Abstract
Background: Artemether-lumefantrine is a highly effective artemisinin-based combination therapy that was adopted in Mali as first-line treatment for uncomplicated Plasmodium falciparum malaria. This study was designed to measure the efficacy of artemether-lumefantrine and to assess the selection of the P. falciparum chloroquine resistance transporter (pfcrt) and P. falciparum multi-drug resistance 1 (pfmdr1) genotypes that have been associated with drug resistance. Methods: A 28-day follow-up efficacy trial of artemether-lumefantrine was conducted in patients aged 6 months and older suffering from uncomplicated falciparum malaria in four different Malian areas during the 2009 malaria transmission season. The polymorphic genetic markers MSP2, MSP1, and Ca1 were used to distinguish between recrudescence and reinfection. Reinfection and recrudescence were then grouped as recurrent infections and analyzed together by PCR-restriction fragment length polymorphism (RFLP) to identify candidate markers for artemether-lumefantrine tolerance in the P. falciparum chloroquine resistance transporter (pfcrt) gene and the P. falciparum multi-drug resistance 1 (pfmdr1) gene. Results: Clinical outcomes in 326 patients (96.7%) were analyzed and the 28-day uncorrected adequate clinical and parasitological response (ACPR) rate was 73.9%. The total PCR-corrected 28-day ACPR was 97.2%. The pfcrt 76T and pfmdr1 86Y population prevalence decreased from 49.3% and 11.0% at baseline (n = 337) to 38.8% and 0% in patients with recurrent infection (n = 85); p = 0.001), respectively. Conclusion: Parasite populations exposed to artemether-lumefantrine in this study were selected toward chloroquine-sensitivity and showed a promising trend that may warrant future targeted reintroduction of chloroquine or/and amodiaquine.
1. Introduction
Resistance of Plasmodium falciparum to chloroquine is present in almost all malaria-endemic countries [1]. Resistance to most other antimalarial drugs emerged after these therapies were introduced to replace chloroquine [2,3,4]. Artemisinins are structurally distinct from all other antimalarials and have so far been effective against multidrug-resistant strains of P. falciparum [5]. The World Health Organization (WHO) recommends artemisinin-based combination therapies (ACTs) as first-line treatment for uncomplicated malaria worldwide [6]. In Mali, the National Malaria Control Program (NMCP) recommends treating uncomplicated malaria with artesunate-amodiaquine or artemether-lumefantrine [7]. Although treatment policy in Mali changed from chloroquine to these ACTs in 2006, artemether-lumefantrine was not widely implemented as first-line therapy until 2007 [7]. Studies in Mali in 2005 revealed high efficacy of artemether-lumefantrine [8,9,10].
Artemether-lumefantrine combines the rapid-acting, short half-life synthetic artemisinin with the long-acting, more slowly cleared aryl amino alcohol, lumefantrine [11]. Efforts have been made in past years to identify mechanisms of decreased sensitivity to antimalarial drugs by searching for the selection of particular genetic polymorphisms in parasites after treatment. We previously showed results of treatment with chloroquine, pyrimethamine, pyrimethamine-sulfadoxine, artesunate-amodiaquine, and artesunate-sulfadoxine-pyrimethamine that were respectively selected for parasites carrying the molecular markers of resistance to each drug [12,13,14,15].
The Plasmodium falciparum multi-drug resistance 1 (pfmdr1) gene on chromosome 5 encodes a digestive vacuole transmembrane glycoprotein (Pgh1, for P-glycoprotein homologue 1). Point mutations at codons 86, 184 (predominant in Asia and Africa) and 1034, 1042, and 1246 (predominant in South America) and increased gene copy numbers of pfmdr1 are associated with resistance to many drugs, including chloroquine, quinine, mefloquine, and the aryl amino alcohols (halofantrine and lumefantrine) [16,17,18].
Mutations in the P. falciparum chloroquine resistance transporter (pfcrt) gene on chromosome 7, which also encodes a digestive vacuole transmembrane protein, are associated with chloroquine resistance [19]. Point mutations at codon 76 may play a role in mefloquine resistance and are now suggested to contribute to parasite susceptibility to other aryl amino alcohols, particularly lumefantrine [20].
