The Role of Biodegradable Poly-(L-lactide)-Based Polymers in Blood Cell Activation and Platelet-Monocyte Interaction
Abstract
1. Introduction
2. Results
2.1. Polymer Wettability
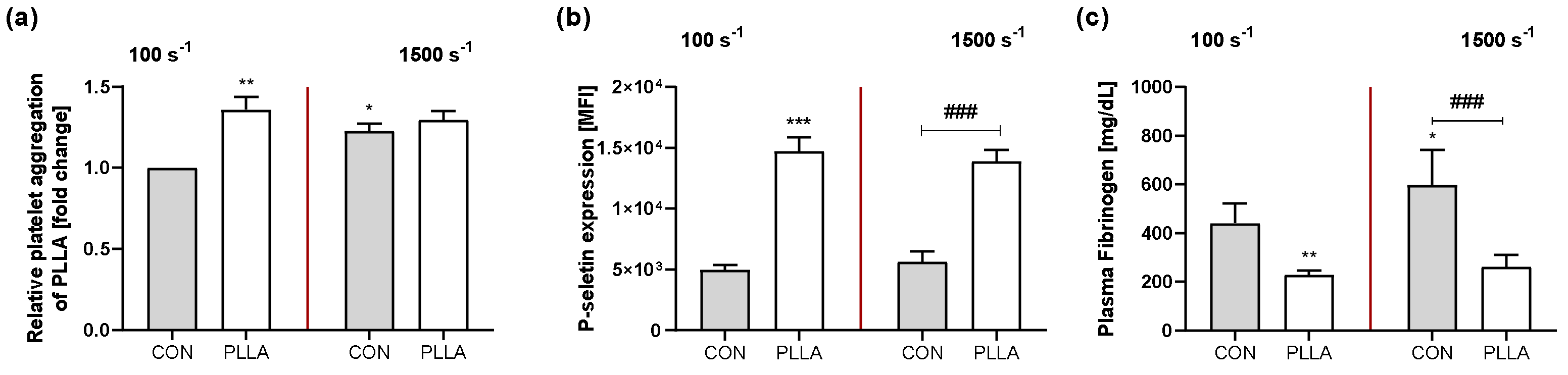
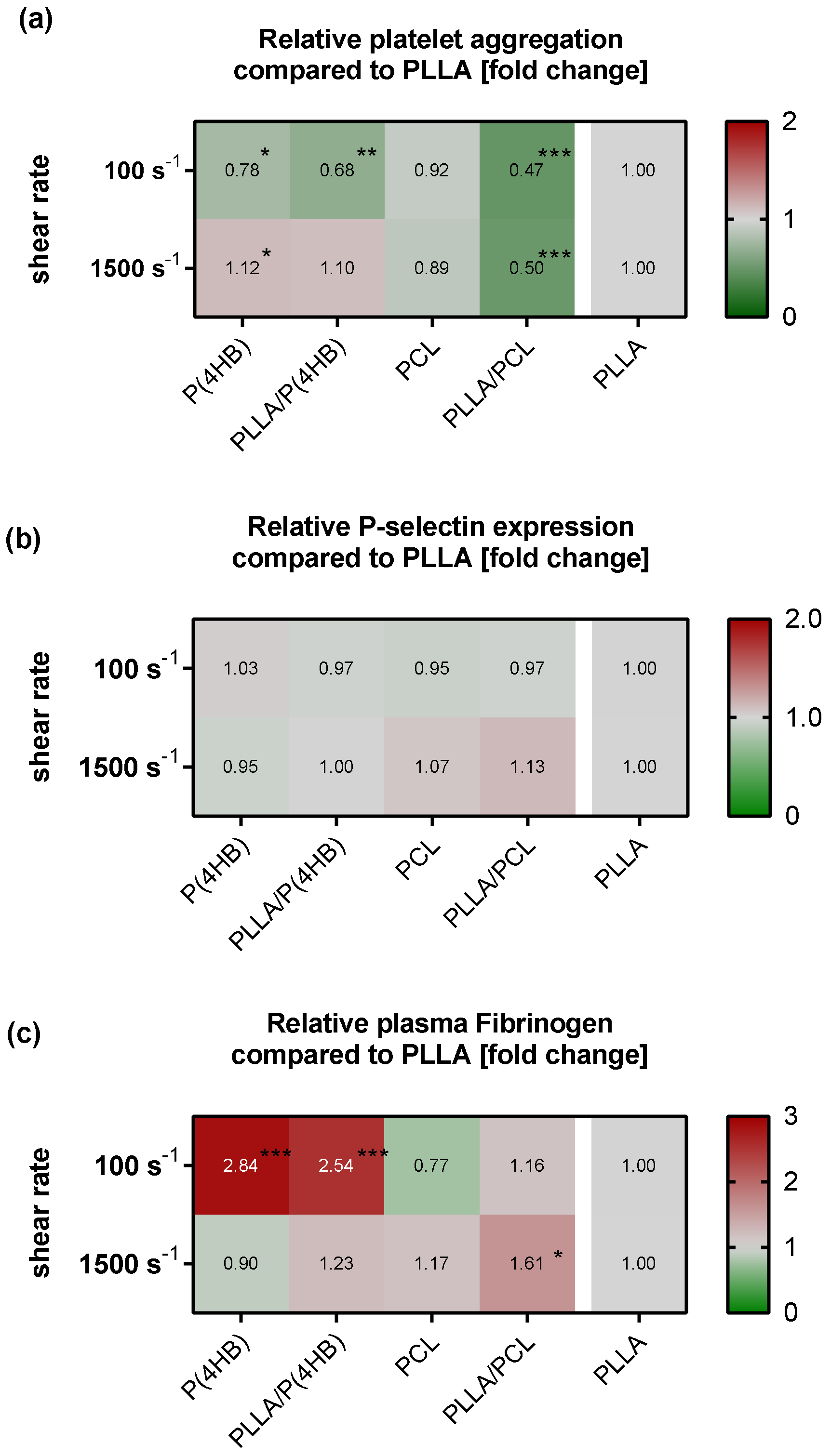
2.2. Platelet–Polymer Interaction
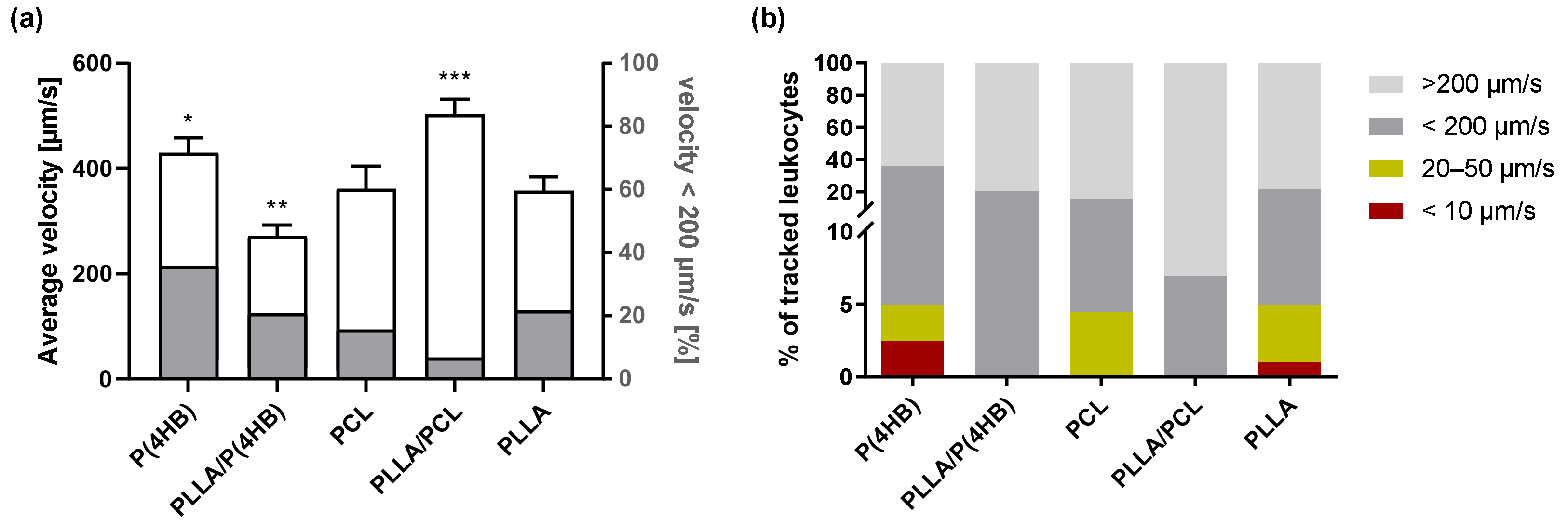
2.3. Leukocyte–Polymer Interaction
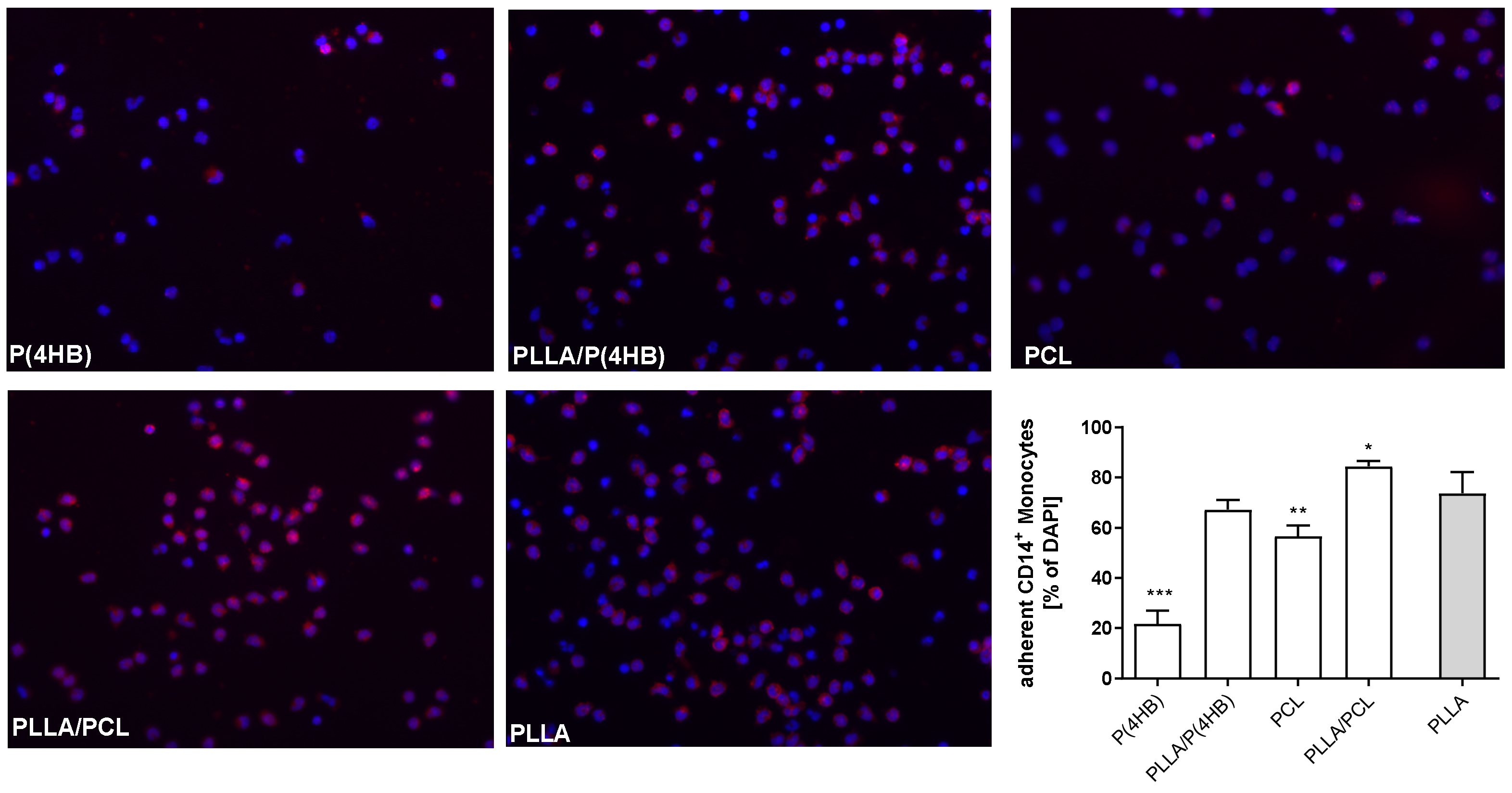
2.4. Monocyte Activation
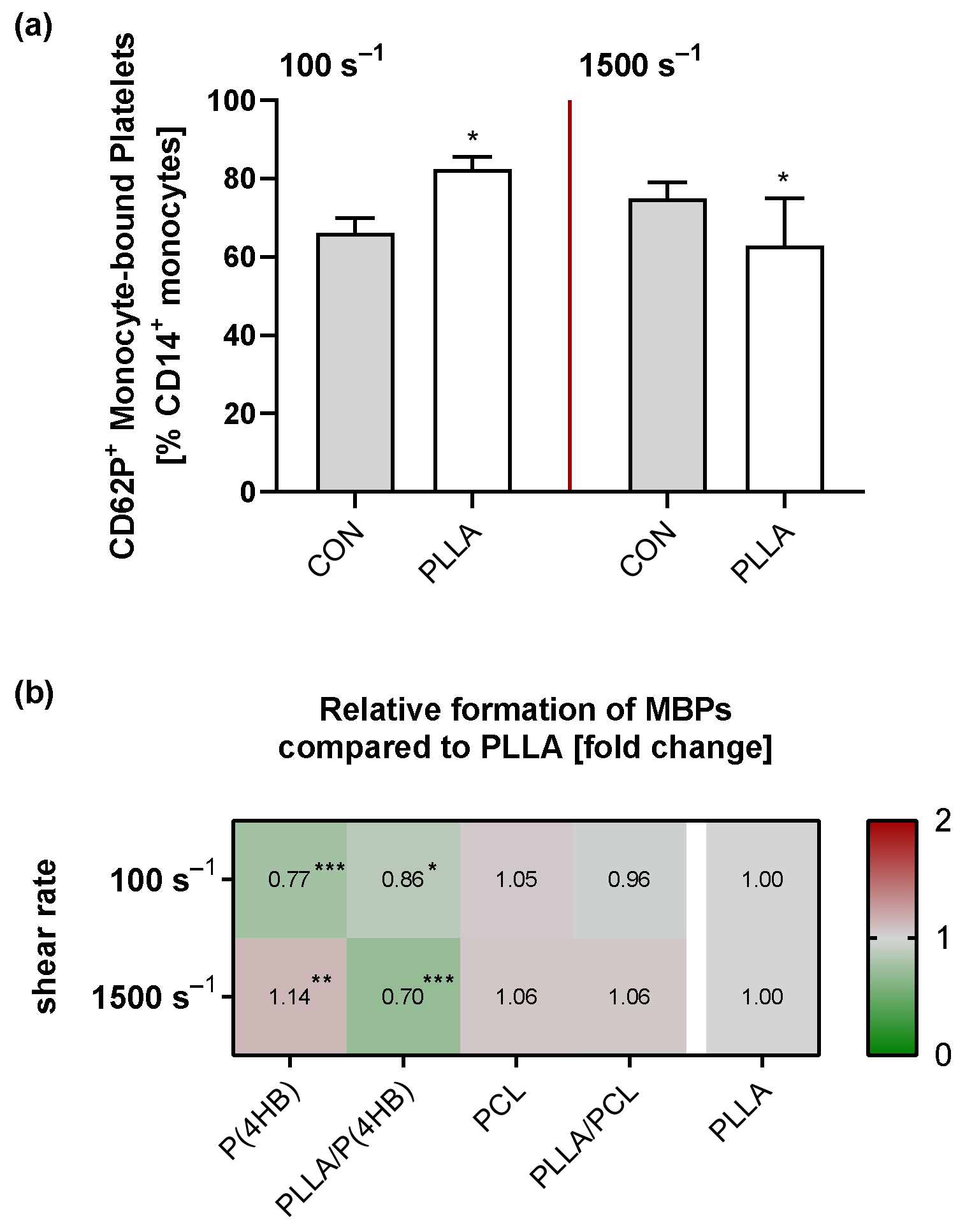
2.5. Platelet–Monocyte Aggregation
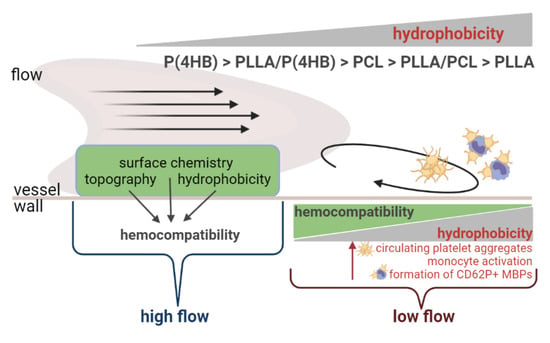
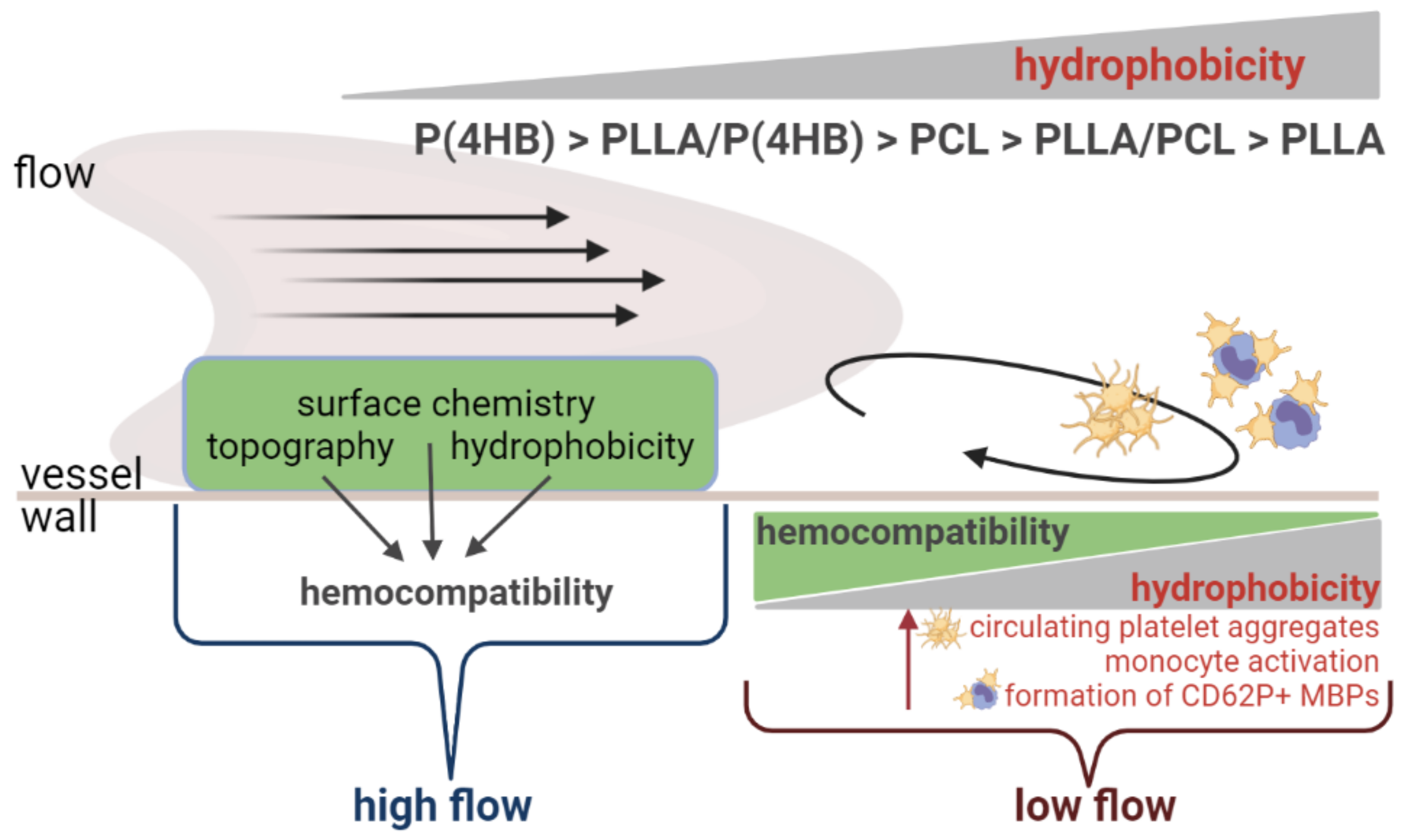
3. Discussion
3.1. Platelet–Polymer Interactions Are Influenced by Local Hemodynamic Conditions
3.2. Biomaterials Facilitate Leucocyte–Polymer Interactions
3.3. Biomaterials Influence the Formation of CD62P+ Monocyte-Bound Platelets (MBPs)
4. Materials and Methods
4.1. Polymer Film Preparation
4.2. Surface Characterization
4.3. Isolation of Human Blood Cells
4.4. Flow Experiments
4.5. Platelet Aggregation
4.6. Measurement of Surface P-Selectin and Plasma Fibrinogen (Fg)
4.7. Leukocyte Tracking
4.8. Monocyte Adhesion
4.9. Flow Cytometry for the Detection of Monocytes Activation and Platelet–Monocyte Aggregates
4.10. Data Collection and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fischer, M.; Maitz, M.F.; Werner, C. Coatings for biomaterials to improve hemocompatibility. In Hemocompatibility of Biomaterials for Clinical Applications; Siedlecki, C.A., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 163–190. [Google Scholar]
- Recek, N. Biocompatibility of Plasma-Treated Polymeric Implants. Materials 2019, 12, 240. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, J.S.; Hayman, D. An Overview of Mechanical Properties and Material Modeling of Polylactide (PLA) for Medical Applications. Ann. Biomed. Eng. 2016, 44, 330–340. [Google Scholar] [CrossRef]
