Toxicological Profile of Plasmonic Nanoparticles in Zebrafish Model
Abstract
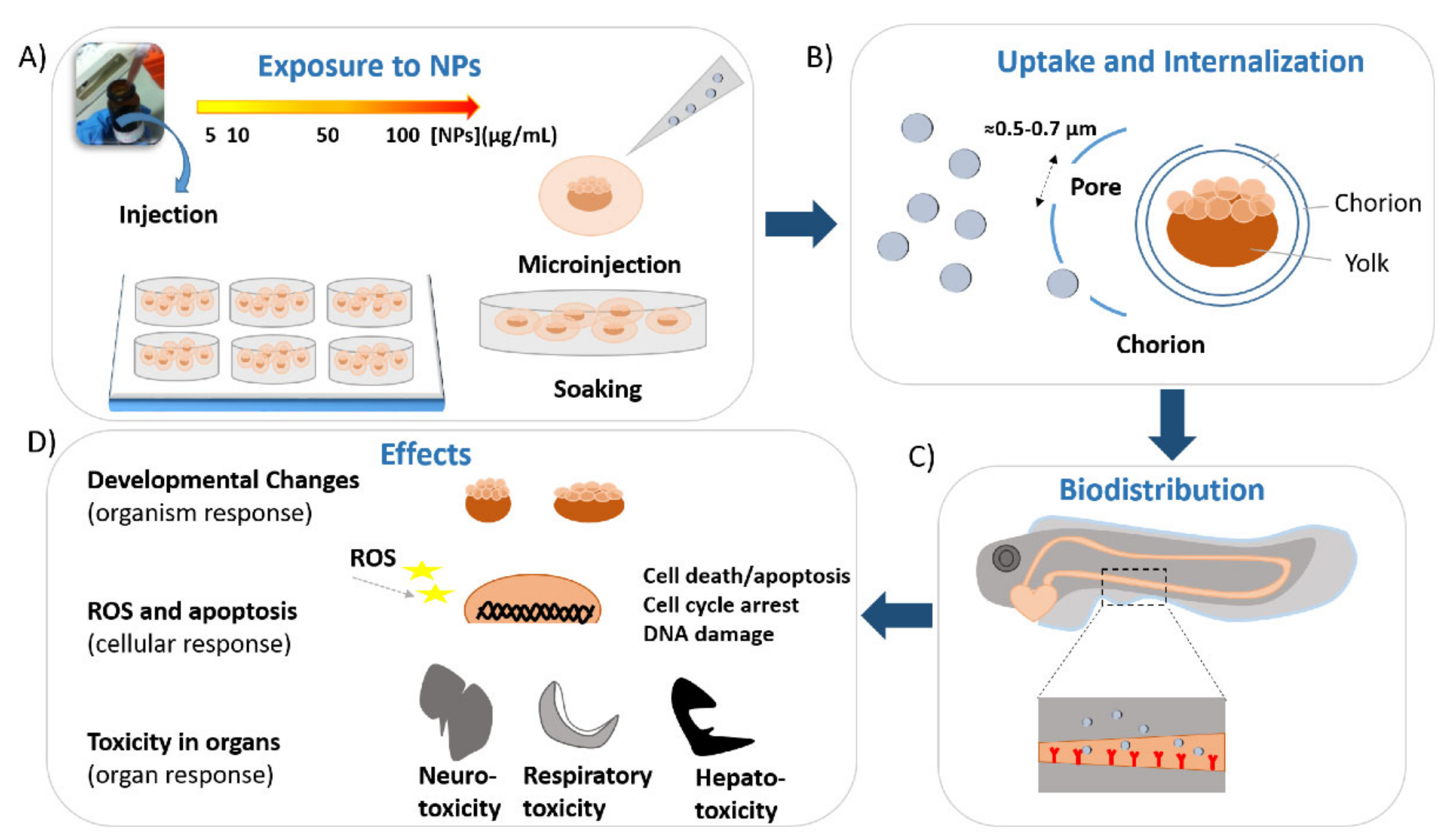
1. Introduction
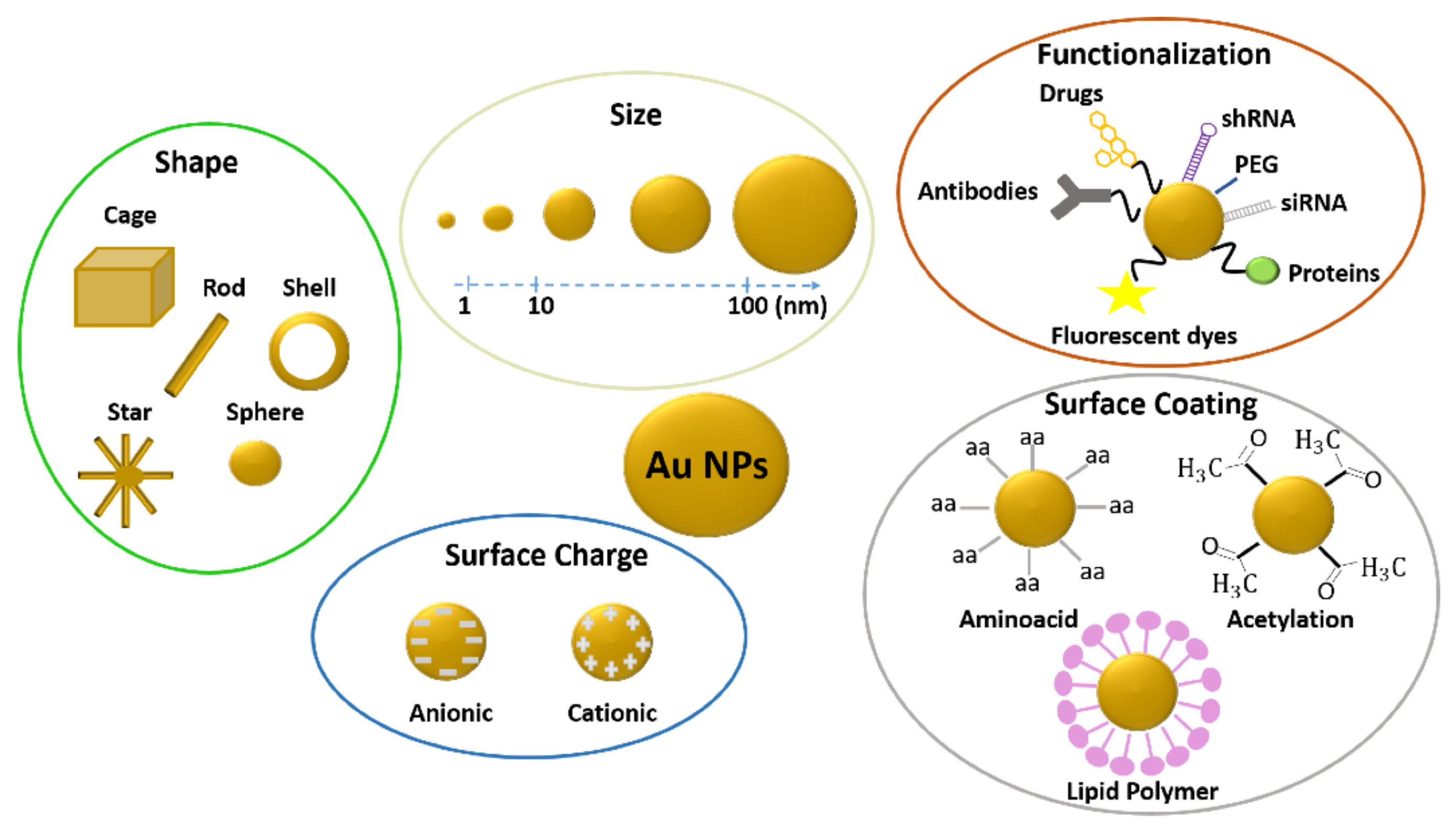
2. Gold Nanoparticles
2.1. Toxicity of Gold Nanoparticles in Zebrafish during Development
2.1.1. Uptake and Biodistribution of Gold Nanoparticles
2.1.2. Influence of Gold Nanoparticle Size on Toxicity
2.1.3. Influence of Gold Nanoparticle Surface Chemistry on Toxicity
2.1.4. Influence of Gold Nanoparticle Surface Charge on Toxicity
2.1.5. Influence of Gold Nanoparticle Shape on Toxicity
2.2. Toxicity of Gold Nanoparticles in Adult Zebrafish
2.2.1. Effects of Gold Nanoparticles Chemically Synthetized
2.2.2. Effects of Gold Nanoparticles Biologically Synthetized
3. Silver Nanoparticles
3.1. Toxicity of Silver Nanoparticles in Zebrafish during Development
3.1.1. Uptake and Biodistribution of Silver Nanoparticles
3.1.2. Different Effects of Silver Nanoparticles
3.1.3. Influence of Silver Nanoparticle Size on Toxicity
3.1.4. Effects of Silver Nanoparticle Surface Chemistry and Charge on Toxicity
3.1.5. Influence of Sediment, Aquatic Plants, and Medium Composition on Silver Nanoparticle Toxicity
3.1.6. Influence of Silver Dissolution on Toxicity
3.2. Toxicity of Silver Nanoparticles in Adult Zebrafish
3.2.1. Influence of Silver Nanoparticle Surface Chemistry on Toxicity
3.2.2 Influence of Silver Nanoparticle Dissolution on Toxicity
3.2.3 Effects of Silver Nanoparticles Biologically Synthetized
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 25. [Google Scholar]
- Maier, S.A. Plasmonics: Fundamentals and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Lee, K.-S.; El-Sayed, M.A. Gold and Silver Nanoparticles in Sensing and Imaging: Sensitivity of Plasmon Response to Size, Shape, and Metal Composition. J. Phys. Chem. B 2006, 110, 19220–19225. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Rycenga, M.; Cobley, C.M.; Zeng, J.; Li, W.; Moran, C.H.; Zhang, Q.; Qin, D.; Xia, Y. Controlling the Synthesis and Assembly of Silver Nanostructures for Plasmonic Applications. Chem. Rev. 2011, 111, 3669–3712. [Google Scholar] [CrossRef] [PubMed]
- Stockman, M.I. Nanoplasmonics: The physics behind the applications. Phys. Today 2011, 64, 39–44. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, E. Metal nanoclusters: New fluorescent probes for sensors and bioimaging. Nano Today 2014, 9, 132–157. [Google Scholar] [CrossRef]
- Slepička, P.; Slepičková Kasálková, N.; Siegel, J.; Kolská, Z.; Švorčík, V. Methods of Gold and Silver Nanoparticles Preparation. Materials 2019, 13, 1. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Rizzo, L.Y.; Golombek, S.K.; Mertens, M.E.; Pan, Y.; Laaf, D.; Broda, J.; Jayapaul, J.; Möckel, D.; Subr, V.; Hennink, W.E.; et al. In Vivo Nanotoxicity Testing using the Zebrafish Embryo Assay. J. Mater. Chem. B 2013, 1. [Google Scholar] [CrossRef]
- d’Amora, M.; Giordani, S. The Utility of Zebrafish as a Model for Screening Developmental Neurotoxicity. Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef]
- Beliaeva, N.F.; Kashirtseva, V.N.; Medvedeva, N.V.; Khudoklinova, I.; Ipatova, O.M.; Archakov, A.I. [Zebrafish as a model organism for biomedical studies]. Biomed. Khim. 2010, 56, 120–131. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.-S. Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. J. Nanobiotechnol. 2016, 14, 1–13. [Google Scholar] [CrossRef]
- Brand, M.; Granato, M.; Nüsslein-Volhard, C.; Nusslein-Volhard, C.; Dahm, R. Zebrafish: A practical approach. Keep. Rais. Zebrafish 2002, 7, 39. [Google Scholar]
- Amacher, S.L. Zebrafish embryo as a developmental system. e LS 2001. [Google Scholar] [CrossRef]
- Parichy, D.M.; Elizondo, M.R.; Mills, M.G.; Gordon, T.N.; Engeszer, R.E. Normal table of postembryonic zebrafish development: Staging by externally visible anatomy of the living fish. Dev. Dyn. 2009, 238, 2975–3015. [Google Scholar] [CrossRef]
- Fishman, M.C. Zebrafish genetics: The enigma of arrival. Proc. Natl. Acad. Sci. USA 1999, 96, 10554–10556. [Google Scholar] [CrossRef]
- Hsu, C.H.; Wen, Z.H.; Lin, C.S.; Chakraborty, C. The zebrafish model: Use in studying cellular mechanisms for a spectrum of clinical disease entities. Curr. Neurovasc. Res. 2007, 4, 111–120. [Google Scholar] [CrossRef]
- Howes, P.D.; Rana, S.; Stevens, M.M. Plasmonic nanomaterials for biodiagnostics. Chem. Soc. Rev. 2014, 43, 3835–3853. [Google Scholar] [CrossRef]
- You, J.; Zhang, G.; Li, C. Exceptionally High Payload of Doxorubicin in Hollow Gold Nanospheres for Near-Infrared Light-Triggered Drug Release. ACS Nano 2010, 4, 1033–1041. [Google Scholar] [CrossRef]
- Brown, S.D.; Nativo, P.; Smith, J.-A.; Stirling, D.; Edwards, P.R.; Venugopal, B.; Flint, D.J.; Plumb, J.A.; Graham, D.; Wheate, N.J. Gold Nanoparticles for the Improved Anticancer Drug Delivery of the Active Component of Oxaliplatin. J. Am. Chem. Soc. 2010, 132, 4678–4684. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Thompson, L.B.; Boulos, S.P.; Sisco, P.N.; Murphy, C.J. Gold nanorods: Their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv. Drug. Deliv. Rev. 2012, 64, 190–199. [Google Scholar] [CrossRef]
- Kumar, A.; Zhang, X.; Liang, X.J. Gold nanoparticles: Emerging paradigm for targeted drug delivery system. Biotechnol. Adv. 2013, 31, 593–606. [Google Scholar] [CrossRef]
- Baptista, P.V.; Doria, G.; Quaresma, P.; Cavadas, M.; Neves, C.S.; Gomes, I.; Eaton, P.; Pereira, E.; Franco, R. Nanoparticles in molecular diagnostics. Prog. Mol. Biol. Transl. Sci. 2011, 104, 427–488. [Google Scholar] [CrossRef]
- Zhou, W.; Gao, X.; Liu, D.; Chen, X. Gold Nanoparticles for In Vitro Diagnostics. Chem. Rev. 2015, 115, 10575–10636. [Google Scholar] [CrossRef]
- Karthick, V.; Kumar, V.G.; Dhas, T.S.; Singaravelu, G.; Sadiq, A.M.; Govindaraju, K. Effect of biologically synthesized gold nanoparticles on alloxan-induced diabetic rats-an in vivo approach. Colloids Surf. B Biointerfaces 2014, 122, 505–511. [Google Scholar] [CrossRef]
- Gao, N.; Sun, H.; Dong, K.; Ren, J.; Qu, X. Gold-nanoparticle-based multifunctional amyloid-β inhibitor against Alzheimer’s disease. Chemistry 2015, 21, 829–835. [Google Scholar] [CrossRef]
- Lee, H.; Lee, M.-Y.; Bhang, S.H.; Kim, B.-S.; Kim, Y.S.; Ju, J.H.; Kim, K.S.; Hahn, S.K. Hyaluronate–Gold Nanoparticle/Tocilizumab Complex for the Treatment of Rheumatoid Arthritis. ACS Nano 2014, 8, 4790–4798. [Google Scholar] [CrossRef] [PubMed]
- Huff, T.B.; Tong, L.; Zhao, Y.; Hansen, M.N.; Cheng, J.X.; Wei, A. Hyperthermic effects of gold nanorods on tumor cells. Nanomedicine 2007, 2, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xia, Y. Gold and silver nanoparticles: A class of chromophores with colors tunable in the range from 400 to 750 nm. Analyst 2003, 128, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.A.; Khlebtsov, N.G. Gold nanoparticles in biology and medicine: Recent advances and prospects. Acta. Naturae. 2011, 3, 34–55. [Google Scholar] [CrossRef] [PubMed]