Several countries in West Africa, including Mali, use artemether-lumefantrine as a first-line treatment for uncomplicated malaria. A number of other studies from Africa have investigated whether artemether-lumefantrine administration selects for particular pfcrt and pfmdr1 SNPs, but just a few were from West Africa [21] and Mali. Here, a clinical study was performed at four different Malian field sites, with different malaria transmission patterns and different drug-resistance rates, to monitor the efficacy of artemether-lumefantrine and its potential impact on pfmdr1 and pfcrt polymorphisms three years after the wide use of artemether-lumefantrine in Mali.
2. Results
2.1. Trial Profile
Of 400 recruited patients, 337 were enrolled: 77 in Kolle (n = 77), 88 in Faladje (n = 88), 100 in Bandiagara (n = 100), and 72 in Pongonon (n = 72). After 28 days of follow-up, 327 (97%) participants completed the study: 70 in Kolle (91%), 88 in Faladje (100%), 99 in Bandiagara (99%), and 70 in Pongonon (97.2%), as shown in Figure 1.
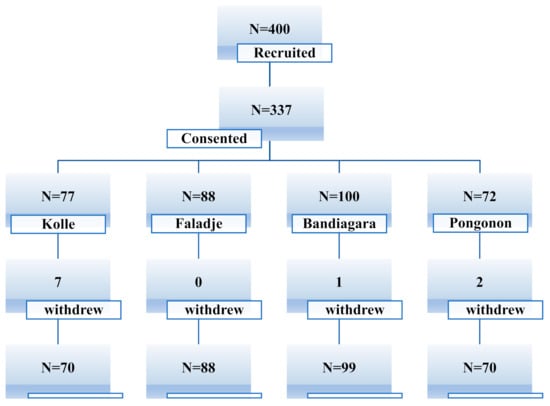
Figure 1.
Study profile.
2.2. Characteristics of Participants at Inclusion
Table 1 shows some differences exist among the four study sites, notably gametocyte carriage and parasite density at enrollment. Average parasite density at enrollment was 37,250 and average gametocyte carriage was 6.5% to 25% at one study site.

Table 1.
Sociodemographic, clinical, and parasitological variables at enrollment at each study site.
2.3. Treatment Efficacy for Uncomplicated Falciparum Malaria
Table 2 shows the 28-day treatment outcomes. Only one early treatment failure was observed: a child developed convulsions on Day 1 with a persistent fever (38.6 °C) despite diminished parasitemia (45,275/μL to 100/μL), and no death was observed in this study. The earliest recurrent infections were detected on Day 14. Between Days 14 and 28, 86 cases of parasitemia occurred, resulting in a cumulative risk of failure of 26.1%. Genotyping using the three polymorphic genes (MSP2, MSP1, and Ca1) showed that nearly all recurrent infections were new infections, rather than recrudescence. Considering only recrudescence, eight (2.5%) participants were classified as having genuine treatment failures, and risk of recrudescence did not differ significantly among the four sites (p > 0.78).

Table 2.
Primary treatment outcomes.
2.4. Secondary Outcomes
The clearance of fever was not statistically different among the four sites (data not shown). By Day 2, nearly all fevers had resolved. Parasitemia was statistically different among sites and highest in Pongonon on Day 1, but parasites were undetectable in all patients by Day 3 (Figure 2). Hemoglobin concentrations improved equally in all four sites (data not shown). Gametocytemia was highest in Pongonon at enrollment, but the levels became statistically similar to other sites by Day 1 (Figure 3). Gametocytes were not detected on any peripheral blood smears from Bandiagara.
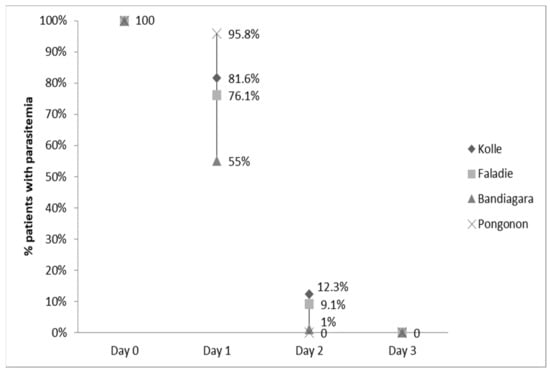
Figure 2.
Parasitemia clearance.