- Virmani, R.; Guagliumi, G.; Farb, A.; Musumeci, G.; Grieco, N.; Motta, T.; Mihalcsik, L.; Tespili, M.; Valsecchi, O.; Kolodgie, F.D. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent should we be cautious? Circulation 2004, 109, 701–705. [Google Scholar] [CrossRef]
- Mann, G.S.; Singh, L.P.; Kumar, P.; Singh, S.; Prakash, C. On briefing the surface modifications of polylactic acid: A scope for betterment of biomedical structures. J. Thermoplast. Compos. Mater. 2019. [Google Scholar] [CrossRef]
- Bagheri, M.; Mohammadi, M.; Steele, T.W.; Ramezani, M. Nanomaterial coatings applied on stent surfaces. Nanomedicine 2016, 11, 1309–1326. [Google Scholar] [CrossRef] [PubMed]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef]
- Evans-Nguyen, K.M.; Schoenfisch, M.H. Fibrin proliferation at model surfaces: Influence of surface properties. Langmuir 2005, 21, 1691–1694. [Google Scholar] [CrossRef]
- Kolar, M.; Mozetic, M.; Stana-Kleinschek, K.; Frohlich, M.; Turk, B.; Vesel, A. Covalent Binding of Heparin to Functionalized PET Materials for Improved Haemocompatibility. Materials 2015, 8, 1526–1544. [Google Scholar] [CrossRef]
- Otto, M.; Franzen, A.; Hansen, T.; Kirkpatrick, C.J. Modification of human platelet adhesion on biomaterial surfaces by protein preadsorption under static and flow conditions. J. Mater. Sci. Mater. Med. 2004, 15, 35–42. [Google Scholar] [CrossRef]
- Qi, P.K.; Maitz, M.F.; Huang, N. Surface modification of cardiovascular materials and implants. Surf. Coat. Technol. 2013, 233, 80–90. [Google Scholar] [CrossRef]
- Sivaraman, B.; Latour, R.A. The relationship between platelet adhesion on surfaces and the structure versus the amount of adsorbed fibrinogen. Biomaterials 2010, 31, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Asif, S.; Asawa, K.; Inoue, Y.; Ishihara, K.; Lindell, B.; Holmgren, R.; Nilsson, B.; Ryden, A.; Jensen-Waern, M.; Teramura, Y.; et al. Validation of an MPC Polymer Coating to Attenuate Surface-Induced Crosstalk between the Complement and Coagulation Systems in Whole Blood in In Vitro and In Vivo Models. Macromol. Biosci. 2019, 19, e1800485. [Google Scholar] [CrossRef]