- Adewale, O.B.; Davids, H.; Cairncross, L.; Roux, S. Toxicological Behavior of Gold Nanoparticles on Various Models: Influence of Physicochemical Properties and Other Factors. Int. J. Toxicol. 2019, 38, 357–384. [Google Scholar] [CrossRef]
- Jia, Y.-P.; Ma, B.-Y.; Wei, X.-W.; Qian, Z.-Y. The in vitro and in vivo toxicity of gold nanoparticles. Chin. Chem. Lett. 2017, 28, 691–702. [Google Scholar] [CrossRef]
- Yang, L.; Kuang, H.; Zhang, W.; Aguilar, Z.P.; Wei, H.; Xu, H. Comparisons of the biodistribution and toxicological examinations after repeated intravenous administration of silver and gold nanoparticles in mice. Sci. Rep. 2017, 7, 3303. [Google Scholar] [CrossRef]
- Browning, L.M.; Lee, K.J.; Huang, T.; Nallathamby, P.D.; Lowman, J.E.; Xu, X.H. Random walk of single gold nanoparticles in zebrafish embryos leading to stochastic toxic effects on embryonic developments. Nanoscale 2009, 1, 138–152. [Google Scholar] [CrossRef]
- Browning, L.M.; Huang, T.; Xu, X.-H.N. Real-time in vivo imaging of size-dependent transport and toxicity of gold nanoparticles in zebrafish embryos using single nanoparticle plasmonic spectroscopy. Interface Focus 2013, 3, 20120098. [Google Scholar] [CrossRef]
- Harper, S.L.; Carriere, J.L.; Miller, J.M.; Hutchison, J.E.; Maddux, B.L.S.; Tanguay, R.L. Systematic Evaluation of Nanomaterial Toxicity: Utility of Standardized Materials and Rapid Assays. ACS Nano 2011, 5, 4688–4697. [Google Scholar] [CrossRef]
- Truong, L.; Tilton, S.C.; Zaikova, T.; Richman, E.; Waters, K.M.; Hutchison, J.E.; Tanguay, R.L. Surface functionalities of gold nanoparticles impact embryonic gene expression responses. Nanotoxicology 2013, 7, 192–201. [Google Scholar] [CrossRef]
- Asharani, P.V.; Lianwu, Y.; Gong, Z.; Valiyaveettil, S. Comparison of the toxicity of silver, gold and platinum nanoparticles in developing zebrafish embryos. Nanotoxicology 2011, 5, 43–54. [Google Scholar] [CrossRef]
- Mesquita, B.; Lopes, I.; Silva, S.; Bessa, M.J.; Starykevich, M.; Carneiro, J.; Galvão, T.L.P.; Ferreira, M.G.S.; Tedim, J.; Teixeira, J.P.; et al. Gold nanorods induce early embryonic developmental delay and lethality in zebrafish (Danio rerio). J. Toxicol. Environ. Health Part A 2017, 80, 672–687. [Google Scholar] [CrossRef]
- van Pomeren, M.; Peijnenburg, W.; Vlieg, R.C.; van Noort, S.J.T.; Vijver, M.G. The biodistribution and immuno-responses of differently shaped non-modified gold particles in zebrafish embryos. Nanotoxicology 2019, 13, 558–571. [Google Scholar] [CrossRef]
- Ganeshkumar, M.; Sastry, T.P.; Sathish Kumar, M.; Dinesh, M.G.; Kannappan, S.; Suguna, L. Sun light mediated synthesis of gold nanoparticles as carrier for 6-mercaptopurine: Preparation, characterization and toxicity studies in zebrafish embryo model. Mater. Res. Bull. 2012, 47, 2113–2119. [Google Scholar] [CrossRef]
- Wang, Y.; Seebald, J.L.; Szeto, D.P.; Irudayaraj, J. Biocompatibility and Biodistribution of Surface-Enhanced Raman Scattering Nanoprobes in Zebrafish Embryos: In vivo and Multiplex Imaging. ACS Nano 2010, 4, 4039–4053. [Google Scholar] [CrossRef]
- Liu, X.; Dumitrescu, E.; Kumar, A.; Austin, D.; Goia, D.; Wallace, K.N.; Andreescu, S. Differential lethal and sublethal effects in embryonic zebrafish exposed to different sizes of silver nanoparticles. Environ. Pollut. 2019, 248, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Bar-Ilan, O.; Albrecht, R.M.; Fako, V.E.; Furgeson, D.Y. Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos. Small 2009, 5, 1897–1910. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-T.; Zaikova, T.; Hutchison, J.E.; Tanguay, R.L. Gold nanoparticles disrupt zebrafish eye development and pigmentation. Toxicol. Sci. 2013, 133, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Truong, L.; Saili, K.S.; Miller, J.M.; Hutchison, J.E.; Tanguay, R.L. Persistent adult zebrafish behavioral deficits results from acute embryonic exposure to gold nanoparticles. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2012, 155, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Leifert, A.; Pan-Bartnek, Y.; Simon, U.; Jahnen-Dechent, W. Molecularly stabilised ultrasmall gold nanoparticles: Synthesis, characterization and bioactivity. Nanoscale 2013, 5, 6224–6242. [Google Scholar] [CrossRef]
- Pan, Y.; Leifert, A.; Ruau, D.; Neuss, S.; Bornemann, J.; Schmid, G.; Brandau, W.; Simon, U.; Jahnen-Dechent, W. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small 2009, 5, 2067–2076. [Google Scholar] [CrossRef]
- Ginzburg, A.L.; Truong, L.; Tanguay, R.L.; Hutchison, J.E. Synergistic Toxicity Produced by Mixtures of Biocompatible Gold Nanoparticles and Widely Used Surfactants. ACS Nano 2018, 12, 5312–5322. [Google Scholar] [CrossRef]
- Abdelrasoul, G.N.; Magrassi, R.; Dante, S.; d’Amora, M.; d’Abbusco, M.S.; Pellegrino, T.; Diaspro, A. PEGylated gold nanorods as optical trackers for biomedical applications: An in vivo and in vitro comparative study. Nanotechnology 2016, 27, 255101. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, D.; Liu, H.; Bao, Z.; Wang, Y. Toxicity assessment of precise engineered gold nanoparticles with different shapes in zebrafish embryos. RSC Adv. 2016, 6, 33009–33013. [Google Scholar] [CrossRef]
- Patibandla, S.; Zhang, Y.; Tohari, A.M.; Gu, P.; Reilly, J.; Chen, Y.; Shu, X. Comparative analysis of the toxicity of gold nanoparticles in zebrafish. J. Appl. Toxicol. 2018, 38, 1153–1161. [Google Scholar] [CrossRef]
- Peer Reviewed: Internal Exposure: Linking Bioavailability to Effects. Environ. Sci. Technol. 2004, 38, 455A–462A. [CrossRef]
- Rawson, D.M.; Zhang, T.; Kalicharan, D.; Jongebloed, W.L. Field emission scanning electron microscopy and transmission electron microscopy studies of the chorion, plasma membrane and syncytial layers of the gastrula-stage embryo of the zebrafish Brachydanio rerio: A consideration of the structural and functional relationships with respect to cryoprotectant penetration. Aquac. Res. 2000, 31, 325–336. [Google Scholar] [CrossRef]
- Hart, N.H.; Donovan, M. Fine structure of the chorion and site of sperm entry in the egg of Brachydanio. J. Exp. Zool. 1983, 227, 277–296. [Google Scholar] [CrossRef]
- Truong, L.; Zaikova, T.; Richman, E.K.; Hutchison, J.E.; Tanguay, R.L. Media ionic strength impacts embryonic responses to engineered nanoparticle exposure. Nanotoxicology 2012, 6, 691–699. [Google Scholar] [CrossRef]
- Böhme, S.; Baccaro, M.; Schmidt, M.; Potthoff, A.; Stärk, H.-J.; Reemtsma, T.; Kühnel, D. Metal uptake and distribution in the zebrafish (Danio rerio) embryo: Differences between nanoparticles and metal ions. Environ. Sci. Nano 2017, 4, 1005–1015. [Google Scholar] [CrossRef]