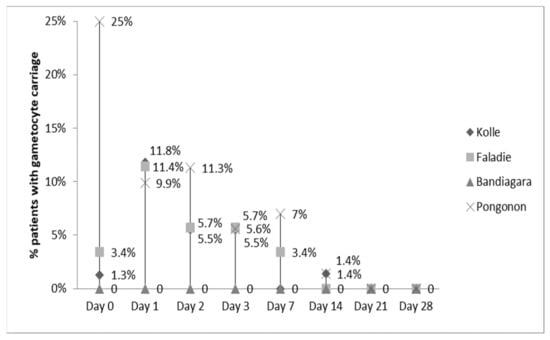
Figure 3.
Gametocytemia clearance.
2.5. Baseline Allele-Prevalence and Treatment Outcome
A total of 337 infections before treatment were successfully analyzed. Figure 4 and Figure 5 show the prevalence of the studied pfcrt 76T and pfmdr1 86Y mutations in all participants at baseline (Day 0) and re-treatment (Day R). Carriage of the pfmdr1 N86 allele and/or the pfcrt K76 allele (wild types) was not associated with blood smear positivity after initial parasite clearance in participants at any site within the 28-day follow-up period (p > 0.05). Additionally, we conducted an analysis of directional selection acting on alleles pfmdr1 codon 86 and pfcrt codon 76 from infected patients following therapy with artemether-lumefantrine. The frequencies of the pfmdr1 N86 and the pfcrt K76 alleles in recurrent infections increased from 84.7% to 100% (p = 0.0002) and 43.5% to 61.2% (p = 0.02), respectively (Table 3). The direction of the allele changes from baseline to recurrence was assessed using McNemar’s χ2 test for paired samples.
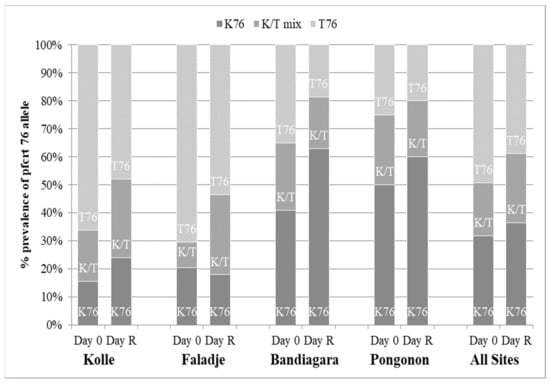
Figure 4.
Pfcrt allele frequency. D0 = date of enrollment; Day R = date of recurrent infection.
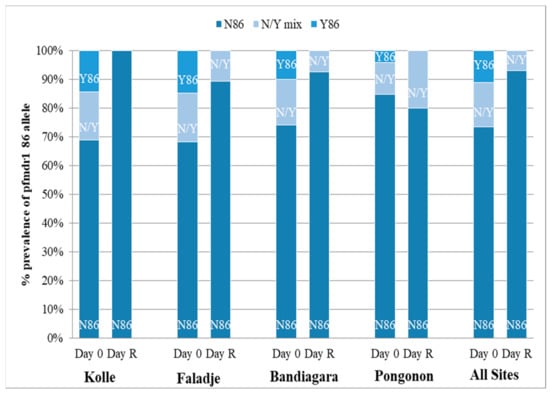
Figure 5.
Pfmdr1 allele frequency. D0 = date of enrollment; Day R = date of recurrent infection.

Table 3.
Matched post-treatment allele frequencies.
Examples of gel photo illustrations for MSP2, MSP1, and Ca1 (Figure 6).

Figure 6.
Gel photo illustrations for MSP2, MSP1, and Ca1 (D0 = Day 0 and DF = Failure Day). (Plate 1). DNA band of Plasmodium falciparum MSP2. (Plate 2). DNA band of P. falciparum MSP1. (Plate 3). DNA band of microsatellite Ca1.
3. Discussion
We found that both pfcrt K76 and pfmdr1 N86 (wild types) were selected in vivo after treatment with artemether-lumefantrine. Statistically significant pfcrt allele-selection has not been observed as often as pfmdr1 allele-selection. Our study, in addition to those by Sisowath, et. al. [22], and Otienoburu et al. [23], confirmed that the selection of the K76 SNP occurs in response after artemether-lumefantrine treatment. Veiga et al. showed the selection of K76 and N86 with lumefantrine treatment using gene-edited parasites [24]. All these studies described a decreasing chloroquine exposure and increased lumefantrine exposure and that this contributed to the selection of the wild types of pfmdr1 and pfcrt.
