Fetal Zone Steroids and Estrogen Show Sex Specific Effects on Oligodendrocyte Precursor Cells in Response to Oxidative Damage
Abstract
1. Introduction
2. Results
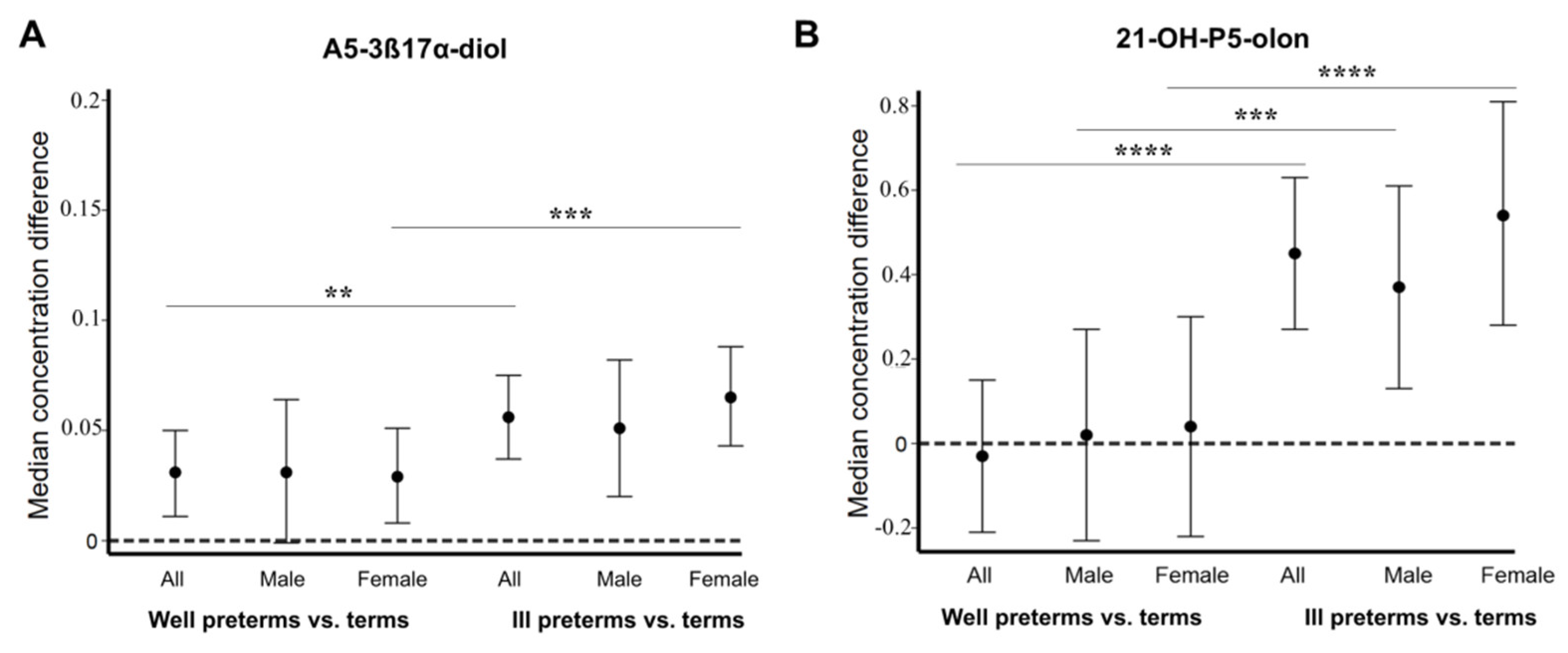
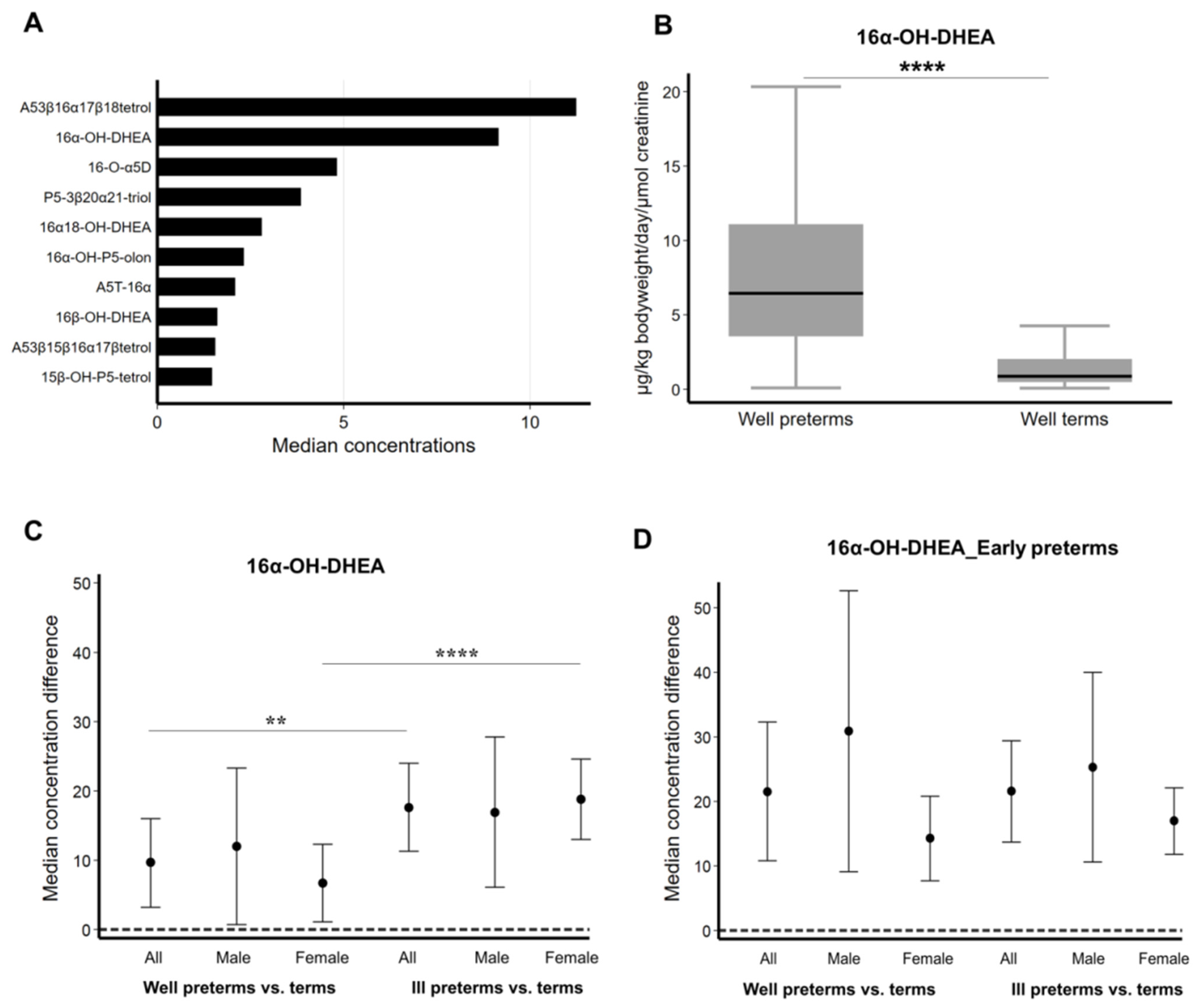
2.1. Fetal Zone Steroid Amounts Differ in the Urine of Preterm Male and Female Infants
2.2. Concentration of 16α-OH-DHEA and Other FZS Increases in Ill Female Preterm Infants
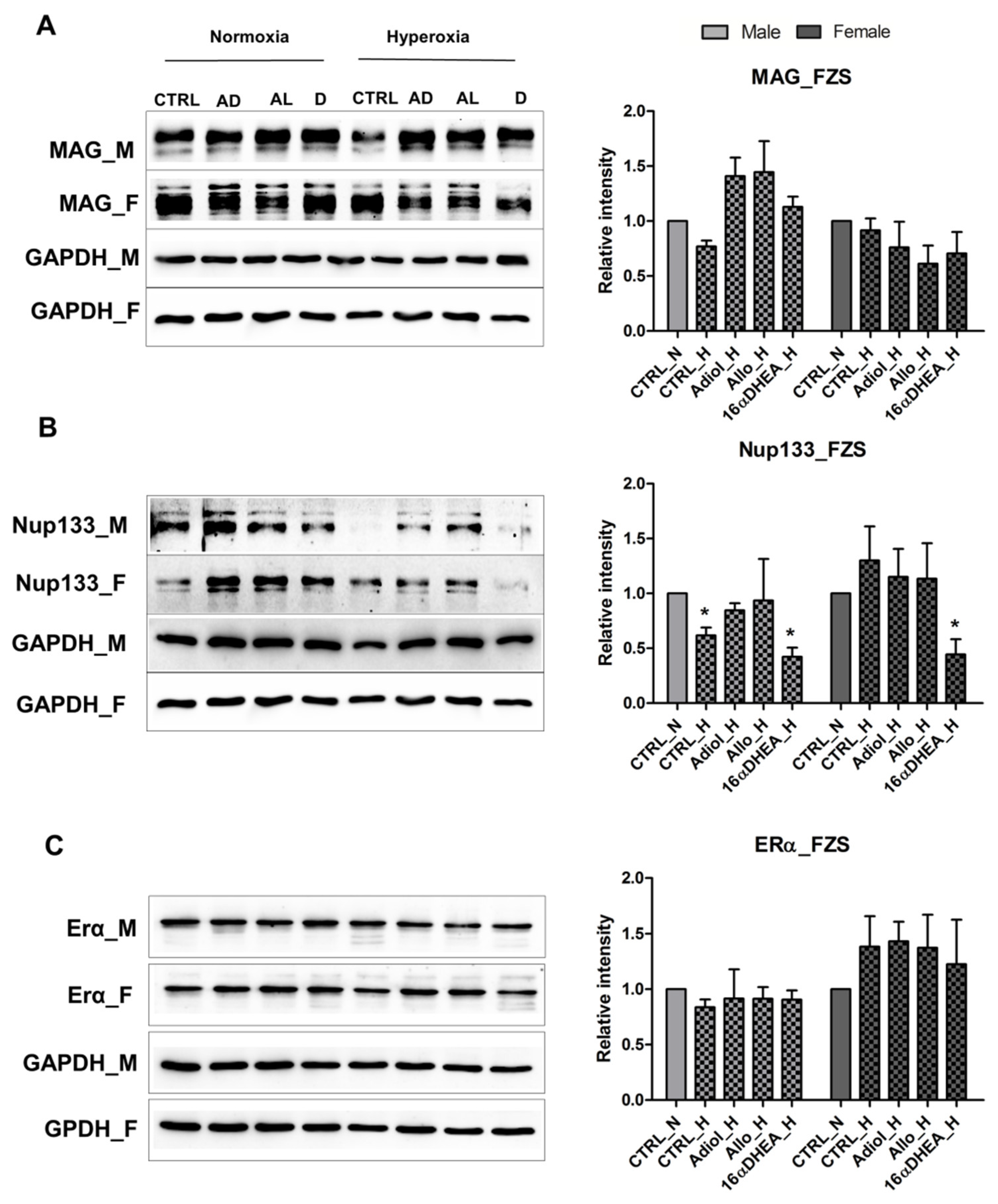
2.3. FZS and Allopregnanolone Have a Protective Effect on Both Male and Female OPCs
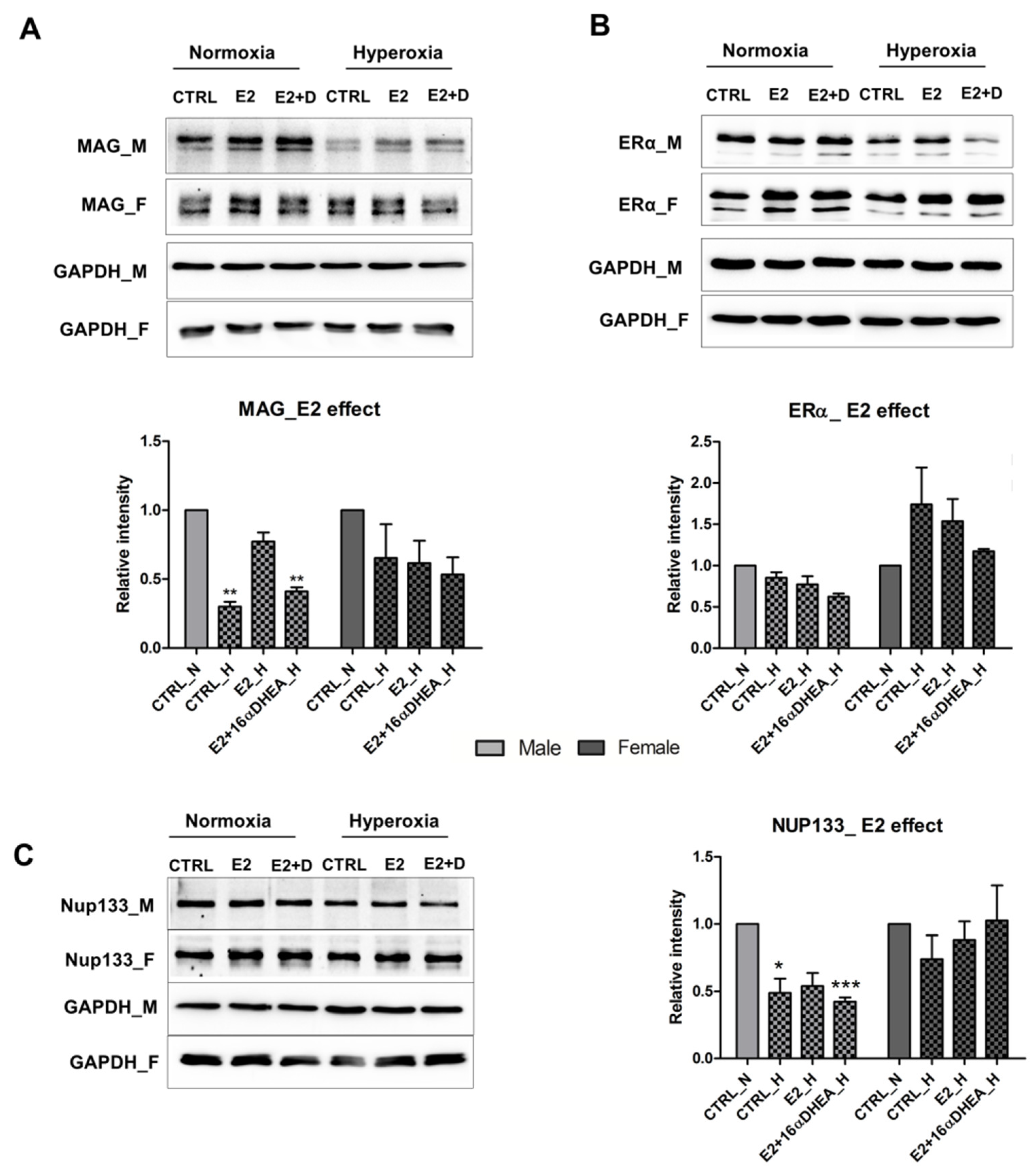
2.4. Estradiol in Presence of 16αOH-DHEA Exerts a Negative Effect on the Maturation of Male Derived Cells
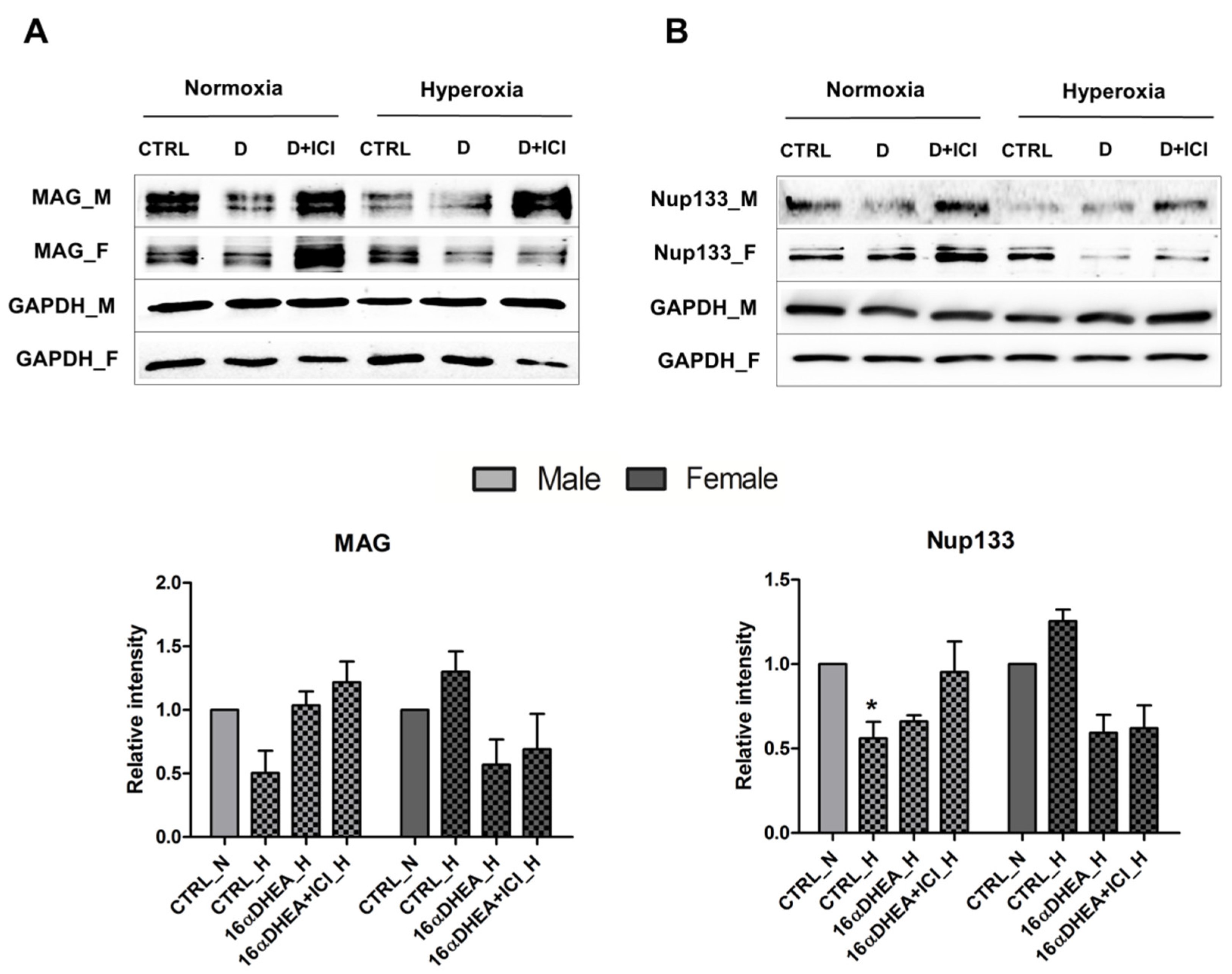
2.5. 16α-, OH-DHEA Activates Different Mechanisms in Male and Female Cells
3. Discussion
4. Materials and Methods
4.1. Human Infant Urine Study
4.2. Animals
4.3. Isolation and Culture of Mouse OPCs
4.4. Treatment with Hyperoxia
4.5. Treatment with Steroids
4.6. Cell Lines
4.7. Immunoblot Analysis
4.8. Experimental Design and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Linsell, L.; Johnson, S.; Wolke, D.; O’Reilly, H.; Morris, J.K.; Kurinczuk, J.J.; Marlow, N. Cognitive trajectories from infancy to early adulthood following birth before 26 weeks of gestation: A prospective, population-based cohort study. Arch. Dis. Child. 2018, 103, 363–370. [Google Scholar] [CrossRef]
- Woythaler, M. Neurodevelopmental outcomes of the late preterm infant. Semin. Fetal Neonatal Med. 2019, 24, 54–59. [Google Scholar] [CrossRef]
- Novak, C.M.; Ozen, M.; Burd, I. Perinatal Brain Injury: Mechanisms, Prevention, and Outcomes. Clin. Perinatol. 2018, 45, 357–375. