Development of Radiofluorinated Nicotinamide/Picolinamide Derivatives as Diagnostic Probes for the Detection of Melanoma
Abstract
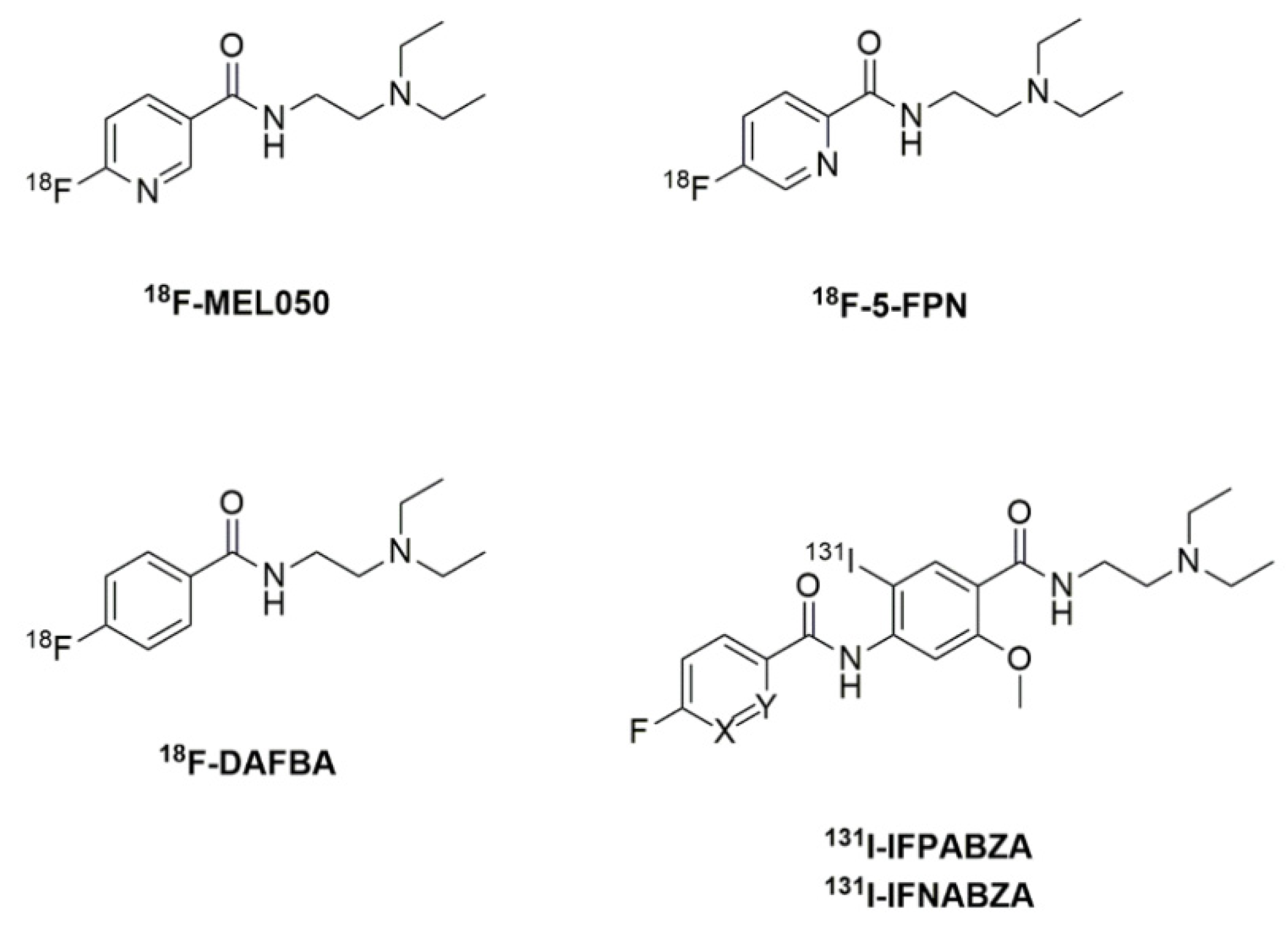
:1. Introduction
2. Results
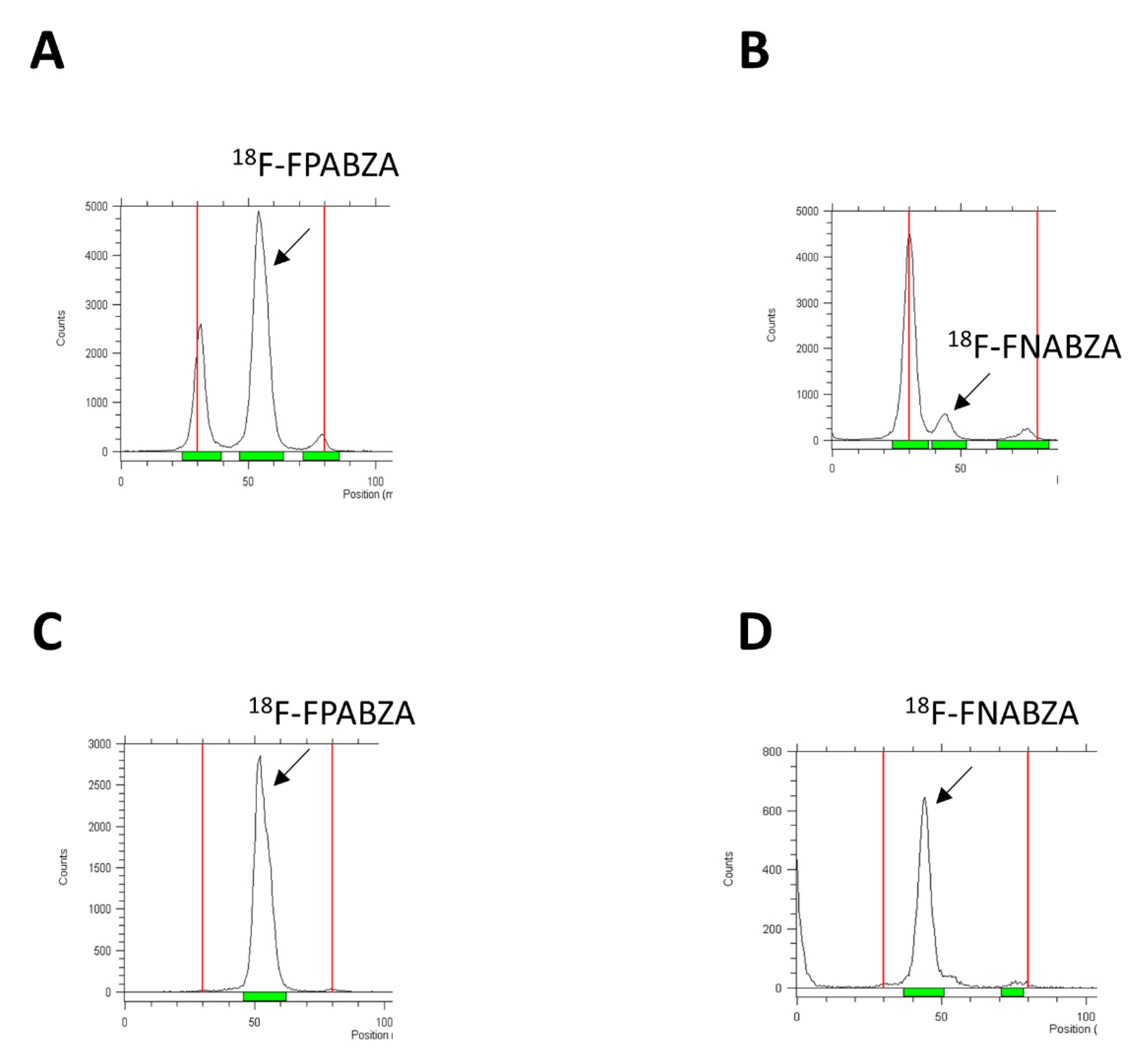
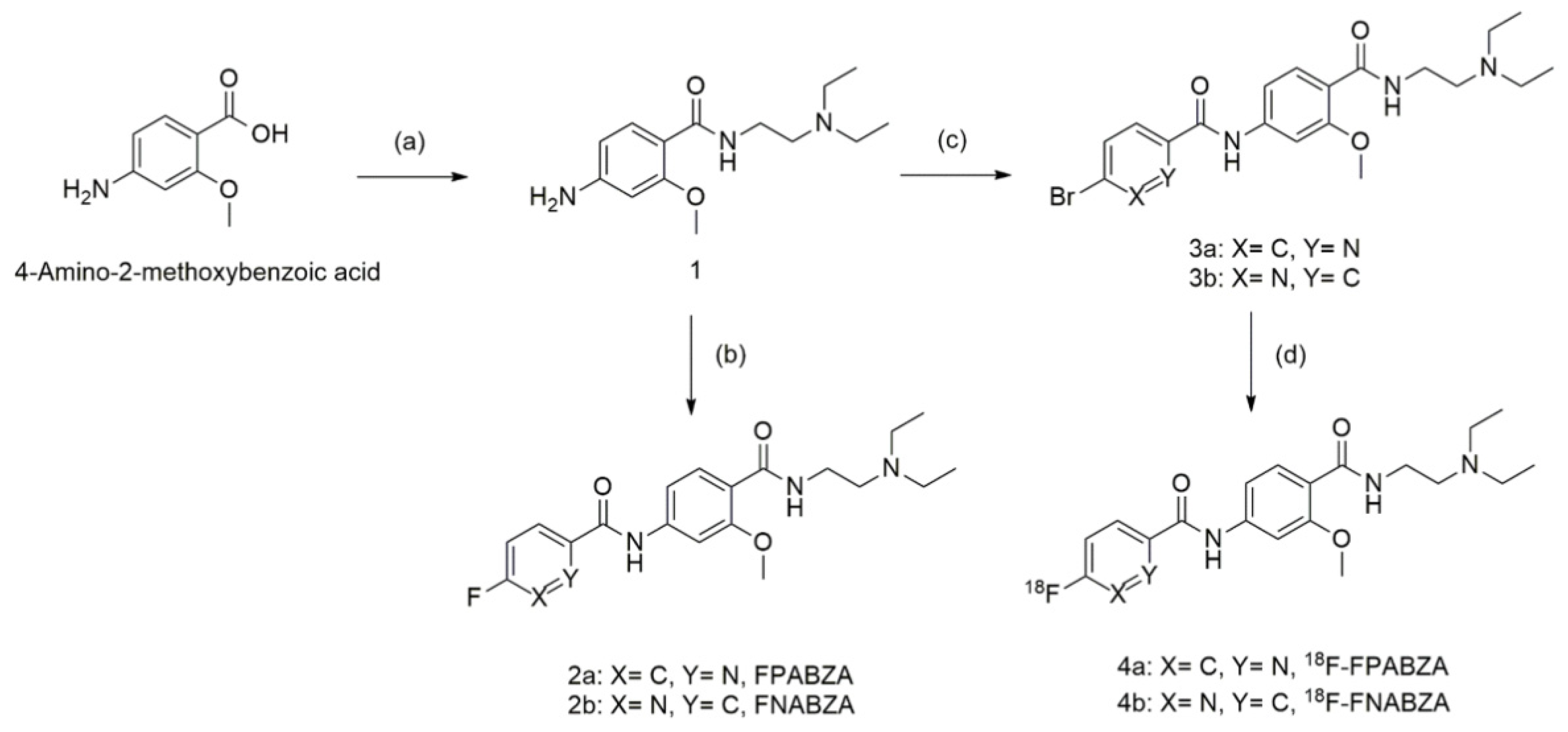
2.1. The Preparation of 18F-FPABZA and 18F-FNABZA
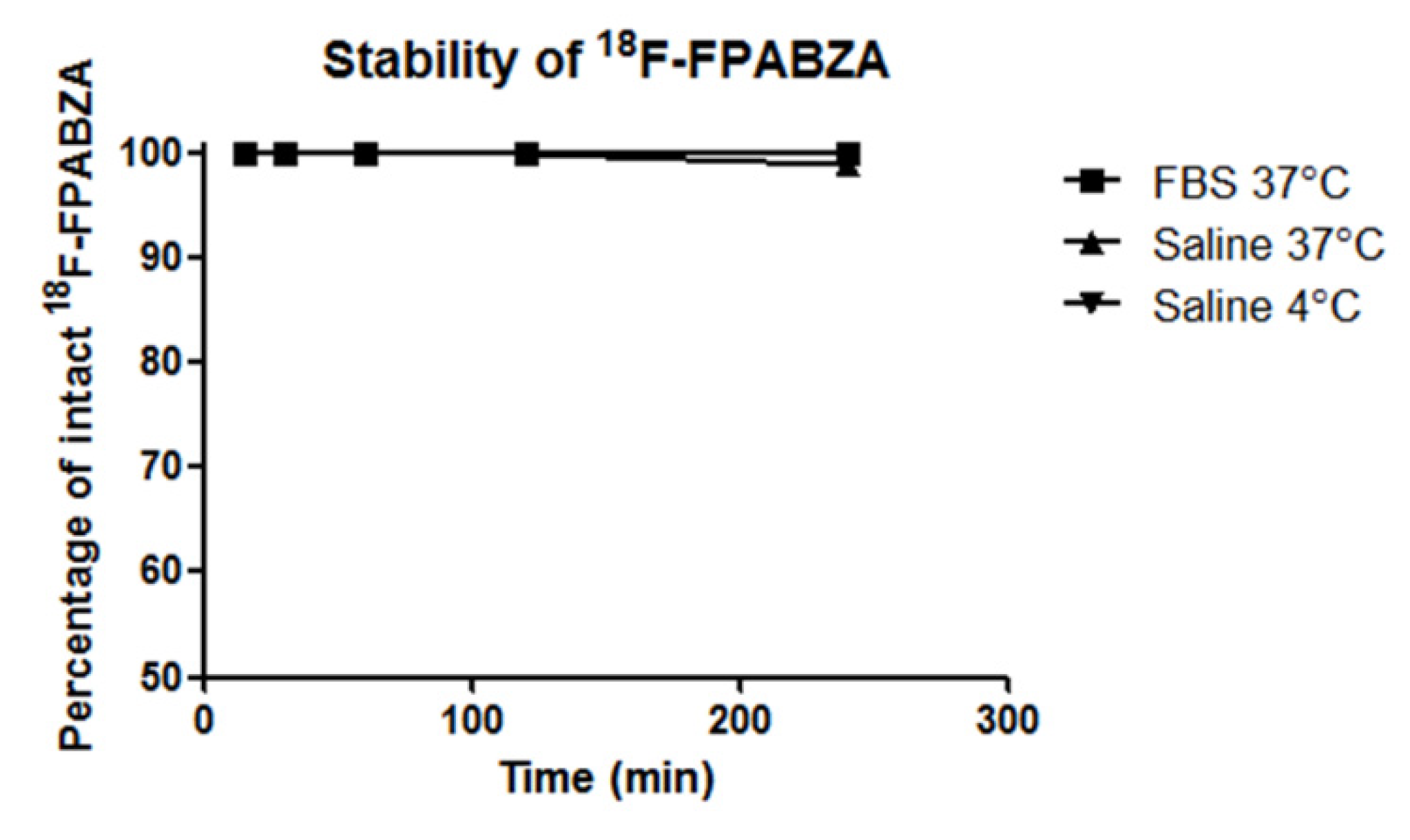
2.2. Partition Coefficient Determination and In Vitro Stability of 18F-FPABZA or 18F-FNABZA
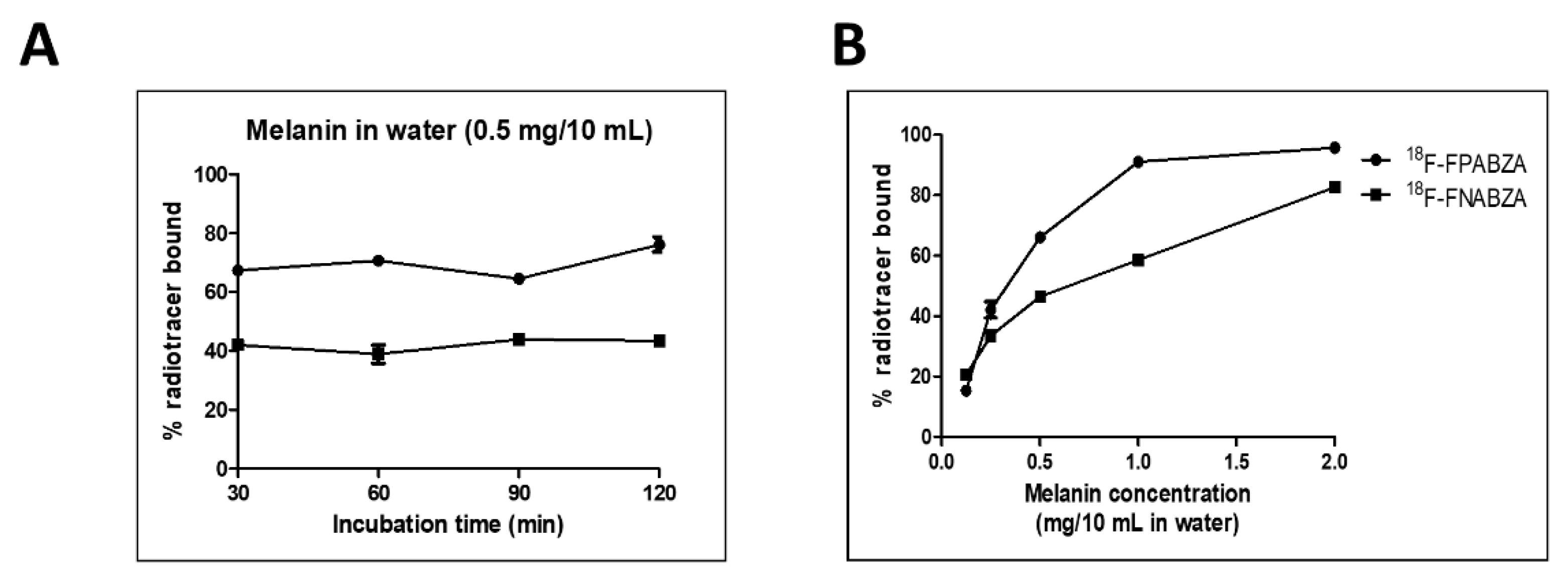
2.3. In Vitro Binding of 18F-FPABZA and 18F-FNABZA to Melanin
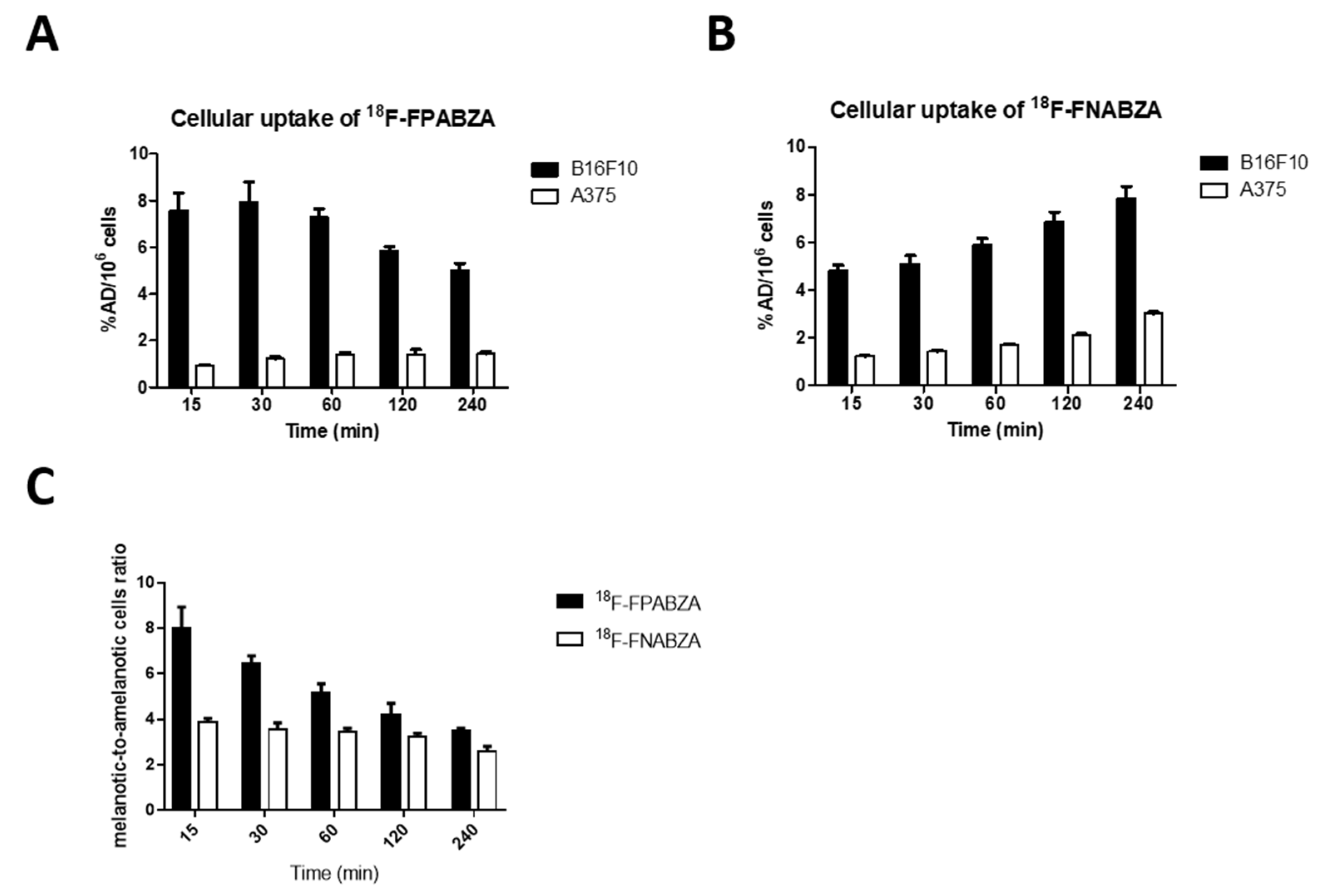
2.4. In Vitro Cellular Uptake Study of 18F-FPABZA and 18F-FNABZA in Melanoma Cells
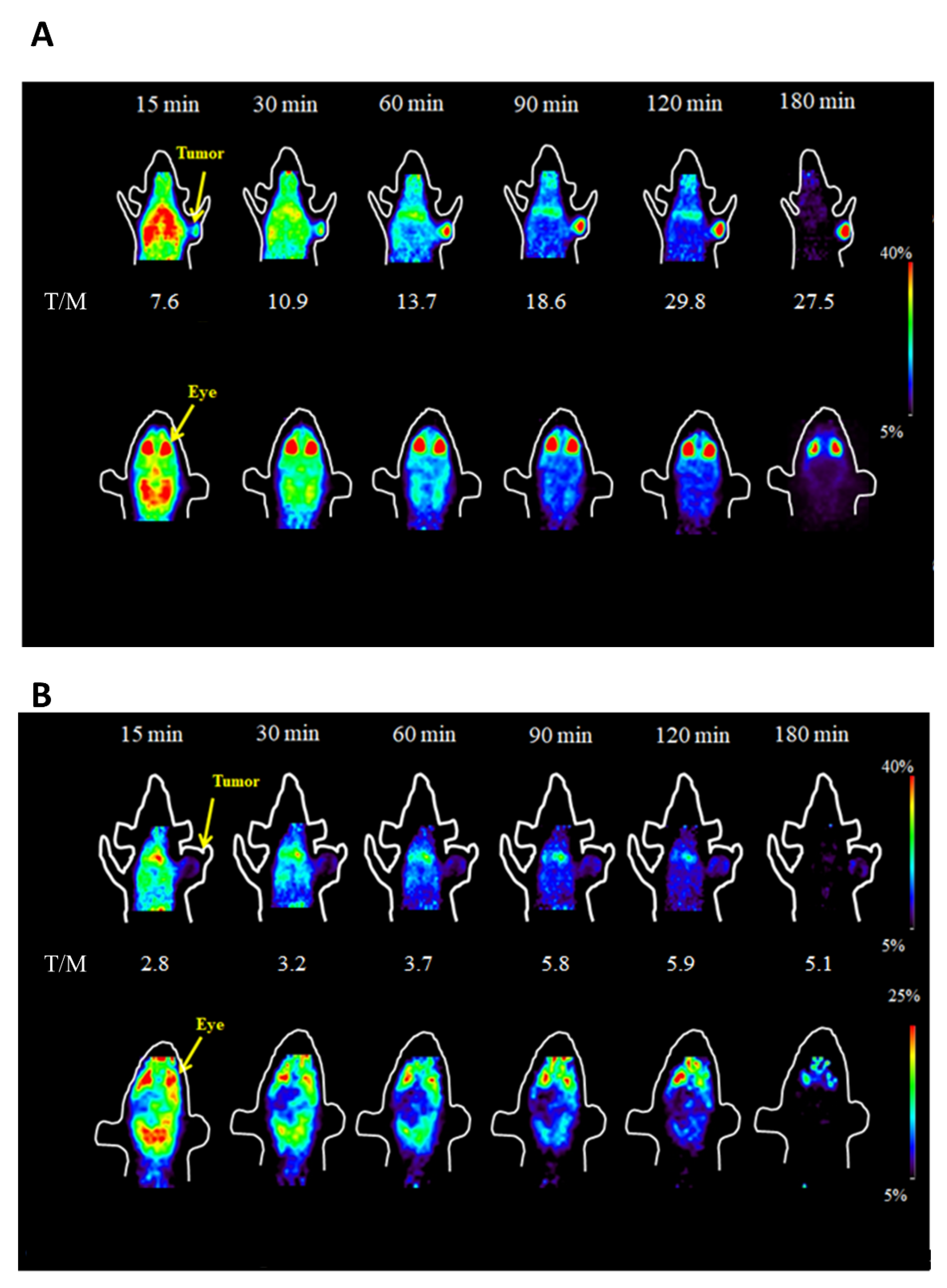
2.5. MicroPET Imaging
2.6. Biodistribution Study
3. Discussion
4. Materials and Methods
4.1. Reagents and Instruments
4.2. Preparation of 18F-FPABZA and 18F-FNABZA
4.2.1. Synthesis of 4-Amino-N-(2-(diethylamine)ethyl)-2-methoxybenzamide (1)
4.2.2. Synthesis of N-(4-((2-Diethylamino)ethyl)carbamoyl-5-methoxyphenyl)-picolinamide (2a) and Synthesis of N-(4-((2-diethylamino)ethyl)carbamoyl-5-methoxyphenyl)-nicotinamide (2b)
4.2.3. Radiolabeling of 18F-FPABZA (3a) and 18F-FNABZA (3b)