- Otto, M.; Wahn, B.; Kirkpatrick, C.J. Modification of human polymorphonuclear neutrophilic cell (PMN)-adhesion on biomaterial surfaces by protein preadsorption under static and flow conditions. J. Mater. Sci. Mater. Med. 2003, 14, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Sperling, C.; Tengvall, P.; Werner, C. The ability of surface characteristics of materials to trigger leukocyte tissue factor expression. Biomaterials 2010, 31, 2498–2507. [Google Scholar] [CrossRef] [PubMed]
- Markiewski, M.M.; Nilsson, B.; Ekdahl, K.N.; Mollnes, T.E.; Lambris, J.D. Complement and coagulation: Strangers or partners in crime? Trends Immunol. 2007, 28, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Holme, P.A.; Orvim, U.; Hamers, M.J.; Solum, N.O.; Brosstad, F.R.; Barstad, R.M.; Sakariassen, K.S. Shear-induced platelet activation and platelet microparticle formation at blood flow conditions as in arteries with a severe stenosis. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 646–653. [Google Scholar] [CrossRef]
- Alcaide, P.; Auerbach, S.; Luscinskas, F.W. Neutrophil recruitment under shear flow: It’s all about endothelial cell rings and gaps. Microcirculation 2009, 16, 43–57. [Google Scholar] [CrossRef]
- Braune, S.; Grunze, M.; Straub, A.; Jung, F. Are there sufficient standards for the in vitro hemocompatibility testing of biomaterials? Biointerphases 2013, 8, 33. [Google Scholar] [CrossRef]
- Inoue, T.; Uchida, T.; Yaguchi, I.; Sakai, Y.; Takayanagi, K.; Morooka, S. Stent-induced expression and activation of the leukocyte integrin Mac-1 is associated with neointimal thickening and restenosis. Circulation 2003, 107, 1757–1763. [Google Scholar] [CrossRef]
- Yu, T.; Tutwiler, V.K.S. The Role of Macrophages in the Foreign Body Response to Implanted Biomaterials. In Biomaterials in Regenerative Medicine and the Immune System; Santambrogio, L., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 17–34. [Google Scholar]
