2004, 323, 512–517. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Campo, M.; Fernández-Calle, R.; Vicente-Rodríguez, M.; Martín Martínez, S.; Gramage, E.; Zapico, J.M.; Haro, M.; Herradon, G. Role of Receptor Protein Tyrosine Phosphatase β/ζ in Neuron–Microglia Communication in a Cellular Model of Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 6646. https://doi.org/10.3390/ijms22136646
del Campo M, Fernández-Calle R, Vicente-Rodríguez M, Martín Martínez S, Gramage E, Zapico JM, Haro M, Herradon G. Role of Receptor Protein Tyrosine Phosphatase β/ζ in Neuron–Microglia Communication in a Cellular Model of Parkinson’s Disease. International Journal of Molecular Sciences. 2021; 22(13):6646. https://doi.org/10.3390/ijms22136646
Chicago/Turabian Styledel Campo, Marta, Rosalía Fernández-Calle, Marta Vicente-Rodríguez, Sara Martín Martínez, Esther Gramage, José María Zapico, María Haro, and Gonzalo Herradon. 2021. "Role of Receptor Protein Tyrosine Phosphatase β/ζ in Neuron–Microglia Communication in a Cellular Model of Parkinson’s Disease" International Journal of Molecular Sciences 22, no. 13: 6646. https://doi.org/10.3390/ijms22136646
APA Styledel Campo, M., Fernández-Calle, R., Vicente-Rodríguez, M., Martín Martínez, S., Gramage, E., Zapico, J. M., Haro, M., & Herradon, G. (2021). Role of Receptor Protein Tyrosine Phosphatase β/ζ in Neuron–Microglia Communication in a Cellular Model of Parkinson’s Disease. International Journal of Molecular Sciences, 22(13), 6646. https://doi.org/10.3390/ijms22136646