Whether pfcrt K76 and pfmdr1 N86 are selected for under the same mechanism remains to be determined. The reason for recurrent infections is primarily reinfection and allele selection occurs in reinfecting parasites. The concentration of lumefantrine is high enough to eliminate mutated parasites, but insufficient to eliminate wild-type parasites without the combined protection of artemether. Hastings and Ward have suggested that the selection of N86 may represent a marker of tolerance to lumefantrine [25].
In our study, treatment with artemether-lumefantrine (AL) was selected for pfcrt K76 and pfmdr1 N86 alleles (wild types) among recurrent infections. In this analysis, allele prevalence on Day 0 was compared with allele prevalence on Day R among the 85 patients who had recurrent infection. Recurrent parasitemia was statistically similar among three of the four sites (Kolle, Faladje, and Bandiagara), though recurrent malaria was most commonly observed in Bandiagara (15.2%). Recurrent malaria (4.3%) and parasitemia (2.9%) were both statistically lower in Pongonon.
Carriage of the pfmdr1 N86 allele has been associated with treatment failure in patients given artemether-lumefantrine [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47]. Prevalence of the pfmdr1 N86 allele also increased in all isolates, which shows a selection in favor of the wild-type allele, as seen previously with the use of artemether-lumefantrine [48]. The pattern of the pfmdr1 allele prevalence change was similar to that reported recently by Dokomajilar [49] from their study of Ugandan children, from Humphreys in Tanzania [26], and from Sisowath in Unguja Island and Micheweni [20]. Prevalence of the pfcrt K76 allele (wild type) increased in all isolates after treatment. We found evidence for significant selection of both pfcrt K76 and pfmdr1 N86 SNPs (wild types) after treatment with artemether-lumefantrine.
The selection of a SNP previously associated with quinoline antimalarial drug resistance was first documented in 2005. Our results regarding pfmdr1 N86 allele-selection (wild type) are consistent with previous studies; however, the risk of new infections among these studies has varied. In areas of Tanzania with intermediate transmission, the risk of new infection after artemether-lumefantrine treatment was only 5%; a similar study performed in an area of high transmission in Tanzania showed new infection rates of about 50%. In our study, risk of reinfection also varied by site, from Pongonon to Faladje/Bandiagara/Kolle. As transmission intensity increased from one site to another, so did the proportion of treated patients selecting for parasites harboring a particular allele. In vitro studies propose that gene selection occurs during the elimination phase of lumefantrine (t ½ = 3–6 days) [50], but this effect is unclear at the population level, where the proportion of parasites exposed to drug-selective pressures will vary geographically.
The eight (2.5%) recrudescent infections in this study could not be explained by pfmdr1 or pfcrt selection alone, albeit the sample size may have been insufficient to identify any associations. We thus relied on the combined outcomes of reinfection and recrudescence, as previous studies have done. However, grouping these outcomes involves the risk of seeing relationships that may not exist. Unless an increased frequency of recrudescence occurs in patients treated with artemether-lumefantrine, insights regarding tolerance and resistance will remain limited.
The presence of these wild-type alleles has been associated with reduced sensitivity to lumefantrine in vitro [51]. Other studies have associated the pfmdr1 haplotype of N86-184F-1246 with treatment failure [52,53]. We hypothesize that treatment with artesunate-amodiaquine (ASAQ) may lead to a selection of molecular markers of drug resistance (pfcrt 76T and pfmdr1 86Y (mutant types)) against artemisinin partner molecules [54,55]. AL and ASAQ could have opposite effects in selecting for pfcrt K76 and pfmdr1 N86 alleles (wild types), suggesting a potential benefit of using different ACTs simultaneously as first-line treatment to reduce selective pressures by each regimen. Alternating the use of AL versus ASAQ could maintain drug effectiveness, while decreasing the risk of tolerance/resistance. Larger studies examining this hypothesis could inform NMCPs to adjust malaria treatment policies.
In conclusion, parasite populations exposed to artemether-lumefantrine in this study were selected toward chloroquine-sensitivity and showed a promising trend that may warrant future targeted reintroduction of chloroquine or/and amodiaquine in areas where P. falciparum was previously resistant to these drugs.