- Hu, H.; Varon, D.; Hjemdahl, P.; Savion, N.; Schulman, S.; Li, N.L. Platelet-leukocyte aggregation under shear stress: Differential involvement of selectins and integrins. Thromb. Haemost. 2003, 90, 679–687. [Google Scholar] [CrossRef]
- Puranik, A.S.; Dawson, E.R.; Peppas, N.A. Recent advances in drug eluting stents. Int. J. Pharm. 2013, 441, 665–679. [Google Scholar] [CrossRef]
- Sperling, C.; Fischer, M.; Maitz, M.F.; Werner, C. Blood coagulation on biomaterials requires the combination of distinct activation processes. Biomaterials 2009, 30, 4447–4456. [Google Scholar] [CrossRef] [PubMed]
- Jung, F.; Braune, S. Thrombogenicity and hemocompatibility of biomaterials. Biointerphases 2015, 11, 029601. [Google Scholar] [CrossRef] [PubMed]
- Slepian, M.J.; Sheriff, J.; Hutchinson, M.; Tran, P.; Bajaj, N.; Garcia, J.G.N.; Scott Saavedra, S.; Bluestein, D. Shear-mediated platelet activation in the free flow: Perspectives on the emerging spectrum of cell mechanobiological mechanisms mediating cardiovascular implant thrombosis. J. Biomech. 2017, 50, 20–25. [Google Scholar] [CrossRef]
- Rahman, S.M.; Eichinger, C.D.; Hlady, V. Effects of upstream shear forces on priming of platelets for downstream adhesion and activation. Acta Biomater. 2018, 73, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Gentile, F.; Tirinato, L.; Battista, E.; Causa, F.; Liberale, C.; di Fabrizio, E.M.; Decuzzi, P. Cells preferentially grow on rough substrates. Biomaterials 2010, 31, 7205–7212. [Google Scholar] [CrossRef] [PubMed]
- Keselowsky, B.G.; Collard, D.M.; Garcia, A.J. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials 2004, 25, 5947–5954. [Google Scholar] [CrossRef] [PubMed]
- Grunkemeier, J.M.; Tsai, W.B.; McFarland, C.D.; Horbett, T.A. The effect of adsorbed fibrinogen, fibronectin, von Willebrand factor and vitronectin on the procoagulant state of adherent platelets. Biomaterials 2000, 21, 2243–2252. [Google Scholar] [CrossRef]
