Role of Chiral Configuration in the Photoinduced Interaction of D- and L-Tryptophan with Optical Isomers of Ketoprofen in Linked Systems
Abstract
1. Introduction
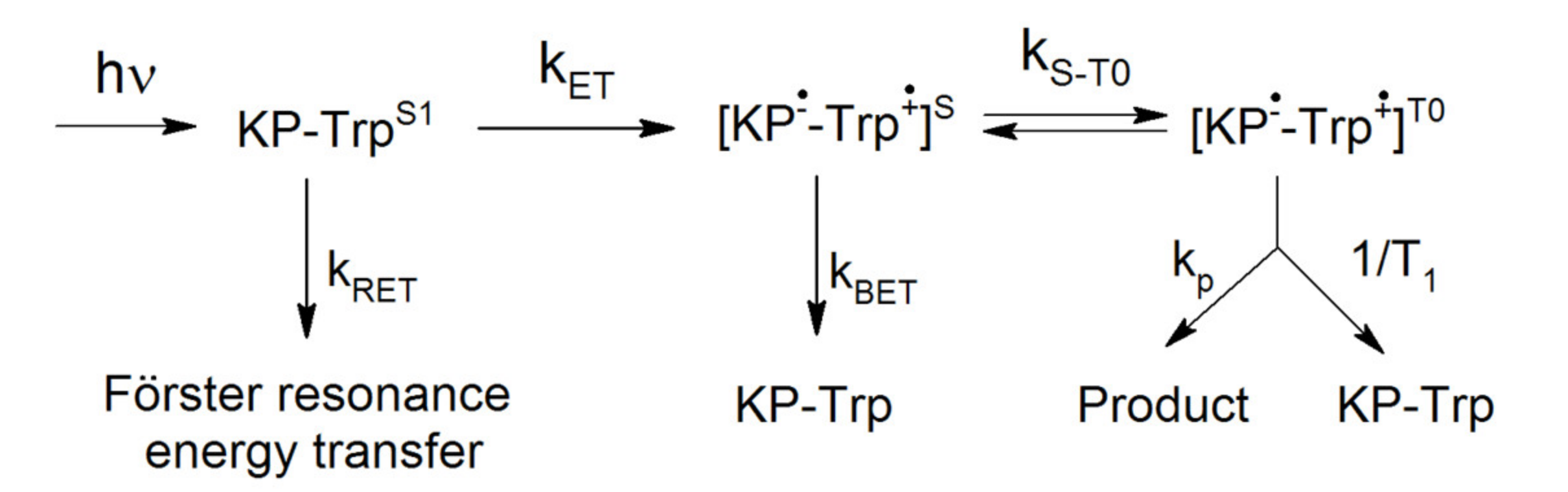
2. Results and Discussions
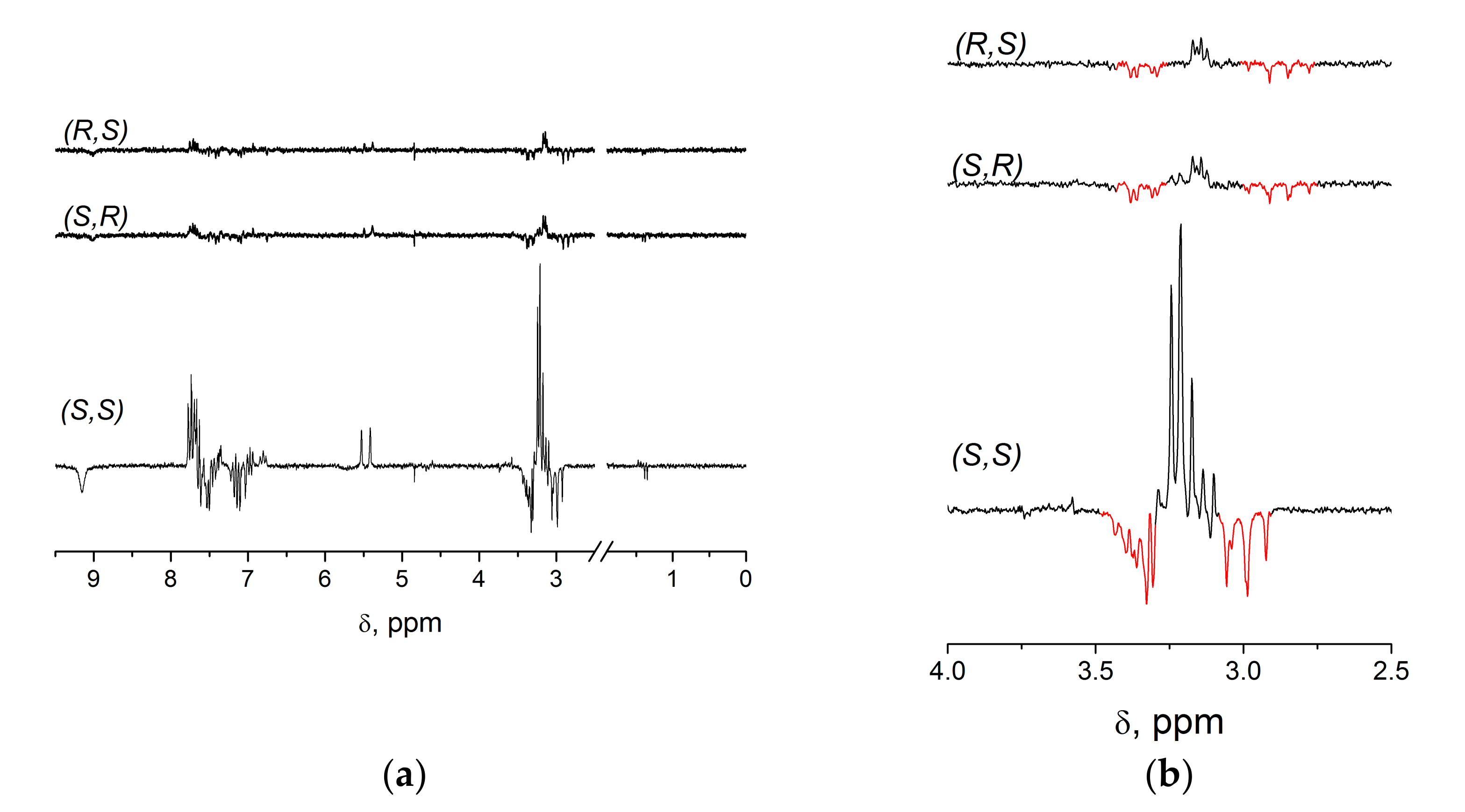
2.1. NMR and CIDNP Study
2.2. Electron Dipole–Dipole Interaction as a Factor of the Chiral Centers’ Influence on CIDNP (Theoretical Part)
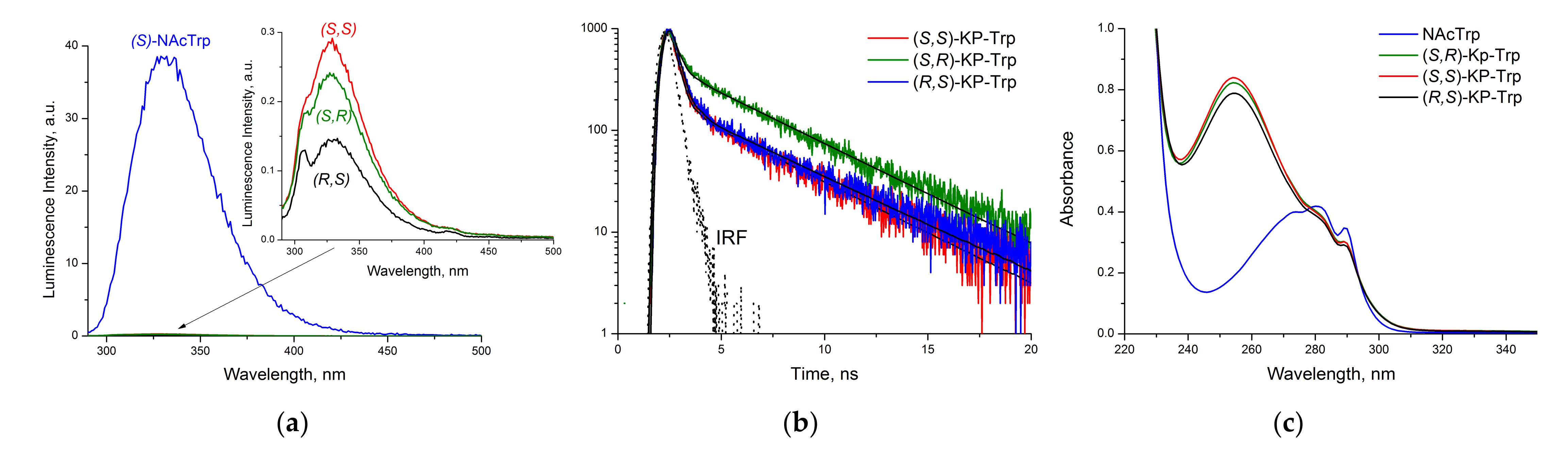
2.3. Fluorescence Measurement
2.4. Modern Concepts of the Mutual Influence of Chiral Centers
3. Materials and Methods
3.1. Synthesis
3.2. Optical Spectroscopy
3.3. NMR Measurements
3.4. Pseudo Steady-State Photo-CIDNP (PSS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tverdislov, V.A.; Yakovenko, L.V.; Zhavoronkov, A.A. Chirality as a problem of biochemical physics. Russ. J. Gen. Chem. 2007, 77, 1994–2005. [Google Scholar] [CrossRef]
- Zerze, G.H.; Stillinger, F.H.; Debenedetti, P.G. Effect of heterochiral inversions on the structure of a β-hairpin peptide. Proteins 2019, 87, 569–578. [Google Scholar] [CrossRef]
- Raskatov, J.A.; Teplow, D.B. Using chirality to probe the conformational dynamics and assembly of intrinsically disordered amyloid proteins. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, B.; Symoens, S.; Vanderheyden, S.; Engelborghs, Y.; Joliot, A.; Prochiantz, A.; Vandekerckhove, J.; Rosseneu, M.; Vanloo, B. Tryptophan fluorescence study of the interaction of penetratin peptides with model membranes. JBIC J. Biol. Inorg. Chem. 2002, 269, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Khramtsova, E.A.; Sosnovsky, D.; Ageeva, A.; Nuin, E.; Marin, M.L.; Purtov, P.A.; Borisevich, S.S.; Khursan, S.; Roth, H.D.; Miranda, M.A.; et al. Impact of chirality on the photoinduced charge transfer in linked systems containing naproxen enantiomers. Phys. Chem. Chem. Phys. 2016, 18, 12733–12741. [Google Scholar] [CrossRef] [PubMed]
