Transcriptional Profiling Identifies Upregulation of Neuroprotective Pathways in Retinitis Pigmentosa
Abstract
1. Introduction
2. Results
2.1. Photoreceptor Degeneration in VPP Mice
2.2. Transcriptional Alterations in VPP Retinae: RNAseq and Weighted Correlation Network Analysis (WGCNA)
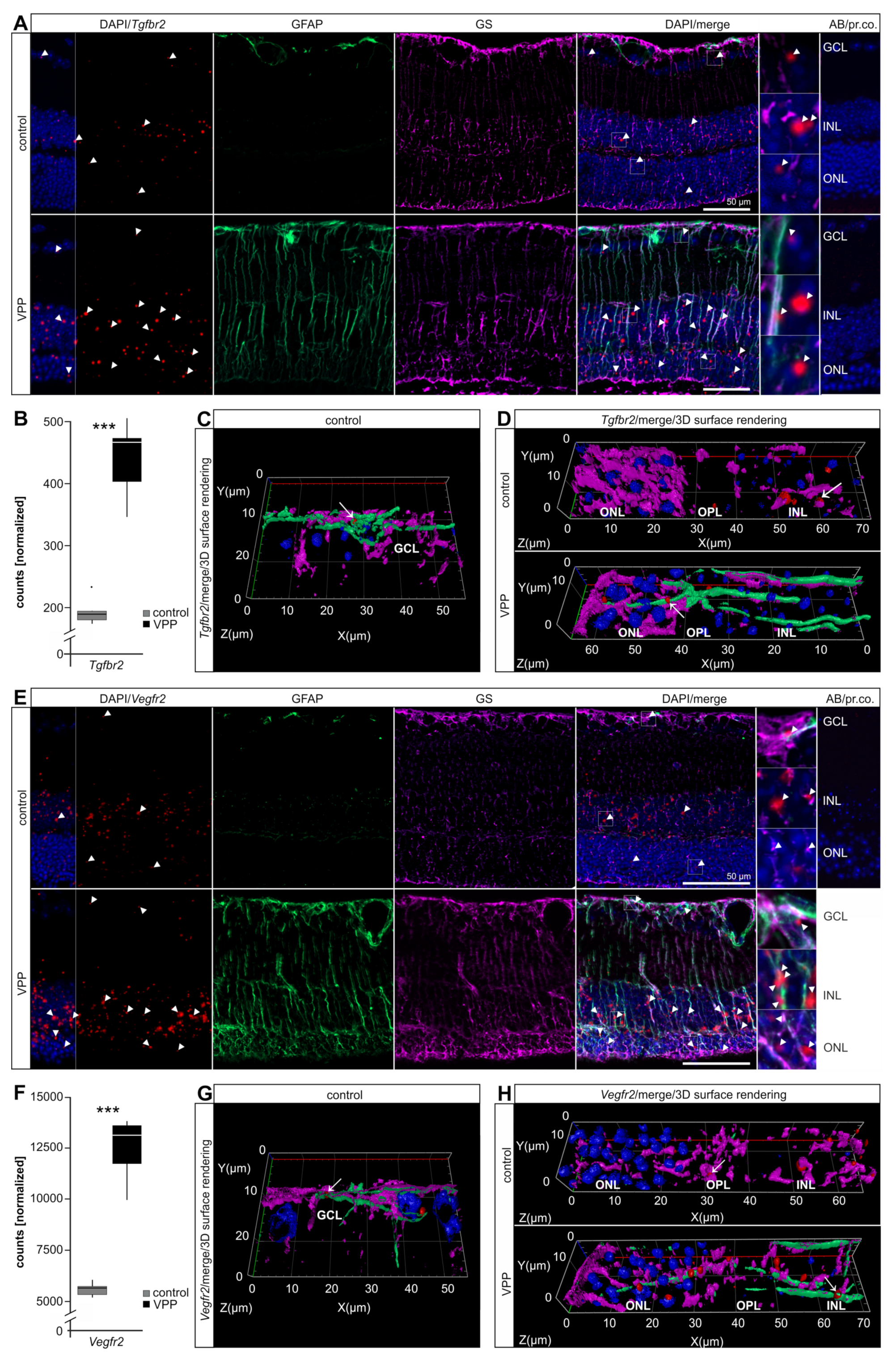
2.3. Dysregulation of Potentially Neuroprotective Pathways in VPP Retinae: VEGF-, TGF-β-, and G-protein Mediated Signaling
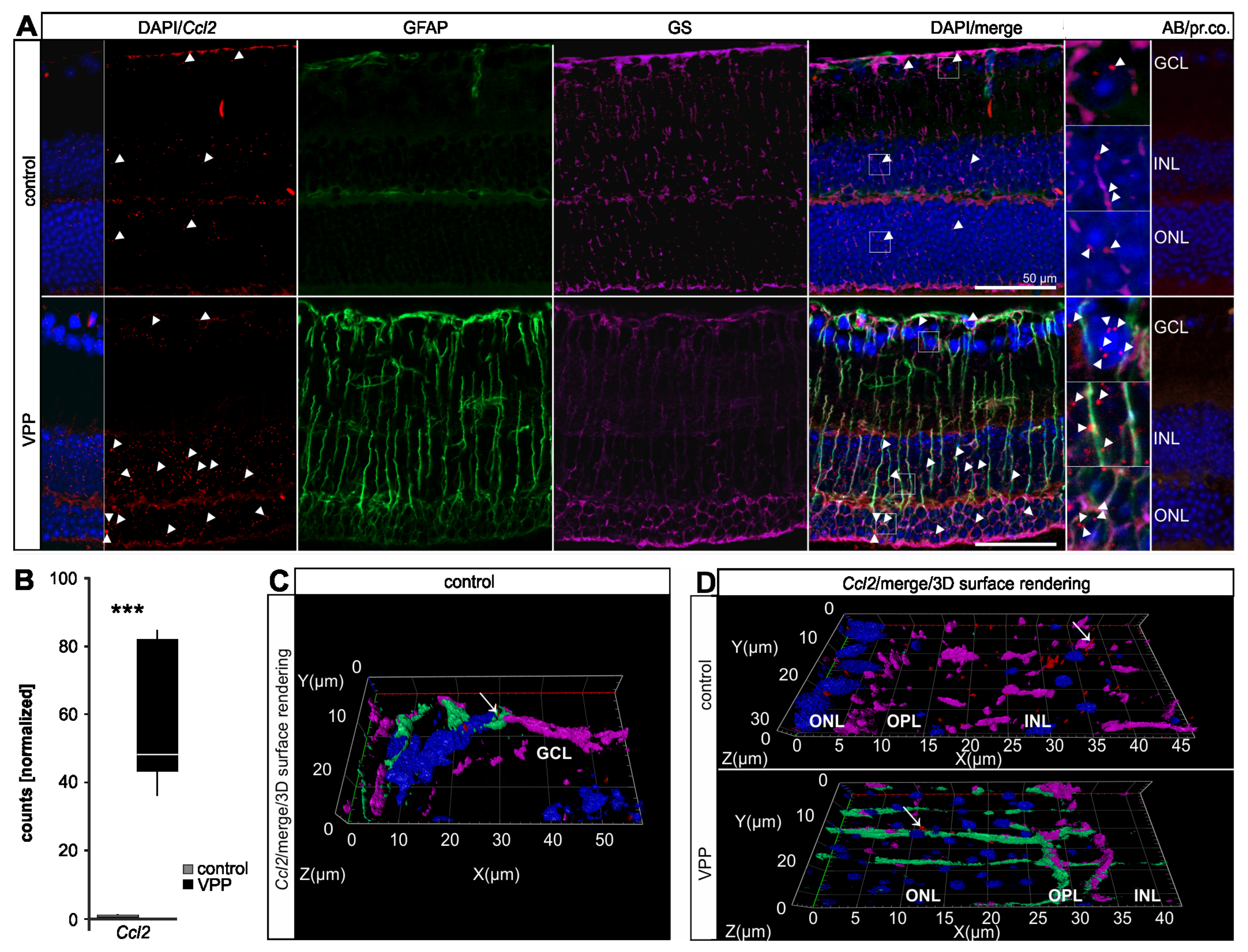
2.4. The Glial Response to Photoreceptor Degeneration in VPP Mice
3. Discussion
3.1. The Transcriptional Response to Photoreceptor Degeneration Leads to Increased Expression of Genes Regulating Inflammatory or Immune Response Functions
3.2. The Transcriptional Response to Photoreceptor Degeneration Leads to the Upregulation of Neuroprotective Factors and Pathways
3.3. The Transcriptional Response to Photoreceptor Degeneration Leads to Expression of Pro-Apoptotic Factors and Extracellular Matrix Organization
4. Material and Methods
4.1. Mice
4.2. Microscopy and Morphometric Analyses (Spider Diagram)
4.3. Apoptosis: TdT-Mediated dUTP-Biotin Nick End Labeling (TUNEL)
4.4. Immunofluorescence and RNA/Basescope® In Situ Hybridization
4.5. RNA Isolation and Quantitative Real-Time RT-PCR (qPCR)
4.6. RNA Sequencing
4.7. Bioinformatics
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Bik | Bcl2-interacting killer |
| BMP | Bone morphogenetic protein |
| C1q | Complement component 1q |
| C3 | Complement component 3 |
| Casp1 | Caspase 1 |
| Ccl2 | Chemokine (C-C motif) ligand 2 |
| Ccl5 | Chemokine (C-C motif) ligand 5 |
| Cd3g | T-cell receptor T3 gamma chain |
| Cfh | Complement factor H |
| Cfi | Complement component factor i |
| Cr3 | Complement receptor 3 |
| Cxcl13 | C-X-C motif chemokine ligand 13 |
| Edn1 | Endothelin 1 |
| Edn2 | Endothelin 2 |
| Ednra | Endothelin receptor type a |
| Ednrb | Endothelin receptor type b |
| Fgf2 | Fibroblast growth factor 2 |
| Fgl2 | Fibrinogen-like 2 |
| GCL | Ganglion cell layer |
| Gfap | Glial fibrillary acidic protein |
| Glb1l3 | Galactosidase beta 1 like 3 |
| Gnb2l1 | Guanine nucleotide binding protein subunit beta2 like 1 |
| GS | Glutamine synthetase |
| Hdc | Histidine decarboxylase |
| Iba1 | Ionized calcium-binding adapter molecule 1 |
| Il-1 | Interleukin-1 |
| INL | Inner nuclear layer |
| IPL | Inner plexiform layer |
| Kdr | Kinase insert domain receptor |
| Lif | Leukemia inhibitory factor |
| Lcn2 | Lipocalin 2 |
| ONH | Optical nerve head |
| ONL | Outer nuclear layer |
| OPL | Outer plexiform layer |
| Prss56 | Serine protease 56 |
| RNAseq | Next generation RNA sequencing |
| RP | Retinitis pigmentosa |
| RPE | Retinal pigment epithelium |
| Serpina3n | Serine protease inhibitor A3N |
| TGF | Transforming growth factor |
| Tgfbr1 | Transforming growth factor -ß receptor type 1 |
| Tgfbr2 | Transforming growth factor -ß receptor type 2 |
| Ubc | Ubiquitin C |
| VEGF | Vascular endothelial growth factor |
| Vegfr1 | Vascular endothelial growth factor receptor type 1 |
| Vegfr2 | Vascular endothelial growth factor receptor type 2 |
| WGCNA | Weighted correlation network analysis |
References