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, D.N.; McGovern, M.; Greene, C.M.; Molloy, E.J. Gender disparities in preterm neonatal outcomes. Acta Paediatr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Hussain, R.; Gago, N.; Oudinet, J.P.; Mattern, C.; Ghoumari, A.M. Progesterone synthesis in the nervous system: Implications for myelination and myelin repair. Front. Neurosci. 2012, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Seo, F.; Hirato, K.; Fukuda, T.; Yanaihara, T.; Araki, H.; Nakayama, T. Changes in placental enzymatic activities in relation to estrogen production during pregnancy. Nihon Sanka Fujinka Gakkai Zasshi 1985, 37, 547–554. [Google Scholar]
- Wood, C.E. Estrogen/hypothalamus-pituitary-adrenal axis interactions in the fetus: The interplay between placenta and fetal brain. J. Soc. Gynecol. Investig. 2005, 12, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Coumailleau, P.; Pellegrini, E.; Adrio, F.; Diotel, N.; Cano-Nicolau, J.; Nasri, A.; Vaillant, C.; Kah, O. Aromatase, estrogen receptors and brain development in fish and amphibians. Biochim. Biophys. Acta 2015, 1849, 152–162. [Google Scholar] [CrossRef]
- Rao, M.L.; Kolsch, H. Effects of estrogen on brain development and neuroprotection--implications for negative symptoms in schizophrenia. Psychoneuroendocrinology 2003, 28 (Suppl. 2), 83–96. [Google Scholar] [CrossRef]
- Gerstner, B.; Lee, J.; DeSilva, T.M.; Jensen, F.E.; Volpe, J.J.; Rosenberg, P.A. 17beta-estradiol protects against hypoxic/ischemic white matter damage in the neonatal rat brain. J. Neurosci. Res. 2009, 87, 2078–2086. [Google Scholar] [CrossRef]
- Gerstner, B.; Sifringer, M.; Dzietko, M.; Schuller, A.; Lee, J.; Simons, S.; Obladen, M.; Volpe, J.J.; Rosenberg, P.A.; Felderhoff-Mueser, U. Estradiol attenuates hyperoxia-induced cell death in the developing white matter. Ann. Neurol. 2007, 61, 562–573. [Google Scholar] [CrossRef]