- Rodrigues, S.N.; Goncalves, I.C.; Martins, M.C.; Barbosa, M.A.; Ratner, B.D. Fibrinogen adsorption, platelet adhesion and activation on mixed hydroxyl-/methyl-terminated self-assembled monolayers. Biomaterials 2006, 27, 5357–5367. [Google Scholar] [CrossRef]
- Rana, A.; Westein, E.; Niego, B.; Hagemeyer, C.E. Shear-Dependent Platelet Aggregation: Mechanisms and Therapeutic Opportunities. Front. Cardiovasc. Med. 2019, 6, 141. [Google Scholar] [CrossRef]
- Dunne, J.L.; Ballantyne, C.M.; Beaudet, A.L.; Ley, K. Control of leukocyte rolling velocity in TNF-alpha-induced inflammation by LFA-1 and Mac-1. Blood 2002, 99, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Eaton, J.W. Fibrin(ogen) mediates acute inflammatory responses to biomaterials. J. Exp. Med. 1993, 178, 2147–2156. [Google Scholar] [CrossRef]
- Hylton, D.M.; Shalaby, S.W.; Latour, R.A., Jr. Direct correlation between adsorption-induced changes in protein structure and platelet adhesion. J. Biomed. Mater. Res. A 2005, 73, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Sohma, R.; Miyazaki, T.; Iwasaki, Y.; Yaguchi, I.; Morooka, S. Comparison of activation process of platelets and neutrophils after coronary stent implantation versus balloon angioplasty for stable angina pectoris. Am. J. Cardiol. 2000, 86, 1057–1062. [Google Scholar] [CrossRef]
- Marketou, M.; Kochiadakis, G.E.; Giaouzaki, A.; Sfiridaki, K.; Petousis, S.; Maragoudakis, F.; Roufas, K.; Vougia, D.; Logakis, J.; Chlouverakis, G.; et al. Long-term serial changes in platelet activation indices following sirolimus elution and bare metal stent implantation in patients with stable coronary artery disease. Hell. J. Cardiol. 2017, 58, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Witzenbichler, B.; Weisz, G.; Rinaldi, M.J.; Neumann, F.J.; Metzger, D.C.; Henry, T.D.; Cox, D.A.; Duffy, P.L.; Mazzaferri, E.; et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): A prospective multicentre registry study. Lancet 2013, 382, 614–623. [Google Scholar] [CrossRef]
