Myositis/Myasthenia after Pembrolizumab in a Bladder Cancer Patient with an Autoimmunity-Associated HLA: Immune–Biological Evaluation and Case Report
Abstract
1. Introduction
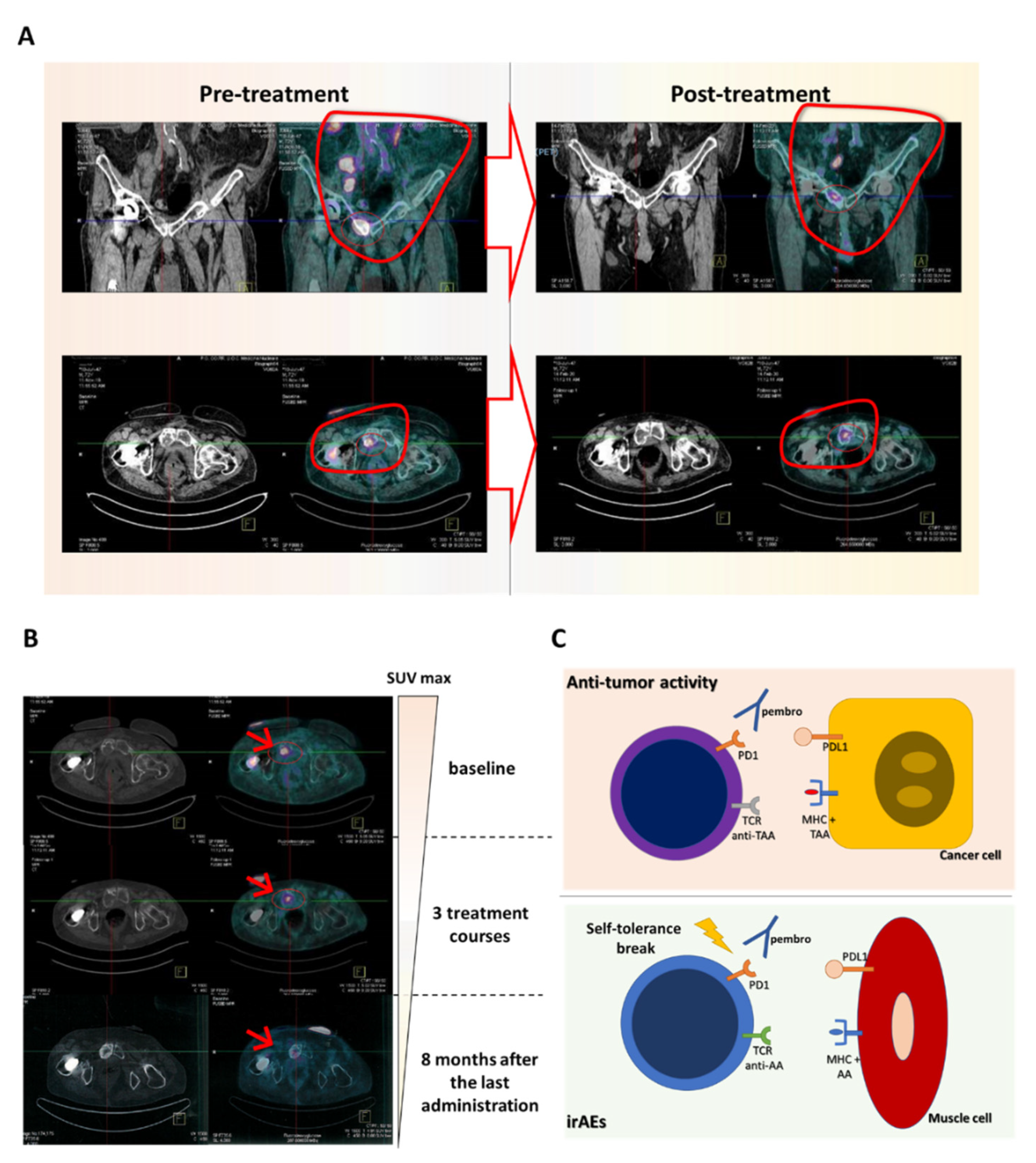
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Xia, L.; Liu, Y.; Wang, Y. PD-1/PD-L1 Blockade Therapy in Advanced Non-Small-Cell Lung Cancer: Current Status and Future Directions. Oncologist 2019, 24, S31–S41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, Z.; Liang, J.; Zhang, F.; Wu, K.; Zhou, C.; Lu, Y.; Wang, X. The efficacy and safety of immunotherapy targeting the PD-1 pathway for advanced urothelial carcinoma: A meta-analysis of published clinical trials. Clin. Transl. Oncol. 2020. [CrossRef]
- Day, D.; Hansen, A.R. Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitors. BioDrugs 2016, 30, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Correale, P.; Tagliaferri, P.; Fioravanti, A.; Del Vecchio, M.T.; Remondo, C.; Montagnani, F.; Rotundo, M.S.; Ginanneschi, C.; Martellucci, I.; Francini, E.; et al. Immunity feedback and clinical outcome in colon cancer patients undergoing chemoimmunotherapy with gemcitabine + FOLFOX followed by subcutaneous granulocyte macrophage colony-stimulating factor and aldesleukin (GOLFIG-1 Trial). Clin. Cancer Res. 2008, 14, 4192–4199. [Google Scholar] [CrossRef] [PubMed]
- Giannicola, R.; D’Arrigo, G.; Botta, C.; Agostino, R.; Del Medico, P.; Falzea, A.C.; Barbieri, V.; Staropoli, N.; Del Giudice, T.; Pastina, P.; et al. Early blood rise in auto-antibodies to nuclear and smooth muscle antigens is predictive of prolonged survival and autoimmunity in metastatic-non-small cell lung cancer patients treated with PD-1 immune-check point blockade by nivolumab. Mol. Clin. Oncol. 2019, 11, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, G.C.; Candido, S.; Falzone, L.; Spandidos, D.A.; Libra, M. Cutaneous melanoma and the immunotherapy revolution (Review). Int. J. Oncol. 2020, 57, 609–618. [Google Scholar] [CrossRef]
- Rodriguez-Cerdeira, C.; Carnero Gregorio, M.; Lopez-Barcenas, A.; Sanchez-Blanco, E.; Sanchez-Blanco, B.; Fabbrocini, G.; Bardhi, B.; Sinani, A.; Guzman, R.A. Advances in Immunotherapy for Melanoma: A Comprehensive Review. Mediat. Inflamm. 2017, 2017, 3264217. [Google Scholar] [CrossRef]
- Maher, V.E.; Fernandes, L.L.; Weinstock, C.; Tang, S.; Agarwal, S.; Brave, M.; Ning, Y.M.; Singh, H.; Suzman, D.; Xu, J.; et al. Analysis of the Association Between Adverse Events and Outcome in Patients Receiving a Programmed Death Protein 1 or Programmed Death Ligand 1 Antibody. J. Clin. Oncol. 2019, 37, 2730–2737. [Google Scholar] [CrossRef]
- Mohn, N.; Beutel, G.; Gutzmer, R.; Ivanyi, P.; Satzger, I.; Skripuletz, T. Neurological Immune Related Adverse Events Associated with Nivolumab, Ipilimumab, and Pembrolizumab Therapy-Review of the Literature and Future Outlook. J. Clin. Med. 2019, 8, 1777. [Google Scholar] [CrossRef]