- Guo, H.; Liu, M.; Zhang, L.; Wang, L.; Hou, W.; Ma, Y. The Critical Period for Neuroprotection by Estrogen Replacement Therapy and the Potential Underlying Mechanisms. Curr. Neuropharmacol. 2020, 18, 485–500. [Google Scholar] [CrossRef]
- Trotter, A.; Maier, L.; Pohlandt, F. Management of the extremely preterm infant: Is the replacement of estradiol and progesterone beneficial? Paediatr. Drugs 2001, 3, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Siiteri, P.K.; MacDonald, P.C. Placental estrogen biosynthesis during human pregnancy. J. Clin. Endocrinol. Metab. 1966, 26, 751–761. [Google Scholar] [CrossRef]
- Hubner, S.; Reich, B.; Heckmann, M. Role of sex steroids and their receptors in human preterm infants: Impacts on future treatment strategies for cerebral development. Biochem. Pharmacol. 2015, 98, 556–563. [Google Scholar] [CrossRef]
- Ishimoto, H.; Jaffe, R.B. Development and function of the human fetal adrenal cortex: A key component in the feto-placental unit. Endocr. Rev. 2011, 32, 317–355. [Google Scholar] [CrossRef]
- Midgley, P.C.; Russell, K.; Oates, N.; Holownia, P.; Shaw, J.C.; Honour, J.W. Adrenal function in preterm infants: ACTH may not be the sole regulator of the fetal zone. Pediatr. Res. 1998, 44, 887–893. [Google Scholar] [CrossRef][Green Version]
- Heckmann, M.; Hartmann, M.F.; Kampschulte, B.; Gack, H.; Bodeker, R.H.; Gortner, L.; Wudy, S.A. Persistent high activity of the fetal adrenal cortex in preterm infants: Is there a clinical significance? J. Pediatr. Endocrinol. Metab. 2006, 19, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Grueters, A.; Korth-Schutz, S. Longitudinal study of plasma dehydroepiandrosterone sulfate in preterm and fullterm infants. J. Clin. Endocrinol. Metab. 1982, 55, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Kari, M.A.; Raivio, K.O.; Stenman, U.H.; Voutilainen, R. Serum cortisol, dehydroepiandrosterone sulfate, and steroid-binding globulins in preterm neonates: Effect of gestational age and dexamethasone therapy. Pediatr. Res. 1996, 40, 319–324. [Google Scholar] [CrossRef]