4. Methods
4.1. Study Area and Population
This study was conducted during the malaria transmission season, from October 2009 to January 2010 in four different Malian areas: Kolle, Faladje, Bandiagara, and Pongonon. Kolle is a rural village of ~3000 inhabitants located 57 km southwest of Bamako. The climate is Sudanian savanna with two distinct seasons, the dry season (January–June) and the rainy season (July–December). Malaria is hyperendemic during 3 to 4 months of the rainy season with a parasite index (PI) of 70%–85%, and of 40%–50% during the dry season [56]. The entomological inoculation rate during the high transmission season was 5.2 infective bites per person per month and the pfcrt K76T mutation prevalence was previously recorded in 1999 and 2002 at 28.3% and 85%, respectively [57,58]. Faladje is located 80 km northwest of Bamako and its health center serves ~23,000 inhabitants. Malaria is seasonal (July–November) with a hyperendemic peak in October. The pfcrt K76T mutation prevalence was previously recorded in 2003 at 80% [57]. Bandiagara is a town of ~13,364 inhabitants located 700 km northeast of Bamako. Malaria is endemic with a transmission period from July to November. The pfcrt K76T mutation prevalence was previously recorded in 2002 at 39% [57]. Pongonon is a village of ~1400 inhabitants located 20 km from the rural town of Koro and 785 km from Bamako in northeastern Mali. Malaria is endemic with a transmission period from July to October. The entomological inoculation rate was one infective bite per person per month. Pongonon is located in an ecological transition zone between the Savanna and Sahel regions. The pfcrt K76T mutation prevalence was previously recorded in 2002 at 13% (unpublished data).
4.2. Study Volunteers
Inclusion criteria consisted of the following: (i) age ≥6 months, (ii) microscopic detection of P. falciparum at a density ≥2000 asexual parasites/µL to 200,000/μL of blood, (iii) axillary temperature ≥37.5 °C or reported fever in the preceding 24 h, (iv) ability to swallow oral medication, and (v) willingness to consent and comply with the study protocol for the duration of the study (parental consent was required for children <18 years). Exclusion criteria consisted of the following: (i) signs of severe malaria according to 2003 WHO definitions, (ii) a concomitant febrile condition (i.e., measles, acute respiratory infection, severe diarrhea), (iii) severe malnutrition, (iv) disclosed or clinically patent pregnancy, (v) other known underlying chronic or severe diseases, or (vi) history of hypersensitivity to artemether-lumefantrine or the rescue treatment, artesunate-amodiaquine.
4.3. Sample Collection
Finger-prick blood samples were collected from enrolled patients to assess the parasite density and hemoglobin level on-site, and to be preserved on the filter papers (Whatman 3MM, Whatman Lab Sales Ltd., Maidstone, Kent, UK), which were stored in self-sealing plastic bags with desiccant for later analysis. Patients received a twice-daily dose of artemether-lumefantrine of 20 mg/120 mg as indicated by weight for three consecutive days: one tablet per dose for patients weighing 5–14 kg, two tablets per dose for those weighing 15–24 kg, three tablets per dose for those weighing 25–34 kg, and four tablets per dose for those weighing ≥35 kg. All doses were administered on-site under supervision. A full dose was re-administered if a participant vomited within 30 min of the initial drug administration. After treatment initiation, patients were examined and finger-prick blood samples were collected routinely on Days 1, 2, 3, 7, 14, 21 and 28 of the treatment and on any day of recurrent illness during the 28-day follow-up period. In cases of treatment failure, quinine was administered if the patient was re-diagnosed with uncomplicated malaria or parasitemia. As per the national guidelines, patients were referred to a hospital and intravenous quinine was administered if severe malaria was diagnosed at any time during the follow-up period. Treatment was also given for any other conditions diagnosed (Supplementary File).
4.4. Laboratory
Specimen analysis was completed at the Malaria Research and Training Center (MRTC) lab in Bamako, Mali. Parasite density and rate of gametocyte carriage were measured, as number of parasites per microliter, from Giemsa-stained thick blood smears. A positive smear was defined as a thick smear with at least one asexual parasite per 100 fields under 1000× magnification (standardized technique from the Malian Ministry of Health). Blood was collected for hemoglobin measurement (HemoCue AB) on Day 0 prior to treatment and on Days 14 and 28 of treatment. Children with hemoglobin <7 g/dL were treated with oral iron therapy according to national policy [59]. For quality control purposes, 10% of smears were read by two independent microscopists blinded from the results of the first reading. On the basis of the results of these assessments, patients were classified as having therapeutic failure (early or late) or an adequate clinical and therapeutic response.