- Khramtsova, E.; Ageeva, A.; Stepanov, A.; Plyusnin, V.; Leshina, T. Photoinduced Electron Transfer in Dyads with (R)-/(S)-Naproxen and (S)-Tryptophan. Z. Phys. Chem. 2017, 231, 609–623. [Google Scholar] [CrossRef]
- Magin, I.M.; Polyakov, N.E.; Kruppa, A.I.; Purtov, P.A.; Leshina, T.V.; Kiryutin, A.; Miranda, M.A.; Nuin, E.; Marin, M.L. Low field photo-CIDNP in the intramolecular electron transfer of naproxen–pyrrolidine dyads. Phys. Chem. Chem. Phys. 2015, 18, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Ageeva, A.A.; Khramtsova, E.A.; Magin, I.M.; Purtov, P.A.; Miranda, M.A.; Leshina, T.V. Role of association in chiral catalysis: From asymmetric synthesis to spin selectivity. Chem. Eur. J. 2018, 24, 18587–18600. [Google Scholar] [CrossRef] [PubMed]
- Ageeva, A.A.; Khramtsova, E.A.; Magin, I.M.; Rychkov, D.A.; Purtov, P.A.; Miranda, M.A.; Leshina, T.V. Spin Selectivity in Chiral Linked Systems. Chem. A Eur. J. 2018, 24, 3882–3892. [Google Scholar] [CrossRef] [PubMed]
- Ageeva, A.A.; Khramtsova, E.A.; Plyusnin, V.F.; Miranda, M.A.; Leshina, T.V. Physico chemical Approach to the Study of Naproxen Enantiomers Activity Difference. In Naproxen Chemistry, Clinical Aspects and Effects; Corner, J., Ed.; Nova: New York, NY, USA, 2018; Volume 2, pp. 35–67. [Google Scholar]
- Ageeva, A.A.; Babenko, S.V.; Polyakov, N.E.; Leshina, T.V. NMR investigation of photoinduced chiral inversion in (R)/(S)-naproxen–(S)-tryptophan linked system. Mendeleev Commun. 2019, 29, 260–262. [Google Scholar] [CrossRef]