- Hlavkova, D.; Caloudova, H.; Palikova, P.; Kopel, P.; Plhalova, L.; Beklova, M.; Havelkova, B. Effect of Gold Nanoparticles and Ions Exposure on the Aquatic Organisms. Bull. Environ. Contam. Toxicol. 2020, 105, 530–537. [Google Scholar] [CrossRef]
- Ramachandran, R.; Krishnaraj, C.; Sivakumar, A.S.; Prasannakumar, P.; Abhay Kumar, V.K.; Shim, K.S.; Song, C.G.; Yun, S.I. Anticancer activity of biologically synthesized silver and gold nanoparticles on mouse myoblast cancer cells and their toxicity against embryonic zebrafish. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 674–683. [Google Scholar] [CrossRef]
- Geffroy, B.; Ladhar, C.; Cambier, S.; Treguer-Delapierre, M.; Brèthes, D.; Bourdineaud, J.P. Impact of dietary gold nanoparticles in zebrafish at very low contamination pressure: The role of size, concentration and exposure time. Nanotoxicology 2012, 6, 144–160. [Google Scholar] [CrossRef]
- Dedeh, A.; Ciutat, A.; Treguer-Delapierre, M.; Bourdineaud, J.P. Impact of gold nanoparticles on zebrafish exposed to a spiked sediment. Nanotoxicology 2015, 9, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Dayal, N.; Thakur, M.; Patil, P.; Singh, D.; Vanage, G.; Joshi, D.S. Histological and genotoxic evaluation of gold nanoparticles in ovarian cells of zebrafish (Danio rerio). J. Nanoparticle Res. 2016, 18, 291. [Google Scholar] [CrossRef]
- Ramachandran, R.; Krishnaraj, C.; Kumar, V.K.A.; Harper, S.L.; Kalaichelvan, T.P.; Yun, S.I. In vivo toxicity evaluation of biologically synthesized silver nanoparticles and gold nanoparticles on adult zebrafish: A comparative study. 3 Biotech 2018, 8, 441. [Google Scholar] [CrossRef] [PubMed]
- Sangabathuni, S.; Murthy, R.V.; Chaudhary, P.M.; Subramani, B.; Toraskar, S.; Kikkeri, R. Mapping the Glyco-Gold Nanoparticles of Different Shapes Toxicity, Biodistribution and Sequestration in Adult Zebrafish. Sci. Rep. 2017, 7, 4239. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-j.; Yin, Y.-g.; Liu, J.-f. Silver nanoparticles in the environment. Environ. Sci. Process. Impacts 2013, 15, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Majdalawieh, A.; Kanan, M.C.; El-Kadri, O.; Kanan, S.M. Recent advances in gold and silver nanoparticles: Synthesis and applications. J. Nanosci. Nanotechnol. 2014, 14, 4757–4780. [Google Scholar] [CrossRef] [PubMed]
- Capek, I. Preparation of metal nanoparticles in water-in-oil (w/o) microemulsions. Adv. Colloid Interface Sci. 2004, 110, 49–74. [Google Scholar] [CrossRef]
- Frattini, A.; Pellegri, N.; Nicastro, D.; Sanctis, O.d. Effect of amine groups in the synthesis of Ag nanoparticles using aminosilanes. Mater. Chem. Phys. 2005, 94, 148–152. [Google Scholar] [CrossRef]
- Dipankar, C.; Murugan, S. The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf. B: Biointerfaces 2012, 98, 112–119. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Jagan, E.G.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.T.; Mohan, N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B Biointerfaces 2010, 76, 50–56. [Google Scholar] [CrossRef]
- Dziendzikowska, K.; Gromadzka-Ostrowska, J.; Lankoff, A.; Oczkowski, M.; Krawczyńska, A.; Chwastowska, J.; Sadowska-Bratek, M.; Chajduk, E.; Wojewódzka, M.; Dušinská, M.; et al. Time-dependent biodistribution and excretion of silver nanoparticles in male Wistar rats. J. Appl. Toxicol. 2012, 32, 920–928. [Google Scholar] [CrossRef]
- van der Zande, M.; Vandebriel, R.J.; Van Doren, E.; Kramer, E.; Herrera Rivera, Z.; Serrano-Rojero, C.S.; Gremmer, E.R.; Mast, J.; Peters, R.J.; Hollman, P.C.; et al. Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure. ACS Nano 2012, 6, 7427–7442. [Google Scholar] [CrossRef]
- Naidu, K.S.B.; Adam, J.K.; Govender, P. Biomedical applications and toxicity of nanosilver: A review: Review. Med. Technol. SA 2015, 29, 13–19. [Google Scholar] [CrossRef]
- Edwards-Jones, V. The benefits of silver in hygiene, personal care and healthcare. Lett. Appl. Microbiol. 2009, 49, 147–152. [Google Scholar] [CrossRef]
- Benn, T.M.; Westerhoff, P. Nanoparticle Silver Released into Water from Commercially Available Sock Fabrics. Environ. Sci. Technol. 2008, 42, 4133–4139. [Google Scholar] [CrossRef]
- Geranio, L.; Heuberger, M.; Nowack, B. The Behavior of Silver Nanotextiles during Washing. Environ. Sci. Technol. 2009, 43, 8113–8118. [Google Scholar] [CrossRef]
- Abramenko, N.B.; Demidova, T.B.; Abkhalimov, Е.V.; Ershov, B.G.; Krysanov, E.Y.; Kustov, L.M. Ecotoxicity of different-shaped silver nanoparticles: Case of zebrafish embryos. J. Hazard. Mater. 2018, 347, 89–94. [Google Scholar] [CrossRef]
- Lee, K.J.; Nallathamby, P.D.; Browning, L.M.; Osgood, C.J.; Xu, X.-H.N. In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos. ACS Nano 2007, 1, 133–143. [Google Scholar] [CrossRef]
- Kim, K.T.; Truong, L.; Wehmas, L.; Tanguay, R.L. Silver nanoparticle toxicity in the embryonic zebrafish is governed by particle dispersion and ionic environment. Nanotechnology 2013, 24, 115101. [Google Scholar] [CrossRef]
- Asharani, P.V.; Lian Wu, Y.; Gong, Z.; Valiyaveettil, S. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology 2008, 19, 255102. [Google Scholar] [CrossRef]
- van Aerle, R.; Lange, A.; Moorhouse, A.; Paszkiewicz, K.; Ball, K.; Johnston, B.D.; de-Bastos, E.; Booth, T.; Tyler, C.R.; Santos, E.M. Molecular mechanisms of toxicity of silver nanoparticles in zebrafish embryos. Environ. Sci. Technol. 2013, 47, 8005–8014. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Liu, T.; Wang, Z.; Hou, Y.; Dong, L.; Zhu, J.; Qi, J. The effect of silver nanoparticles on zebrafish embryonic development and toxicology. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Browning, L.M.; Nallathamby, P.D.; Desai, T.; Cherukuri, P.K.; Xu, X.H. In vivo quantitative study of sized-dependent transport and toxicity of single silver nanoparticles using zebrafish embryos. Chem. Res. Toxicol. 2012, 25, 1029–1046. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Browning, L.M.; Nallathamby, P.D.; Xu, X.H. Study of charge-dependent transport and toxicity of peptide-functionalized silver nanoparticles using zebrafish embryos and single nanoparticle plasmonic spectroscopy. Chem. Res. Toxicol. 2013, 26, 904–917. [Google Scholar] [CrossRef]
- Xin, Q.; Rotchell, J.M.; Cheng, J.; Yi, J.; Zhang, Q. Silver nanoparticles affect the neural development of zebrafish embryos. J. Appl. Toxicol. 2015, 35, 1481–1492. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Chen, I.H.; Chen, C.-J. The surface modification of silver nanoparticles by phosphoryl disulfides for improved biocompatibility and intracellular uptake. Biomaterials 2008, 29, 1807–1816. [Google Scholar] [CrossRef]