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Ruzickova, S.; Stanek, D. Mutations in spliceosomal proteins and retina degeneration. RNA Biol. 2017, 14, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Buch, H.; Vinding, T. Prevalence and causes of visual impairment and blindness among 9980 Scandinavian adults: The Copenhagen City Eye Study. Ophthalmology 2004, 111, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Farrar, G.; Kenna, P.F.; Humphries, P.; La Cour, M.; Appleyard, M.; Jensen, G.B.; Nielsen., N.V. NEW EMBO MEMBER’S REVIEW: On the genetics of retinitis pigmentosa and on mutation-independent approaches to therapeutic intervention. EMBO J. 2002, 21, 857–864. [Google Scholar] [CrossRef]
- Kolb, H. Simple Anatomy of the Retina. In Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernandez, E., Nelson, R., Eds.; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 1995. [Google Scholar]
- Naash, M.I.; Hollyfield, J.G.; Al-Ubaidi, M.R.; Baehr, W. Simulation of human autosomal dominant retinitis pigmentosa in transgenic mice expressing a mutated murine opsin gene. Proc. Natl. Acad. Sci. USA 1993, 90, 5499–5503. [Google Scholar] [CrossRef]
- Sung, C.H.; Davenport, C.M.; Hennessey, J.C.; Maumenee, I.H.; Jacobson, S.G.; Heckenlively, J.R.; Nowakowski, R.; Fishman, G.; Gouras, P.; Nathans, J. Rhodopsin mutations in autosomal dominant retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 1991, 88, 6481–6485. [Google Scholar] [CrossRef]
- Berson, E.L.; Rosner, B.; Sandberg, M.A.; Dryja, T.P. Ocular Findings in Patients With Autosomal Dominant Retinitis Pigmentosa and a Rhodopsin Gene Defect (Pro-23-His). Arch. Ophthalmol. 1991, 109, 92–101. [Google Scholar] [CrossRef]
- Dryja, T.P.; McGee, T.L.; Reichel, E.; Hahn, L.B.; Cowley, G.S.; Yandell, D.W.; Sandberg, M.A.; Berson, E.L. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nat. Cell Biol. 1990, 343, 364–366. [Google Scholar] [CrossRef]
- Braunger, B.M.; Ohlmann, A.; Koch, M.; Tanimoto, N.; Volz, C.; Yang, Y.; Bösl, M.R.; Cvekl, A.; Jägle, H.; Seeliger, M.W.; et al. Constitutive overexpression of Norrin activates Wnt/β-catenin and endothelin-2 signaling to protect photoreceptors from light damage. Neurobiol. Dis. 2013, 50, 1–12. [Google Scholar] [CrossRef]
- Joly, S.; Lange, C.; Thiersch, M.; Samardzija, M.; Grimm, C. Leukemia inhibitory factor extends the lifespan of injured photoreceptors in vivo. J. Neurosci. 2008, 28, 13765–13774. [Google Scholar] [CrossRef]
- Rattner, A.; Nathans, J. The genomic response to retinal disease and injury: Evidence for endothelin signaling from photoreceptors to glia. J. Neurosci. 2005, 25, 4540–4549. [Google Scholar] [CrossRef]
- Braunger, B.M.; Leimbeck, S.V.; Schlecht, A.; Volz, C.; Jägle, H.; Tamm, E.R. Deletion of Ocular Transforming Growth Factor β Signaling Mimics Essential Characteristics of Diabetic Retinopathy. Am. J. Pathol. 2015, 185, 1749–1768. [Google Scholar] [CrossRef]
- Braunger, B.M.; Pielmeier, S.; Demmer, C.; Landstorfer, V.; Kawall, D.; Abramov, N.; Leibinger, M.; Kleiter, I.; Fischer, D.; Jägle, H.; et al. TGF-β Signaling Protects Retinal Neurons from Programmed Cell Death during the Development of the Mammalian Eye. J. Neurosci. 2013, 33, 14246–14258. [Google Scholar] [CrossRef]
- Schmitt, S.I.; Bielmeier, C.B.; Braunger, B.M. New Insights into Endothelin Signaling and Its Diverse Roles in the Retina. Adv. Exp. Med. Biol. 2019, 1185, 519–523. [Google Scholar]