- Polyakov, N.; Ageeva, A.; Kiryutin, A.; Timoshnikov, V.; Magin, I.; Babenko, S.; Kuznetsova, P.; Kruppa, A.; Purtov, P.; Stepanov, A.; et al. Spin effects as a tool to study photoinduced processes in (S/R)-ketoprofen-(S)-N-methylpyrrolidine dyads. J. Chem. Phys. 2019, 151, 245101. [Google Scholar] [CrossRef] [PubMed]
- Neshchadin, D.; Palumbo, F.; Sinicropi, M.S.; Andreu, I.; Gescheidt, G.; Miranda, M.A. Topological control in radical reactions of cholesterol in model dyads. Chem. Sci. 2013, 4, 1608–1614. [Google Scholar] [CrossRef]
- Ageeva, A.A.; Babenko, S.V.; Magin, I.M.; Plyusnin, V.F.; Kuznetsova, P.S.; Stepanov, A.A.; Vasilevsky, S.F.; Polyakov, N.E.; Doktorov, A.B.; Leshina, T.V. Stereoselectivity of Electron and Energy Transfer in the Quenching of (S/R)-Ketoprofen-(S)-Tryptophan Dyad Excited State. Int. J. Mol. Sci. 2020, 21, 5370. [Google Scholar] [CrossRef] [PubMed]
- Vivian, J.T.; Callis, P. Mechanisms of Tryptophan Fluorescence Shifts in Proteins. Biophys. J. 2001, 80, 2093–2109. [Google Scholar] [CrossRef]
- Gorokhov, V.V.; Knox, P.P.; Korvatovskiy, B.N.; Seifullina, N.K.; Goryachev, S.N.; Paschenko, V.Z. Temperature dependence of tryptophan fluorescence lifetime in aqueous glycerol and trehalose solutions. Biochemistry (Moscow) 2017, 82, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Ghisaidoobe, A.B.T.; Chung, S.J. Intrinsic Tryptophan Fluorescence in the Detection and Analysis of Proteins: A Focus on Förster Resonance Energy Transfer Techniques. Int. J. Mol. Sci. 2014, 15, 22518–22538. [Google Scholar] [CrossRef] [PubMed]
- Guida, W.C.; Daniel, K.G. The Significance of Chirality in Drug Design and Development. Curr. Top. Med. Chem. 2011, 11, 760–770. [Google Scholar] [CrossRef]
- Ignarro, L.J. Different Pharmacological Properties of Two Enantiomers in a Unique β-Blocker, Nebivolol. Cardiovasc. Ther. 2008, 26, 115–134. [Google Scholar] [CrossRef]

