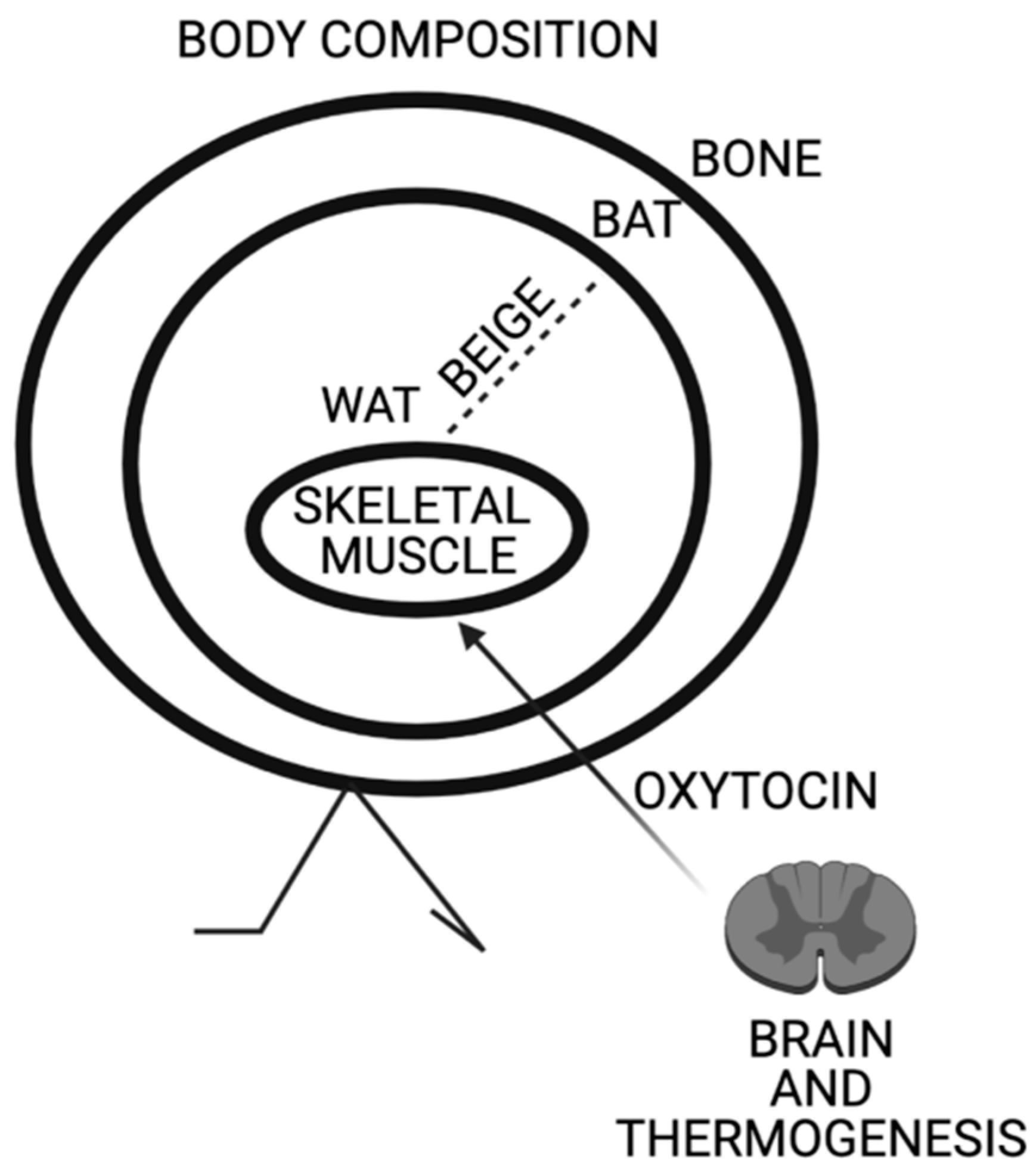
Oxytocin Involvement in Body Composition Unveils the True Identity of Oxytocin
Abstract
1. Introduction
2. Oxytocin Functions Outside of Pregnancy: A Step Back, Oxytocin–Vasopressin System in Invertebrates
3. Yuan et al.’s Theory and Oxytocin Regulation of Thermogenesis in Skeletal Muscle
4. Sun et al.’s Theory and Oxytocin Regulation of Bone Mass and Brown Fat
5. Conte et al.’s Theory and Oxytocin Driven Increased Tonicity of Skeletal Muscle and the “Oxytonic Effect”
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| BAT | Brown adipose tissue |
| WAT | White adipose tissue |
| iWAT | Inguinal adipose tissue |
| CS | Cold stress |
| CSF | Cerebrospinal fluid |
| BBB | Blood brain barrier |
| HIPP | Hippocampus |
| MyHC | Myosin heavy chain |
| Oxt | Oxytocin |
| Oxtr | Oxytocin receptor |
| PVN | Paraventricular nucleus |
| PWS | Prader–Willi syndrome |
| SON | Supraoptical nucleus |
| Sol | Soleus muscle |
| TA | Tibialis anterioris |
| TRPV1 | Transient receptor potential vanilloid 1 |
| PAI1 | Plasminogen activator inhibitor 1 |
| AVpr1a | Vasopressin receptor |
References
- Geraerts, W.P.M.; Smit, A.B.; Li, K.W. Flexibility and Constraints in Behavioral Systems; Greenspan, R.J., Kyriacou, C.P., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 1994; pp. 209–235. [Google Scholar]
- Van Kesteren, R.E.; Tensen, C.P.; Smit, A.B.; van Minnen, J.; Kolakowski, L.F.; Meyerhof, W.; Richter, D.; van Heerikhuizen, H.; Vreugdenhil, E.; Geraerts, W.P. Co-evolution of ligand-receptor pairs in the vasopressin/oxytocin superfamily of bioactive peptides. J. Biol. Chem. 1996, 271, 3619–3626. [Google Scholar] [CrossRef]
- Moyle, W.R.; Campbell, R.K.; Myers, R.V.; Bernard, M.P.; Han, Y.; Wang, X. Co-evolution of ligand-receptor pairs. Nature 1994, 368, 251–255. [Google Scholar] [CrossRef]
- Odekunle, E.A.; Elphick, M.R. Comparative and Evolutionary Physiology of Vasopressin/ Oxytocin-Type Neuropeptide Signaling in Invertebrates. Front. Endocrinol. 2020, 11, 225. [Google Scholar] [CrossRef]
- Oliver, G.; Schäfer, E.A. The Physiological Effects of Extracts of the Suprarenal Capsules. J. Physiol. 1895, 18, 230–276. [Google Scholar] [CrossRef] [PubMed]
- Howell, W.H. The proteids of the blood with especial reference to the existence of a non-coagulable proteid. J. Physiol. 1906, 280–296. [Google Scholar] [CrossRef]
- Dale, H.H. On some physiological actions of ergot. J. Physiol. 1906, 34, 163–206. [Google Scholar] [CrossRef] [PubMed]
- Dale, H.H. The Action of Extracts of the Pituitary Body. Biochem. J. 1909, 4, 427–447. [Google Scholar] [CrossRef] [PubMed]
- Farini, F. Diabete insipido ed opoterapia ipofisaria. Gazz. Osp. Clin. 1913, 34, 1135–1139. [Google Scholar]
- Kamm, O.; Aldrich, T.B.; Grote, I.W.; Rowe, L.W.; Bugbee, E.P. The active principles of the posterior lobe of the pituitary gland.1 i. the demonstration of the presence of two active principles. ii. the separation of the two principles and their concentration in the form of potent solid preparation. J. Am. Chem. Soc. 1928, 50, 573–601. [Google Scholar] [CrossRef]
- Turner, R.A.; Pierce, J.G.; Du Vigneaud, V. The desulfurization of oxytocin. J. Biol. Chem. 1951, 193, 359–361. [Google Scholar] [CrossRef]
- Acher, R.; Chauvet, J. The structure of bovine vasopressin. Biochim. Biophys. Acta 1953, 487–488. [Google Scholar] [CrossRef]
- Tuppy, H. The amino-acid sequence in oxytocin. Biochim. Biophys. Acta 1953, 11, 449–450. [Google Scholar] [CrossRef]