4.2.4. Synthesis of N-(4-((2-(Diethylamino)ethyl)carbamoyl)-3-methoxyphenyl)-5-fluoropicolinamide (4a) and N-(4-((2-(diethylamino)ethyl)carbamoyl)-3-methoxyphenyl)-6-fluoronicotinamide (4b)
4.3. Partition Coefficient Determination of 18F-FPABZA or 18F-FNABZA
4.4. In Vitro Stability of 18F-FPABZA
4.5. Binding Affinity to Melanin Assays
4.6. Cellular Uptake Studies
4.7. Establishment of Melanoma-Bearing Mouse Models
4.8. MicroPET Scanning
4.9. MicroPET Scanning
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chia, A.L.K.; Simonova, G.; Dutta, B.; Lim, A.; Shumack, S. Melanoma diagnosis: Australian dermatologists’ number needed to treat. Australas. J. Dermatol. 2008, 49, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Linos, E.; Swetter, S.M.; Cockburn, M.G.; Colditz, G.; Clarke, C.A. Increasing Burden of Melanoma in the United States. J. Investig. Dermatol. 2009, 129, 1666–1674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimbow, K.; Miyake, Y.; Homma, K.; Yasuda, K.; Izumi, Y.; Tsutsumi, A.; Ito, S. Characterization of melanogenesis and morphogene-sis of melanosomes by physicochemical properties of melanin and melanosomes in malignant melanoma. Cancer Res. 1984, 44, 1128–1134. [Google Scholar]