- Mohn, N.; Suhs, K.W.; Gingele, S.; Angela, Y.; Stangel, M.; Gutzmer, R.; Satzger, I.; Skripuletz, T. Acute progressive neuropathy-myositis-myasthenia-like syndrome associated with immune-checkpoint inhibitor therapy in patients with metastatic melanoma. Melanoma Res. 2019, 29, 435–440. [Google Scholar] [CrossRef]
- Cuzzubbo, S.; Javeri, F.; Tissier, M.; Roumi, A.; Barlog, C.; Doridam, J.; Lebbe, C.; Belin, C.; Ursu, R.; Carpentier, A.F. Neurological adverse events associated with immune checkpoint inhibitors: Review of the literature. Eur. J. Cancer 2017, 73, 1–8. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- Correale, P.; Botta, C.; Martino, E.C.; Ulivieri, C.; Battaglia, G.; Carfagno, T.; Rossetti, M.G.; Fioravanti, A.; Guidelli, G.M.; Cheleschi, S.; et al. Phase Ib study of poly-epitope peptide vaccination to thymidylate synthase (TSPP) and GOLFIG chemo-immunotherapy for treatment of metastatic colorectal cancer patients. Oncoimmunology 2016, 5, e1101205. [Google Scholar] [CrossRef]
- Correale, P.; Saladino, R.E.; Giannarelli, D.; Sergi, A.; Mazzei, M.A.; Bianco, G.; Giannicola, R.; Iuliano, E.; Forte, I.M.; Calandruccio, N.D.; et al. HLA Expression Correlates to the Risk of Immune Checkpoint Inhibitor-Induced Pneumonitis. Cells 2020, 9, 1964. [Google Scholar] [CrossRef]
- Cusi, M.G.; Botta, C.; Pastina, P.; Rossetti, M.G.; Dreassi, E.; Guidelli, G.M.; Fioravanti, A.; Martino, E.C.; Gandolfo, C.; Pagliuchi, M.; et al. Phase I trial of thymidylate synthase poly-epitope peptide (TSPP) vaccine in advanced cancer patients. Cancer Immunol. Immunother. 2015, 64, 1159–1173. [Google Scholar] [CrossRef]
- Nardone, V.; Pastina, P.; Giannicola, R.; Agostino, R.; Croci, S.; Tini, P.; Pirtoli, L.; Giordano, A.; Tagliaferri, P.; Correale, P. How to Increase the Efficacy of Immunotherapy in NSCLC and HNSCC: Role of Radiation Therapy, Chemotherapy, and Other Strategies. Front. Immunol. 2018, 9, 2941. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, L.C.; Gutierrez, A.K.; Bingham, C.O., 3rd; Shah, A.A. Rheumatic and Musculoskeletal Immune-Related Adverse Events Due to Immune Checkpoint Inhibitors: A Systematic Review of the Literature. Arthritis Care Res. (Hoboken) 2017, 69, 1751–1763. [Google Scholar] [CrossRef]
- Kennedy, L.B.; Salama, A.K.S. A Review of Immune-Mediated Adverse Events in Melanoma. Oncol. Ther. 2019, 7, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Zang, X.Y.; Wang, J.C.; Huang, S.S.; Xu, J.; Zhang, P. Diagnosis and Management of Immune Related Adverse Events (irAEs) in Cancer Immunotherapy. Biomed. Pharmacother. 2019, 120, 109437. [Google Scholar] [CrossRef] [PubMed]
- Simeone, E.; Grimaldi, A.M.; Festino, L.; Trojaniello, C.; Vitale, M.G.; Vanella, V.; Palla, M.; Ascierto, P.A. Immunotherapy in metastatic melanoma: A novel scenario of new toxicities and their management. Melanoma Manag. 2019, 6, MMT30. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zeng, L.; Shen, Q.; Zhou, Z.; Mao, Z.; Wang, Q.; Zhang, X.; Li, Y.; Yao, W. Diagnosis and Treatment of Rheumatic Adverse Events Related to Immune Checkpoint Inhibitors. J. Immunol. Res. 2020, 2020, 2640273. [Google Scholar] [CrossRef]