- Du Vigneaud, V.; Ressler, C.; Swan, J.M.; Roberts, C.W.; Katsoyannis, P.G. The Synthesis of Oxytocin. J. Am. Chem. Soc. 1954, 76, 3115–3121. [Google Scholar] [CrossRef]
- Takayanagi, Y.; Kasahara, Y.; Onaka, T.; Takahashi, N.; Kawada, T.; Nishimori, K. Oxytocin receptor-deficient mice developed late- onset obesity. NeuroReport 2008, 19, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Camerino, C. Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity 2009, 17, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Kublaoui, B.M.; Gemelli, T.; Tolson, K.P.; Wang, Y.; Zinn, A.R. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol. Endocrinol. 2008, 22, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Chen, H.; Weingarth, D.; Trumbauer, M.E.; Novi, D.E.; Guan, X.; Yu, H.; Shen, Z.; Feng, Y.; Frazier, E.; et al. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol. Cell Biol. 2002, 22, 5027–5035. [Google Scholar] [CrossRef]
- Conte, E.; Romano, A.; De Bellis, M.; de Ceglia, M.; Carratù, M.R.; Gaetani, S.; Maqoud, F.; Tricarico, D.; Camerino, C. Oxtr/TRPV1 expression and acclimation of skeletal muscle to cold-stress in male mice. J. Endocrinol. 2021, 249, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lipschitz, D.L.; Crowley, W.R.; Bealer, S.L. Differential sensitivity of intranuclear and systemic oxytocin release to central noradrenergic receptor stimulation during mid- and late gestation in rats. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E523–E528. [Google Scholar] [CrossRef]
- Lawson, E.A.; Marengi, D.A.; DeSanti, R.L.; Holmes, T.M.; Schoenfeld, D.A.; Tolley, C.J. Oxytocin reduces caloric intake in men. Obesity 2015, 23, 950–956. [Google Scholar] [CrossRef]
- Thienel, M.; Fritsche, A.; Heinrichs, M.; Peter, A.; Ewers, M.; Lehnert, H.; Born, J.; Hallschmid, M. Oxytocin’s inhibitory effect on food intake is stronger in obese than normal-weight men. Int. J. Obes. 2016, 40, 1707–1714. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, R.; Wu, R.; Gu, Y.; Lu, Y. The effects of oxytocin to rectify metabolic dysfunction in obese mice are associated with increased thermogenesis. Mol. Cell. Endocrinol. 2020, 514, 110903. [Google Scholar] [CrossRef]
- Sun, L.; Lizneva, D.; Ji, Y.; Colaianni, G.; Hadelia, E.; Gumerova, A.; Ievleva, K.; Kuo, T.C.; Korkmaz, F.; Ryu, V.; et al. Oxytocin regulates body composition. Proc. Natl. Acad. Sci. USA 2019, 116, 26808–26815. [Google Scholar] [CrossRef] [PubMed]
- Odekunle, E.A.; Semmens, D.C.; Martynyuk, N.; Tinoco, A.B.; Garewal, A.K.; Patel, R.R.; Blowes, L.M.; Zandawala, M.; Delroiss, J.; Slade, S.E.; et al. Ancient role of vasopressin/oxytocin-type neuropeptides as regulators of feeding revealed in an echinoderm. BMC Biol. 2019, 17, 60. [Google Scholar] [CrossRef] [PubMed]
- Elphick, M.R.; Mirabeau, O.; Larhammar, D. Evolution of neuropeptide signalling systems. J. Exp. Biol. 2018, 221, jeb151092. [Google Scholar] [CrossRef]
- Mirabeau, O.; Joly, J.S. Molecular evolution of peptidergic signaling systems in bilaterians. Proc. Natl. Acad. Sci. USA 2013, 110, E2028–E2037. [Google Scholar] [CrossRef] [PubMed]
- Jékely, G. Global view of the evolution and diversity of metazoan neuropeptide signaling. Proc. Natl. Acad. Sci. USA 2013, 110, 8702–8707. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, C.H. Neuropeptide families and their receptors, evolutionary perspectives. Brain Res. 1999, 848, 1–25. [Google Scholar] [CrossRef]