- Wu, G.; Feng, X.; Stein, L. A human functional protein interaction network and its application to cancer data analysis. Genome Biol. 2010, 11, R53. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef]
- Lewis, G.P.; Erickson, P.A.; Kaska, D.D.; Fisher, S.K. An immunocytochemical comparison of Müller cells and astrocytes in the cat retina. Exp. Eye Res. 1988, 47, 839–853. [Google Scholar] [CrossRef]
- Feng, C.; Wang, X.; Liu, T.; Zhang, M.; Xu, G.; Ni, Y. Expression of CCL2 and its receptor in activation and migration of microglia and monocytes induced by photoreceptor apoptosis. Mol. Vis. 2017, 23, 765–777. [Google Scholar]
- Rutar, M.; Natoli, R.; Valter, K.; Provis, J.M. Early focal expression of the chemokine Ccl2 by Muller cells during exposure to damage-inducing bright continuous light. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2379–2388. [Google Scholar] [CrossRef]
- Chen, W.-W.; Zhang, X.I.A.; Huang, W.-J. Role of neuroinflammation in neurodegenerative diseases (Review). Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, N.; Fernandez-Sanchez, L.; Campello, L.; Maneu, V.; De la Villa, P.; Lax, P.; Pinilla, I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog. Retin. Eye Res. 2014, 43, 17–75. [Google Scholar] [CrossRef] [PubMed]
- Mullins, R.F.; Kuehn, M.H.; Radu, R.A.; Enriquez, G.S.; East, J.S.; Schindler, E.I.; Travis, G.H.; Stone, E.M. Autosomal recessive retinitis pigmentosa due to ABCA4 mutations: Clinical, pathologic, and molecular characterization. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1883–1894. [Google Scholar] [CrossRef] [PubMed]
- Stoll, G.; Jander, S.; Schroeter, M. Cytokines in CNS disorders: Neurotoxicity versus neuroprotection. J. Neural Transm. Suppl. 2000, 59, 81–89. [Google Scholar] [PubMed]
- Hanisch, U.-K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Langmann, T. Microglia activation in retinal degeneration. J. Leukoc. Biol. 2007, 81, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Samardzija, M.; Wenzel, A.; Aufenberg, S.; Thiersch, M.; Reme, C.; Grimm, C. Differential role of Jak-STAT signaling in retinal degenerations. FASEB J. 2006, 20, 2411–2413. [Google Scholar] [CrossRef]
- Samardzija, M.; Wenzel, A.; Thiersch, M.; Frigg, R.; Reme, C.; Grimm, C. Caspase-1 ablation protects photoreceptors in a model of autosomal dominant retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5181–5190. [Google Scholar] [CrossRef]
- Rohrer, B.; Guo, Y.; Kunchithapautham, K.; Gilkeson, G.S. Eliminating Complement Factor D Reduces Photoreceptor Susceptibility to Light-Induced Damage. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5282–5289. [Google Scholar] [CrossRef]
- Lohr, H.R.; Kuntchithapautham, K.; Sharma, A.K.; Rohrer, B. Multiple, parallel cellular suicide mechanisms participate in photoreceptor cell death. Exp. Eye Res. 2006, 83, 380–389. [Google Scholar] [CrossRef]
- Rohrer, B.; Demos, C.; Frigg, R.; Grimm, C. Classical complement activation and acquired immune response pathways are not essential for retinal degeneration in the rd1 mouse. Exp. Eye Res. 2007, 84, 82–91. [Google Scholar] [CrossRef][Green Version]
- Bales, K.L.; Ianov, L.; Kennedy, A.J.; Sweatt, J.D.; Gross, A.K. Autosomal dominant retinitis pigmentosa rhodopsin mutant Q344X drives specific alterations in chromatin complex gene transcription. Mol. Vis. 2018, 24, 153–164. [Google Scholar]