- Kunkel, E.J.; Chomas, J.E.; Ley, K. Role of primary and secondary capture for leukocyte accumulation in vivo. Circ. Res. 1998, 82, 30–38. [Google Scholar] [CrossRef]
- Bauer, G.J.; Arbabi, S.; Garcia, I.A.; de Hingh, I.; Rosengart, M.R.; Maier, R.V. Adherence regulates macrophage signal transduction and primes tumor necrosis factor production. Shock 2000, 14, 435–440. [Google Scholar] [CrossRef]
- Dinnes, D.L.; Marcal, H.; Mahler, S.M.; Santerre, J.P.; Labow, R.S. Material surfaces affect the protein expression patterns of human macrophages: A proteomics approach. J. Biomed. Mater. Res. A 2007, 80, 895–908. [Google Scholar] [CrossRef]
- Jones, J.A.; Chang, D.T.; Meyerson, H.; Colton, E.; Kwon, I.K.; Matsuda, T.; Anderson, J.M. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J. Biomed. Mater. Res. A 2007, 83, 585–596. [Google Scholar] [CrossRef]
- Ahn, K.C.; Jun, A.J.; Pawar, P.; Jadhav, S.; Napier, S.; McCarty, O.J.; Konstantopoulos, K. Preferential binding of platelets to monocytes over neutrophils under flow. Biochem. Biophys. Res. Commun. 2005, 329, 345–355. [Google Scholar] [CrossRef]
- Busch, G.; Steppich, B.; Sibbing, D.; Braun, S.L.; Stein, A.; Groha, P.; Schomig, A.; Kastrati, A.; von Beckerath, N.; Ott, I. Bivalirudin reduces platelet and monocyte activation after elective percutaneous coronary intervention. Thromb. Haemost. 2009, 101, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Kato, T.; Hikichi, Y.; Hashimoto, S.; Hirase, T.; Morooka, T.; Imoto, Y.; Takeda, Y.; Sendo, F.; Node, K. Stent-induced neutrophil activation is associated with an oxidative burst in the inflammatory process, leading to neointimal thickening. Thromb. Haemost. 2006, 95, 43–48. [Google Scholar]
- Sukegawa, H.; Maekawa, Y.; Yuasa, S.; Anzai, A.; Kodaira, M.; Takei, M.; Sano, F.; Ueda, I.; Kawakami, T.; Hayashida, K.; et al. Intensive statin therapy stabilizes C-reactive protein, but not chemokine in stable coronary artery disease treated with an everolimus-eluting stent. Coron. Artery Dis. 2016, 27, 405–411. