Neurobiology of Cancer: Introduction of New Drugs in the Treatment and Prevention of Cancer
Abstract
1. Introduction
2. Propranolol
2.1. Propranolol and Cancer Incidence
2.2. Propranolol and Cancer Progression
2.3. Propranolol and Efficiency of Conventional Anti-Cancer Treatment
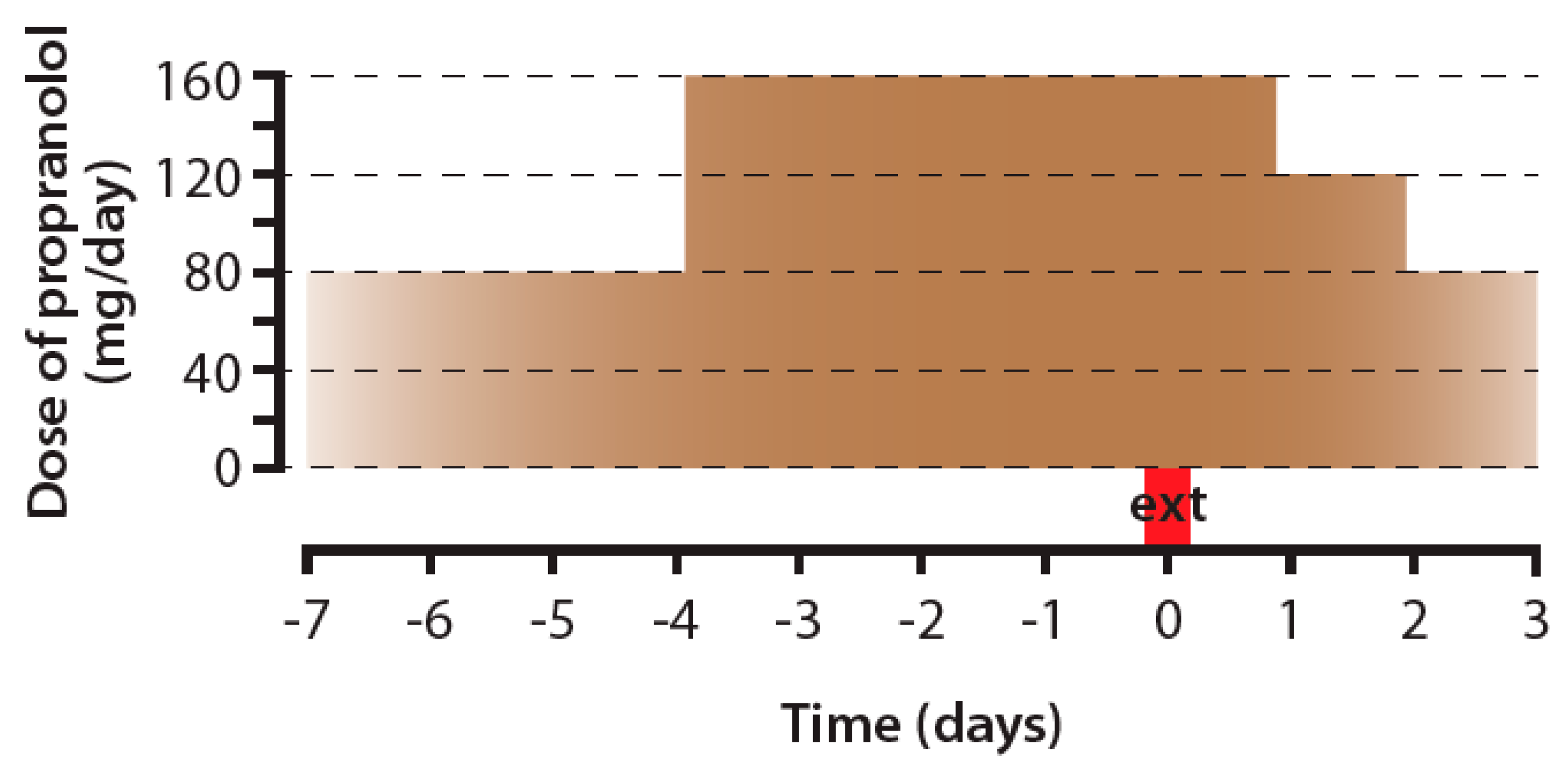
2.4. The Issues Related to Propranolol Usage in Oncology
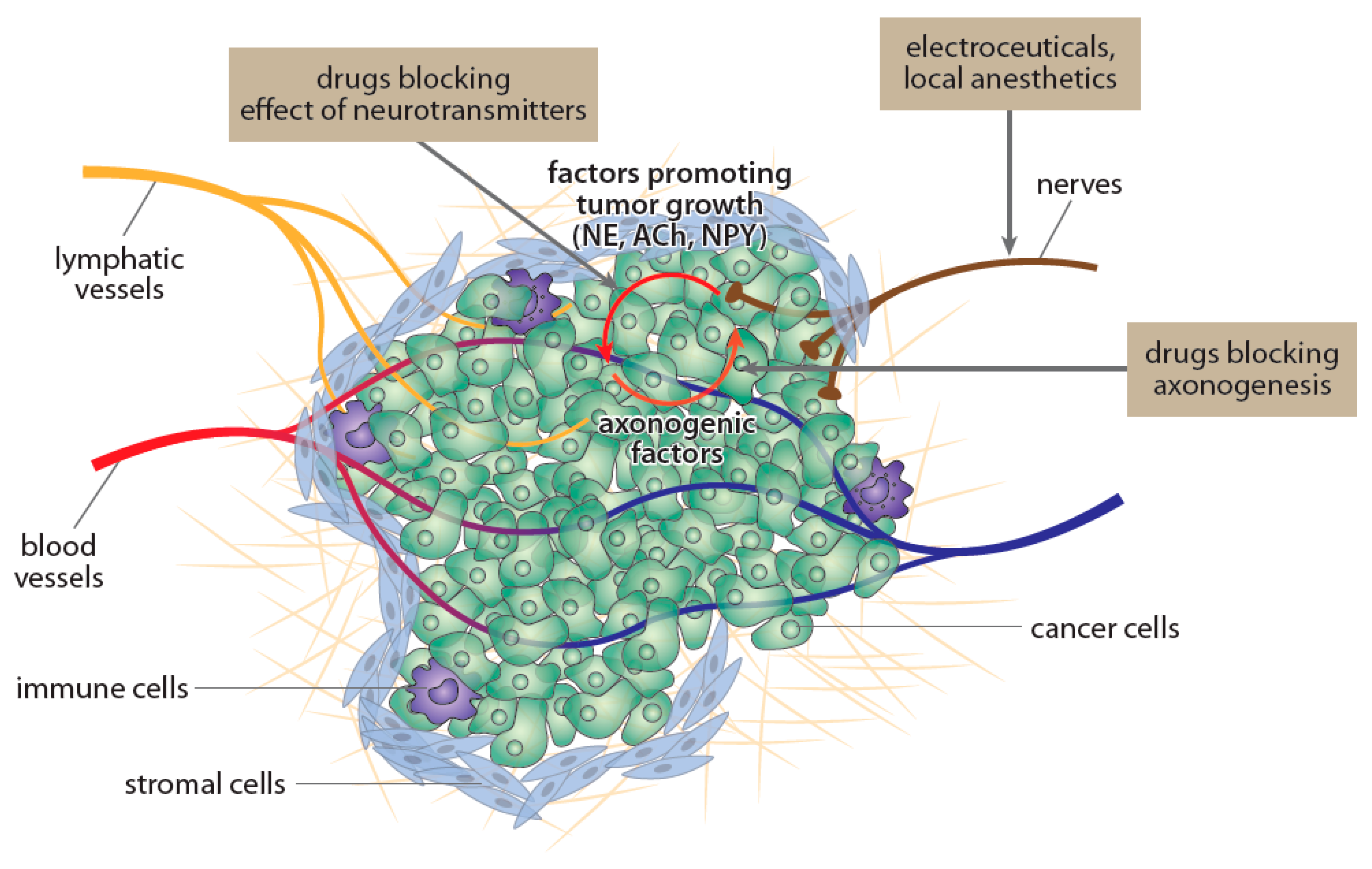
3. Drugs Reducing Density or Activity of Nerves Innervating Cancer Tissue
3.1. Drugs Reducing Nerve Growth Factor-Related Signaling
3.2. Botulotoxin
3.3. Electroceuticals
3.4. Local Anesthetics
4. Drugs Interfering with Cancer Effects on the Brain
4.1. Aspirin
4.2. Metformin
5. Conclusions
Funding
Conflicts of Interest
References
- Mravec, B.; Gidron, Y.; Hulin, I. Neurobiology of cancer: Interactions between nervous, endocrine and immune systems as a base for monitoring and modulating the tumorigenesis by the brain. Semin. Cancer Biol. 2008, 18, 150–163. [Google Scholar] [CrossRef]
- Cole, S.W.; Sood, A.K. Molecular pathways: Beta-adrenergic signaling in cancer. Clin. Cancer Res. 2012, 18, 1201–1206. [Google Scholar] [CrossRef]
- Cole, S.W.; Nagaraja, A.S.; Lutgendorf, S.K.; Green, P.A.; Sood, A.K. Sympathetic nervous system regulation of the tumour microenvironment. Nat. Rev. Cancer 2015, 15, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef]
- Alexander, S.P.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Marrion, N.V.; Peters, J.A.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; Sharman, J.L.; et al. THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: G protein-coupled receptors. Br. J. Pharm. 2017, 174 (Suppl. S1), S17–S129. [Google Scholar] [CrossRef]
- Mravec, B.; Horvathova, L.; Hunakova, L. Neurobiology of cancer: The role of β-adrenergic receptor signaling in various tumor environments. Int. J. Mol. Sci. 2020, 21, 7958. [Google Scholar] [CrossRef] [PubMed]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef]
- Allen, J.K.; Armaiz-Pena, G.N.; Nagaraja, A.S.; Sadaoui, N.C.; Ortiz, T.; Dood, R.; Ozcan, M.; Herder, D.M.; Haemmerle, M.; Gharpure, K.M.; et al. Sustained Adrenergic Signaling Promotes Intratumoral Innervation through BDNF Induction. Cancer Res. 2018, 78, 3233–3242. [Google Scholar] [CrossRef] [PubMed]
- Calvani, M.; Pelon, F.; Comito, G.; Taddei, M.L.; Moretti, S.; Innocenti, S.; Nassini, R.; Gerlini, G.; Borgognoni, L.; Bambi, F.; et al. Norepinephrine promotes tumor microenvironment reactivity through beta3-adrenoreceptors during melanoma progression. Oncotarget 2015, 6, 4615–4632. [Google Scholar] [CrossRef] [PubMed]
- Lamkin, D.M.; Sung, H.Y.; Yang, G.S.; David, J.M.; Ma, J.C.; Cole, S.W.; Sloan, E.K. alpha2-Adrenergic blockade mimics the enhancing effect of chronic stress on breast cancer progression. Psychoneuroendocrinology 2015, 51, 262–270. [Google Scholar] [CrossRef]
- Calvani, M.; Bruno, G.; Dabraio, A.; Subbiani, A.; Bianchini, F.; Fontani, F.; Casazza, G.; Vignoli, M.; De Logu, F.; Frenos, S.; et al. beta3-Adrenoreceptor Blockade Induces Stem Cells Differentiation in Melanoma Microenvironment. Int. J. Mol. Sci. 2020, 21, 1420. [Google Scholar] [CrossRef]
- Florent, R.; Poulain, L.; N’Diaye, M. Drug Repositioning of the alpha1-Adrenergic Receptor Antagonist Naftopidil: A Potential New Anti-Cancer Drug? Int. J. Mol. Sci. 2020, 21, 5339. [Google Scholar] [CrossRef]
- Fitzgerald, P.J. Norepinephrine release may play a critical role in the Warburg effect: An integrative model of tumorigenesis. Neoplasma 2020, 67, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Reeder, A.; Attar, M.; Nazario, L.; Bathula, C.; Zhang, A.; Hochbaum, D.; Roy, E.; Cooper, K.L.; Oesterreich, S.; Davidson, N.E.; et al. Stress hormones reduce the efficacy of paclitaxel in triple negative breast cancer through induction of DNA damage. Br. J. Cancer 2015, 112, 1461–1470. [Google Scholar] [CrossRef]
- Hara, M.R.; Kovacs, J.J.; Whalen, E.J.; Rajagopal, S.; Strachan, R.T.; Grant, W.; Towers, A.J.; Williams, B.; Lam, C.M.; Xiao, K.; et al. A stress response pathway regulates DNA damage through beta2-adrenoreceptors and beta-arrestin-1. Nature 2011, 477, 349–353. [Google Scholar] [CrossRef]
- Hara, M.R.; Sachs, B.D.; Caron, M.G.; Lefkowitz, R.J. Pharmacological blockade of a beta(2)AR-beta-arrestin-1 signaling cascade prevents the accumulation of DNA damage in a behavioral stress model. Cell Cycle 2013, 12, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Ding, X.P.; An, S.M.; Tang, Y.B.; Yang, X.J.; Teng, L.; Zhang, C.; Shen, Y.; Chen, H.Z.; Zhu, L. Adrenergic DNA damage of embryonic pluripotent cells via beta2 receptor signalling. Sci. Rep. 2015, 5, 15950. [Google Scholar] [CrossRef]
- Armaiz-Pena, G.N.; Allen, J.K.; Cruz, A.; Stone, R.L.; Nick, A.M.; Lin, Y.G.; Han, L.Y.; Mangala, L.S.; Villares, G.J.; Vivas-Mejia, P.; et al. Src activation by beta-adrenoreceptors is a key switch for tumour metastasis. Nat. Commun 2013, 4, 1403. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Liu, D.; Duan, H.; Qian, L.; Wang, L.; Niu, L.; Zhang, H.; Yong, Z.; Gong, Z.; Song, L.; et al. The beta2-adrenergic receptor and Her2 comprise a positive feedback loop in human breast cancer cells. Breast Cancer Res. Treat. 2011, 125, 351–362. [Google Scholar] [CrossRef]