- Massarsky, A.; Dupuis, L.; Taylor, J.; Eisa-Beygi, S.; Strek, L.; Trudeau, V.L.; Moon, T.W. Assessment of nanosilver toxicity during zebrafish (Danio rerio) development. Chemosphere 2013, 92, 59–66. [Google Scholar] [CrossRef]
- Park, H.-G.; Yeo, M.-K. Comparison of gene expression changes induced by exposure to Ag, Cu-TiO2, and TiO2 nanoparticles in zebrafish embryos. Mol. Cell. Toxicol. 2013, 9, 129–139. [Google Scholar] [CrossRef]
- González, E.A.; Carty, D.R.; Tran, F.D.; Cole, A.M.; Lein, P.J. Developmental exposure to silver nanoparticles at environmentally relevant concentrations alters swimming behavior in zebrafish (Danio rerio). Environ. Toxicol. Chem. 2018, 37, 3018–3024. [Google Scholar] [CrossRef]
- Myrzakhanova, M.; Gambardella, C.; Falugi, C.; Gatti, A.M.; Tagliafierro, G.; Ramoino, P.; Bianchini, P.; Diaspro, A. Effects of Nanosilver Exposure on Cholinesterase Activities, CD41, and CDF/LIF-Like Expression in ZebraFish (Danio rerio) Larvae. BioMed. Res. Int. 2013, 2013, 205183. [Google Scholar] [CrossRef]
- Cambier, S.; Røgeberg, M.; Georgantzopoulou, A.; Serchi, T.; Karlsson, C.; Verhaegen, S.; Iversen, T.G.; Guignard, C.; Kruszewski, M.; Hoffmann, L.; et al. Fate and effects of silver nanoparticles on early life-stage development of zebrafish (Danio rerio) in comparison to silver nitrate. Sci. Total Environ. 2018, 610–611, 972–982. [Google Scholar] [CrossRef]
- Gao, J.; Sepúlveda, M.S.; Klinkhamer, C.; Wei, A.; Gao, Y.; Mahapatra, C.T. Nanosilver-coated socks and their toxicity to zebrafish (Danio rerio) embryos. Chemosphere 2015, 119, 948–952. [Google Scholar] [CrossRef]
- Powers, C.M.; Slotkin, T.A.; Seidler, F.J.; Badireddy, A.R.; Padilla, S. Silver nanoparticles alter zebrafish development and larval behavior: Distinct roles for particle size, coating and composition. Neurotoxicol. Teratol. 2011, 33, 708–714. [Google Scholar] [CrossRef]
- Christen, V.; Capelle, M.; Fent, K. Silver nanoparticles induce endoplasmatic reticulum stress response in zebrafish. Toxicol. Appl. Pharmacol. 2013, 272, 519–528. [Google Scholar] [CrossRef]
- Yeo, M.-K.; Yoon, J.-W. Comparison of the effects of nano-silver antibacterial coatings and silver ions on zebrafish embryogenesis. Mol. Cell. Toxicol. 2009, 5, 23–31. [Google Scholar]
- Groh, K.J.; Dalkvist, T.; Piccapietra, F.; Behra, R.; Suter, M.J.; Schirmer, K. Critical influence of chloride ions on silver ion-mediated acute toxicity of silver nanoparticles to zebrafish embryos. Nanotoxicology 2015, 9, 81–91. [Google Scholar] [CrossRef]
- Cunningham, S.; Brennan-Fournet, M.E.; Ledwith, D.; Byrnes, L.; Joshi, L. Effect of Nanoparticle Stabilization and Physicochemical Properties on Exposure Outcome: Acute Toxicity of Silver Nanoparticle Preparations in Zebrafish (Danio rerio). Environ. Sci. Technol. 2013, 47, 3883–3892. [Google Scholar] [CrossRef]
- Griffitt, R.J.; Luo, J.; Gao, J.; Bonzongo, J.C.; Barber, D.S. Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ. Toxicol. Chem. 2008, 27, 1972–1978. [Google Scholar] [CrossRef]
- Orbea, A.; González-Soto, N.; Lacave, J.M.; Barrio, I.; Cajaraville, M.P. Developmental and reproductive toxicity of PVP/PEI-coated silver nanoparticles to zebrafish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 199, 59–68. [Google Scholar] [CrossRef]
- Boyle, D.; Goss, G.G. Effects of silver nanoparticles in early life-stage zebrafish are associated with particle dissolution and the toxicity of soluble silver. NanoImpact 2018, 12, 1–8. [Google Scholar] [CrossRef]
- Verma, S.K.; Jha, E.; Panda, P.K.; Mishra, A.; Thirumurugan, A.; Das, B.; Parashar, S.; Suar, M. Rapid novel facile biosynthesized silver nanoparticles from bacterial release induce biogenicity and concentration dependent in vivo cytotoxicity with embryonic zebrafish—A mechanistic insight. Toxicol. Sci. 2018, 161, 125–138. [Google Scholar] [CrossRef]
- Qiang, L.; Arabeyyat, Z.H.; Xin, Q.; Paunov, V.N.; Dale, I.J.; Lloyd Mills, R.I.; Rotchell, J.M.; Cheng, J. Silver nanoparticles in Zebrafish (Danio rerio) embryos: Uptake, growth and molecular responses. Int. J. Mol. Sci. 2020, 21, 1876. [Google Scholar] [CrossRef]
- Mosselhy, D.A.; He, W.; Li, D.; Meng, Y.; Feng, Q. Silver nanoparticles: In vivo toxicity in zebrafish embryos and a comparison to silver nitrate. J. Nanoparticle Res. 2016, 18, 1–15. [Google Scholar] [CrossRef]
- Bone, A.J.; Colman, B.P.; Gondikas, A.P.; Newton, K.M.; Harrold, K.H.; Cory, R.M.; Unrine, J.M.; Klaine, S.J.; Matson, C.W.; Di Giulio, R.T. Biotic and Abiotic Interactions in Aquatic Microcosms Determine Fate and Toxicity of Ag Nanoparticles: Part 2–Toxicity and Ag Speciation. Environ. Sci. Technol. 2012, 46, 6925–6933. [Google Scholar] [CrossRef]
- Osborne, O.J.; Johnston, B.D.; Moger, J.; Balousha, M.; Lead, J.R.; Kudoh, T.; Tyler, C.R. Effects of particle size and coating on nanoscale Ag and TiO2 exposure in zebrafish (Danio rerio) embryos. Nanotoxicology 2013, 7, 1315–1324. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Li, X.; Shao, J.; Peijnenburg, W.J. Aquatic toxicity of nanosilver colloids to different trophic organisms: Contributions of particles and free silver ion. Environ. Toxicol. Chem. 2012, 31, 2408–2413. [Google Scholar] [CrossRef]
- Xu, L.; Xu, Q.H.; Zhou, X.Y.; Yin, L.Y.; Guan, P.P.; Zhang, T.; Liu, J.X. Mechanisms of silver_nanoparticles induced hypopigmentation in embryonic zebrafish. Aquat. Toxicol. 2017, 184, 49–60. [Google Scholar] [CrossRef]
- Choi, J.E.; Kim, S.; Ahn, J.H.; Youn, P.; Kang, J.S.; Park, K.; Yi, J.; Ryu, D.Y. Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish. Aquat. Toxicol. 2010, 100, 151–159. [Google Scholar] [CrossRef]
- Cui, B.; Ren, L.; Xu, Q.H.; Yin, L.Y.; Zhou, X.Y.; Liu, J.X. Silver_ nanoparticles inhibited erythrogenesis during zebrafish embryogenesis. Aquat. Toxicol. 2016, 177, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Bilberg, K.; Hovgaard, M.B.; Besenbacher, F.; Baatrup, E. In Vivo Toxicity of Silver Nanoparticles and Silver Ions in Zebrafish (Danio rerio). J. Toxicol. 2012, 2012, 293784. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Netam, S.P.; Mahanty, A.; Saha, A.; Bosu, R.; Krishnani, K.K. Toxicity Evaluation of Chemically and Plant Derived Silver Nanoparticles on Zebrafish (Danio rerio). Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2014, 84, 885–892. [Google Scholar] [CrossRef]
- Girilal, M.; Krishnakumar, V.; Poornima, P.; Mohammed Fayaz, A.; Kalaichelvan, P.T. A comparative study on biologically and chemically synthesized silver nanoparticles induced Heat Shock Proteins on fresh water fish Oreochromis niloticus. Chemosphere 2015, 139, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Devi, G.P.; Ahmed, K.B.A.; Varsha, M.K.N.S.; Shrijha, B.S.; Lal, K.K.S.; Anbazhagan, V.; Thiagarajan, R. Sulfidation of silver nanoparticle reduces its toxicity in zebrafish. Aquat. Toxicol. 2015, 158, 149–156. [Google Scholar] [CrossRef] [PubMed]