4.5. DNA Extraction
Three methods were used for DNA extraction. (1) Methanol was used to extract parasite DNA from all filter paper blood samples. Samples that did not yield DNA by this method were extracted using (2) the Chelex method [60]. For a single day 0 sample from Bandiagara, a filter paper blood sample was not available; thus, parasites were extracted from the thick smear according to a modified dried blood protocol using (3) QIAmp DNA blood mini kit (Qiagen, Valencia, CA, USA). DNA samples were stored at −20 °C until use.
4.6. Genotyping of Plasmodium Falciparum Isolates
MSP2, MSP1, and Ca1 were used to compare genotypic profiles between pre- and post-treatment P. falciparum isolates, in order to distinguish between episodes of recrudescence and reinfection [61,62,63]. The number of clones in pre- and post-treatment P. falciparum isolates were denoted by the number of different-sized PCR products [61]. Length differences were determined using a 100 bp molecular weight ladder (Promega, Madison, WI, USA). Allele-specific positive controls and DNA-free negative controls were included in each round of reactions. Primers for MSP1, MSP2 and Ca1 were synthesized by Integrated DNA Technologies, Coralville, IA, USA.
4.7. Pfcrt Codon 76-Point and Pfmdr1 Codon 86-Point Mutations Analysis
Mutations in pfcrt and pfmdr1 were assessed for all baseline infections (Day 0) and all recurring infections post-treatment (Day R) to examine any association between the artemether-lumefantrine treatment and the development of tolerance/resistance, as has been previously suggested [21]. The pfcrt K76T SNP was analyzed by restriction fragment length polymorphism (RFLP)-PCR and mutation-specific MS-PCR [13]. DNA from P. falciparum isolates Dd2 (containing the 76T mutation) and 3D7 (lacking this mutation) were used as the positive controls, while a DNA-free reaction mix was used as negative control. The pfmdr1 N86Y SNP was analyzed by PCR-RFLP, as described previously [64]. The negative control without parasite DNA and the positive controls were processed alongside the samples. HB3 and Dd2 P. falciparum isolates, representing wild-type (N86) and mutant genotypes (86Y), respectively, were used as the positive controls. PCR was performed in a tetrad 2 thermocycler (BIO-RAD, Marnes-la-Coquette, France). Primers for pfcrt were synthesized by Integrated DNA Technologies, Coralville, IA, USA. Primers for pfmdr1 were synthesized by the UMD Biopolymer core facility. RFLP and MS-PCR products and digestions were separated by electrophoresis in 2% agarose gels with ethidium bromide and were visualized under UV transillumination (SynGene; GeneSnap v.6.05).
4.8. Data Management and Analysis
Data were collected on case report forms and double entered using MS Access and analyzed using Stata (Stata Corp 11). The prevalence of single mutations and of various genotypes including the double mutant (pfcrt 76T + pfmdr1 86Y) were calculated with 95% confidence interval (CI). Chi-square or Fisher exact probability tests and McNemar’s χ2 test were used for comparisons as appropriate with statistical significance set at p value < 0.05.
4.9. Ethics
The study was approved by the ethical committee of the Faculty of Medicine, Pharmacy and Dentistry of Bamako, University of Sciences, Techniques, and Technologies of Bamako, Mali #No9/63/FMPOS#. Community permission was obtained from each locality prior to the study. Written informed consent was obtained from all adults and parents/guardians of children enrolled prior to screening.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms22116057/s1.
Author Contributions
H.M. is a study PI, developed the study concept, wrote the protocol, conducted the study in Kolle and edited paper, A.G. conducted the study in Bandiagara and wrote the first draft paper. A.A.D. designed the study and edited the paper. I.S. performed statistical data analysis and edited the paper. C.V.P. edited the paper. O.B.T., K.T., Y.T., A.A.D., A.T., A.B., Z.I.T. and K.S. were involved in protocol development, data collection, and clinical investigations. A.A.D. and O.K.D. validated the final consent and ethical procedures. All authors have read and agreed to the published version of the manuscript.