- Zhong, H.; Zhou, J.; Xu, D.; Zeng, X. Rheumatic immune-related adverse events induced by immune checkpoint inhibitors. Asia Pac. J. Clin. Oncol. 2020. [CrossRef] [PubMed]
- Solimando, A.G.; Crudele, L.; Leone, P.; Argentiero, A.; Guarascio, M.; Silvestris, N.; Vacca, A.; Racanelli, V. Immune Checkpoint Inhibitor-Related Myositis: From Biology to Bedside. Int. J. Mol. Sci. 2020, 21, 3054. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Calle, N.; Rodriguez-Otero, P.; Villar, S.; Mejias, L.; Melero, I.; Prosper, F.; Marinello, P.; Paiva, B.; Idoate, M.; San-Miguel, J. Anti-PD1 associated fulminant myocarditis after a single pembrolizumab dose: The role of occult pre-existing autoimmunity. Haematologica 2018, 103, e318–e321. [Google Scholar] [CrossRef]
- Cuce, M.; Gallo Cantafio, M.E.; Siciliano, M.A.; Riillo, C.; Caracciolo, D.; Scionti, F.; Staropoli, N.; Zuccala, V.; Maltese, L.; Di Vito, A.; et al. Trabectedin triggers direct and NK-mediated cytotoxicity in multiple myeloma. J. Hematol. Oncol. 2019, 12, 32. [Google Scholar] [CrossRef]
- Botta, C.; Misso, G.; Martino, E.C.; Pirtoli, L.; Cusi, M.G.; Tassone, P.; Tagliaferri, P.; Caraglia, M.; Correale, P. The route to solve the interplay between inflammation, angiogenesis and anti-cancer immune response. Cell Death Dis. 2016, 7, e2299. [Google Scholar] [CrossRef]
- Botta, C.; Di Martino, M.T.; Ciliberto, D.; Cuce, M.; Correale, P.; Rossi, M.; Tagliaferri, P.; Tassone, P. A gene expression inflammatory signature specifically predicts multiple myeloma evolution and patients survival. Blood Cancer J. 2016, 6, e511. [Google Scholar] [CrossRef] [PubMed]
- Botta, C.; Cuce, M.; Caracciolo, D.; Fiorillo, L.; Tagliaferri, P.; Tassone, P. Immunomodulatory Activity of MicroRNAs: Potential Implications for Multiple Myeloma Treatment. Curr. Cancer Drug Targets 2017, 17, 819–838. [Google Scholar] [CrossRef] [PubMed]
- Botta, C.; Bestoso, E.; Apollinari, S.; Cusi, M.G.; Pastina, P.; Abbruzzese, A.; Sperlongano, P.; Misso, G.; Caraglia, M.; Tassone, P.; et al. Immune-modulating effects of the newest cetuximab-based chemoimmunotherapy regimen in advanced colorectal cancer patients. J. Immunother. 2012, 35, 440–447. [Google Scholar] [CrossRef]
- Botta, C.; Barbieri, V.; Ciliberto, D.; Rossi, A.; Rocco, D.; Addeo, R.; Staropoli, N.; Pastina, P.; Marvaso, G.; Martellucci, I.; et al. Systemic inflammatory status at baseline predicts bevacizumab benefit in advanced non-small cell lung cancer patients. Cancer Biol. Ther. 2013, 14, 469–475. [Google Scholar] [CrossRef]
- Botta, C.; Gulla, A.; Correale, P.; Tagliaferri, P.; Tassone, P. Myeloid-derived suppressor cells in multiple myeloma: Pre-clinical research and translational opportunities. Front. Oncol. 2014, 4, 348. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.; Botta, C.; Zabaleta, A.; Puig, N.; Cedena, M.T.; Goicoechea, I.; Alameda, D.; San Jose-Eneriz, E.; Merino, J.; Rodriguez-Otero, P.; et al. Immunogenomic identification and characterization of granulocytic myeloid-derived suppressor cells in multiple myeloma. Blood 2020, 136, 199–209. [Google Scholar] [CrossRef]