| K(CH2) | K(NH) | |
|---|---|---|
| (S,S) | 1.91 | 2.08 |
| (S,R), (R,S) | 0.195 | 0.255 |
| KSS/KSR,RS | 9.8 | 8.2 |
| Average | 9.0 | |
| (R,S)-KP-Trp | (S,R)-KP-Trp | (S,S)-KP-Trp | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ε | τ1, ns | A1, % | τ2, ns | A2, % | τ1, ns | A1, % | τ2, ns | A2, % | τ1, ns | A1, % | τ2, ns | A2, % |
| 36.8 | 0.2 ± 0.1 | 49 | 4.5 ± 0.5 | 51 | 0.1 ± 0.1 | 27 | 4.4 ± 0.4 | 73 | 0.1 ± 0.1 | 50 | 4.3 ± 0.4 | 50 |
| 30.25 | 0.2 ± 0.1 | 55 | 4.6 ± 0.5 | 45 | 0.2 ± 0.1 | 27 | 4.3 ± 0.4 | 73 | 0.1 ± 0.1 | 44 | 4.1 ± 0.4 | 56 |
| 23.85 | 0.1 ± 0.1 | 57 | 4.4 ± 0.5 | 43 | 0.2 ± 0.1 | 27 | 4.2 ± 0.4 | 73 | 0.1 ± 0.1 | 49 | 4.2 ± 0.4 | 51 |
| 17.7 | 0.2 ± 0.1 | 57 | 4.4 ± 0.4 | 43 | 0.2 ± 0.1 | 26 | 4.1 ± 0.4 | 74 | 0.1 ± 0.1 | 51 | 4.1 ± 0.4 | 49 |
| 11.9 | 0.2 ± 0.1 | 56 | 4.4 ± 0.4 | 44 | 0.2 ± 0.1 | 24 | 4.0 ± 0.4 | 76 | 0.2 ± 0.1 | 45 | 4.0 ± 0.4 | 55 |
| 6.19 | 0.2 ± 0.1 | 51 | 4.1 ± 0.4 | 49 | 0.3 ± 0.1 | 26 | 3.7 ± 0.4 | 74 | 0.2 ± 0.1 | 50 | 3.9 ± 0.4 | 50 |
| (R,S)-KP-Trp | (S,R)-KP-Trp | (S,S)-KP-Trp | ||||
|---|---|---|---|---|---|---|
| ε | φfl | λem, nm | φfl | λem, nm | φfl | λem, nm |
| 36.8 | 0.0027 ± 0.0003 | 327 | 0.0043 ± 0.0004 | 328 | 0.0052 ± 0.0005 | 328 |
| 30.25 | 0.0025 ± 0.0003 | 325 | 0.0049 ± 0.0005 | 328 | 0.0061 ± 0.0006 | 328 |
| 23.85 | 0.0025 ± 0.0003 | 325 | 0.0049 ± 0.0005 | 328 | 0.0055 ± 0.0006 | 328 |
| 17.7 | 0.0026 ± 0.0003 | 326 | 0.0049 ± 0.0005 | 328 | 0.0052 ± 0.0005 | 328 |
| 11.9 | 0.0026 ± 0.0003 | 326 | 0.0054 ± 0.0005 | 326 | 0.0058 ± 0.0006 | 327 |
| 6.19 | 0.0026 ± 0.0003 | 325 | 0.0049 ± 0.0005 | 326 | 0.0052 ± 0.0005 | 325 |
| kET | kRET | |
|---|---|---|
| (R,S)/(S,R) | 1 | 1.6 |
| (S,S)/(R,S) | (4.7 ± 3.4) * | 0.5 |