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H.; Williams, M.C.; Raina, R.; Loushine, R.J.; Weller, R.N.; Kimbrough, W.F.; King, N.M. Susceptibility of a polycaprolactone-based root canal filling material to degradation. I. Alkaline hydrolysis. J. Endod. 2005, 31, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Hui, H.; Fuller, K.; Erber, W.N.; Linden, M.D. Measurement of monocyte-platelet aggregates by imaging flow cytometry. Cytometry A 2015, 87, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Koh, L.B.; Rodriguez, I.; Venkatraman, S.S. The effect of topography of polymer surfaces on platelet adhesion. Biomaterials 2010, 31, 1533–1545. [Google Scholar] [CrossRef]
- Bunger, C.M.; Grabow, N.; Sternberg, K.; Goosmann, M.; Schmitz, K.P.; Kreutzer, H.J.; Ince, H.; Kische, S.; Nienaber, C.A.; Martin, D.P.; et al. A biodegradable stent based on poly(L-lactide) and poly(4-hydroxybutyrate) for peripheral vascular application: Preliminary experience in the pig. J. Endovasc. Ther. 2007, 14, 725–733. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Su, S.H.; Sheng, A.; Wawro, D.; Schwade, N.D.; Brouse, C.F.; Greilich, P.E.; Tang, L.; Eberhart, R.C. In vitro hemocompatibility studies of drug-loaded poly-(L-lactic acid) fibers. Biomaterials 2003, 24, 5191–5201. [Google Scholar] [CrossRef]
- Su, S.H.; Landau, C.; Chao, R.Y.N.; Timmons, R.; Meidell, R.; Tang, L.P.; Eberhart, R. Expandable, bioresorbable endovascular stent with anti-platelet and anti-inflammation treatments. Circulation 2001, 104, 507. [Google Scholar]
- Van der Giessen, W.J.; Lincoff, A.M.; Schwartz, R.S.; van Beusekom, H.M.; Serruys, P.W.; Holmes, D.R.; Jr Ellis, S.G.; Topol, E.J. Marked inflammatory sequelae to implantation of biodegradable and nonbiodegradable polymers in porcine coronary arteries. Circulation 1996, 94, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Busch, R.; Strohbach, A.; Rethfeldt, S.; Walz, S.; Busch, M.; Petersen, S.; Felix, S.; Sternberg, K. New stent surface materials: The impact of polymer-dependent interactions of human endothelial cells, smooth muscle cells, and platelets. Acta Biomater. 