- Sacco, A.; Battaglia, A.M.; Botta, C.; Aversa, I.; Mancuso, S.; Costanzo, F.; Biamonte, F. Iron Metabolism in the Tumor Microenvironment-Implications for Anti-Cancer Immune Response. Cells 2021, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Nerli, S.; Sgourakis, N.G. Structure-based modeling of SARS-CoV-2 peptide/HLA-A02 antigens. bioRxiv 2020. [CrossRef]
- Chinoy, H.; Payne, D.; Poulton, K.V.; Fertig, N.; Betteridge, Z.; Gunawardena, H.; Davidson, J.E.; Oddis, C.V.; McHugh, N.J.; Wedderburn, L.R.; et al. HLA-DPB1 associations differ between DRB1*03 positive anti-Jo-1 and anti-PM-Scl antibody positive idiopathic inflammatory myopathy. Rheumatology 2009, 48, 1213–1217. [Google Scholar] [CrossRef][Green Version]
- Maniaol, A.H.; Elsais, A.; Lorentzen, A.R.; Owe, J.F.; Viken, M.K.; Saether, H.; Flam, S.T.; Brathen, G.; Kampman, M.T.; Midgard, R.; et al. Late onset myasthenia gravis is associated with HLA DRB1*15:01 in the Norwegian population. PLoS ONE 2012, 7, e36603. [Google Scholar] [CrossRef]
- Rothwell, S.; Chinoy, H.; Lamb, J.A.; Miller, F.W.; Rider, L.G.; Wedderburn, L.R.; McHugh, N.J.; Mammen, A.L.; Betteridge, Z.E.; Tansley, S.L.; et al. Focused HLA analysis in Caucasians with myositis identifies significant associations with autoantibody subgroups. Ann. Rheum. Dis. 2019, 78, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.S.; Cardwell, C.R.; McCarron, P.O.; McConville, J. A systematic review of population based epidemiological studies in Myasthenia Gravis. BMC Neurol. 2010, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Osserman, K.E.; Genkins, G. Studies in myasthenia gravis: Review of a twenty-year experience in over 1200 patients. Mt. Sinai J. Med. 1971, 38, 497–537. [Google Scholar]
- Hoch, W.; McConville, J.; Helms, S.; Newsom-Davis, J.; Melms, A.; Vincent, A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat. Med. 2001, 7, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, J.M.; Seybold, M.E.; Lennon, V.A.; Whittingham, S.; Duane, D.D. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology 1976, 26, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chmielowski, B.; Lao, C.D.; Hodi, F.S.; Sharfman, W.; Weber, J.; Suijkerbuijk, K.P.M.; Azevedo, S.; Li, H.; Reshef, D.; et al. Neurologic Serious Adverse Events Associated with Nivolumab Plus Ipilimumab or Nivolumab Alone in Advanced Melanoma, Including a Case Series of Encephalitis. Oncologist 2017, 22, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Safa, H.; Johnson, D.H.; Trinh, V.A.; Rodgers, T.E.; Lin, H.; Suarez-Almazor, M.E.; Fa’ak, F.; Saberian, C.; Yee, C.; Davies, M.A.; et al. Immune checkpoint inhibitor related myasthenia gravis: Single center experience and systematic review of the literature. J. Immunother. Cancer 2019, 7, 319. [Google Scholar] [CrossRef]