- Prota, G. Melanins, Melanogenesis and Melanocytes: Looking at Their Functional Significance from the Chemist’s Viewpoint. Pigment. Cell Res. 2000, 13, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Pankovich, J.M.; Jimbow, K. Tyrosine transport in a human melanoma cell line as a basis for selective transport of cytotoxic analogues. Biochem. J. 1991, 280, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Michelot, J.M.; Moreau, M.F.; Labarre, P.G.; Madelmont, J.C.; Veyre, A.J.; Papon, J.M.; Parry, D.F.; Bonafous, J.F.; Boire, J.Y.; Desplanches, G.G. Synthesis and evaluation of new iodine-125 radiopharmaceuticals as potential tracers for malignant melanoma. J. Nucl. Med. 1991, 32, 1573–1580. [Google Scholar] [PubMed]
- Michelot, J.M.; Moreau, M.F.C.; Veyre, A.J.; Bonafous, J.F.; Bacin, F.J.; Madelmont, J.C. Phase II scintigraphic clinical trial of malig-nant melanoma and metastases with iodine-123-N-(2-diethylaminoethyl 4-iodobenzamide). J. Nucl. Med. 1993, 34, 1260–1266. [Google Scholar] [PubMed]
- Garg, S.; Kothari, K.; Thopate, S.R.; Doke, A.K.; Garg, P.K. Design, Synthesis, and Preliminary in Vitro and in Vivo Evaluation of N-(2-diethylaminoethyl)-4-[18F]fluorobenzamide ([18F]-DAFBA): A Novel Potential PET Probe to Image Melanoma Tumors. Bioconjugate Chem. 2009, 20, 