2014, 10, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Grabow, N.; Bunger, C.M.; Schultze, C.; Schmohl, K.; Martin, D.P.; Williams, S.F.; Sternberg, K.; Schmitz, K.P. A biodegradable slotted tube stent based on poly(L-lactide) and poly(4-hydroxybutyrate) for rapid balloon-expansion. Ann. Biomed. Eng. 2007, 35, 2031–2038. [Google Scholar] [CrossRef]

| Polymer | Θ W [°] Mean±Standard Deviation | p-Value |
|---|---|---|
| P(4HB) | 72.1 ± 3.9 | <0.001 |
| PLLA/P(4HB) | 76.7 ± 2.8 | 0.001 |
| PCL | 78.6 ± 3.2 | 0.043 |
| PLLA/PCL | 79.9 ± 3.4 | 0.295 |
| PLLA | 82.2 ± 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strohbach, A.; Maess, F.; Wulf, K.; Petersen, S.; Grabow, N.; Schmitz, K.-P.; Felix, S.B.; Busch, R. The Role of Biodegradable Poly-(L-lactide)-Based Polymers in Blood Cell Activation and Platelet-Monocyte Interaction. Int. J. Mol. Sci. 2021, 22, 6340. https://doi.org/10.3390/ijms22126340
Strohbach A, Maess F, Wulf K, Petersen S, Grabow N, Schmitz K-P, Felix SB, Busch R. The Role of Biodegradable Poly-(L-lactide)-Based Polymers in Blood Cell Activation and Platelet-Monocyte Interaction. International Journal of Molecular Sciences. 2021; 22(12):6340. https://doi.org/10.3390/ijms22126340
Chicago/Turabian StyleStrohbach, Anne, Friedemann Maess, Katharina Wulf, Svea Petersen, Niels Grabow, Klaus-Peter Schmitz, Stephan B. Felix, and Raila Busch. 2021. "The Role of Biodegradable Poly-(L-lactide)-Based Polymers in Blood Cell Activation and Platelet-Monocyte Interaction" International Journal of Molecular Sciences 22, no. 12: 6340. https://doi.org/10.3390/ijms22126340
APA StyleStrohbach, A., Maess, F., Wulf, K., Petersen, S., Grabow, N., Schmitz, K.-P., Felix, S. B., & Busch, R. (2021). The Role of Biodegradable Poly-(L-lactide)-Based Polymers in Blood Cell Activation and Platelet-Monocyte Interaction. International Journal of Molecular Sciences, 22(12), 6340. https://doi.org/10.3390/ijms22126340