- Elphick, M.R.; Rowe, M.L. NGFFFamide and echinotocin, structurally unrelated myoactive neuropeptides derived from neurophysin-containing precursors in sea urchins. J. Exp. Biol. 2009, 212, 1067–1077. [Google Scholar] [CrossRef]
- Beets, I.; Temmerman, L.; Janssen, T.; Liliane Schoofs, L. Ancient neuromodulation by vasopressin/oxytocin-related peptides. Worm 2013, 2, e24246. [Google Scholar] [CrossRef]
- Gruber, C.W. Physiology of invertebrate oxytocin and vasopressin neuropeptides. Exp. Physiol. 2014, 99, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Roch, G.J.; Busby, E.R.; Sherwood, N.M. Evolution of GnRH, diving deeper. Gen. Comp. Endocrinol. 2011, 171, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lindemans, M.; Liu, F.; Janssen, T.; Husson, S.J.; Mertens, I.; Gäde, G.; Schoofs, L. Adipokinetic hormone signaling through the gonadotropin-releasing hormone receptor modulates egg-laying in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2009, 106, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Lockard, M.A.; Ebert, M.S.; Bargmann, C.I. Oxytocin mediated behavior in invertebrates, An evolutionary perspective. Dev. Neurobiol. 2017, 77, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Oumi, T.; Ukena, K.; Matsushima, O.; Ikeda, T.; Fujita, T.; Minakata, H.; Nomoto, K. Annetocin, an annelid oxytocin-related peptide, induces egg-laying behavior in the earthworm, Eisenia foetida. J. Exp. Zool. 1996, 276, 151–156. [Google Scholar] [CrossRef]
- Aikins, M.J.; Schooley, D.A.; Begum, K.; Detheux, M.; Beeman, R.W.; Park, Y. Vasopressin-like peptide and its receptor function in an indirect diuretic signaling pathway in the red flour beetle. Insect Biochem. Mol. Biol 2008, 38, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Van Kesteren, R.E.; Smit, A.B.; Dirks, R.W.; de With, N.D.; Geraerts, W.P.; Joosse, J. Evolution of the vasopressin/oxytocin superfamily, characterization of a cDNA encoding a vasopressin-related precursor, preproconopressin, from the mollusc Lymnaea stagnalis. Proc. Natl. Acad. Sci. USA 1992, 89, 4593–4597. [Google Scholar] [CrossRef]
- Van Kesteren, R.E.; Smit, A.B.; De Lange, R.P.; Kits, K.S.; Van Golen, F.A.; Van Der Schors, R.C.; De With, N.D.; Burke, J.F.; Geraerts, W.P. Structural and functional evolution of the vasopressin/oxytocin superfamily, vasopressin-related conopressin is the only member present in Lymnaea, and is involved in the control of sexual behavior. J. Neurosci. 1995, 15, 5989–5998. [Google Scholar] [CrossRef]
- Camerino, C.; Conte, E.; Carratù, M.R.; Fonzino, A.; Lograno, M.D.; Tricarico, D. Oxytocin/osteocalcin/IL-6 and NGF/BDNF mRNA levels in response to cold stress challenge in mice, possible oxytonic brain- bone-muscle-interaction. Front. Physiol. 2019, 10, 1437. [Google Scholar] [CrossRef]
- Sawyer, W.H. Evolution of neurohypophyseal hormones and their receptors. Fed. Proc. 1977, 36, 1842–1847. [Google Scholar]
- Johnson, Z.V.; Young, L.J. Oxytocin and vasopressin neural networks, Implications for social behavioral diversity and translational neuroscience. Neurosci. Biobehav. Rev. 2017, 76, 87–98. [Google Scholar] [CrossRef]