- Hubner, S.; Sunny, D.E.; Pohlke, C.; Ruhnau, J.; Vogelgesang, A.; Reich, B.; Heckmann, M. Protective Effects of Fetal Zone Steroids Are Comparable to Estradiol in Hyperoxia-Induced Cell Death of Immature Glia. Endocrinology 2017, 158, 1419–1435. [Google Scholar] [CrossRef][Green Version]
- Sunny, D.E.; Hammer, E.; Strempel, S.; Joseph, C.; Manchanda, H.; Ittermann, T.; Hubner, S.; Weiss, F.U.; Volker, U.; Heckmann, M. Nup133 and ERalpha mediate the differential effects of hyperoxia-induced damage in male and female OPCs. Mol. Cell Pediatr 2020, 7, 10. [Google Scholar] [CrossRef]
- Greaves, R.F.; Wudy, S.A.; Badoer, E.; Zacharin, M.; Hirst, J.J.; Quinn, T.; Walker, D.W. A tale of two steroids: The importance of the androgens DHEA and DHEAS for early neurodevelopment. J. Steroid Biochem Mol. Biol 2019, 188, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Hampl, R.; Starka, L. Mini review: 16alpha-hydroxylated metabolites of dehydroepiandrosterone and their biological significance. Endocr. Regul. 2000, 34, 161–163. [Google Scholar]
- Schweigmann, H.; Sanchez-Guijo, A.; Ugele, B.; Hartmann, K.; Hartmann, M.F.; Bergmann, M.; Pfarrer, C.; Doring, B.; Wudy, S.A.; Petzinger, E.; et al. Transport of the placental estriol precursor 16alpha-hydroxy-dehydroepiandrosterone sulfate (16alpha-OH-DHEAS) by stably transfected OAT4-, SOAT-, and NTCP-HEK293 cells. J. Steroid Biochem. Mol. Biol. 2014, 143, 259–265. [Google Scholar] [CrossRef]