- Gu, L.; Lau, S.K.; Loera, S.; Somlo, G.; Kane, S.E. Protein kinase A activation confers resistance to trastuzumab in human breast cancer cell lines. Clin. Cancer Res. 2009, 15, 7196–7206. [Google Scholar] [CrossRef] [PubMed]
- Dimitroglou, E.; Zafiropoulou, M.; Messini-Nikolaki, N.; Doudounakis, S.; Tsilimigaki, S.; Piperakis, S.M. DNA damage in a human population affected by chronic psychogenic stress. Int. J. Hyg. Environ. Health 2003, 206, 39–44. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, C.R.; Bucsek, M.J.; Qiao, G.; Chen, M.; Evans, L.; Greenberg, D.J.; Uccello, T.P.; Battaglia, N.G.; Hylander, B.L.; Singh, A.K.; et al. Adrenergic Receptor Signaling Regulates the Response of Tumors to Ionizing Radiation. Radiat. Res. 2019, 191, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Renz, B.W.; Takahashi, R.; Tanaka, T.; Macchini, M.; Hayakawa, Y.; Dantes, Z.; Maurer, H.C.; Chen, X.; Jiang, Z.; Westphalen, C.B.; et al. beta2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer. Cancer Cell 2018, 33, 75–90 e77. [Google Scholar] [CrossRef] [PubMed]
- Huan, H.B.; Wen, X.D.; Chen, X.J.; Wu, L.; Wu, L.L.; Zhang, L.; Yang, D.P.; Zhang, X.; Bie, P.; Qian, C.; et al. Sympathetic nervous system promotes hepatocarcinogenesis by modulating inflammation through activation of alpha1-adrenergic receptors of Kupffer cells. Brain. Behav. Immun. 2017, 59, 118–134. [Google Scholar] [CrossRef]
- Schuller, H.M.; Cole, B. Regulation of cell proliferation by beta-adrenergic receptors in a human lung adenocarcinoma cell line. Carcinogenesis 1989, 10, 1753–1755. [Google Scholar] [CrossRef]
- Huang, X.Y.; Wang, H.C.; Yuan, Z.; Huang, J.; Zheng, Q. Norepinephrine stimulates pancreatic cancer cell proliferation, migration and invasion via beta-adrenergic receptor-dependent activation of P38/MAPK pathway. Hepatogastroenterology 2012, 59, 889–893. [Google Scholar]
- Lackovicova, L.; Banovska, L.; Bundzikova, J.; Janega, P.; Bizik, J.; Kiss, A.; Mravec, B. Chemical sympathectomy suppresses fibrosarcoma development and improves survival of tumor-bearing rats. Neoplasma 2011, 58, 424–429. [Google Scholar] [CrossRef]
- Lucido, C.T.; Callejas-Valera, J.L.; Colbert, P.L.; Vermeer, D.W.; Miskimins, W.K.; Spanos, W.C.; Vermeer, P.D. beta2-Adrenergic receptor modulates mitochondrial metabolism and disease progression in recurrent/metastatic HPV(+) HNSCC. Oncogenesis 2018, 7, 81. [Google Scholar] [CrossRef]
- Park, S.Y.; Kang, J.H.; Jeong, K.J.; Lee, J.; Han, J.W.; Choi, W.S.; Kim, Y.K.; Kang, J.; Park, C.G.; Lee, H.Y. Norepinephrine induces VEGF expression and angiogenesis by a hypoxia-inducible factor-1alpha protein-dependent mechanism. Int. J. Cancer 2011, 128, 2306–2316. [Google Scholar] [CrossRef]
- Yang, E.V.; Kim, S.J.; Donovan, E.L.; Chen, M.; Gross, A.C.; Webster Marketon, J.I.; Barsky, S.H.; Glaser, R. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: Implications for stress-related enhancement of tumor progression. Brain. Behav. Immun. 2009, 23, 267–275. [Google Scholar] [CrossRef]
- Hulsurkar, M.; Li, Z.; Zhang, Y.; Li, X.; Zheng, D.; Li, W. Beta-adrenergic signaling promotes tumor angiogenesis and prostate cancer progression through HDAC2-mediated suppression of thrombospondin-1. Oncogene 2017, 36, 1525–1536. [Google Scholar] [CrossRef]
- Yang, E.V.; Sood, A.K.; Chen, M.; Li, Y.; Eubank, T.D.; Marsh, C.B.; Jewell, S.; Flavahan, N.A.; Morrison, C.; Yeh, P.E.; et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006, 66, 10357–10364. [Google Scholar] [CrossRef]
- Le, C.P.; Nowell, C.J.; Kim-Fuchs, C.; Botteri, E.; Hiller, J.G.; Ismail, H.; Pimentel, M.A.; Chai, M.G.; Karnezis, T.; Rotmensz, N.; et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat. Commun. 2016, 7, 10634. [Google Scholar] [CrossRef]
- Kim, T.H.; Gill, N.K.; Nyberg, K.D.; Nguyen, A.V.; Hohlbauch, S.V.; Geisse, N.A.; Nowell, C.J.; Sloan, E.K.; Rowat, A.C. Cancer cells become less deformable and more invasive with activation of beta-adrenergic signaling. J. Cell Sci. 2016, 129, 4563–4575. [Google Scholar]
- Masur, K.; Niggemann, B.; Zanker, K.S.; Entschladen, F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. 2001, 61, 2866–2869. [Google Scholar] [PubMed]
- Iseri, O.D.; Sahin, F.I.; Terzi, Y.K.; Yurtcu, E.; Erdem, S.R.; Sarialioglu, F. beta-Adrenoreceptor antagonists reduce cancer cell proliferation, invasion, and migration. Pharm. Biol. 2014, 52, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Sloan, E.K.; Priceman, S.J.; Cox, B.F.; Yu, S.; Pimentel, M.A.; Tangkanangnukul, V.; Arevalo, J.M.; Morizono, K.; Karanikolas, B.D.; Wu, L.; et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010, 70, 7042–7052. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; He, Z.; Yin, K.; Li, B.; Zhang, L.; Xu, Z. Chronic stress promotes gastric cancer progression and metastasis: An essential role for ADRB2. Cell Death Dis. 2019, 10, 788. [Google Scholar] [CrossRef] [PubMed]