- Leslie, M.; Silva, P.; Paloyelis, Y.; Blevins, J.; Treasure, J. A Systematic Review and Quantitative Meta-Analysis of Oxytocin’s Effects on Feeding. J. Neuroendocrinol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lawson, E.A. The effects of oxytocin on eating behaviour and metabolism in humans. Nat. Rev. Endocrinol. 2017, 13, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Oumi, T.; Ukena, K.; Matsushima, O.; Ikeda, T.; Fujita, T.; Minakata, H.; Nomoto, K. Annetocin, an oxytocin-related peptide isolated from the earthworm, Eisenia foetida. Biochem. Biophys. Res. Commun. 1994, 198, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, D.A.; Hamilton, M.S.; Huang, T.; Kristan, W.B.; French, K.A. A hormone-activated central pattern generator for courtship. Curr. Biol. 2010, 20, 487–495. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fujino, Y.; Nagahama, T.; Oumi, T.; Ukena, K.; Morishita, F.; Furukawa, Y.; Matsushima, O.; Ando, M.; Takahama, H.; Satake, H.; et al. Possible functions of oxytocin/vasopressin-superfamily peptides in annelids with special reference to reproduction and osmoregulation. J. Exp. Zool. 1999, 284, 401–406. [Google Scholar] [CrossRef]
- Kawada, T.; Kanda, A.; Minakata, H.; Matsushima, O.; Satake, H. Identification of a novel receptor for an invertebrate oxytocin/vasopressin superfamily peptide, molecular and functional evolution of the oxytocin/vasopressin superfamily. Biochem. J. 2004, 382, 231–237. [Google Scholar] [CrossRef]
- Kanda, A.; Satake, H.; Kawada, T.; Minakata, H. Novel evolutionary lineages of the invertebrate oxytocin/vasopressin superfamily peptides and their receptors in the common octopus (Octopus vulgaris). Biochem. J. 2005, 387, 85–91. [Google Scholar] [CrossRef][Green Version]
- Takuwa-Kuroda, K.; Iwakoshi-Ukena, E.; Kanda, A.; Minakata, H. Octopus, which owns the most advanced brain in invertebrates, has two members of vasopressin/oxytocin superfamily as in vertebrates. Regul. Pept. 2003, 115, 139–149. [Google Scholar] [CrossRef]
- Kanda, A.; Takuwa-Kuroda, K.; Iwakoshi-Ukena, E.; Furukawa, Y.; Matsushima, O.; Minakata, H. Cloning of Octopus cephalotocin receptor, a member of the oxytocin/vasopressin superfamily. J. Endocrinol. 2003, 179, 281–291. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Garrison, J.L.; Macosko, E.Z.; Bernstein, S.; Pokala, N.; Albrecht, D.R.; Bargmann, C.I. Oxytocin/vasopressin-related peptides have an ancient role in reproductive behavior. Science 2012, 338, 540–543. [Google Scholar] [CrossRef]
- Beets, I.; Janssen, T.; Meelkop, E.; Temmerman, L.; Suetens, N.; Rademakers, S.; Jansen, G.; Schoofs, L. Vasopressin/oxytocin-related signaling regulates gustatory associative learning in C. elegans. Science 2012, 338, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Colbert, H.A.; Smith, T.L.; Bargmann, C.I. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J. Neurosci. 1997, 17, 8259–8269. [Google Scholar] [CrossRef] [PubMed]
- Ukena, K.; Iwakoshi-Ukena, E.; Hikosaka, A. Unique form and osmoregulatory function of a neurohypophysial hormone in a urochordate. Endocrinology 2008, 149, 5254–5261. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, E.S.; Uhm, K.O.; Lee, Y.M.; Kwon, J.; Park, S.H.; Soo, K.H. Oxytocin stimulates glucose uptake in skeletal muscle cells through the calcium-CaMKK-AMPK pathway. Regul. Pept. 2008, 151, 71–74. [Google Scholar] [CrossRef] [PubMed]
- McGonigle, P. Peptide therapeutics for CNS indications. Biochem. Pharm. 2012, 83, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scimè, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. What we talk about when we talk about fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef]
- Bertholet, A.M.; Kirichok, Y. UCP1, A transporter for H+ and fatty acid anions. Biochimie 2017, 134, 28–34. [Google Scholar] [CrossRef]
- Badenes, M.; Amin, A.; González-García, I.; Félix, I.; Burbridge, E.; Cavadas, M.; Ortega, F.J.; de Carvalho, E.; Faísca, P.; Carobbio, S.; et al. Deletion of iRhom2 protects against diet-induced obesity by increasing thermogenesis. Mol. Metab. 2020, 31, 67–84. [Google Scholar] [CrossRef]