| (R,S) | (S,R) | (S,S) | |
|---|---|---|---|
| Distance, Å | 4.11 | 4.19 | 4.13 |
| Interplane angle, ° | 32.3 | 56.3 | 30.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ageeva, A.A.; Magin, I.M.; Doktorov, A.B.; Plyusnin, V.F.; Kuznetsova, P.S.; Stepanov, A.A.; Alekseev, A.A.; Polyakov, N.E.; Leshina, T.V. Role of Chiral Configuration in the Photoinduced Interaction of D- and L-Tryptophan with Optical Isomers of Ketoprofen in Linked Systems. Int. J. Mol. Sci. 2021, 22, 6198. https://doi.org/10.3390/ijms22126198
Ageeva AA, Magin IM, Doktorov AB, Plyusnin VF, Kuznetsova PS, Stepanov AA, Alekseev AA, Polyakov NE, Leshina TV. Role of Chiral Configuration in the Photoinduced Interaction of D- and L-Tryptophan with Optical Isomers of Ketoprofen in Linked Systems. International Journal of Molecular Sciences. 2021; 22(12):6198. https://doi.org/10.3390/ijms22126198
Chicago/Turabian StyleAgeeva, Aleksandra A., Ilya M. Magin, Alexander B. Doktorov, Victor F. Plyusnin, Polina S. Kuznetsova, Alexander A. Stepanov, Alexander A. Alekseev, Nikolay E. Polyakov, and Tatyana V. Leshina. 2021. "Role of Chiral Configuration in the Photoinduced Interaction of D- and L-Tryptophan with Optical Isomers of Ketoprofen in Linked Systems" International Journal of Molecular Sciences 22, no. 12: 6198. https://doi.org/10.3390/ijms22126198
APA StyleAgeeva, A. A., Magin, I. M., Doktorov, A. B., Plyusnin, V. F., Kuznetsova, P. S., Stepanov, A. A., Alekseev, A. A., Polyakov, N. E., & Leshina, T. V. (2021). Role of Chiral Configuration in the Photoinduced Interaction of D- and L-Tryptophan with Optical Isomers of Ketoprofen in Linked Systems. International Journal of Molecular Sciences, 22(12), 6198. https://doi.org/10.3390/ijms22126198