- Rivero, E.M.; Pinero, C.P.; Gargiulo, L.; Entschladen, F.; Zanker, K.; Bruzzone, A.; Luthy, I.A. The beta 2-Adrenergic Agonist Salbutamol Inhibits Migration, Invasion and Metastasis of the Human Breast Cancer MDA-MB- 231 Cell Line. Curr. Cancer Drug Targets 2017, 17, 756–766. [Google Scholar] [CrossRef]
- Gillis, R.D.; Botteri, E.; Chang, A.; Ziegler, A.I.; Chung, N.C.; Pon, C.K.; Shackleford, D.M.; Andreassen, B.K.; Halls, M.L.; Baker, J.G.; et al. Carvedilol blocks neural regulation of breast cancer progression in vivo and is associated with reduced breast cancer mortality in patients. Eur. J. Cancer 2021, 147, 106–116. [Google Scholar] [CrossRef]
- Benowitz, N. Antihypertensive Agents. In Basic & Clinical Pharmacology, 14th ed.; Katzung, B., Ed.; McGraw-Hill: New York, NY, USA, 2017; pp. 173–193. [Google Scholar]
- Al-Majed, A.A.; Bakheit, A.H.H.; Abdel Aziz, H.A.; Alajmi, F.M.; AlRabiah, H. Propranolol. Profiles Drug Subst. Excip. Relat. Methodol. 2017, 42, 287–338. [Google Scholar]
- Chang, P.Y.; Huang, W.Y.; Lin, C.L.; Huang, T.C.; Wu, Y.Y.; Chen, J.H.; Kao, C.H. Propranolol Reduces Cancer Risk: A Population-Based Cohort Study. Medicine (Baltim. ) 2015, 94, e1097. [Google Scholar] [CrossRef] [PubMed]
- Diaz, E.S.; Karlan, B.Y.; Li, A.J. Impact of beta blockers on epithelial ovarian cancer survival. Gynecol. Oncol. 2012, 127, 375–378. [Google Scholar] [CrossRef]
- Wang, H.M.; Liao, Z.X.; Komaki, R.; Welsh, J.W.; O’Reilly, M.S.; Chang, J.Y.; Zhuang, Y.; Levy, L.B.; Lu, C.; Gomez, D.R. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann. Oncol. 2013, 24, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Grytli, H.H.; Fagerland, M.W.; Fossa, S.D.; Tasken, K.A. Association between use of beta-blockers and prostate cancer-specific survival: A cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur. Urol. 2014, 65, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Powe, D.G.; Voss, M.J.; Zanker, K.S.; Habashy, H.O.; Green, A.R.; Ellis, I.O.; Entschladen, F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 2010, 1, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Lemeshow, S.; Sorensen, H.T.; Phillips, G.; Yang, E.V.; Antonsen, S.; Riis, A.H.; Lesinski, G.B.; Jackson, R.; Glaser, R. beta-Blockers and survival among Danish patients with malignant melanoma: A population-based cohort study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2273–2279. [Google Scholar] [CrossRef]
- Baek, M.H.; Kim, D.Y.; Kim, S.O.; Kim, Y.J.; Park, Y.H. Impact of beta blockers on survival outcomes in ovarian cancer: A nationwide population-based cohort study. J. Gynecol. Oncol. 2018, 29, e82. [Google Scholar] [CrossRef]
- Spera, G.; Fresco, R.; Fung, H.; Dyck, J.R.B.; Pituskin, E.; Paterson, I.; Mackey, J.R. Beta blockers and improved progression-free survival in patients with advanced HER2 negative breast cancer: A retrospective analysis of the ROSE/TRIO-012 study. Ann. Oncol. 2017, 28, 1836–1841. [Google Scholar] [CrossRef] [PubMed]
- Udumyan, R.; Montgomery, S.; Fang, F.; Almroth, H.; Valdimarsdottir, U.; Ekbom, A.; Smedby, K.E.; Fall, K. Beta-Blocker Drug Use and Survival among Patients with Pancreatic Adenocarcinoma. Cancer Res. 2017, 77, 3700–3707. [Google Scholar] [CrossRef] [PubMed]
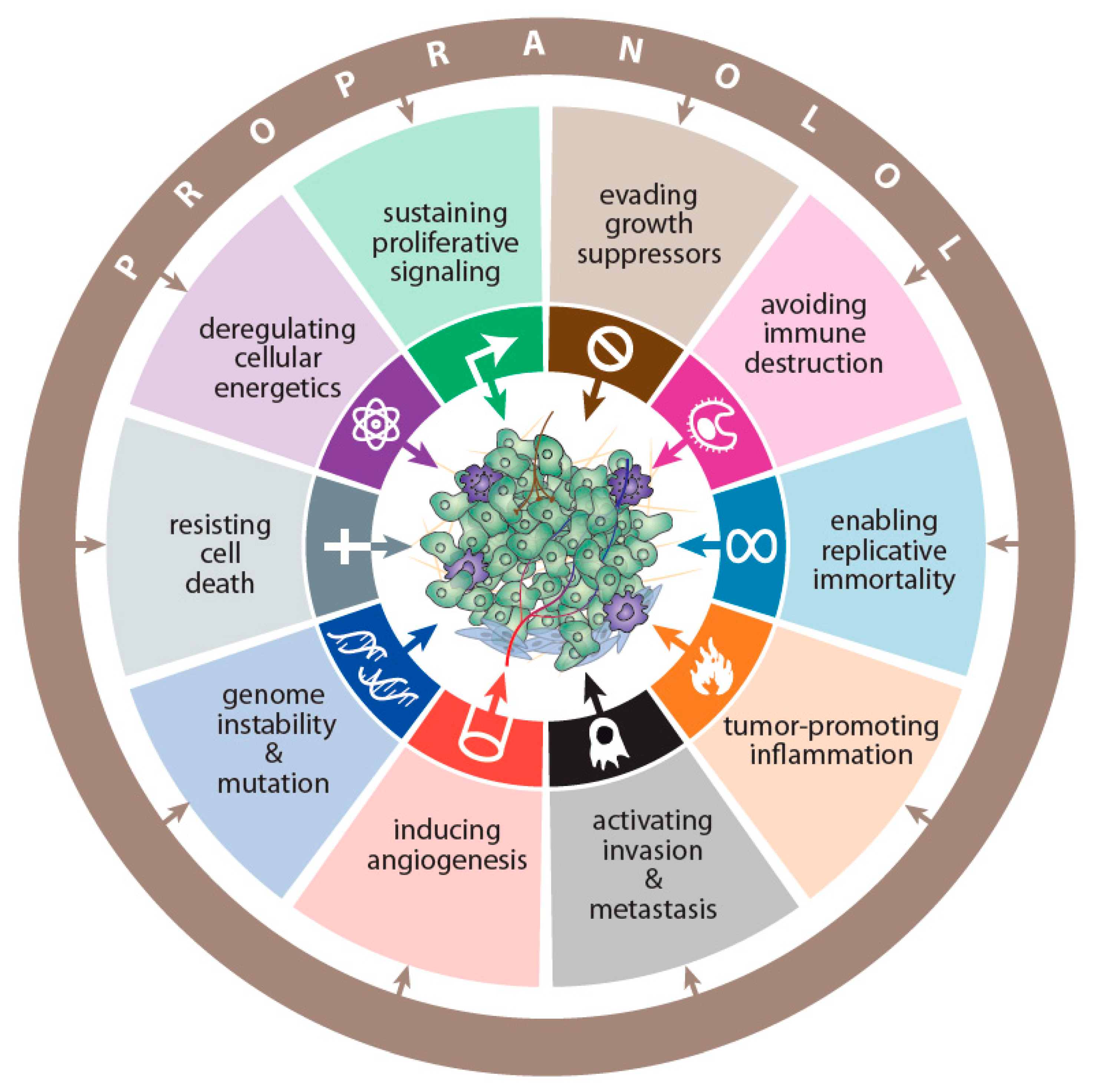
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Cubuk, S.; Uckan, S.; Ozdemir, H.; Taslica, Z.F.; Bacanli, D. The efficiency of propranolol on occurrence and development of 4-nitroquinoline 1-oxide-induced squamous cell carcinoma of the tongue in rats. J. Oral Maxillofac. Pathol. 2020, 24, 400. [Google Scholar] [CrossRef]
- Al-Wadei, H.A.; Al-Wadei, M.H.; Schuller, H.M. Prevention of pancreatic cancer by the beta-blocker propranolol. Anticancer Drugs 2009, 20, 477–482. [Google Scholar] [CrossRef]
- Tibensky, M.; Cernackova, A.; Horvathova, L.; Macejova, D.; Tillinger, A.; Mravec, B. Chronic propranolol treatment moderately attenuated development of MNU-induced mammary carcinoma in female rats. Anticancer Drugs 2021, in press. [Google Scholar]
- Suna, N.; Ozer Etik, D.; Ocal, S.; Selcuk, H. Effect of Propranolol Treatment on the Incidence of Hepatocellular Carcinoma in Patients Waiting for Liver Transplant With Cirrhosis: A Retrospective, Surveillance Study in a Tertiary Center. Exp. Clin. Transpl. 2019, 17, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Herrera, I.; Pascual, S.; Zapater, P.; Carnicer, F.; Bellot, P.; Maria Palazon, J. The use of beta-blockers is associated with a lower risk of developing hepatocellular carcinoma in patients with cirrhosis. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1194–1197. [Google Scholar] [CrossRef] [PubMed]