- Crane, J.D.; Palanivel, R.; Mottillo, E.P.; Bujak, A.L.; Wang, H.; Ford, R.J.; Collins, A.; Blümer, R.M.; Fullerton, M.D.; Yabut, J.M.; et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat. Med. 2015, 21, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Carreira, M.C.; Andrade, S.; Gonzalez-Izquierdo, A.; Amil, M.; Folgueira, C.; Monteiro, M.P.; Sanz, E.; Crujeiras, A.B.; Casanueva, F.F. Anti-obesity activity of OBEX is regulated by activation of thermogenesis and decreasing adiposity gain. Sci. Rep. 2018, 8, 17155. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.H.; Kang, Y.H.; Kim, J.S.; Yun, S.C.; Kang, S.W.; Song, Y. Suppression of Brown Adipocyte Autophagy Improves Energy Metabolism by Regulating Mitochondrial Turnover. Int. J. Mol. Sci. 2019, 20, 3520. [Google Scholar] [CrossRef]
- Altirriba, J.; Poher, A.L.; Caillon, A.; Arsenijevic, D.; Veyrat-Durebex, C.; Lyautey, J.; Dulloo, A.; Rohner-Jeanrenaud, F. Divergent effects of oxytocin treatment of obese diabetic mice on adiposity and diabetes. Endocrinology 2014, 155, 4189–4201. [Google Scholar] [CrossRef] [PubMed]
- Blevins, J.E.; Baskin, D.G. Translational and therapeutic potential of oxytocin as an anti-obesity strategy, Insights from rodents, nonhuman primates and humans. Physiol. Behav. 2015, 152, 438–449. [Google Scholar] [CrossRef]
- Deblon, N.; Veyrat-Durebex, C.; Bourgoin, L.; Caillon, A.; Bussier, A.L.; Petrosino, S.; Piscitelli, F.; Legros, J.-J.; Geenen, V.; Foti, M.; et al. Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PLoS ONE 2011, 6, e25565. [Google Scholar] [CrossRef]
- Plante, E.; Menaouar, A.; Danalache, B.A.; Yip, D.; Broderick, T.L.; Chiasson, J.L.; Jankowski, M.; Gutkowska, J. Oxytocin treatment prevents the cardiomyopathy observed in obese diabetic male db/db mice. Endocrinology 2015, 156, 1416–1428. [Google Scholar] [CrossRef] [PubMed]
- Noble, E.E.; Billington, C.J.; Kotz, C.M.; Wang, C. Oxytocin in the ventromedial hypothalamic nucleus reduces feeding and acutely increases energy expenditure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R737–R745. [Google Scholar] [CrossRef]
- Zhang, G.; Cai, D. Circadian intervention of obesity development via resting-stage feeding manipulation or oxytocin treatment. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1004–E1012. [Google Scholar] [CrossRef] [PubMed]
- Blevins, J.E.; Thompson, B.W.; Anekonda, V.T.; Ho, J.M.; Graham, J.L.; Roberts, Z.S.; Hwang, B.H.; Ogimoto, K.; Wolden-Hanson, T.; Nelson, J.; et al. Chronic CNS oxytocin signaling preferentially induces fat loss in high-fat diet-fed rats by enhancing satiety responses and increasing lipid utilization. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R640–R658. [Google Scholar] [CrossRef]
- Maejima, Y.; Iwasaki, Y.; Yamahara, Y.; Kodaira, M.; Sedbazar, U.; Yada, T. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging 2011, 3, 1169–1177. [Google Scholar] [CrossRef]
- Morton, G.J.; Thatcher, B.S.; Reidelberger, R.D.; Ogimoto, K.; Wolden-Hanson, T.; Baskin, D.G.; Schwartz, M.W.; Blevins, J.E. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E134–E144. [Google Scholar] [CrossRef]
- Kajimura, S.; Spiegelman, B.M.; Seale, P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 2015, 22, 546–559. [Google Scholar] [CrossRef]
- Xi, D.; Long, C.; Lai, M.; Casella, A.; O’Lear, L.; Kublaoui, B.; Roizen, J.D. Ablation of oxytocin neurons causes a deficit in cold stress response. J. Endocr. Soc. 2017, 1, 1041–1055. [Google Scholar] [CrossRef]