- Rutar, M.; Natoli, R.; Provis, J.M. Small interfering RNA-mediated suppression of Ccl2 in Muller cells attenuates microglial recruitment and photoreceptor death following retinal degeneration. J. Neuroinflamm. 2012, 9, 221. [Google Scholar] [CrossRef]
- Rutar, M.; Natoli, R.; Kozulin, P.; Valter, K.; Gatenby, P.; Provis, J.M. Analysis of Complement Expression in Light-Induced Retinal Degeneration: Synthesis and Deposition of C3 by Microglia/Macrophages Is Associated with Focal Photoreceptor Degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5347–5358. [Google Scholar] [CrossRef]
- Hadziahmetovic, M.; Kumar, U.; Song, Y.; Grieco, S.; Song, D.; Li, Y.; Tobias, J.W.; Dunaief, J.L. Microarray Analysis of Murine Retinal Light Damage Reveals Changes in Iron Regulatory, Complement, and Antioxidant Genes in the Neurosensory Retina and Isolated RPE. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5231–5241. [Google Scholar] [CrossRef]
- Schäfer, N.; Grosche, A.; Schmitt, S.I.; Braunger, B.M.; Pauly, D. Complement Components Showed a Time-Dependent Local Expression Pattern in Constant and Acute White Light-Induced Photoreceptor Damage. Front. Mol. Neurosci. 2017, 10. [Google Scholar] [CrossRef]
- Silverman, S.M.; Ma, W.; Wang, X.; Zhao, L.; Wong, W.T. C3-and CR3-dependent microglial clearance protects photoreceptors in retinitis pigmentosa. J. Exp. Med. 2019, 216, 1925–1943. [Google Scholar] [CrossRef]
- Saint-Geniez, M.; Maharaj, A.S.R.; Walshe, T.E.; Tucker, B.A.; Sekiyama, E.; Kurihara, T.; Darland, D.C.; Young, M.J.; D’Amore, P.A. Endogenous VEGF is required for visual function: Evidence for a survival role on müller cells and photoreceptors. PLoS ONE 2008, 3, e3554. [Google Scholar] [CrossRef]
- Boneva, S.; Groß, T.; Schlecht, A.; Schmitt, S.; Sippl, C.; Jägle, H.; Volz, C.; Neueder, A.; Tamm, E.; Braunger, B. Cre recombinase expression or topical tamoxifen treatment do not affect retinal structure and function, neuronal vulnerability or glial reactivity in the mouse eye. Neuroscience 2016, 325, 188–201. [Google Scholar] [CrossRef][Green Version]
- Penn, J.W.; Grobbelaar, A.O.; Rolfe, K.J. The role of the TGF-β family in wound healing, burns and scarring: A review. Int. J. Burn. Trauma 2012, 2, 18–28. [Google Scholar]
- Saika, S. TGFbeta pathobiology in the eye. Laboratory investigation. J. Tech. Methods Pathol. 2006, 86, 106–115. [Google Scholar]
- Goumans, M.-J.; Liu, Z.; ten Dijke, P. TGF-beta signaling in vascular biology and dysfunction. Cell Res. 2009, 19, 116–127. [Google Scholar] [CrossRef]
- Goumans, M.J.; Mummery, C. Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int. J. Dev. Biol. 2000, 44, 253–265. [Google Scholar]
- Massagué, J. G1 cell-cycle control and cancer. Nature 2004, 432, 298–306. [Google Scholar] [CrossRef]
- Schlecht, A.; Leimbeck, S.V.; Jägle, H.; Feuchtinger, A.; Tamm, E.R.; Braunger, B.M. Deletion of Endothelial Transforming Growth Factor–β Signaling Leads to Choroidal Neovascularization. Am. J. Pathol. 2017, 187, 2570–2589. [Google Scholar] [CrossRef]
- Fuchshofer, R. The pathogenic role of transforming growth factor-β2 in glaucomatous damage to the optic nerve head. Exp. Eye Res. 2010. [Google Scholar] [CrossRef]