583–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Noyer, D.; Greguric, I.; Roselt, P.; Neels, O.; Aide, N.; Taylor, S.R.; Katsifis, A.; Dorow, D.S.; Hicks, R.J. High-contrast PET of melanoma using (18)F-MEL050, a selective probe for melanin with predominantly renal clearance. J. Nucl. Med. 2010, 51, 441–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greguric, I.; Taylor, S.R.; Denoyer, D.; Ballantyne, P.; Berghofer, P.; Roselt, P.; Pham, T.Q.; Mattner, F.; Bourdier, T.; Neels, O.; et al. Discovery of [18F]N-(2-(Diethylamino)ethyl)-6-fluoronicotinamide: A Melanoma Positron Emission Tomography Imaging Radiotracer with High Tumor to Body Contrast Ratio and Rapid Renal Clearance. J. Med. Chem. 2009, 52, 5299–5302. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, S.; Miao, Z.; Deng, Z.; Shen, B.; Hong, X.; Cheng, Z. Development of 18F-Labeled Picolinamide Probes for PET Imaging of Malignant Melanoma. J. Med. Chem. 2013, 56, 895–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Pham, T.Q.; Berghofer, P.; Chapman, J.; Greguric, I.; Mitchell, P.; Mattner, F.; Loc’H, C.; Katsifis, A. Synthesis and evaluation of novel radioiodinated nicotinamides for malignant melanoma. Nucl. Med. Biol. 2008, 35, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Shen, C.-C.; Chen, C.-L.; Liu, R.-S.; Lin, M.