Funding
Standard diagnosis (SD) and the Malaria Research and Training Centre (MRTC of University of Sciences, Techniques and Technologies of Bamako (USTTB) of Mali supported this work. A.G. was supported by NIH-Fogarty International Center (FIC) grant to Vanderbilt University (Number: 5R24 TW007988-03).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Faculty of Medicine Pharmacy and Odonto-Stomatology of Bamako (No9/63/FMPOS, 9/24/2009)
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Acknowledgments
We thank all the patients, parents, community leaders and health center staff that participated in the study. We thank Hamidou Niangaly, Nouhoum Diallo, Aly Kodio, Sekou Koumare, Mahamadou Aly Thera, Mody Sissoko and Demba Dembele for their participation. We thank J. Patrick Gorres of the National Institute of Health (NIH) for assistance in reviewing and editing the manuscript. We also thank APTI program and Patrick Duffy at LMIV/NIAID/NIH for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ursos, L.M.; Roepe, P.D. Chloroquine resistance in the malarial parasite, Plasmodium falciparum. Med. Res. Rev. 2002, 22, 465–491. [Google Scholar] [CrossRef] [PubMed]
- Wongsrichanalai, C.; Pickard, A.L.; Wernsdorfer, W.H.; Meshnick, S.R. Epidemiology of drug-resistant malaria. Lancet Infect. Dis. 2002, 2, 209–218. [Google Scholar] [CrossRef]
- Edeson, J.F.; Field, J.W. Proguanil-resistant falciparum malaria in Malaya. Br. Med. J. 1950, 1, 147–151. [Google Scholar] [CrossRef]
- White, N.J. Antimalarial drug resistance. J. Clin. Investig. 2004, 113, 1084–1092. [Google Scholar] [CrossRef]
- White, N.J. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob. Agents Chemother. 1997, 41, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for the Treatment of Malaria, 2nd ed.; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- WHO. Global Technical Strategy for Malaria Control 2016–2030; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Sagara, I.; Dicko, A.; Djimde, A.; Guindo, O.; Kone, M.; Tolo, Y.; Thera, M.A.; Sogoba, M.; Fofana, M.; Ouattara, A.; et al. A randomized trial of artesunate-sulfamethoxypyrazine-pyrimethamine versus artemether-lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in Mali. Am. J. Trop. Med. Hyg. 2006, 75, 630–636. [Google Scholar] [CrossRef]
- Sagara, I.; Diallo, A.; Kone, M.; Coulibaly, M.; Diawara, S.I.; Guindo, O.; Maiga, H.; Niambele, M.B.; Sissoko, M.; Dicko, A.; et al. A randomized trial of artesunate-mefloquine versus artemether-lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria in Mali. Am. J. Trop. Med. Hyg. 2008, 79, 655–661. [Google Scholar] [CrossRef]
- Diarra, M.; Coulibaly, D.; Tapily, A.; Guindo, B.; Sanogo, K.; Kone, D.; Kone, Y.; Kone, K.; Bathily, A.; Yattara, O.; et al. Monitoring of the Sensitivity In Vivo of Plasmodium falciparum to Artemether-Lumefantrine in Mali. Trop. Med. Infect. Dis. 2021, 6, 13. [Google Scholar] [CrossRef]
- White, N.J.; van Vugt, M.; Ezzet, F. Clinical pharmacokinetics and pharmacodynamics and pharmacodynamics of artemether-lumefantrine. Clin. Pharmacokinet. 1999, 37, 105–125. [Google Scholar] [CrossRef]
- Djimde, A.A.; Fofana, B.; Sagara, I.; Sidibe, B.; Toure, S.; Dembele, D.; Dama, S.; Ouologuem, D.; Dicko, A.; Doumbo, O.K. Efficacy, safety, and selection of molecular markers of drug resistance by two ACTs in Mali. Am. J. Trop. Med. Hyg. 2008, 78, 455–461. [Google Scholar] [CrossRef]
- Djimde, A.; Doumbo, O.K.; Cortese, J.F.; Kayentao, K.; Doumbo, S.; Diourte, Y.; Coulibaly, D.; Dicko, A.; Su, X.Z.; Nomura, T.; et al. A molecular marker for chloroquine-resistant falciparum malaria. N. Engl. J. Med. 2001, 344, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Doumbo, O.K.; Kayentao, K.; Djimde, A.; Cortese, J.F.; Diourte, Y.; Konare, A.; Kublin, J.G.; Plowe, C.V. Rapid selection of Plasmodium falciparum dihydrofolate reductase mutants by pyrimethamine prophylaxis. J. Infect. Dis. 2000, 182, 993–996. [Google Scholar] [CrossRef]