- Fuchshofer, R.; Tamm, E.R. Modulation of extracellular matrix turnover in the trabecular meshwork. Exp. Eye Res. 2009, 88, 683–688. [Google Scholar] [CrossRef]
- Fuchshofer, R.; Tamm, E.R. The role of TGF-β in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012, 347, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Xue, Y.; Cepko, C.L. Microglia modulation by TGF-beta1 protects cones in mouse models of retinal degeneration. J. Clin. Investig. 2020, 130, 4360–4369. [Google Scholar] [PubMed]
- Tesseur, I.; Nguyen, A.; Chang, B.; Li, L.; Woodling, N.S.; Wyss-Coray, T.; Luo, J. Deficiency in neuronal TGF-β signaling leads to nigrostriatal degeneration and activation of TGF-β signaling protects against MPTP neurotoxicity in mice. J. Neurosci. Off. J. Soc. Neurosci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Giaume, C.; Kirchhoff, F.; Matute, C.; Reichenbach, A.; Verkhratsky, A. Glia: The fulcrum of brain diseases. Cell Death Differ. 2007, 14, 1324–1335. [Google Scholar] [CrossRef]
- Sylvain, N.J.; Salman, M.M.; Pushie, M.J.; Hou, H.; Meher, V.; Herlo, R.; Peeling, L.; Kelly, M.E. The effects of trifluoperazine on brain edema, aquaporin-4 expression and metabolic markers during the acute phase of stroke using photothrombotic mouse model. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183573. [Google Scholar] [CrossRef]
- Kitchen, P.; Salman, M.M.; Halsey, A.M.; Clarke-Bland, C.; MacDonald, J.A.; Ishida, H.; Vogel, H.J.; Almutiri, S.; Logan, A.; Kreida, S.; et al. Targeting Aquaporin-4 Subcellular Localization to Treat Central Nervous System Edema. Cell 2020, 181, 784–799.e19. [Google Scholar] [CrossRef]
- Moffitt, J.R.; Hao, J.; Wang, G.; Chen, K.H.; Babcock, H.P.; Zhuang, X. High-throughput single-cell gene-expression profiling with multiplexed error-robust fluorescence in situ hybridization. Proc. Natl. Acad. Sci. USA 2016, 113, 11046–11051. [Google Scholar] [CrossRef]
- Moffitt, J.R.; Bambah-Mukku, D.; Eichhorn, S.W.; Vaughn, E.; Shekhar, K.; Perez, J.D.; Rubinstein, N.D.; Hao, J.; Regev, A.; Dulac, C.; et al. Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science 2018, 362. [Google Scholar] [CrossRef]
- Aldewachi, H.; Al-Zidan, R.N.; Conner, M.T.; Salman, M.M. High-Throughput Screening Platforms in the Discovery of Novel Drugs for Neurodegenerative Diseases. Bioengineering 2021, 8, 30. [Google Scholar] [CrossRef]
- Salman, M.M.; Al-Obaidi, Z.; Kitchen, P.; Loreto, A.; Bill, R.M.; Wade-Martins, R. Advances in Applying Computer-Aided Drug Design for Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 4688. [Google Scholar] [CrossRef]
- Chytil, A.; Magnuson, M.A.; Wright, C.V.; Moses, H.L. Conditional inactivation of the TGF-beta type II receptor using Cre:Lox. Genesis 2002, 32, 73–75. [Google Scholar] [CrossRef]
- Kugler, M.; Schlecht, A.; Fuchshofer, R.; Schmitt, S.I.; Kleiter, I.; Aigner, L.; Tamm, E.R.; Braunger, B.M. SMAD7 deficiency stimulates Müller progenitor cell proliferation during the development of the mammalian retina. Histochem. Cell Biol. 2017, 148, 21–32. [Google Scholar] [CrossRef]
- Richardson, K.C.; Jarett, L.; Finke, E.H. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol. 1960, 35, 313–323. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 11 June 2021).