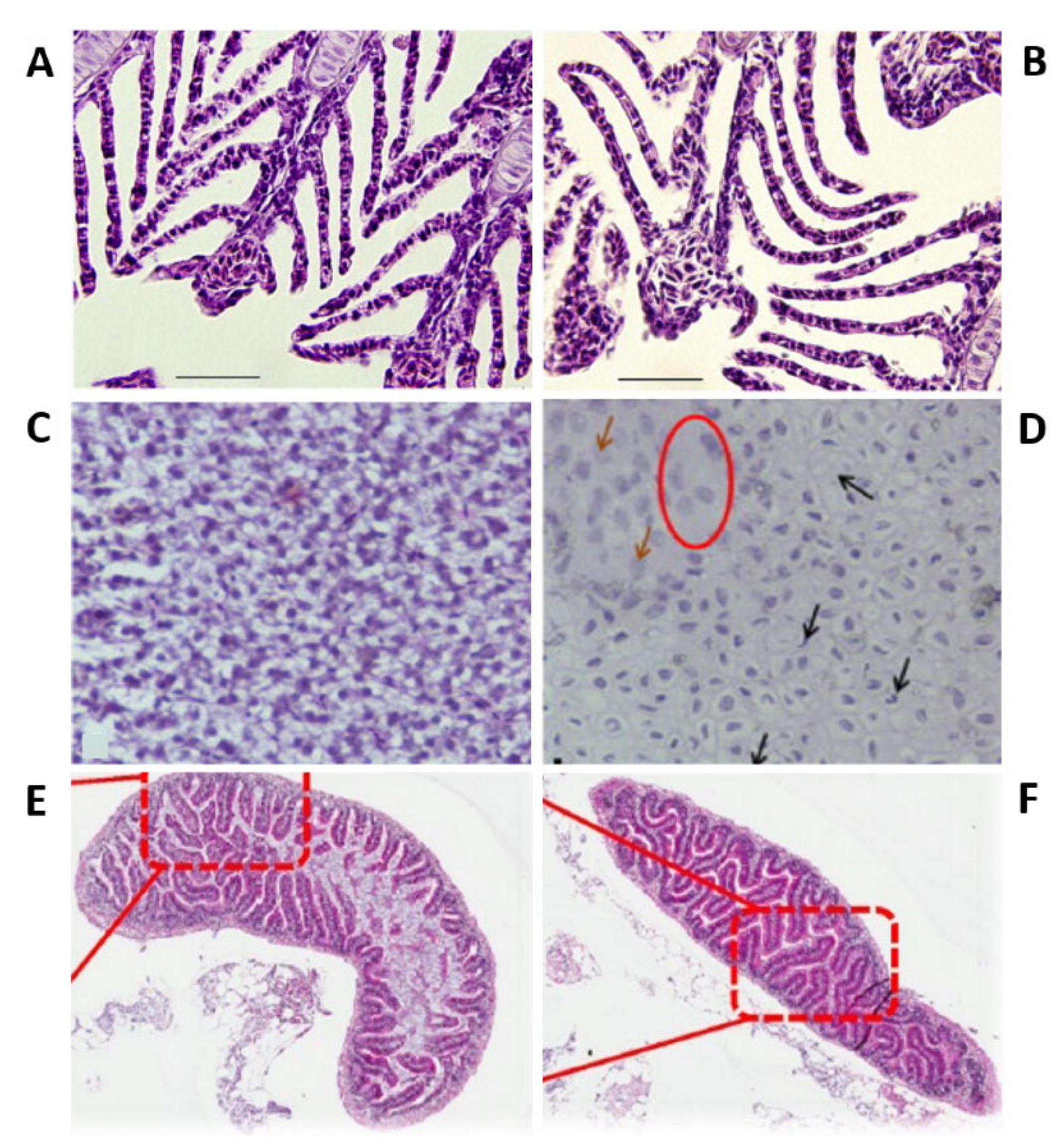
- Griffitt, R.J.; Hyndman, K.; Denslow, N.D.; Barber, D.S. Comparison of molecular and histological changes in zebrafish gills exposed to metallic nanoparticles. Toxicol. Sci. 2009, 107, 404–415. [Google Scholar] [CrossRef]
- Griffitt, R.J.; Lavelle, C.M.; Kane, A.S.; Denslow, N.D.; Barber, D.S. Chronic nanoparticulate silver exposure results in tissue accumulation and transcriptomic changes in zebrafish. Aquat. Toxicol. 2013, 130–131, 192–200. [Google Scholar] [CrossRef]
- Pecoraro, R.; Marino, F.; Salvaggio, A.; Capparucci, F.; Di Caro, G.; Iaria, C.; Salvo, A.; Rotondo, A.; Tibullo, D.; Guerriero, G. Evaluation of chronic nanosilver toxicity to adult zebrafish. Front. Physiol. 2017, 8, 1011. [Google Scholar] [CrossRef]
- Katuli, K.K.; Massarsky, A.; Hadadi, A.; Pourmehran, Z. Silver nanoparticles inhibit the gill Na⁺/K⁺-ATPase and erythrocyte AChE activities and induce the stress response in adult zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2014, 106, 173–180. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Harper, S.L.; Yun, S.I. In Vivo toxicological assessment of biologically synthesized silver nanoparticles in adult Zebrafish (Danio rerio). J. Hazard Mater. 2016, 301, 480–491. [Google Scholar] [CrossRef]
- Ma, Y.; Song, L.; Lei, Y.; Jia, P.; Lu, C.; Wu, J.; Xi, C.; Strauss, P.R.; Pei, D.-S. Sex dependent effects of silver nanoparticles on the zebrafish gut microbiota. Environ. Sci. Nano 2018, 5, 740–751. [Google Scholar] [CrossRef]
- Ma, Y.-B.; Lu, C.-J.; Junaid, M.; Jia, P.-P.; Yang, L.; Zhang, J.-H.; Pei, D.-S. Potential adverse outcome pathway (AOP) of silver nanoparticles mediated reproductive toxicity in zebrafish. Chemosphere 2018, 207, 320–328. [Google Scholar] [CrossRef]
- Speshock, J.L.; Elrod, N.; Sadoski, D.K.; Maurer, E.; Braydich-Stolle, L.K.; Brady, J.; Hussain, S. Differential organ toxicity in the adult zebra fish following exposure to acute sub-lethal doses of 10 nm silver nanoparticles. Front. Nanosci. Nanotech. 2016, 2, 114–120. [Google Scholar] [CrossRef][Green Version]
- Osborne, O.J.; Lin, S.; Chang, C.H.; Ji, Z.; Yu, X.; Wang, X.; Lin, S.; Xia, T.; Nel, A.E. Organ-Specific and Size-Dependent Ag Nanoparticle Toxicity in Gills and Intestines of Adult Zebrafish. ACS Nano 2015, 9, 9573–9584. [Google Scholar] [CrossRef]
- Santoro, C.M.; Duchsherer, N.L.; Grainger, D.W. Antimicrobial efficacy and ocular cell toxicity from silver nanoparticles. Nanobiotechnology 2007, 3, 55–65. [Google Scholar] [CrossRef]
| Life Stage | Shape | Size | Surface Coatings | Concentration and Time of Exposure | Mortality Value vs. Dosesor LC50 | Results | Ref. |
|---|---|---|---|---|---|---|---|
| Embryos | Sphere | 12 nm | Citrate | 0.025, 0.05, 0.10, 0.20, 0.40, 0.60, 0.80, 1.0, 1.2 nM, from cleavage-stage to 120 hpf | 31% at 1.2 nM | Effects stochastic dependent from concentration—low mortality—different malformations (fin fold abnormality, tail and spinal cord flexure and truncation, cardiac malformation, yolk sac edema, and acephaly). | [36] |
| Embryos | Ellipse | 86 nm | Citrate | 0–78 µg/mL from 0.75/2.25 to 120 hpf | 3% at 78 µg/mL | Normal development—effects not concentration dependent—larger Au-NPs (86 nm) more biocompatible than smaller (12–35 nm). | [37] |
| Embryos | Sphere | 0.8, 1.5, and 15 nm | TMAT MES MEE MEEE | 0.016, 0.08, 0.4, 2, 10, 50 and 250 mg/L, from 2 to 120 hpf (5 days) |
| TMAT-Au-NPs: increased mortality—negligible malformations. MES-Au-NPs: no significant lethality—increased incidence of abnormalities. MEE-Au-NPs and MEEE-Au-NPs: no adverse effects. | [38] |
| Embryos | Sphere | 1.5 nm | TMAT MES MEEE | 0.016, 0.08, 0.4, 2, 10, 50 and 250 ppm, from 6 to 48 hpf |
| TMAT-Au-NPs: high mortality—misregulations of genes associated with immune and inflammation responses. MES-Au-NPs: high percentages of abnormalities—misregulations of genes associated with immune and inflammation responses. MEE-Au-NPs: no biological responses. | [39] |
| Embryos | Sphere and oval | 15–35 nm | PVA | 10, 25, 50, 75, and 100 mg/mL, from 8-cell stage to 72 hpf | ≤3 at 100 mg/mL | No increase in mortality—no hatching delay—no defects in the development—no effects on the heartbeat rate—no perturbations in the touch response for all the tested concentrations. | [40] |
| Embryos | Rod | 20 nm × 7 nm | CTAB | 50, 60, 72, 87, 104, 125, and 150 μg/L, from 6 to 96 hpf | L5096 h= 110.2 μg/L | Toxicity concentration dependent—at low doses (50 and 87 μg/L) no developmental perturbation—at high doses (125 and 150 μg/L) delay in the development (eye and brain) and different morphological abnormalities (tail deformities and pericardial edema, tail elongation, and body size)—no DNA damage. | [41] |
| Embryos | Sphere Rod Urchin Bipyramid | 79 79 78 5014 | - PVP - PEG | 5 mg/L, from 3 to 5 dpf | - | Spheres: reduced amount of the neutrophils. Rods and urchins: no effects on the immune system—increased total swimming distance. Bipyramids: no effect on immune systems—under stress-induced, reduction of the swimming distance. | [42] |
| Embryos | Sphere | 33–346 nm | Folic acid | 0.325, 0.65, 0.97, 1.3, 1.62, 1.95, 2.27 and 2.6 ng/100 µL, from 2 hpf to 96 hpf | - | Toxicity concentration-dependent—decrease of survival rate—delay in the hatching rate—presence of different malformations (length of larvae and poor pigmentation). | [43] |
| Embryos | Sphere | 25 and 40 nm | Citrate | Microinjection at one-cell stage with 1 nL (5 × 10−18 mole) | - | Normal development of somites—normal response to touch at 4 dpf—normal function of the cardiovascular system and other organs—normal expression of different genes (ntl, gsc, myoD, and β-globin). | [44] |
| Embryos | Sphere | 3, 10, 50, and 100 nm |
Bare
TPPMS | 50, 5, 0.5 and 0.05 mg/L, from 4 to 120 hpf |
cAu3 negligible cAu10 negligible cAu 50 negligible cAu 100 negligible | No increase in mortality—no significant morphological defects—no differences in effects between unfunctionalized and functionalized NPs. | [46] |
| Embryos | Sphere | 1.3 nm | TMAT | 0.08, 0.4, 2, 10 and 50 mg/L, from 4 hpf to 120 hpf | LC50120 hpf =30 mg/L | High mortality rate and high incidence of abnormalities—perturbations in the eye development, with grey and small eye—downregulation of genes involved in the eye formation and apoptosis processes—abnormal axon development. | [47] |
| Embryos/adults | Sphere | 1.5 nm |
TMAT MES MEEE | 50 mg/L, embryos from 6 to 120 hpf, adult from 4 hpf to 122 days | 20% TMAT-Au-NPs 50% MES-Au-NPs | MES and TMATAu NPs: Embryos: hypo-locomotor activity. Adults: low survivorship into adulthood and abnormal behavior. MEEEAu NPs: Embryos: normal locomotor activity. | [48] |