-H.; Wang, H.-E. Synthesis and evaluation of 123/131I-Iochlonicotinamide as a novel SPECT probe for malignant melanoma. Bioorg. Med. Chem. 2015, 23, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Chen, Y.-Y.; Lo, Y.-H.; Lin, M.-H.; Chang, C.-H.; Chen, C.-L.; Wang, H.-E.; Wu, C.-Y. Evaluation of Radioiodinated Fluoronicotinamide/Fluoropicolinamide-Benzamide Derivatives as Theranostic Agents for Melanoma. Int. J. Mol. Sci. 2020, 21, 6597. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.Q.; Greguric, I.; Liu, X.; Berghofer, P.; Ballantyne, P.; Chapman, J.; Mattner, F.; Dikic, B.; Jackson, T.; Loc’, C.; et al. Synthesis and Evaluation of Novel Radioiodinated Benzamides for Malignant Melanoma. J. Med. Chem. 2007, 50, 3561–3572. [Google Scholar] [CrossRef] [PubMed]
- Amaro, A.; Gangemi, R.; Piaggio, F.; Angelini, G.; Barisione, G.; Ferrini, S.; Pfeffer, U. The biology of uveal melanoma. Cancer Metastasis Rev. 2017, 36, 109–140. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Di Crescenzo, R.M.; Broggi, G.; Merolla, F.; Martino, F.; Varricchio, S.; Ilardi, G.; Borzillo, A.; Carandente, R.; Pignatiello, S.; et al. Expression of P16INK4a in Uveal Melanoma: New Perspectives. Front. Oncol. 2020, 10, 562074. [Google Scholar] [CrossRef] [PubMed]