- Gajdosechova, L.; Krskova, K.; Olszanecki, R.; Zorad, S. Differential regulation of oxytocin receptor in various adipose tissue depots and skeletal muscle types in obese Zucker rats. Horm. Metab. Res. 2015, 47, 600–604. [Google Scholar] [CrossRef]
- Fuchs, A.R.; Fuchs, F.; Husslein, P.; Soloff, M.S.; Fernström, M.J. Oxytocin receptors and human parturition: A dual role for oxytocin in the initiation of labor. Science 1982, 215, 1396–1398. [Google Scholar] [CrossRef]
- Takagi, T.; Tanizawa, O.; Otsuki, Y.; Sugita, N.; Haruta, M.; Yamaji, K. Oxytocin in the cerebrospinal fluid and plasma of pregnant and nonpregnant subjects. Horm. Metab. Res. 1985, 17, 308–310. [Google Scholar] [CrossRef]
- Murata, T.; Murata, E.; Liu, C.X.; Narita, K.; Honda, K.; Higuchi, T. Oxytocin receptor gene expression in rat uterus, regulation by ovarian steroids. J. Endocrinol. 2000, 166, 45–52. [Google Scholar] [CrossRef]
- Colucci, S.; Colaianni, G.; Mori, G.; Grano, M.; Zallone, A. Human osteoclasts express oxytocin receptor. Biochem. Biophys. Res. Commun. 2002, 297, 442–445. [Google Scholar] [CrossRef]
- Elabd, C.; Basillais, A.; Beaupied, H.; Breuil, V.; Wagner, N.; Scheideler, M.; Zaragosi, L.E.; Massiéra, F.; Lemichez, E.; Trajanoski, Z.; et al. Oxytocin controls differentiation of human mesenchymal stem cells and reverses osteoporosis. Stem Cells 2008, 26, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Tamma, R.; Colaianni, G.; Zhu, L.; DiBenedetto, A.; Greco, G.; Montemurro, G.; Patano, N.; Strippoli, M.; Vergari, R.; Mancini, L.; et al. Oxytocin is an anabolic bone hormone. Proc. Natl. Acad. Sci. USA 2009, 106, 7149–7154. [Google Scholar] [CrossRef]
- Camerino, C. Oxytocin inhibits bone formation through the activation of the sympathetic tone, A new candidate in the central regulation of bone formation. J. Bone Miner. Res. 2008, 23 (Suppl. S1), S56. [Google Scholar]
- MacIntyre, I.; Zaidi, M.; Alam, A.S.; Datta, H.K.; Moonga, B.S.; Lidbury, P.S.; Hecker, M.; Vane, J.R. Osteoclastic inhibition, an action of nitric oxide not mediated by cyclic GMP. Proc. Natl. Acad. Sci. USA 1991, 88, 2936–2940. [Google Scholar] [CrossRef]
- Liu, X.S.; Bevill, G.; Keaveny, T.M.; Sajda, P.; Guo, X.E. Micromechanical analyses of vertebral trabecular bone based on individual trabeculae segmentation of plates and rods. J. Biomech. 2009, 42, 249–256. [Google Scholar] [CrossRef][Green Version]
- Colaianni, G.; Sun, L.; Di Benedetto, A.; Tamma, R.; Zhu, L.L.; Cao, J.; Grano, M.; Yuen, T.; Colucci, S.; Cuscito, C.; et al. Bone marrow oxytocin mediates the anabolic action of estrogen on the skeleton. J. Biol. Chem. 2012, 287, 29159–29167. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Sun, L.; Zambonin, C.G.; Tamma, R.; Nico, B.; Calvano, C.D.; Colaianni, G.; Ji, Y.; Mori, G.; Grano, M.; et al. Osteoblast regulation via ligand-activated nuclear trafficking of the oxytocin receptor. Proc. Natl. Acad. Sci. USA 2014, 111, 16502–16507. [Google Scholar] [CrossRef]
- Ardeshirpour, L.; Brian, S.; Dann, P.; VanHouten, J.; Wysolmerski, J. Increased PTHrP and decreased estrogens alter bone turnover but do not reproduce the full effects of lactation on the skeleton. Endocrinology 2010, 151, 5591–5601. [Google Scholar] [CrossRef]
- Sun, L.; Peng, Y.; Sharrow, A.C.; Iqbal, J.; Zhang, Z.; Papachristou, D.J.; Zaidi, S.; Zhu, L.L.; Yaroslavskiy, B.B.; Zhou, H.; et al. FSH directly regulates bone mass. Cell 2006, 125, 247–260. [Google Scholar] [CrossRef]