| Embryos | Sphere | 1, 2.8, 3.1, 3.6, and 3.9 nm | MEEE | 2.3, 5, 10.7, 23.2, 50 µg/mL, from 6 hpf to 5 dpf | 88% at 50 µg/mL Au-NPs + 0.003 PS20Lily Zhao 100% at 23.2 µg/mL Au-NPs + 0.3 PS20 | Au-NPs and PS20 alone have low toxicity—mixtures of Au-NPs and PS20 presented increased toxicity. | [51] |
| Embryos | Rod | 48 × 16 nm and 51 × 13 nm | CTAB | 1, 5, 10, 20 50 nM, from 4 to 120 hpf | - | Toxicity concentration dependent—up to middle dose, no perturbation in mortality and hatching rates and incidence of malformations—at the highest dose tested increased mortality, decreased hatching rate and presence of severe malformations (pericardial edema, yolk sac edema, tail flexure, and fin-fold abnormality). | [52] |
| Embryos | Sphere Rod Polyhedron | 46 nm 76 × 23 nm 38 nm | CTAB | 0–15.7 µM, from 4 to 80 hpf | LC5080 hpf Sphere: 0.11 nM Rod: 1.54 µM Polyhedron: 0.13 µM | Mortality and hatching rates concentration dependent—order of induced mortality: Au nanospheres > nanopolyhedrons > nanorods—different malformations (several yolk sac edema, cardiac edema, bleeding, skeletal defects, lack of pigmentation, and tail/spinal cord flexure) with the highest percentages induced by polyhedrons. | [53] |
| Embryos | Sphere Rod | 38.1 nm × 12 nm × 52 nm | Sphere: PSS Rods: PSS PAH/PSS | 0.01, 0.025, 0.05 and 0.1 nM, from 2 to 72 hpf | - | Spheres: no important changes in hatching, mortality, or heartbeat rates. Rods: increased mortality—decreased hatching and heartbeat rates—perturbations in the expression of oxidative stress genes. Different shapes of nanoparticles and surface coatings affected the toxicity. | [54] |
| Embryos | Sphere | 1.2 nm | MPA | 0.08, 0.4, 2, 10 and 50 mg/L, from 6 to 120 hpf | <13% at 50 mg/mL | The ionic concentration of the EM influenced the toxicological profile—low mortality and percentage of malformations at highest ionic concentrations tested—normal motor activity. | [58] |
| Embryos | Sphere | 1.16 and 11.6 nm | Citrate PVP | 0, 1, 12.5, 25,50, 75, and 100 mg/L, from 4 to 96 hpf | LC50 above 100 mg/mL | Low mortality at the highest concentration of Au-NPs-CIT and Au-NPs-PVP tested—no hatching rate delay—no significant incidence of malformations. | [60] |
| Embryos | Sphere | 5–50 nm | - | 100–300 mg/mL, from 4 to 96 hpf | 100% at 300 mg/mL | 100% mortality rate (concentration dependent) at the highest concentration tested—one abnormality (tail malformations). | [61] |
| Adults | Sphere | 12 and 50 nm | Citrate | 0.04 and 0.1 mg/day/g fish body weight, 36 or 60 days | No mortality | No mortality—different impairment at the subcellular level, dependent on the exposure time and Au-NPs sizes and doses—mitochondrial perturbations in the muscle and the brain—perturbations in the expression of the genes associated with oxidative stress, apoptosis, and DNA repair. | [62] |
| Adults | Sphere | 14 nm | Citrate | 0.25 and 0.8 mg/L, 20 days | - | Variations of brain and muscle AchE activity—DNA damage and alternations—variations in the expression of genes associated with oxidative stress, apoptosis, and DNA repair. | [63] |
| Adults | Sphere | 10–20 nm and 40–50 nm | Citrate | 20 µg/g/day for 28 days | - | Histological alterations in ovarian tissue | [64] |
| Adults | Sphere | <30 nm | - | 9.7, 19.4, 29.1, 38.8, 43.65, 48.5 and 58.2 mg/L for 96 h | LC5096 h = 41 mg/L | Toxicity concentration dependent—100% mortality at the highest concentration tested after 24 h—aggressive behavior after 12 h—no cytological changes—no genotoxicity. | [65] |
| Adults | Sphere Rod Star | 16.5 nm 47 × 12 nm 42 × 16 nm | Mannose PEG | 2 µL for intraperitoneal injection (5 µ/g), 120 h | - | Very low toxicity. | [66] |
| Life Stage | Shape | Size | Surface Coatings | Concentration and Time of Exposure | Mortality Value vs. Doses or LC50 | Results | Ref. |
|---|---|---|---|---|---|---|---|
| Embryos | Sphere | 5–35 nm | PVA | 10, 25, 50, 75, and 100 µg/mL, from 8-cell stage to 72 hpf | 43% at 100 µg/mL | Toxicity concentration dependent—increase in mortality rate—drop in hatching rate- different phenotypic defects (absence of eyes, growth retardation, undulated notochord, larvae shorter in length)—drop in the heartbeat—decrease of the touch response. | [40] |
| Embryos | Sphere | 3, 10, 50, and 100 nm | No coatings or TPPMS | 50, 5, 0.5 and 0.05 mg/L, from 4 to 120 hpf | LC50: cAg3: 93.31µM cAg10: 125.66 µM cAg50: 126.96µM cAg100: 37.26µM | High mortality rate, time and concentration dependent—not hatched embryos—problems in the circulatory system (i.e., blood clots and hemorrhages)—high incidence of malformations, most pronounced at 120 hpf (tail malformations, pericardial edema, bent spine, and small head). | [46] |
| Embryos | Sphere | 2–20 nm | - | 1–3 µg/mL from 4 to 96 hpf | 45% at 3 µg/mL | 100% mortality at the highest concentration (3 µg/mL) tested—yolk sac edema and tail malformation at middle concentrations. | [61] |
| Embryos | Sphere | 11 nm | Citrate | 0.04–0.06–0.07–0.08–0.19–0.38–0.57–0.66–0.71 nM, from 8-cell stage to 120 hpf | - | Toxicity concentration dependent—increase of the mortality—different types of malformations. | [80] |
| Embryos | Sphere | 20 or 110 nm | Citrate (C) or PVP (P) | 0.8, 4, 20, 10, and 50 mg/L from 4 to 120 hpf | LC50: 20 Ag-NPs-C: 44.78 mg/mL 110 Ag-NPs-C: N/A 20 Ag-NPs-P: 42.49 mg/mL | Ag-NPs dissolved in UP and CaCl2 solutions more toxic than the one dissolved in EM—smaller NPs (20 nm) more toxic than larger (100 nm)—order of toxicity: 20 Ag-NPs-C > 20 Ag-NPs-P > 110 Ag-NPs-C ≈ 110 Ag-NPs-P. | [81] |
| Embryos | Sphere | 5–20 nm | BSA | 5, 10, 25, 50 and 100 mg/L from 4 to 72 hpf | LC50: 5–50 μg /mL | Toxicity concentration dependent—increased mortality rate—hatching rate delay—severe malformations (pericardial edema, twisted notochord, abnormal body axes)—bradycardia. | [82] |
| Embryos | Sphere | 10 nm | - | 3.9–8000 µg/L, from 4 to 48 hpf | No mortality | No mortality—at 24 and 48 hpf alteration in expression of genes associated with protein synthesis and oxidative phosphorylation (downregulation after 24 h of treatment and recovery after 48 h). | [83] |
| Embryos | Sphere | 10–20 nm | DL-a-aminopropanoic acid | 5, 10, 25, 50 and 100 mg/L, from 4 to 48 hpf | 100% at 50–100 mg/L | Toxicity concentration dependent—100% mortality at the highest concentrations tested at 24 and 48 hpf—no alteration of heart beat rate—severe malformations (degeneration of the body, bent and twisted notochord, pericardial edema)—alteration in expression of relevant genes (sox17, gsc, ntl, otx2). | [84] |
| Embryos | Sphere | 42 nm | Citrate | 0.02–0.05–0.1–0.2 0.3–0.4–0.5 and 0.7 nM, from 4 to 120 hpf | - | Toxicity concentration dependent—100% of mortality at the high doses (≥ 0.2 nM)—different malformations (abnormal tail and spinal cord flexure, fin-fold deformities, eye/head defects, yolk sac edema, cardiac abnormalities) at low and middle doses (0.02–0.2 nM). | [85] |