- Trotter, A.; Pohlandt, F. The replacement of oestradiol and progesterone in very premature infants. Ann. Med. 2000, 32, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Trotter, A.; Bokelmann, B.; Sorgo, W.; Bechinger-Kornhuber, D.; Heinemann, H.; Schmucker, G.; Oesterle, M.; Kohntop, B.; Brisch, K.H.; Pohlandt, F. Follow-up examination at the age of 15 months of extremely preterm infants after postnatal estradiol and progesterone replacement. J. Clin. Endocrinol. Metab. 2001, 86, 601–603. [Google Scholar] [CrossRef]
- Trotter, A.; Steinmacher, J.; Kron, M.; Pohlandt, F. Neurodevelopmental follow-up at five years corrected age of extremely low birth weight infants after postnatal replacement of 17beta-estradiol and progesterone. J. Clin. Endocrinol. Metab. 2012, 97, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Alur, P. Sex Differences in Nutrition, Growth, and Metabolism in Preterm Infants. Front. Pediatr. 2019, 7, 22. [Google Scholar] [CrossRef]
- Shaw, J.C.; Dyson, R.M.; Palliser, H.K.; Gray, C.; Berry, M.J.; Hirst, J.J. Neurosteroid replacement therapy using the allopregnanolone-analogue ganaxolone following preterm birth in male guinea pigs. Pediatr. Res. 2019, 85, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Warner, M.; Gustafsson, J.A. DHEA—A precursor of ERbeta ligands. J. Steroid Biochem. Mol. Biol. 2015, 145, 245–247. [Google Scholar] [CrossRef]
- Friess, E.; Schiffelholz, T.; Steckler, T.; Steiger, A. Dehydroepiandrosterone—A neurosteroid. Eur J. Clin. Investig. 2000, 30 (Suppl. 3), 46–50. [Google Scholar] [CrossRef] [PubMed]
- Pinna, G. Allopregnanolone, the Neuromodulator Turned Therapeutic Agent: Thank You, Next? Front. Endocrinol (Lausanne) 2020, 11, 236. [Google Scholar] [CrossRef]
- Bennett, G.A.; Palliser, H.K.; Walker, D.; Hirst, J. Severity and timing: How prenatal stress exposure affects glial developmental, emotional behavioural and plasma neurosteroid responses in guinea pig offspring. Psychoneuroendocrinology 2016, 70, 47–57. [Google Scholar] [CrossRef]