| Blood Tests | Baseline | Post-Treatment | Follow-Up |
|---|---|---|---|
| Inflammatory markers | |||
| CRP (mg/L) | 40.7 (#) | 15.5 (#) | 6.59 (#) |
| ESR (mm/h) | 104 (#) | 50 (#) | 24 (#) |
| Cell lysis enzymes | |||
| AST (U/L) | 35 | 445 (#) | 19 |
| ALT (U/L) | 15 | 109 (#) | 7 |
| LDH (U/L) | 154 | 4403 (#) | 177 |
| CK (U/L) | ND | 4403 (#) | 100 |
| CK MB(U/L) | ND | 189 (#) | 9 |
| Troponin I (ng/mL) | ND | 5.42 (#) | <0.012 |
| Auto-antibodies | |||
| ASMA | ND | negative | negative |
| ENA | ND | negative | negative |
| ANA | ND | 1/1280 (#) | 1/160 (#) |
| Anti-peroxidase (U/l) | ND | 94.64 (#) | 125.80 (#) |
| Anti-ChR-abs (U/mL) | ND | 2.8 (#) | 2.68 (#) |
| Anti-MuSK (U/mL) | ND | <0.4 | <0.4 |
| CD4+/CD8+ T cell ratio | 1.50 | 1.1 | ND |
| Activated T cells % (HLA-DR+) | 21 | 27 | ND |
| Activated T cells % (HLA-DR+) |
| Patient’s Characteristics | |
|---|---|
| Contrast-enhanced brain MRI | No CNS metastases |
| Single-fiber EMG and repetitive nerve stimulation test | Within normal ranges |
| Cerebrospinal fluid | Within normal ranges |
| hearth ultrasonography | Within normal ranges |
| HLA haplotype | HLA-A*02/*29 HLA-B*08/*35 HLA-C*04/*07 DRB1*03/*04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botta, C.; Agostino, R.M.; Dattola, V.; Cianci, V.; Calandruccio, N.D.; Bianco, G.; Mafodda, A.; Maisano, R.; Iuliano, E.; Orizzonte, G.; et al. Myositis/Myasthenia after Pembrolizumab in a Bladder Cancer Patient with an Autoimmunity-Associated HLA: Immune–Biological Evaluation and Case Report. Int. J. Mol. Sci. 2021, 22, 6246. https://doi.org/10.3390/ijms22126246
Botta C, Agostino RM, Dattola V, Cianci V, Calandruccio ND, Bianco G, Mafodda A, Maisano R, Iuliano E, Orizzonte G, et al. Myositis/Myasthenia after Pembrolizumab in a Bladder Cancer Patient with an Autoimmunity-Associated HLA: Immune–Biological Evaluation and Case Report. International Journal of Molecular Sciences. 2021; 22(12):6246. https://doi.org/10.3390/ijms22126246
Chicago/Turabian StyleBotta, Cirino, Rita Maria Agostino, Vincenzo Dattola, Vittoria Cianci, Natale Daniele Calandruccio, Giovanna Bianco, Antonino Mafodda, Roberto Maisano, Eleonora Iuliano, Giovanna Orizzonte, and et al. 2021. "Myositis/Myasthenia after Pembrolizumab in a Bladder Cancer Patient with an Autoimmunity-Associated HLA: Immune–Biological Evaluation and Case Report" International Journal of Molecular Sciences 22, no. 12: 6246. https://doi.org/10.3390/ijms22126246
APA StyleBotta, C., Agostino, R. M., Dattola, V., Cianci, V., Calandruccio, N. D., Bianco, G., Mafodda, A., Maisano, R., Iuliano, E., Orizzonte, G., Mazzacuva, D., Falzea, A. C., Saladino, R. E., Giannicola, R., Restifo, G., Aguglia, U., Caraglia, M., & Correale, P. (2021). Myositis/Myasthenia after Pembrolizumab in a Bladder Cancer Patient with an Autoimmunity-Associated HLA: Immune–Biological Evaluation and Case Report. International Journal of Molecular Sciences, 22(12), 6246. https://doi.org/10.3390/ijms22126246