- Nkontchou, G.; Aout, M.; Mahmoudi, A.; Roulot, D.; Bourcier, V.; Grando-Lemaire, V.; Ganne-Carrie, N.; Trinchet, J.C.; Vicaut, E.; Beaugrand, M. Effect of long-term propranolol treatment on hepatocellular carcinoma incidence in patients with HCV-associated cirrhosis. Cancer Prev. Res. (Phila.) 2012, 5, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Mravec, B.; Tibensky, M.; Horvathova, L. Stress and cancer. Part I: Mechanisms mediating the effect of stressors on cancer. J. Neuroimmunol. 2020, 346, 577311. [Google Scholar] [CrossRef]
- Cardwell, C.R.; Pottegard, A.; Vaes, E.; Garmo, H.; Murray, L.J.; Brown, C.; Vissers, P.A.; O’Rorke, M.; Visvanathan, K.; Cronin-Fenton, D.; et al. Propranolol and survival from breast cancer: A pooled analysis of European breast cancer cohorts. Breast Cancer Res. 2016, 18, 119. [Google Scholar] [CrossRef] [PubMed]
- Siltari, A.; Murtola, T.J.; Talala, K.; Taari, K.; Tammela, T.L.J.; Auvinen, A. Antihypertensive drugs and prostate cancer risk in a Finnish population-based cohort. Scand. J. Urol. 2018, 52, 321–327. [Google Scholar] [CrossRef]
- Huang, T.; Poole, E.M.; Eliassen, A.H.; Okereke, O.I.; Kubzansky, L.D.; Sood, A.K.; Forman, J.P.; Tworoger, S.S. Hypertension, use of antihypertensive medications, and risk of epithelial ovarian cancer. Int. J. Cancer 2016, 139, 291–299. [Google Scholar] [CrossRef]
- Drucker, A.M.; Hollestein, L.; Na, Y.; Weinstock, M.A.; Li, W.Q.; Abdel-Qadir, H.; Chan, A.W. Association between antihypertensive medications and risk of skin cancer in people older than 65 years: A population-based study. CMAJ 2021, 193, E508–E516. [Google Scholar] [CrossRef]
- Copland, E.; Canoy, D.; Nazarzadeh, M.; Bidel, Z.; Ramakrishnan, R.; Woodward, M.; Chalmers, J.; Teo, K.K.; Pepine, C.J.; Davis, B.R.; et al. Antihypertensive treatment and risk of cancer: An individual participant data meta-analysis. Lancet Oncol. 2021, 22, 558–570. [Google Scholar] [CrossRef]
- Cho, I.J.; Shin, J.H.; Jung, M.H.; Kang, C.Y.; Hwang, J.; Kwon, C.H.; Kim, W.; Kim, D.H.; Lee, C.J.; Kang, S.H.; et al. Antihypertensive Drugs and the Risk of Cancer: A Nationwide Cohort Study. J. Clin. Med. 2021, 10, 771. [Google Scholar] [CrossRef]
- Han, H.; Guo, W.; Shi, W.; Yu, Y.; Zhang, Y.; Ye, X.; He, J. Hypertension and breast cancer risk: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 44877. [Google Scholar] [CrossRef]
- Vatten, L.J.; Trichopoulos, D.; Holmen, J.; Nilsen, T.I. Blood pressure and renal cancer risk: The HUNT Study in Norway. Br. J. Cancer 2007, 97, 112–114. [Google Scholar] [CrossRef]
- Sanfilippo, K.M.; McTigue, K.M.; Fidler, C.J.; Neaton, J.D.; Chang, Y.; Fried, L.F.; Liu, S.; Kuller, L.H. Hypertension and obesity and the risk of kidney cancer in 2 large cohorts of US men and women. Hypertension 2014, 63, 934–941. [Google Scholar] [CrossRef]
- Mravec, B.; Tibensky, M. Increased cancer risk in polycystic ovary syndrome: An (un)sympathetic connection? Med. Hypotheses 2020, 134, 109437. [Google Scholar] [CrossRef] [PubMed]
- Mravec, B.; Tibensky, M.; Horvathova, L.; Babal, P. E-Cigarettes and Cancer Risk. Cancer Prev. Res. (Phila.) 2020, 13, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.P.; Leadbitter, P.; Smithers, F.; Tan, S.T. beta-blocker therapy for infantile hemangioma. Expert Rev. Clin. Pharm. 2020, 13, 899–915. [Google Scholar] [CrossRef]
- Thaker, P.H.; Han, L.Y.; Kamat, A.A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.; Bankson, J.A.; Ravoori, M.; et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 2006, 12, 939–944. [Google Scholar] [CrossRef]
- Sastry, K.S.; Karpova, Y.; Prokopovich, S.; Smith, A.J.; Essau, B.; Gersappe, A.; Carson, J.P.; Weber, M.J.; Register, T.C.; Chen, Y.Q.; et al. Epinephrine protects cancer cells from apoptosis via activation of cAMP-dependent protein kinase and BAD phosphorylation. J. Biol. Chem. 2007, 282, 14094–14100. [Google Scholar] [CrossRef]
- Chin, C.C.; Li, J.M.; Lee, K.F.; Huang, Y.C.; Wang, K.C.; Lai, H.C.; Cheng, C.C.; Kuo, Y.H.; Shi, C.S. Selective beta2-AR Blockage Suppresses Colorectal Cancer Growth Through Regulation of EGFR-Akt/ERK1/2 Signaling, G1-Phase Arrest, and Apoptosis. J. Cell. Physiol. 2016, 231, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Ben-Eliyahu, S.; Shakhar, G.; Page, G.G.; Stefanski, V.; Shakhar, K. Suppression of NK cell activity and of resistance to metastasis by stress: A role for adrenal catecholamines and beta-adrenoceptors. Neuroimmunomodulation 2000, 8, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.K.; Bhatty, R.; Kamat, A.A.; Landen, C.N.; Han, L.; Thaker, P.H.; Li, Y.; Gershenson, D.M.; Lutgendorf, S.; Cole, S.W. Stress hormone-mediated invasion of ovarian cancer cells. Clin. Cancer Res. 2006, 12, 369–375. [Google Scholar] [CrossRef]
- Hassan, S.; Karpova, Y.; Baiz, D.; Yancey, D.; Pullikuth, A.; Flores, A.; Register, T.; Cline, J.M.; D’Agostino, R., Jr.; Danial, N.; et al. Behavioral stress accelerates prostate cancer development in mice. J. Clin. Invest. 2013, 123, 874–886. [Google Scholar] [CrossRef]