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Soneson, C.; Love, M.I.; Robinson, M.D. Differential analyses for RNA-seq: Transcript-level estimates improve gene-level inferences. F1000Research 2015, 4, 1521. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Stephens, M. False discovery rates: A new deal. Biostatistics 2017, 18, 275–294. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Langfelder, P.; Mischel, P.S.; Horvath, S. When is hub gene selection better than standard meta-analysis? PLoS ONE 2013, 8, e61505. [Google Scholar] [CrossRef]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’Ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]



| Dysregulation Analysis | Enriched Pathways 1: BioPlanet 2019, 2: Reactome 2016, 3: NCI-Nature 2016 | Gene Ontology Enrichment (Biological Process 2018) | Potential Regulators 1: ChEA 2016, 2: ENCODE TF ChIP-seq 2015 |
|---|---|---|---|
| 4620 upregulated genes (padj < 0.05) | 1: Platelet activation, signaling, and aggregation 98.77; Axon guidance 92.59; TGF-beta regulation of extracellular matrix 89.40; Integrin cell surface interactions 80.66; PI3K class IB pathway in neutrophils 69.39 2: Platelet activation, signaling, and aggregation 98.99; Integrin cell surface interactions 84.30; Hemostasis 73.18, EPH-Ephrin 62.44; Axon guidance 56.40 3: Integrin family cell surface interactions 61.52, S1P3 pathway 54.91, CXCR4-mediated signaling events 53.30, LPA receptor mediated events 52.65, S1P1 pathway 48.70 | extracellular matrix organization 93.21, neutrophil activation involved in immune response 78.28, cellular response to cytokine stimulus 72.40, regulation of cell migration 69.84, vascular endothelial growth factor receptor signaling pathway 68.22 | 1: SUZ12 268.86, MTF2 160.06, WT1 96.47 2: EZH2 98.33, EP300 96.32, MYOD1 30.74 |
| 4636 downregulated genes (padj < 0.05) | 1: Messenger RNA processing 141.05, Visual signal transduction: rods 57.92, Global genomic nucleotide excision repair 45.63, Mitotic G2-G2/M phases 42.06, RNA polymerase II transcription 40.22, 2: Assembly of the primary cilium 107.65, mRNA splicing—major pathway 73.55, Activation of the phototransduction cascade 69.44, DNA repair 61.73, Cell cycle 58.51 3: Visual signal transduction: Rods 57.92, Fanconi anemia pathway 27.00, ATR signaling pathway 16.33, Regulation of Telomerase 13.58, ATM pathway 12.73, p38 MAPK signaling pathway 11.53 | mRNA processing 168.47, DNA repair 93.72, cilium assembly 68.84, termination of RNA polymerase II transcription 55.78, rhodopsin mediated signaling pathway 48.90 | 1: CREM 212.80, FOXO3 172.49, KDM5B 152.04 2: KAT2A 233.47, GABPA 199.23, E2F4 196.15 |
| WGCNA Module | Enriched Pathways 1: BioPlanet 2019, 2: Reactome 2016, 3: NCI-Nature 2016 | Gene Ontology Enrichment (Biological Process 2018) | Potential Regulators 1: ChEA 2016, 2: ENCODE TF ChIP-seq 2015 |
|---|---|---|---|
| Pos1 (7705 genes) | 1: Axon guidance 36.18; T helper cell surface molecules 32.25; Platelet activation, signaling, and aggregation 29.28; Alpha-V beta-3 integrin/OPN pathway 27.52; PI3K class IB pathway in neutrophils 25.55 2: Integrin cell surface interactions 31.84; Platelet activation, signaling, and aggregation 29.71; Ephrin signaling 27.88; Extracellular matrix organization 27.76; Signal amplification 24.50; Semaphorin interactions 20.52 3: Osteopontin-mediated events 27.52, Beta3 integrin cell surface interactions 25.55, S1P2 pathway 25.22, S1P3 pathway 24.00, LPA receptor mediated events 20.74 | extracellular matrix organization 44.07, sprouting angiogenesis 27.06, ephrin receptor signaling pathway 24.92, response to cytokine 24.09, vascular endothelial growth factor receptor signaling 23.91 | 1: SUZ12 67.13, MTF2 35.24, JARID2 25.08 2: EZH2 14.15, EP300 6.68 |