- Bacin, F.; Michelot, J.; Bonafous, J.; Veyre, A.; Moreau, M.-F.; Kemeny, J.-L.; Chossat, F.; Bekhechi, D. Clinical study of [123 I] N-(2-diethylaminoethyl)-4- iodobenzamide in the diagnosis of primary and metastatic ocular melanoma. Acta Ophthalmol. Scand. 1998, 76, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; De Koning, L.; Coupland, S.E.; Jochemsen, A.G.; Marais, R.; Stern, M.-H.; Valente, A.; Barnhill, R.; Cassoux, N.; Evans, A.; et al. So Close, yet so Far: Discrepancies between Uveal and Other Melanomas. A Position Paper from UM Cure 2020. Cancers 2019, 11, 1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broggi, G.; Ieni, A.; Russo, D.; Varricchio, S.; Puzzo, L.; Russo, A.; Reibaldi, M.; Longo, A.; Tuccari, G.; Staibano, S.; et al. The Macro-Autophagy-Related Protein Beclin-1 Immunohistochemical Expression Correlates with Tumor Cell Type and Clinical Behavior of Uveal Melanoma. Front. Oncol. 2020, 10, 2515. [Google Scholar] [CrossRef] [PubMed]
| Organ | 15 min | 30 min | 60 min | 120 min |
|---|---|---|---|---|
| Blood | 3.70 ± 0.80 | 2.67 ± 0.83 | 0.95 ± 0.24 | 0.24 ± 0.17 |
| Heart | 4.74 ± 0.80 | 2.69 ± 0.75 | 1.00 ± 0.26 | 0.68 ± 0.69 |
| Lung | 8.22 ± 1.87 | 4.58 ± 1.82 | 1.82 ± 0.56 | 1.24 ± 1.34 |