- Zhu, L.L.; Blair, H.; Cao, J.; Yuen, T.; Latif, R.; Guo, L.; Tourkova, I.L.; Li, J.; Davies, T.F.; Sun, L.; et al. Blocking antibody to the β-subunit of FSH prevents bone loss by inhibiting bone resorption and stimulating bone synthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 14574–14579. [Google Scholar] [CrossRef]
- Takeda, S.; Elefteriou, F.; Levasseur, R.; Liu, X.; Zhao, L.; Parker, K.L.; Armstrong, D.; Ducy, P.; Karsenty, G. Leptin regulates bone formation via the sympathetic nervous system. Cell 2002, 111, 305–317. [Google Scholar] [CrossRef]
- Ding, C.; Magkos, F. Oxytocin and Vasopressin Systems in Obesity and Metabolic Health: Mechanisms and Perspectives. Curr. Obes. Rep. 2019, 8, 301–316. [Google Scholar] [CrossRef]
- Holder, I.L., Jr.; Butte, N.F.; Zinn, A.R. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum. Mol. Genet. 2000, 9, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Swaab, D.F.; Purba, J.S.; Hofman, M.A. Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: A study of five cases. J. Clin. Endocrinol. Metab. 1995, 80, 573–579. [Google Scholar] [CrossRef]
- Mizunoya, W.; Iwamoto, Y.; Sato, Y.; Tatsumi, R.; Ikeuchi, Y. Cold exposure increases slow-type myosin heavy chain 1 (MyHC1) composition of Soleus muscle in rats. Anim. Sci. J. 2014, 85, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Camerino, C.; Conte, E.; Cannone, M.; Caloiero, R.; Fonzino, A.; Tricarico, D. Nerve growth factor, brain-derived neurotrophic factor and osteocalcin gene relationship in energy regulation, bone homeostasis and reproductive organs analyzed by mRNA quantitative evaluation and linear correlation analysis. Front. Physiol. 2016, 7, 456. [Google Scholar] [CrossRef]
- Scala, R.; Maqoud, F.; Angelelli, M.; Latorre, R.; Perrone, M.G.; Scilimati, A.; Tricarico, D. Zoledronic acid modulation of TRPV1 channel currents in osteoblast cell line and native rat and mouse bone marrow-derived osteoblasts: Cell proliferation and mineralization effect. Cancers 2019, 11, 206. [Google Scholar] [CrossRef]
- Nersesyan, Y.; Demirkhanyan, L.; Cabezas-Bratesco, D.; Oakes, V.; Kusuda, R.; Dawson, T.; Sun, X.; Cao, C.; Cohen, A.M.; Chelluboina, B.; et al. Oxytocin modulates nociception as an agonist of pain-sensing TRPV1. Cell Rep. 2017, 21, 1681–1691. [Google Scholar] [CrossRef]
- Gonzalez-Hernandez, A.; Charlet, A. Oxytocin, GABA, and TRPV1, the analgesic triad? Front. Mol. Neurosci. 2018, 11, 398. [Google Scholar] [CrossRef]
- Trayhurn, P. Origins and early development of the concept that brown adipose tissue thermogenesis is linked to energy balance and obesity. Biochimie 2017, 134, 62–70. [Google Scholar] [CrossRef]
- Kasahara, Y.; Sato, K.; Takayanagi, Y.; Mizukami, H.; Ozawa, K.; Hidema, S.; So, K.H.; Kawada, T.; Inoue, N.; Ikeda, I.; et al. Oxytocin receptor in the hypothalamus is sufficient to rescue normal thermoregulatory function in male oxytocin receptor knockout mice. Endocrinology 2013, 154, 4305–4315. [Google Scholar] [CrossRef] [PubMed]
- Blondin, D.P.; Haman, F. Shivering and nonshivering thermogenesis in skeletal muscle. Handb. Clin. Neurol. 2018, 156, 153–173. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J. Non-shivering thermogenesis as a mechanism to facilitate sustainable weight loss. Obes. Rev. 2017, 18, 819–831. [Google Scholar] [CrossRef]
- Jurek, B.; Neumann, I.D. The oxytocin receptor: From intracellular signaling to behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef]