- Montoya, A.; Amaya, C.N.; Belmont, A.; Diab, N.; Trevino, R.; Villanueva, G.; Rains, S.; Sanchez, L.A.; Badri, N.; Otoukesh, S.; et al. Use of non-selective beta-blockers is associated with decreased tumor proliferative indices in early stage breast cancer. Oncotarget 2017, 8, 6446–6460. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.Y.; Chung, C.H.; Chang, W.C.; Lin, C.S.; Lin, H.H.; Dai, M.S.; Ho, C.L.; Chien, W.C. The effect of propranolol on the prognosis of hepatocellular carcinoma: A nationwide population-based study. PLoS ONE 2019, 14, e0216828. [Google Scholar] [CrossRef]
- Hwa, Y.L.; Shi, Q.; Kumar, S.K.; Lacy, M.Q.; Gertz, M.A.; Kapoor, P.; Buadi, F.K.; Leung, N.; Dingli, D.; Go, R.S.; et al. Beta-blockers improve survival outcomes in patients with multiple myeloma: A retrospective evaluation. Am. J. Hematol. 2017, 92, 50–55. [Google Scholar] [CrossRef]
- Barron, T.I.; Connolly, R.M.; Sharp, L.; Bennett, K.; Visvanathan, K. Beta blockers and breast cancer mortality: A population- based study. J. Clin. Oncol. 2011, 29, 2635–2644. [Google Scholar] [CrossRef]
- Kim, S.A.; Moon, H.; Roh, J.L.; Kim, S.B.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Postdiagnostic use of beta-blockers and other antihypertensive drugs and the risk of recurrence and mortality in head and neck cancer patients: An observational study of 10,414 person-years of follow-up. Clin. Transl. Oncol. 2017, 19, 826–833. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, V.; Grazzini, M.; Benemei, S.; Marchionni, N.; Botteri, E.; Pennacchioli, E.; Geppetti, P.; Gandini, S. Propranolol for Off-label Treatment of Patients With Melanoma: Results From a Cohort Study. Jama Oncol. 2018, 4, e172908. [Google Scholar] [CrossRef]
- Montoya, A.; Varela-Ramirez, A.; Dickerson, E.; Pasquier, E.; Torabi, A.; Aguilera, R.; Nahleh, Z.; Bryan, B. The beta adrenergic receptor antagonist propranolol alters mitogenic and apoptotic signaling in late stage breast cancer. Biomed. J. 2019, 42, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Hopson, M.B.; Lee, S.; Accordino, M.; Trivedi, M.; Maurer, M.; Crew, K.D.; Hershman, D.L.; Kalinsky, K. Phase II study of propranolol feasibility with neoadjuvant chemotherapy in patients with newly diagnosed breast cancer. Breast Cancer Res. Treat. 2021. [Google Scholar] [CrossRef]
- Hewitt, M.; Rowland, J.H.; Yancik, R. Cancer survivors in the United States: Age, health, and disability. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 82–91. [Google Scholar] [CrossRef]
- Thornton, L.M.; Andersen, B.L.; Blakely, W.P. The pain, depression, and fatigue symptom cluster in advanced breast cancer: Covariation with the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system. Health Psychol. 2010, 29, 333–337. [Google Scholar] [CrossRef]
- Neeman, E.; Ben-Eliyahu, S. Surgery and stress promote cancer metastasis: New outlooks on perioperative mediating mechanisms and immune involvement. Brain. Behav. Immun. 2013, 30, S32–S40. [Google Scholar] [CrossRef]
- Hiller, J.G.; Perry, N.J.; Poulogiannis, G.; Riedel, B.; Sloan, E.K. Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol. 2018, 15, 205–218. [Google Scholar] [CrossRef]
- Benish, M.; Bartal, I.; Goldfarb, Y.; Levi, B.; Avraham, R.; Raz, A.; Ben-Eliyahu, S. Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann. Surg. Oncol. 2008, 15, 2042–2052. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, Y.; Sorski, L.; Benish, M.; Levi, B.; Melamed, R.; Ben-Eliyahu, S. Improving postoperative immune status and resistance to cancer metastasis: A combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann. Surg. 2011, 253, 798–810. [Google Scholar] [CrossRef]
- Choy, C.; Raytis, J.L.; Smith, D.D.; Duenas, M.; Neman, J.; Jandial, R.; Lew, M.W. Inhibition of beta2-adrenergic receptor reduces triple-negative breast cancer brain metastases: The potential benefit of perioperative beta-blockade. Oncol. Rep. 2016, 35, 3135–3142. [Google Scholar] [CrossRef]
- Jang, H.I.; Lim, S.H.; Lee, Y.Y.; Kim, T.J.; Choi, C.H.; Lee, J.W.; Kim, B.G.; Bae, D.S. Perioperative administration of propranolol to women undergoing ovarian cancer surgery: A pilot study. Obs. Gynecol. Sci. 2017, 60, 170–177. [Google Scholar] [CrossRef]
- Shaashua, L.; Shabat-Simon, M.; Haldar, R.; Matzner, P.; Zmora, O.; Shabtai, M.; Sharon, E.; Allweis, T.; Barshack, I.; Hayman, L.; et al. Perioperative COX-2 and beta-Adrenergic Blockade Improves Metastatic Biomarkers in Breast Cancer Patients in a Phase-II Randomized Trial. Clin. Cancer Res. 2017, 23, 4651–4661. [Google Scholar] [CrossRef] [PubMed]
- Haldar, R.; Shaashua, L.; Lavon, H.; Lyons, Y.A.; Zmora, O.; Sharon, E.; Birnbaum, Y.; Allweis, T.; Sood, A.K.; Barshack, I.; et al. Perioperative inhibition of beta-adrenergic and COX2 signaling in a clinical trial in breast cancer patients improves tumor Ki-67 expression, serum cytokine levels, and PBMCs transcriptome. Brain. Behav. Immun. 2018, 73, 294–309. [Google Scholar] [CrossRef]