- Travers, S.; Martinerie, L.; Boileau, P.; Lombes, M.; Pussard, E. Alterations of adrenal steroidomic profiles in preterm infants at birth. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F143–F151. [Google Scholar] [CrossRef] [PubMed]
- Midgley, P.C.; Russell, K.; Oates, N.; Shaw, J.C.; Honour, J.W. Activity of the adrenal fetal zone in preterm infants continues to term. Endocr. Res. 1996, 22, 729–733. [Google Scholar] [CrossRef]
- Hirst, J.J.; Cumberland, A.L.; Shaw, J.C.; Bennett, G.A.; Kelleher, M.A.; Walker, D.W.; Palliser, H.K. Loss of neurosteroid-mediated protection following stress during fetal life. J. Steroid Biochem. Mol. Biol. 2016, 160, 181–188. [Google Scholar] [CrossRef]
- Battarbee, A.N.; Glover, A.V.; Vladutiu, C.J.; Gyamfi-Bannerman, C.; Aliaga, S.; Manuck, T.A.; Boggess, K.A. Sex-Specific Differences in Late Preterm Neonatal Outcomes. Am. J. Perinatol. 2019, 36, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Hindmarsh, G.J.; O’Callaghan, M.J.; Mohay, H.A.; Rogers, Y.M. Gender differences in cognitive abilities at 2 years in ELBW infants. Extremely low birth weight. Early Hum. Dev. 2000, 60, 115–122. [Google Scholar] [CrossRef]
- Hintz, S.R.; Kendrick, D.E.; Vohr, B.R.; Kenneth Poole, W.; Higgins, R.D. Gender differences in neurodevelopmental outcomes among extremely preterm, extremely-low-birthweight infants. Acta Paediatr. 2006, 95, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Grofer, B.; Bodeker, R.H.; Gortner, L.; Heckmann, M. Maturation of adrenal function determined by urinary glucocorticoid steroid excretion rates in preterm infants of more than 30 weeks of gestational age. Neonatology 2010, 98, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, M.; Hartmann, M.F.; Kampschulte, B.; Gack, H.; Bodeker, R.H.; Gortner, L.; Wudy, S.A. Cortisol production rates in preterm infants in relation to growth and illness: A noninvasive prospective study using gas chromatography-mass spectrometry. J. Clin. Endocrinol. Metab. 