| Pos2 (560 genes) | 1: Respiratory electron transport 148.50, Ketone body metabolism 70.09, Cap-dependent translation initiation 50.22, Nef-mediated CD8 downregulation 42.88, Cytoplasmic ribosomal proteins 39.30 2: Respiratory electron transport 128.38, Eukaryotic translation elongation 57.24, Nef mediated CD8 down-regulation 42.88, Orexin and neuropeptides FF and QRFP bind to their respective receptors 35.11, Ketone body metabolism 35.11 3: Validated nuclear estrogen receptor alpha network 15.80, JNK signaling in the CD4+ TCR pathway 14.61, PDGF receptor signaling network 11.03, Alpha-synuclein signaling 9.45, Visual signal transduction: Cones 6.22 | respiratory electron transport chain 114.12, SRP-dependent cotranslational protein targeting to membrane 57.24, negative regulation of peptide 56.44, negative regulation of membrane potential 53.81, negative regulation of necroptotic process 53.81 | 1: EKLF 13.21, THRA 7.02, GATA1 6.80 2: HCFC1 6.41 |
| Pos3 (272 genes) | 1: Adrenoceptors 82.98, Phospholipase C delta-1 interactions in phospholipid-associated cell signalling 39.93, Serotonin and melatonin biosynthesis 39.93, FGFR1b ligand binding and activation 39.93, Pyrimidine biosynthesis 31.12 2: Adrenoceptors 82.98, Free fatty acid receptors 39.93, Arachidonate production from DAG 39.93, Serotonin and melatonin biosynthesis 39.93, FGFR1b ligand binding and activation 31.12 3: Signaling events mediated by the Hedgehog family 22.30, IL4-mediated signaling events 11.14, IL23-mediated signaling events 9.57, Circadian rhythm pathway 8.25, BMP receptor signaling 7.68 | spinal cord dorsal/ventral patterning 365.39, osteoblast development 97.81, positive regulation of catenin import into nucleus 97.81, DNA replication-dependent nucleosome organization 71.54, septin ring assembly 39.82 | 1: FOXP1 17.13, BP1 6.21 2: n.s. |
| Neg1 (7248 genes) | 1: Messenger RNA processing 59.45, Global genomic nucleotide excision repair 25.86, RNA polymerase II C-terminal domain phosphorylation and interaction with capping enzyme 22.43, Visual signal transduction: rods 21.50, Non-coding RNA metabolism 19.92 2: Assembly of the primary cilium 57.92, Processing of intronless pre-mRNAs 42.06, Processing of capped intron-containing Pre-mRNA 37.93, Homologous DNA Pairing and Strand Exchange 33.07, Activation of the phototransduction cascade 30.84 3: Fanconi anemia pathway 21.85, Visual signal transduction: Rods 21.50, ATR signaling pathway 5.94, Regulation of Telomerase 5.36, p38 MAPK signaling pathway 5.30 | mRNA processing 70.46, DNA repair 38.32, ciliary basal body-plasma membrane docking 29.59, DNA-templated transcription, termination 26.90, histone lysine demethylation 22.88 | 1: FOXO3 51.83, YY1 27.21, CREB1 23.28 2: KAT2A 105.06, GABPA 89.64, E2F4 56.05 |