| Liver | 5.32 ± 2.04 | 3.69 ± 1.01 | 1.63 ± 0.37 | 1.32 ± 1.00 |
| Stomach | 4.95 ± 1.99 | 3.69 ± 1.35 | 1.78 ± 0.85 | 0.78 ± 0.39 |
| Small int. | 5.60 ± 0.98 | 7.35 ± 1.45 | 5.01 ± 2.64 | 1.39 ± 0.52 |
| Large int. | 4.64 ± 0.65 | 2.71 ± 0.28 | 1.91 ± 1.00 | 1.20 ± 0.32 |
| Spleen | 11.73 ± 1.37 | 5.75 ± 1.85 | 3.16 ± 1.08 | 0.54 ± 0.07 |
| Pancreas | 12.80 ± 5.60 | 4.99 ± 1.96 | 1.93 ± 0.67 | 0.80 ± 0.22 |
| Bone | 1.84 ± 0.39 | 1.85 ± 0.48 | 1.40 ± 0.51 | 1.40 ± 0.17 |
| Muscle | 3.32 ± 1.45 | 2.34 ± 0.81 | 0.71 ± 0.19 | 0.44 ± 0.65 |
| Tumor | 12.32 ± 4.13 | 13.21 ± 3.91 | 20.57 ± 2.22 | 16.89 ± 2.32 |
| Brain | 3.30 ± 0.12 | 1.26 ± 0.28 | 0.39 ± 0.12 | 0.10 ± 0.04 |
| Kidneys | 15.57 ± 1.71 | 10.82 ± 4.29 | 3.78 ± 1.19 | 2.39 ± 2.22 |
| Eye ball | 20.40 ± 4.81 | 25.74 ± 3.23 | 36.91 ± 3.49 | 33.61 ± 2.14 |
| Urine | 103.75 ± 87.59 | 282.89 ± 115.42 | 276.62 ± 152.36 | 90.79 ± 68.19 |
| Fece | 6.92 ± 3.79 | 7.37 ± 1.47 | 12.18 ± 8.56 | 12.13 ± 2.86 |
| Ratios | ||||
| Tumor/muscle | 6.97 ± 1.96 | 6.66 ± 3.16 | 26.47 ± 3.11 | 86.57 ± 5.39 |
| Tumor/blood | 4.40 ± 1.22 | 5.18 ± 1.66 | 19.89 ± 1.19 | 51.93 ± 18.05 |