2005, 90, 5737–5742. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, M.; Hartmann, M.F.; Kampschulte, B.; Gack, H.; Bodeker, R.H.; Gortner, L.; Wudy, S.A. Assessing cortisol production in preterm infants: Do not dispose of the nappies. Pediatr. Res. 2005, 57, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Sunny, D.E.; Muzzio, D.O.; Heckmann, M. Method for generating highly pure culture of mouse derived Oligospheres under hypoxic conditions. Protoc. Exch. 2015. [Google Scholar] [CrossRef]

| Normoxia | Hyperoxia | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adiol | Allo | 16αD | E2 | E2 + 16αD | Adiol | Allo | 16αD | E2 | E2 + 16αD | |
| Impact on maturation | ||||||||||
| Male | ++ | +++ | +++ | ++ | +++ | ++ | + | + | no | – |
| Female | + | + | ++ | + | + | no | no | no | no | no |
| Impact on Nup133 | ||||||||||
| Male | + | + | + | + | no | + | + | – | no | – |
| Female | ++ | ++ | + | + | + | + | + | – | + | + |
| Impact on ERα | ||||||||||
| Male | no | no | no | no | + | no | no | no | no | – |
| Female | + | + | + | ++ | ++ | ++ | ++ | + | ++ | ++ |
| Metabolites | ||
|---|---|---|
| 1 | Adiol | 5-androstene-3β,17β diol |
| 2 | A5-3β17α | 5-androstene-3β,17α diol |
| 3 | DHEA | 5-androstene-3 βol-17-one |
| 4 | 16α-OH-DHEA | 5-androstene-3 β,16α-diol-17-one |
| 5 | 16β-OH-DHEA | 5-androstene-3β,16β-diol-17-one |
| 6 | 15β16α-OH-DHEA | 5-androstene-3β, 15β, 16α-triol-17-one |
| 7 | 16-O-A5D | 5-androstene-3β, 17β-diol-16-one |
| 8 | A5T-16α | 5-androstene-3β,16α,17β-triol |
| 9 | A5T-16β | 5-androstene-3β,16β,17β-triol |
| 10 | 16α18-OH-DHEA | 5-androstene-3β, 16α, 18-triol-17-one |
| 11 | 15β17α-OH-P5-olon | 5-pregnene-3β,15β,17α-triol-20-one |
| 12 | 16α-OH-P5-olon | 5-pregnene-3β,16α-diol-20-one |