| Neg2 (506 genes) | 1: Signaling by FGFR1 fusion mutants 56.25, Activation of NOXA and translocation to mitochondria 16.74, Polo-like kinase 3 (PLK3) pathway 16.74, Cyclin B2-mediated events 16.74, Tachykinin receptors bind tachykinins 16.74 2: Signaling by cytosolic FGFR1 fusion mutants 61.27, Heme biosynthesis 25.15, Golgi cisternae pericentriolar stack reorganization 17.20, Defective ABCA3 causes pulmonary surfactant metabolism dysfunction type 3 (SMDP3) 16.74, Hyaluronan biosynthesis and export 16.74 3: PLK3 signaling events 16.74, Canonical NF-kappaB pathway 7.46, TNF receptor signaling pathway 5.68, TRAIL signaling pathway 5.22, Signaling mediated by p38-gamma and p38-delta 5.04 | left/right pattern formation 49.64, viral RNA genome replication 21.96, DNA replication-independent nucleosome organization 19.36, mRNA splice site selection 18.87, positive regulation of vascular smooth muscle cell proliferation 17.20 | 1: n.s. 2: n.s. |
| Neg3 (586 genes) | 1: Cytoplasmic ribosomal proteins 195.82; Spliceosomal assembly 176.30; Translation 169.55; Respiratory electron transport, ATP biosynthesis by chemiosmotic coupling, and heat production by uncoupling proteins 137.50; Apoptotic factor-mediated response 104.27 2: Eukaryotic translation initiation 251.77; Mitochondrial translation 208.25; Cytochrome c-mediated apoptotic response 170.73; rRNA processing 135.64; Respiratory electron transport, ATP synthesis by chemiosmotic coupling, and heat production by uncoupling proteins 129.02 3: PLK3 signaling events 13.51, DNA-PK pathway in nonhomologous end joining 10.86, HIV-1 Nef: Negative effector of Fas and TNF-alpha 7.31, C-MYC pathway 5.73 | mitochondrial translation 175.43, mitochondrial electron transport, ubiquinol to cytochrome c 125.37, translation 123.40, rRNA metabolic process 122.84, activation of cysteine-type endopeptidase activity involved in apoptotic process by cytochrome c 104.27 | 1: JARID1A 89.00, ETS1 71.54, EKLF 44.12 2: EP300 54.49, GABPA 48.40, KAT2A 46.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielmeier, C.B.; Roth, S.; Schmitt, S.I.; Boneva, S.K.; Schlecht, A.; Vallon, M.; Tamm, E.R.; Ergün, S.; Neueder, A.; Braunger, B.M. Transcriptional Profiling Identifies Upregulation of Neuroprotective Pathways in Retinitis Pigmentosa. Int. J. Mol. Sci. 2021, 22, 6307. https://doi.org/10.3390/ijms22126307
Bielmeier CB, Roth S, Schmitt SI, Boneva SK, Schlecht A, Vallon M, Tamm ER, Ergün S, Neueder A, Braunger BM. Transcriptional Profiling Identifies Upregulation of Neuroprotective Pathways in Retinitis Pigmentosa. International Journal of Molecular Sciences. 2021; 22(12):6307. https://doi.org/10.3390/ijms22126307
Chicago/Turabian StyleBielmeier, Christina B., Saskia Roth, Sabrina I. Schmitt, Stefaniya K. Boneva, Anja Schlecht, Mario Vallon, Ernst R. Tamm, Süleyman Ergün, Andreas Neueder, and Barbara M. Braunger. 2021. "Transcriptional Profiling Identifies Upregulation of Neuroprotective Pathways in Retinitis Pigmentosa" International Journal of Molecular Sciences 22, no. 12: 6307. https://doi.org/10.3390/ijms22126307
APA StyleBielmeier, C. B., Roth, S., Schmitt, S. I., Boneva, S. K., Schlecht, A., Vallon, M., Tamm, E. R., Ergün, S., Neueder, A., & Braunger, B. M. (2021). Transcriptional Profiling Identifies Upregulation of Neuroprotective Pathways in Retinitis Pigmentosa. International Journal of Molecular Sciences, 22(12), 6307. https://doi.org/10.3390/ijms22126307