| Embryos | Sphere | 12 nm | Peptides: CALNNK CALNNS CALNNE | 0.1, 0.2, 0.4, and 0.6 nM, from cleavage stage to 120 hpf | CALNNK+ζ 33% at 0.6 nM CALNNK−4ζ 77% at 0.6 nM CALNNK−2ζ 42% at 0.6 nM | Toxicity concentration and charge dependent—three Ag-peptide NPs caused several malformations (cardiac malformations, severe eye abnormalities, yolk sac edema, tail, and spinal cord flexure)—positively charged NPs (Ag-CALNNK NPs+ζ) less toxic—more negatively charged (Ag-CALNNE NPs−4ζ) most toxic. | [86] |
| Embryos | Sphere | 4 and 10 nm | Oleic acid | 0.481, 0.963, 1.925, 3.850, 7.700, 11.550 and 23.100 mg/L, from 4 to 96 hpf | EC50: 4 nm: 4.120 mg/L 10 nm: 5.909 mg/L | Toxicity concentration dependent—severe malformations (bent notochord, yolk sac edema, eye, and head hypoplasia and disappearing somite)—decreased heartbeat rate, cardiotoxicity—smaller NPs (4 nm) more toxic than larger ones (10 nm)—at 24 hpf alteration in expression of neural genes (gfap, neuroD, ngn1, huC), ABCC genes and of metal responsive MTs—neurotoxicity. | [87] |
| Embryos | Sphere | 10 nm | Polyacrylate sodium | 0.03, 0.16, 0.31, 0.78, and 1.55 µg/mL of Ag, from 3 hpf to 5 dpf | LC5096 h: 1.18 µg/mL | Toxicity concentration dependent—increased mortality—hatching delay—reduced heartbeat rate at 48 hpf—high incidence of malformations (edema, bent tail, not depleted yolk and malformed spine)—increased ROS generation—depletion of GSH—no alteration in antioxidant enzymes activities. | [89] |
| Embryos | Sphere | 20–30 nm | - | 20 ppt, from 2.5 to 72 hpf | Alteration in the expression of genes involved in apoptosis (54%) endocytosis (10%) and immune response (18%). | [90] | |
| Embryos | Sphere | 20–40 nm | - | 0.03, 0.1, 0.3, 1 and 3 ppm, from 4 to 120 hpf | 5% at 3 ppm | No important changes in the mortality and hatching rates or percentage of malformations—perturbation in the behavior with hyperactivity. | [91] |
| Embryos | Sphere | 1–20 nm | Liposomes | 100–0.001 mg/L, from 12 to 72 hpf | 100% at 100 mg/L | Toxicity concentration dependent—100% of mortality at the highest dose tested—scarce response to touch—decrease on the diameter of the eyes and body length—decrease of AChE and PChE activities—impaired recruitment of T-lymphocytes. | [92] |
| Embryos | Sphere | 20 nm | Bare | 0.01, 0.1, 0.5, 1 and 10 mg/L, from 6 to 21 dpf (15 days) | - | Effect on survival rate—normal body length—perturbation in genes associated with photoreception and circadian clock regulation. | [93] |
| Embryos | Sphere | sock-NPs: 37–165 nm spun-NPs: 15–50 nm | - | 0.01–0.83 mg/L, from 6 to 72 hpf | LC50 Sock-Ag-NP: 0.14 mg/L Spun-Ag-NP: 0.26 mg/L | 100% of mortality at the highest concentration tested by 24 h—reduced hatching rate and abnormal development at lower concentrations—perturbation in oxidative gene expression only for high doses (1.2 mg/L) of spun-NPs. | [94] |
| Embryos | Sphere | 10 and 50 nm | Citrate (C) PVP (P) | 0.01–100 µM, from 6 hpf to 5 dpf | - | Ag-NP-C: delay in the hatching—significant mortality—neurobehavioral changes with hyperactivity or low activity. Ag-NP-P: no hatching delay—neurobehavioral changes with hyperactivity or low activity. | [95] |
| Embryos | Sphere | 120 nm | - | 0.01, 0.1, 1 and 5 mg/L, from 2/4 to 120 hpf | - | Hatching delay and morphological defects (curved backbones) at high concentrations—upregulation of ER stress markers (BiP and Synv) and proapoptotic genes (p53, p21, Noxa). | [96] |
| Embryos | Sphere | 20–30 nm | - | 10 and 20 ppt, from 2.5 to 72 hpf | - | Toxicity concentration dependent—severe cardiac malformations at 72 hpf (blood pooling, pericardial edema)—abnormal notochord—apoptosis in the head—alteration of different gene expressions (activin, BMP, TGF-β, GSK3–β). | [97] |
| Embryos | Sphere | 28 nm | Carbonate | 10–200 µM, from 2 to 6 hpf | LC50: 2 hpf: 26.18 µM 4 hpf: 73.58 µM 6 hpf: 169.00 µM | Toxicity dependent on the starting time of treatments and due to the Ag+ action—younger embryos more sensitive. | [98] |
| Embryos | Sphere | 46/110 nm 52/140 nm 48/155 nm 53/108 nm 78/204 nm 14/140 nm 42/77 nm | PVP TSC BIO Thiol | 0.001–100 ppm, from 4 to 72 hpf | LC5048 h PVP: 0.061/0.228 ppm TSC: 2.427/6.922 ppm BIO: 3.043/5.891 ppm Thiol: 0.0345/0.0845 ppm | Order of toxicity PVP > Thiol > TSC > BIO—most significant perturbations in the heart rate of embryos treated with PVP NPs—increased mortality rate and reduced hatching rate and mobility for all the tested nanoparticles. | [99] |
| Embryos and adults | Sphere | 20–30 nm | Sodium citrate | 0–10 mg/L, 48 h | LC5048 h Embryos: 7.20 mg/L Adults: 7.07 mg/L | LC5048h less than 10 mg/L in both embryos and adult organisms. | [100] |
| Embryos/Adults | Sphere | 5 nm | PVP/PEI | Embryos: 10 μg/L–10 mg/L for 5 days Adults: 100 ng/L for 3 weeks | LC50120 h: 50 μg/L | Embryos: 100% of mortality at high doses (0.1 mg/L) after 24 h of exposure—significant effect on the hatching rate. Adult: vacuolization of the liver- reduction of fecundity—resulting embryos with malformations. | [101] |
| Embryos | Sphere | 25–75 nm | PVP | 0–100 mg/L, from 24 to 120 hpf | LC5024–48 hpf: 51 mg/L | Toxicity increase during the development—less sensitivity compared to AgNO3—alteration in expression of metal ion chaperone metallothionein. | [102] |
| Embryos | Sphere | 20–40 nm | - | 10 and 500 µg/mL, from 3/3.5 to 72 hpf | Ec-Ag-NP: 154 µg/mL SA-Ag-NP: 142 µg/mL ST-Ag-NP: 125 µg/mL BT-Ag-NP: 185 µg/mL | Toxicity dependent on concentration biogenicity and exposure time—hatching delay—decreased heartbeat rate—malformations (yolk, notochord, gastrointestinal lumen)—apoptosis in the tail area and head—ROS production. | [103] |
| Embryos | Sphere | 4 and 10 nm | Oleic acid | 0.481, 0.963 and 1.925 mg/L, from 4 to 96 hpf | - | 4 nm: delayed yolk sac absorption, reduced body length—alteration in expression of Pxmp2 and HIF4. 10 nm: no significant adverse effects. | [104] |
| Embryos | Sphere | 9 and 30 nm | PVP and EG | 10 and 20 µg/mL from 4 to 120 hpf | 9 nm: 100% at 20 µg/mL 30 nm: 60% at 20 µg/mL | Toxicity size and concentration dependent—increased mortality rate—decreased hatching rate and heartbeat rates—high incidence of malformations (tail malformation, yolk sac edema, axial deformity, pericardial edema, eye defects). | [105] |
| Embryos | Sphere | 12 and 49 nm | PVP GA | 2 mg/L Ag | PVP-Ag-NPs in WP: 8% PVP-AG-NPs in WPS: 10% PVP-Ag-NPs in W: 74% GA-Ag-NPs in WP:19% GA-Ag-NPs in WPS: 6% GA-Ag-NPs in WS: 35% GA-Ag-NPs in W: 81% | Mortality rates of zebrafish treated with of GA-Ag-NPs or PVP-Ag-NPs in three different matrices lower with respect to GA-Ag-NPs or PVP-Ag-NPs in water-sediments protective against the GA and PVP-Ag-NPs toxicity. | [106] |