| 13 | A53β16α17β18-tetrol | 5-androstene-3β,16α,17β,18-tetrole |
| 14 | A53β15β16α17β-tetrol | 5-androstene-3β,15β,16α,17β-tetrole |
| 15 | 15β-OH-P5-tetrol | 5-pregnene-3 β, 15β, 17α,20α-tetrol |
| 16 | 21-OH-P5-olon | 5-pregnene-3β,21-diol-20-one |
| 17 | P5-3β20α21-triol | 5-pregnene-3β,20α,21-triol |
| 18 | P5-tetrol | 5-pregnene-3β,16α,20α,21-tetrole |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunny, D.E.; Hammer, E.; Ittermann, T.; Krüger, E.L.; Hübner, S.; Hartmann, M.F.; Wudy, S.A.; Völker, U.; Heckmann, M. Fetal Zone Steroids and Estrogen Show Sex Specific Effects on Oligodendrocyte Precursor Cells in Response to Oxidative Damage. Int. J. Mol. Sci. 2021, 22, 6586. https://doi.org/10.3390/ijms22126586
Sunny DE, Hammer E, Ittermann T, Krüger EL, Hübner S, Hartmann MF, Wudy SA, Völker U, Heckmann M. Fetal Zone Steroids and Estrogen Show Sex Specific Effects on Oligodendrocyte Precursor Cells in Response to Oxidative Damage. International Journal of Molecular Sciences. 2021; 22(12):6586. https://doi.org/10.3390/ijms22126586
Chicago/Turabian StyleSunny, Donna Elizabeth, Elke Hammer, Till Ittermann, Elisabeth Luise Krüger, Stephanie Hübner, Michaela Friederike Hartmann, Stefan Alexander Wudy, Uwe Völker, and Matthias Heckmann. 2021. "Fetal Zone Steroids and Estrogen Show Sex Specific Effects on Oligodendrocyte Precursor Cells in Response to Oxidative Damage" International Journal of Molecular Sciences 22, no. 12: 6586. https://doi.org/10.3390/ijms22126586
APA StyleSunny, D. E., Hammer, E., Ittermann, T., Krüger, E. L., Hübner, S., Hartmann, M. F., Wudy, S. A., Völker, U., & Heckmann, M. (2021). Fetal Zone Steroids and Estrogen Show Sex Specific Effects on Oligodendrocyte Precursor Cells in Response to Oxidative Damage. International Journal of Molecular Sciences, 22(12), 6586. https://doi.org/10.3390/ijms22126586