- Hiller, J.G.; Cole, S.W.; Crone, E.M.; Byrne, D.J.; Shackleford, D.M.; Pang, J.B.; Henderson, M.A.; Nightingale, S.S.; Ho, K.M.; Myles, P.S.; et al. Preoperative beta-Blockade with Propranolol Reduces Biomarkers of Metastasis in Breast Cancer: A Phase II Randomized Trial. Clin. Cancer Res. 2020, 26, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Haldar, R.; Ricon-Becker, I.; Radin, A.; Gutman, M.; Cole, S.W.; Zmora, O.; Ben-Eliyahu, S. Perioperative COX2 and beta-adrenergic blockade improves biomarkers of tumor metastasis, immunity, and inflammation in colorectal cancer: A randomized controlled trial. Cancer 2020, 126, 3991–4001. [Google Scholar] [CrossRef]
- Pasquier, E.; Ciccolini, J.; Carre, M.; Giacometti, S.; Fanciullino, R.; Pouchy, C.; Montero, M.P.; Serdjebi, C.; Kavallaris, M.; Andre, N. Propranolol potentiates the anti-angiogenic effects and anti-tumor efficacy of chemotherapy agents: Implication in breast cancer treatment. Oncotarget 2011, 2, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.H.; Liu, J.; Zhang, J.; Wang, Y.; Peng, X.C.; Wei, Y.Q.; Jiang, Y. Exogenous norepinephrine attenuates the efficacy of sunitinib in a mouse cancer model. J. Exp. Clin. Cancer Res. 2014, 33, 21. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, L.; Garofoli, M.; Di Fonte, R.; Fucci, L.; Volpicella, M.; Strippoli, S.; Guida, M.; Azzariti, A. The beta-adrenergic receptor antagonist propranolol offsets resistance mechanisms to chemotherapeutics in diverse sarcoma subtypes: A pilot study. Sci. Rep. 2020, 10, 10465. [Google Scholar] [CrossRef]
- Lucido, C.T.; Miskimins, W.K.; Vermeer, P.D. Propranolol Promotes Glucose Dependence and Synergizes with Dichloroacetate for Anti-Cancer Activity in HNSCC. Cancers (Basel) 2018, 10, 476. [Google Scholar] [CrossRef]
- Kokolus, K.M.; Zhang, Y.; Sivik, J.M.; Schmeck, C.; Zhu, J.; Repasky, E.A.; Drabick, J.J.; Schell, T.D. Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology 2018, 7, e1405205. [Google Scholar] [CrossRef]
- Ashrafi, S.; Shapouri, R.; Shirkhani, A.; Mahdavi, M. Anti-tumor effects of propranolol: Adjuvant activity on a transplanted murine breast cancer model. Biomed. Pharm. 2018, 104, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.M.; Kerswill, S.A.; Hari, P.; Cole, S.W.; Logan, B.R.; D’Souza, A.; Shah, N.N.; Horowitz, M.M.; Stolley, M.R.; Sloan, E.K.; et al. Repurposing existing medications as cancer therapy: Design and feasibility of a randomized pilot investigating propranolol administration in patients receiving hematopoietic cell transplantation. BMC Cancer 2018, 18, 593. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, A.; Hiyama, T.; Fujimura, A.; Yoshikawa, S. Sympathetic and parasympathetic innervation in cancer: Therapeutic implications. Clin. Auton. Res. 2021, 31, 165–178. [Google Scholar] [CrossRef]
- Zhao, C.M.; Hayakawa, Y.; Kodama, Y.; Muthupalani, S.; Westphalen, C.B.; Andersen, G.T.; Flatberg, A.; Johannessen, H.; Friedman, R.A.; Renz, B.W.; et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014, 6, 250ra115. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, A.; Hayama, Y.; Kato, S.; Shimomura, A.; Shimomura, T.; Irie, K.; Kaneko, R.; Yanagawa, Y.; Kobayashi, K.; Ochiya, T. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat. Neurosci. 2019, 22, 1289–1305. [Google Scholar] [CrossRef]
- Maccari, S.; Buoncervello, M.; Rampin, A.; Spada, M.; Macchia, D.; Giordani, L.; Stati, T.; Bearzi, C.; Catalano, L.; Rizzi, R.; et al. Biphasic effects of propranolol on tumour growth in B16F10 melanoma-bearing mice. Br. J. Pharm. 2017, 174, 139–149. [Google Scholar] [CrossRef]
- Zheng, G.; Sundquist, J.; Sundquist, K.; Ji, J. Beta-Blockers Use and Risk of Breast Cancer in Women with Hypertension. Cancer Epidemiol. Biomark. Prev. 2021, 30, 965–973. [Google Scholar] [CrossRef]
- De Giorgi, V.; Grazzini, M.; Gandini, S.; Benemei, S.; Lotti, T.; Marchionni, N.; Geppetti, P. Treatment with beta-blockers and reduced disease progression in patients with thick melanoma. Arch. Intern. Med. 2011, 171, 779–781. [Google Scholar] [CrossRef]
- Brohee, L.; Peulen, O.; Nusgens, B.; Castronovo, V.; Thiry, M.; Colige, A.C.; Deroanne, C.F. Propranolol sensitizes prostate cancer cells to glucose metabolism inhibition and prevents cancer progression. Sci. Rep. 2018, 8, 7050. [Google Scholar] [CrossRef]
- Kast, R.E.; Skuli, N.; Cos, S.; Karpel-Massler, G.; Shiozawa, Y.; Goshen, R.; Halatsch, M.E. The ABC7 regimen: A new approach to metastatic breast cancer using seven common drugs to inhibit epithelial-to-mesenchymal transition and augment capecitabine efficacy. Breast Cancer (Dove Med. Press) 2017, 9, 495–514. [Google Scholar] [CrossRef]
- Mirosevic, S.; Jo, B.; Kraemer, H.C.; Ershadi, M.; Neri, E.; Spiegel, D. "Not just another meta-analysis": Sources of heterogeneity in psychosocial treatment effect on cancer survival. Cancer Med. 2019, 8, 363–373. [Google Scholar] [CrossRef]
- Vermeer, P.D. Exosomal Induction of Tumor Innervation. Cancer Res. 2019, 79, 3529–3535. [Google Scholar] [CrossRef]