- Breton, C.; Haenggeli, C.; Barberis, C.; Heitz, F.; Bader, C.R.; Bernheim, L.; Tribollet, E. Presence of functional oxytocin receptors in cultured human myoblasts. J. Clin. Endocrinol. Metab. 2002, 87, 1415–1418. [Google Scholar] [CrossRef]
- Qin, J.; Feng, M.; Wang, C.; Ye, Y.; Wang, P.S.; Liu, C. Oxytocin receptor expressed on the smooth muscle mediates the excitatory effect of oxytocin on gastric motility in rats. Neurogastroenterol. Motil. 2009, 21, 430–438. [Google Scholar] [CrossRef]
- Baribeau, D.A.; Anagnostou, E. Oxytocin and vasopressin: Linking pituitary neuropeptides and their receptors to social neurocircuits. Front. Neurosci. 2015, 9, 335. [Google Scholar] [CrossRef]
- Camerino, C.; Conte, E.; Caloiero, R.; Fonzino, A.; Carratù, M.; Lograno, M.D.; Tricarico, D. Evaluation of short and long term cold stress challenge of nerve grow factor, brain-derived neurotrophic factor, osteocalcin and oxytocin mRNA expression in BAT, brain, bone and reproductive tissue of male mice using real-time PCR and linear correlation analysis. Front. Physiol. 2017, 8, 1101. [Google Scholar] [CrossRef]
- Dumitru, A.; Radu, B.M.; Radu, M.; Cretoiu, S.M. Muscle changes during atrophy. Adv. Exp. Med. Biol. 2018, 1088, 73–92. [Google Scholar] [CrossRef]
- Martin, A.; State, M.; Anderson, G.M.; Kaye, W.M.; Hanchett, J.M.; McConaha, C.W.; North, W.G.; Leckman, J.F. Cerebrospinal fluid levels of oxytocin in Prader-Willi syndrome: A preliminary report. Biol. Psychiatry 1998, 44, 1349–1352. [Google Scholar] [CrossRef]
- Muscatelli, F.; Abrous, D.N.; Massacrier, A.; Boccaccio, I.; Le Moal, M.; Cau, P.; Cremer, H. Disruption of the mouse necdin gene results in hypothalamic and behavioral alterations reminiscent of the human Prader-Willi syndrome. Hum. Mol. Genet. 2000, 9, 3101–3110. [Google Scholar] [CrossRef]
- Johnson, Z.V.; Walum, H.; Jamal, Y.A.; Xiao, Y.; Keebaugh, A.C.; Inoue, K.; Young, L.J. Central oxytocin receptors mediate mating-induced partner preferences and enhance correlated activation across forebrain nuclei in male prairie voles. Horm. Behav. 2016, 79, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.G. Prader-Willi syndrome: Obesity due to genomic imprinting. Curr. Genomics 2011, 12, 204–215. [Google Scholar] [CrossRef]
- Elefteriou, F.; Ahn, J.D.; Takeda, S.; Starbuck, M.; Yang, X.; Liu, X.; Kondo, H.; Richards, W.G.; Bannon, T.W.; Noda, M.; et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 2005, 434, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Labyb, M.; Chrétien, C.; Caillon, A.; Rohner-Jeanrenaud, F.; Altirriba, J. Oxytocin Administration Alleviates Acute but Not Chronic Leptin Resistance of Diet-Induced Obese Mice. Int. J. Mol. Sci. 2018, 20, 88. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camerino, C. Oxytocin Involvement in Body Composition Unveils the True Identity of Oxytocin. Int. J. Mol. Sci. 2021, 22, 6383. https://doi.org/10.3390/ijms22126383
Camerino C. Oxytocin Involvement in Body Composition Unveils the True Identity of Oxytocin. International Journal of Molecular Sciences. 2021; 22(12):6383. https://doi.org/10.3390/ijms22126383
Chicago/Turabian StyleCamerino, Claudia. 2021. "Oxytocin Involvement in Body Composition Unveils the True Identity of Oxytocin" International Journal of Molecular Sciences 22, no. 12: 6383. https://doi.org/10.3390/ijms22126383
APA StyleCamerino, C. (2021). Oxytocin Involvement in Body Composition Unveils the True Identity of Oxytocin. International Journal of Molecular Sciences, 22(12), 6383. https://doi.org/10.3390/ijms22126383