| Tumor/liver | 2.46 ± 0.38 | 3.73 ± 1.10 | 11.03 ± 2.40 | 23.61 ± 9.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, Y.-H.; Chang, T.-Y.; Chen, C.-L.; Lin, M.-H.; Wang, H.-E.; Chang, C.-W.; Liu, R.-S.; Wu, C.-Y. Development of Radiofluorinated Nicotinamide/Picolinamide Derivatives as Diagnostic Probes for the Detection of Melanoma. Int. J. Mol. Sci. 2021, 22, 6432. https://doi.org/10.3390/ijms22126432
Lo Y-H, Chang T-Y, Chen C-L, Lin M-H, Wang H-E, Chang C-W, Liu R-S, Wu C-Y. Development of Radiofluorinated Nicotinamide/Picolinamide Derivatives as Diagnostic Probes for the Detection of Melanoma. International Journal of Molecular Sciences. 2021; 22(12):6432. https://doi.org/10.3390/ijms22126432
Chicago/Turabian StyleLo, Yi-Hsuan, Ting-Yu Chang, Chuan-Lin Chen, Ming-Hsien Lin, Hsin-Ell Wang, Chi-Wei Chang, Ren-Shyan Liu, and Chun-Yi Wu. 2021. "Development of Radiofluorinated Nicotinamide/Picolinamide Derivatives as Diagnostic Probes for the Detection of Melanoma" International Journal of Molecular Sciences 22, no. 12: 6432. https://doi.org/10.3390/ijms22126432
APA StyleLo, Y.-H., Chang, T.-Y., Chen, C.-L., Lin, M.-H., Wang, H.-E., Chang, C.-W., Liu, R.-S., & Wu, C.-Y. (2021). Development of Radiofluorinated Nicotinamide/Picolinamide Derivatives as Diagnostic Probes for the Detection of Melanoma. International Journal of Molecular Sciences, 22(12), 6432. https://doi.org/10.3390/ijms22126432