| Embryos | Sphere | 10 and 35 nm | Bare Citrate | 5, 50, 500, 5000, and 25.000 µg/L, from 1.5 to 48 hpf | 10 nm Ag-NPs: 79% at 25.000 µg/L CIT-Ag-NPs: 14% at 25.000 µg/L | Toxicity size and concentration dependent—35 nm Ag-NPs more toxic than 10 nm—coated NPs less toxic than the uncoated ones. 35 nm: highest mortality during gastrulation—yolk sac damage—high cell death in the yolk sac. | [107] |
| Embryos | Sphere | 15, 35, and 80 nm | Bare DIS PVP | 0.05–1000 µM, from 4 to 96 hpf | EC50: Ag-NPs: 1.95 µM DIS-Ag-NPs. 0.83 µM PVP-Ag-NPS: 1.50 µM | Decreasing toxicity order: DIS-nAg > PVP-nAg > Bare-nAg. | [108] |
| Embryos | Sphere | 40 nm | Citrate | 0.4 mg/L, from 4 to 72 hpf | - | Hypopigmentation—reduced xanthocytes and melanocytes—alteration in expression of xanthophone (gch2) and melanophore genes (mitfa and dct) and genes associated with the development of neural crest (foxd3 and pax7) | [109] |
| Embryos | Sphere | 10 and 40 nm | Citrate | 0.2, 0.4, 0.75 and 1 mg/L, from 4 to 96 hpf | - | 40 nm: Mortality and incidence of malformations (cardiac defects and yolk sac edema) concentration dependent—high incidence of malformations—downregulation of hemoglobin complex genes (hbae1, hbbe1, hbbe2, scf4 and alas2)—inhibition of erythroid differentiation—no effects on blood vessel formation or mesodermal specification10 nm: low incidence of malformations. | [111] |
| Adults | Sphere | < 30 nm | 15.5, 18.6, 21.7, 24.8, 27.9 and 31 μg/L for 96 h | LC5096 h: 24.5 μg/L | Toxicity concentration dependent—100% mortality at the highest concentration tested after 12 h—aggressive behavior after 6 h—cytological changes in gills and liver (pyknotic nuclei, cell membrane damage)—high levels of AST and ALT—production of ROS—alteration in expression of genes associated with immune response (C/EBP, IL1β and LYZ, MPO, TLR22, NF-κB, and TLR4) and oxidative stress (MTF1, HSP70). | [65] | |
| Adults | Sphere | 5–20 nm | - | 30, 60 and 120 mg/mL, 24 h | - | Cellular disruption in the liver tissue (pyknosis and chromatin condensation)—increase of MDA and GHS levels—alteration in the expression of oxyradical scavenging enzymes (Cat and GPx1 a)—DNA damage—upregulation of proapoptotic genes (Bax and Noxa). | [110] |
| Adults | Sphere | 81 nm | PVP | 18, 36, 54, 72, 89, 107, 125, and 143 μg/L, 48 h | LC5048 h: 84 μg/L | Toxicity concentration dependent—100% mortality after 24 h exposure to the highest concentration tested (143 μg/L)—extravasations of blood in the anterior ventral surface of the body—lost equilibrium—increased respiratory rate. | [112] |
| Adults | Sphere and polygon | 11–45 nm17–40 nm | - | for 28 days | LD5096 h: chemically synthesized Ag-NPs: 80 µg/L biologically synthesized Ag-NPs: 400 µg/L | Biologically synthetized Ag-NPs less toxic in comparison to both the chemical counterparts and Ag NO3—histological damage in ovarian tissue (mild atresia). | [113] |
| Adults | Sphere | 22–26 nm | PVP | 0.1 ppm, 15 days | - | Sulfidation mitigated the toxicity—mitigation of induced oxidative stress—protection of liver and brain biochemical enzymes. | [115] |
| Adults | Sphere | 20–30 nm | - | 1 mg/L, 24 h and 48 h | - | No alteration in gill filament—dramatic alterations in global gene expression patterns, particularly genes associated with mitogenesis, proliferation, and apoptosis. | [116] |
| Adults | Sphere | 3 nm | Citrate | 5, 15, 25, or 50 µg/L, 28 days | No mortality | No mortality—no morphological alterations in gills—significant alterations in the expression of genes associated with morphogenesis and developmental processes. | [117] |
| Adults | Sphere | 25 nm | - | 8, 45, and 70μg/L for 30 days | - | Damage in gills structure (lamellar fusion, subepithelial edema)—necrosis of intestinal villi—high expression of MTs1. | [118] |
| Adults | Sphere | 25–100 nm | Bare | 5, 10, 20, 25, and 30 mg/L for 4 days—2 and 4 mg/L for 1–3 weeks | LC5096 h: 16.76 mg/L | Mortality concentration and time dependent—decrease of gill Na+/K+ ATPase activity only after 14 and 21 days—decreased erythrocyte AChE activity after 4 d—alterations of electrolyte levels in a short time—increase of plasma cortisol and glucose levels. | [119] |
| Adults | Sphere | 5–50 nm | - | 23.7, 47.4, 142.2, 237, 284.4, 331.8 µg/L, for 96 h 71.1 µg/L for 14 days | LC5096 h: 142.2 µg/L | Exposure for 96 h: 100% mortality at the highest concentration tested—increase in respiratory rate (respiratory toxicity). Exposure for 14 days: oxidative stress with morphological changes in liver and gills—genotoxicity with misregulation of genes associated with immune and stress responses. | [120] |
| Adults | Sphere | 50.7 ± 1.8 nm | - | 10, 33 or 100 μg/L for 35 days | - | Alteration of gut microbiota composition in terms of richness and diversity only in male fish—Male: abundance of Proteobacteria—low Fusobacteria, Cetobacterium and Aeromonas. | [121] |
| Adults | Sphere | 20–70 nm | - | 10, 33, and 100 mg/L for 5 weeks | No mortality | No mortality—both in testis and ovary ROS generation—decrease of fecundity—increase of apoptotic cells in gonads—alteration in the expression of genes linked to mitochondrion-mediated apoptosis (bax, bcl-2, caspase-3, and caspase 9). | [122] |
| Adults | Sphere | 10 | PVP | 1 or 5 mg/kg by injection for 24 h | No mortality | No mortality—damage in kidneys, spleen, heart, and gall bladder—alteration in the expression of IL-1 and TNFα, caspase 6 and 9. | [123] |
| Adults | Sphere | 20–110 nm | Citrate | 1 ppm, for 4 h, 4 days, or 4 days plus 7 days | - | Size-dependent toxicity—morphological changes in intestines and gills—decrease of Na+/K+ ATPase activity. 20 nm: hyperplasia, injury, fusion in the gill tissue—not significant effect in the intestine. 110 nm: less significant changes in the gills—vacuolization and damage in the intestine. | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
d’Amora, M.; Raffa, V.; De Angelis, F.; Tantussi, F. Toxicological Profile of Plasmonic Nanoparticles in Zebrafish Model. Int. J. Mol. Sci. 2021, 22, 6372. https://doi.org/10.3390/ijms22126372
d’Amora M, Raffa V, De Angelis F, Tantussi F. Toxicological Profile of Plasmonic Nanoparticles in Zebrafish Model. International Journal of Molecular Sciences. 2021; 22(12):6372. https://doi.org/10.3390/ijms22126372
Chicago/Turabian Styled’Amora, Marta, Vittoria Raffa, Francesco De Angelis, and Francesco Tantussi. 2021. "Toxicological Profile of Plasmonic Nanoparticles in Zebrafish Model" International Journal of Molecular Sciences 22, no. 12: 6372. https://doi.org/10.3390/ijms22126372
APA Styled’Amora, M., Raffa, V., De Angelis, F., & Tantussi, F. (2021). Toxicological Profile of Plasmonic Nanoparticles in Zebrafish Model. International Journal of Molecular Sciences, 22(12), 6372. https://doi.org/10.3390/ijms22126372