- Ayala, G.E.; Dai, H.; Powell, M.; Li, R.; Ding, Y.; Wheeler, T.M.; Shine, D.; Kadmon, D.; Thompson, T.; Miles, B.J.; et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin. Cancer Res. 2008, 14, 7593–7603. [Google Scholar] [CrossRef] [PubMed]
- Albo, D.; Akay, C.L.; Marshall, C.L.; Wilks, J.A.; Verstovsek, G.; Liu, H.; Agarwal, N.; Berger, D.H.; Ayala, G.E. Neurogenesis in colorectal cancer is a marker of aggressive tumor behavior and poor outcomes. Cancer 2011, 117, 4834–4845. [Google Scholar] [CrossRef] [PubMed]
- Rowe, C.W.; Dill, T.; Griffin, N.; Jobling, P.; Faulkner, S.; Paul, J.W.; King, S.; Smith, R.; Hondermarck, H. Innervation of papillary thyroid cancer and its association with extra-thyroidal invasion. Sci. Rep. 2020, 10, 1539. [Google Scholar] [CrossRef]
- Ferdoushi, A.; Griffin, N.; Marsland, M.; Xu, X.; Faulkner, S.; Gao, F.; Liu, H.; King, S.J.; Denham, J.W.; van Helden, D.F.; et al. Tumor innervation and clinical outcome in pancreatic cancer. Sci. Rep. 2021, 11, 7390. [Google Scholar] [CrossRef]
- Amit, M.; Takahashi, H.; Dragomir, M.P.; Lindemann, A.; Gleber-Netto, F.O.; Pickering, C.R.; Anfossi, S.; Osman, A.A.; Cai, Y.; Wang, R.; et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 2020, 578, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Mauffrey, P.; Tchitchek, N.; Barroca, V.; Bemelmans, A.P.; Firlej, V.; Allory, Y.; Romeo, P.H.; Magnon, C. Progenitors from the central nervous system drive neurogenesis in cancer. Nature 2019, 569, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Alfonso, J.; Monyer, H.; Wick, W.; Winkler, F. Neuronal signatures in cancer. Int. J. Cancer 2020, 147, 3281–3291. [Google Scholar] [CrossRef]
- Schuller, H.M. Neurotransmission and cancer: Implications for prevention and therapy. Anticancer Drugs 2008, 19, 655–671. [Google Scholar] [CrossRef]
- Venkatesh, H.; Monje, M. Neuronal Activity in Ontogeny and Oncology. Trends Cancer 2017, 3, 89–112. [Google Scholar] [CrossRef]
- Han, H.; Yang, C.; Zhang, Y.; Han, C.; Zhang, G. Vascular Endothelial Growth Factor Mediates the Sprouted Axonogenesis of Breast Cancer in Rat. Am. J. Pathol. 2021, 191, 515–526. [Google Scholar] [CrossRef]
- Pundavela, J.; Demont, Y.; Jobling, P.; Lincz, L.F.; Roselli, S.; Thorne, R.F.; Bond, D.; Bradshaw, R.A.; Walker, M.M.; Hondermarck, H. ProNGF correlates with Gleason score and is a potential driver of nerve infiltration in prostate cancer. Am. J. Pathol. 2014, 184, 3156–3162. [Google Scholar] [CrossRef]
- Yoneda, T.; Hiasa, M.; Okui, T. Crosstalk Between Sensory Nerves and Cancer in Bone. Curr. Osteoporos Rep. 2018, 16, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Madeo, M.; Colbert, P.L.; Vermeer, D.W.; Lucido, C.T.; Cain, J.T.; Vichaya, E.G.; Grossberg, A.J.; Muirhead, D.; Rickel, A.P.; Hong, Z.; et al. Cancer exosomes induce tumor innervation. Nat. Commun. 2018, 9, 4284. [Google Scholar] [CrossRef]
- He, D.; Manzoni, A.; Florentin, D.; Fisher, W.; Ding, Y.; Lee, M.; Ayala, G. Biologic effect of neurogenesis in pancreatic cancer. Hum. Pathol. 2016, 52, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Coarfa, C.; Florentin, D.; Putluri, N.; Ding, Y.; Au, J.; He, D.; Ragheb, A.; Frolov, A.; Michailidis, G.; Lee, M.; et al. Influence of the neural microenvironment on prostate cancer. Prostate 2018, 78, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Famm, K.; Litt, B.; Tracey, K.J.; Boyden, E.S.; Slaoui, M. Drug discovery: A jump-start for electroceuticals. Nature 2013, 496, 159–161. [Google Scholar] [CrossRef]
- Johnson, R.L.; Wilson, C.G. A review of vagus nerve stimulation as a therapeutic intervention. J. Inflamm. Res. 2018, 11, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Ramadi, K.B.; Srinivasan, S.S.; Traverso, G. Electroceuticals in the Gastrointestinal Tract. Trends Pharm. Sci. 2020, 41, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Payne, S.C.; Furness, J.B.; Stebbing, M.J. Bioelectric neuromodulation for gastrointestinal disorders: Effectiveness and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 89–105. [Google Scholar] [CrossRef]
- Garcia, G.T.; Ribeiro, R.F.; Faria Santos, I.B.; Gomes, F.C.; de Melo-Neto, J.S. Electrical Stimulation of PC 6 to Control Chemotherapy-Induced Nausea and Vomiting in Patients with Cancer: A Systematic Review and Meta-Analysis. Med. Acupunct 2021, 33, 22–44. [Google Scholar] [CrossRef] [PubMed]
- Levin, M. Bioelectric signaling: Reprogrammable circuits underlying embryogenesis, regeneration, and cancer. Cell 2021, 184, 1971–1989. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Selberg, J.; Rolandi, M. Endogenous Bioelectrics in Development, Cancer, and Regeneration: Drugs and Bioelectronic Devices as Electroceuticals for Regenerative Medicine. iScience 2019, 22, 519–533. [Google Scholar] [CrossRef]
- Tuszynski, J.; Tilli, T.M.; Levin, M. Ion Channel and Neurotransmitter Modulators as Electroceutical Approaches to the Control of Cancer. Curr. Pharm. Des. 2017, 23, 4827–4841. [Google Scholar] [CrossRef]
- Angus, M.; Ruben, P. Voltage gated sodium channels in cancer and their potential mechanisms of action. Channels (Austin) 2019, 13, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Roger, S.; Gillet, L.; Le Guennec, J.Y.; Besson, P. Voltage-gated sodium channels and cancer: Is excitability their primary role? Front. Pharm. 2015, 6, 152. [Google Scholar] [CrossRef]
- Zheng, Q.; Peng, X.; Zhang, Y. Cytotoxicity of amide-linked local anesthetics on melanoma cells via inhibition of Ras and RhoA signaling independent of sodium channel blockade. BMC Anesth. 2020, 20, 43. [Google Scholar] [CrossRef]
- Castelli, V.; Piroli, A.; Marinangeli, F.; d’Angelo, M.; Benedetti, E.; Ippoliti, R.; Zis, P.; Varrassi, G.; Giordano, A.; Paladini, A.; et al. Local anesthetics counteract cell proliferation and migration of human triple-negative breast cancer and melanoma cells. J. Cell. Physiol. 2020, 235, 3474–3484. [Google Scholar] [CrossRef]
- Gao, J.; Hu, H.; Wang, X. Clinically relevant concentrations of lidocaine inhibit tumor angiogenesis through suppressing VEGF/VEGFR2 signaling. Cancer Chemother. Pharm. 2019, 83, 1007–1015. [Google Scholar] [CrossRef]
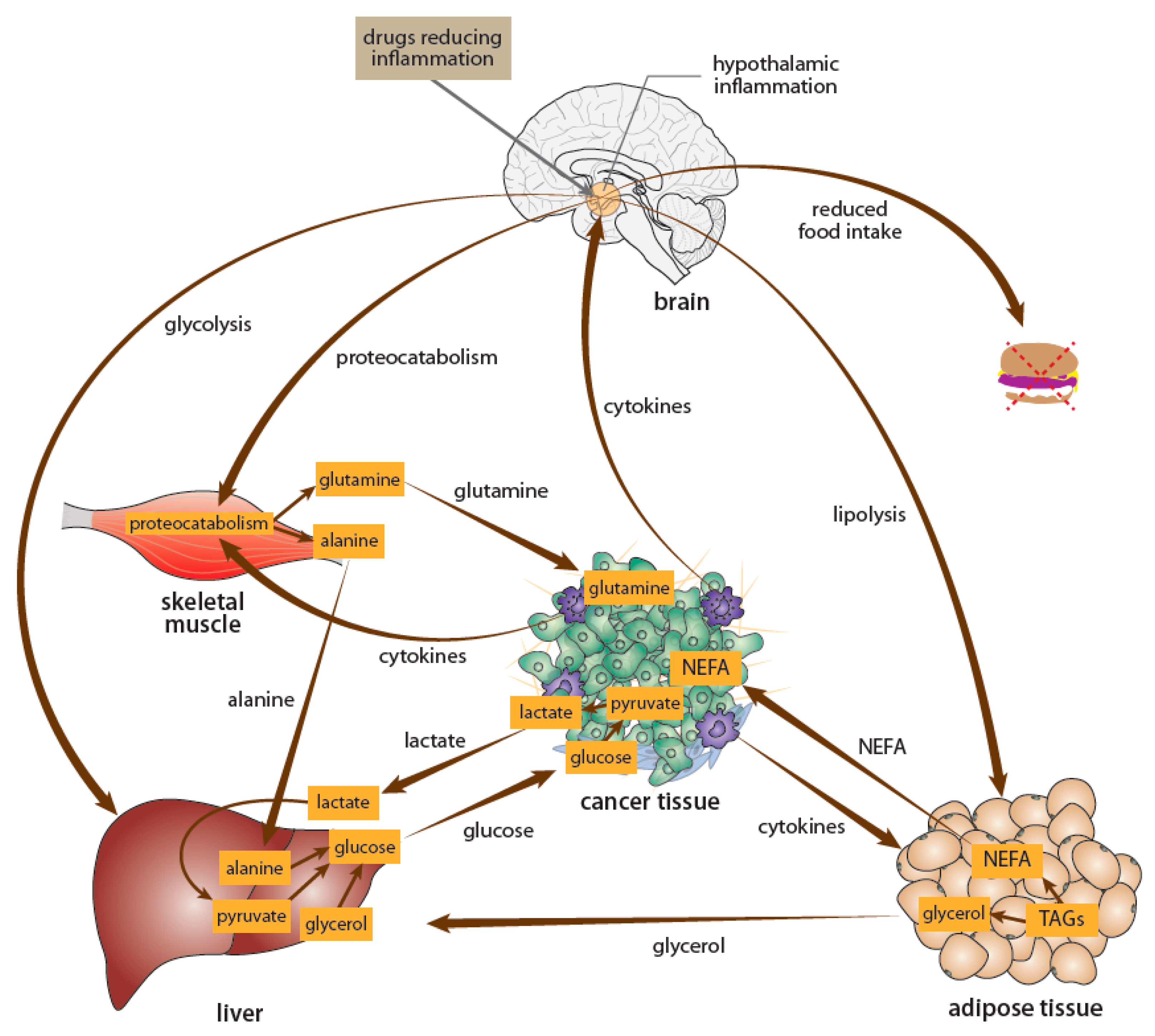
- Grossberg, A.J.; Scarlett, J.M.; Marks, D.L. Hypothalamic mechanisms in cachexia. Physiol. Behav. 2010, 100, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Mravec, B.; Horvathova, L.; Cernackova, A. Hypothalamic Inflammation at a Crossroad of Somatic Diseases. Cell. Mol. Neurobiol. 2019, 39, 11–29. [Google Scholar] [CrossRef] [PubMed]
- van Norren, K.; Dwarkasing, J.T.; Witkamp, R.F. The role of hypothalamic inflammation, the hypothalamic-pituitary-adrenal axis and serotonin in the cancer anorexia-cachexia syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 396–401. [Google Scholar] [CrossRef]
- Dragano, N.R.V.; Solon, C.; Ramalho, A.F.; de Moura, R.F.; Razolli, D.S.; Christiansen, E.; Azevedo, C.; Ulven, T.; Velloso, L.A. Polyunsaturated fatty acid receptors, GPR40 and GPR120, are expressed in the hypothalamus and control energy homeostasis and inflammation. J. Neuroinflamm. 2017, 14. [Google Scholar] [CrossRef]
- Ropelle, E.R.; Flores, M.B.; Cintra, D.E.; Rocha, G.Z.; Pauli, J.R.; Morari, J.; de Souza, C.T.; Moraes, J.C.; Prada, P.O.; Guadagnini, D.; et al. IL-6 and IL-10 Anti-Inflammatory Activity Links Exercise to Hypothalamic Insulin and Leptin Sensitivity through IKK beta and ER Stress Inhibition. PLoS Biol. 2010, 8. [Google Scholar] [CrossRef]
- Laviano, A.; Meguid, M.M.; Rossi-Fanelli, F. Cancer anorexia: Clinical implications, pathogenesis, and therapeutic strategies. Lancet Oncol. 2003, 4, 686–694. [Google Scholar] [CrossRef]
- Argiles, J.M.; Busquets, S.; Stemmler, B.; Lopez-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Vane, J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 1971, 231, 232–235. [Google Scholar] [CrossRef]
- Homem-de-Bittencourt Junior, P.I.; Pontieri, V.; Curi, R.; Lopes, O.U. Effects of aspirin-like drugs on Walker 256 tumor growth and cachexia in rats. Braz. J. Med. Biol. Res. 1989, 22, 1039–1042. [Google Scholar]
- Aronoff, D.M.; Neilson, E.G. Antipyretics: Mechanisms of action and clinical use in fever suppression. Am. J. Med. 2001, 111, 304–315. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-activated protein kinase: Maintaining energy homeostasis at the cellular and whole-body levels. Annu. Rev. Nutr. 2014, 34, 31–55. [Google Scholar] [CrossRef]
- Rena, G.; Pearson, E.R.; Sakamoto, K. Molecular mechanism of action of metformin: Old or new insights? Diabetologia 2013, 56, 1898–1906. [Google Scholar] [CrossRef]
- Moiseeva, O.; Deschenes-Simard, X.; Pollak, M.; Ferbeyre, G. Metformin, aging and cancer. Aging (Albany NY) 2013, 5, 330–331. [Google Scholar] [CrossRef] [PubMed]
- Quinn, B.J.; Kitagawa, H.; Memmott, R.M.; Gills, J.J.; Dennis, P.A. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol. Metab. 2013, 24, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Bora, V.; Patel, B.M. Investigation into the role of anti-diabetic agents in cachexia associated with metastatic cancer. Life Sci. 2021, 274, 119329. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mravec, B. Neurobiology of Cancer: Introduction of New Drugs in the Treatment and Prevention of Cancer. Int. J. Mol. Sci. 2021, 22, 6115. https://doi.org/10.3390/ijms22116115
Mravec B. Neurobiology of Cancer: Introduction of New Drugs in the Treatment and Prevention of Cancer. International Journal of Molecular Sciences. 2021; 22(11):6115. https://doi.org/10.3390/ijms22116115
Chicago/Turabian StyleMravec, Boris. 2021. "Neurobiology of Cancer: Introduction of New Drugs in the Treatment and Prevention of Cancer" International Journal of Molecular Sciences 22, no. 11: 6115. https://doi.org/10.3390/ijms22116115
APA StyleMravec, B. (2021). Neurobiology of Cancer: Introduction of New Drugs in the Treatment and Prevention of Cancer. International Journal of Molecular Sciences, 22(11), 6115. https://doi.org/10.3390/ijms22116115