- Diourte, Y.; Djimde, A.; Doumbo, O.K.; Sagara, I.; Coulibaly, Y.; Dicko, A.; Diallo, M.; Diakite, M.; Cortese, J.F.; Plowe, C.V. Pyrimethamine-sulfadoxine efficacy and selection for mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase in Mali. Am. J. Trop. Med. Hyg. 1999, 60, 475–478. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Basco, L.K.; Le Bras, J.; Rhoades, Z.; Wilson, C.M. Analysis of pfmdr1 and drug susceptibility in fresh isolates of Plasmodium falciparum from subsaharan Africa. Mol. Biochem. Parasitol. 1995, 74, 157–166. [Google Scholar] [CrossRef]
- Pickard, A.L.; Wongsrichanalai, C.; Purfield, A.; Kamwendo, D.; Emery, K.; Zalewski, C.; Kawamoto, F.; Miller, R.S.; Meshnick, S.R. Resistance to antimalarials in Southeast Asia and genetic polymorphisms in pfmdr1. Antimicrob. Agents Chemother. 2003, 47, 2418–2423. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, A.B.; Valderramos, S.G.; Fidock, D.A. pfmdr1 mutations contribute to quinine resistance and enhance mefloquine and artemisinin sensitivity in Plasmodium falciparum. Mol. Microbiol. 2005, 57, 913–926. [Google Scholar] [CrossRef]
- Bray, P.G.; Martin, R.E.; Tilley, L.; Ward, S.A.; Kirk, K.; Fidock, D.A. Defining the role of PfCRT in Plasmodium falciparum chloroquine resistance. Mol. Microbiol. 2005, 56, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Sisowath, C.; Stromberg, J.; Martensson, A.; Msellem, M.; Obondo, C.; Bjorkman, A.; Gil, J.P. In vivo selection of Plasmodium falciparum pfmdr1 86N coding alleles by artemether-lumefantrine (Coartem). J. Infect. Dis. 2005, 191, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Sisowath, C.; Ferreira, P.E.; Bustamante, L.Y.; Dahlstrom, S.; Martensson, A.; Bjorkman, A.; Krishna, S.; Gil, J.P. The role of pfmdr1 in Plasmodium falciparum tolerance to artemether-lumefantrine in Africa. Trop. Med. Int. Health 2007, 12, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Sisowath, C.; Petersen, I.; Veiga, M.I.; Martensson, A.; Premji, Z.; Bjorkman, A.; Fidock, D.A.; Gil, J.P. In vivo selection of Plasmodium falciparum parasites carrying the chloroquine-susceptible pfcrt K76 allele after treatment with artemether-lumefantrine in Africa. J. Infect. Dis. 2009, 199, 750–757. [Google Scholar] [CrossRef]
- Otienoburu, S.D.; Maiga-Ascofare, O.; Schramm, B.; Jullien, V.; Jones, J.J.; Zolia, Y.M.; Houze, P.; Ashley, E.A.; Kiechel, J.R.; Guerin, P.J.; et al. Selection of Plasmodium falciparum pfcrt and pfmdr1 polymorphisms after treatment with artesunate-amodiaquine fixed dose combination or artemether-lumefantrine in Liberia. Malar. J. 2016, 15, 452. [Google Scholar] [CrossRef]
- Veiga, M.I.; Dhingra, S.K.; Henrich, P.P.; Straimer, J.; Gnadig, N.; Uhlemann, A.C.; Martin, R.E.; Lehane, A.M.; Fidock, D.A. Globally prevalent PfMDR1 mutations modulate Plasmodium falciparum susceptibility to artemisinin-based combination therapies. Nat. Commun 2016, 7, 11553. [Google Scholar] [CrossRef] [PubMed]
- Hastings, I.M.; Ward, S.A. Coartem (artemether-lumefantrine) in Africa: The beginning of the end? J. Infect. Dis. 2005, 192, 1303–1304, author reply 1304–1305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Humphreys, G.S.; Merinopoulos, I.; Ahmed, J.; Whitty, C.J.; Mutabingwa, T.K.; Sutherland, C.J.; Hallett, R.L. Amodiaquine and artemether-lumefantrine select distinct alleles of the Plasmodium falciparum mdr1 gene in Tanzanian children treated for uncomplicated malaria. Antimicrob. Agents Chemother. 2007, 51, 991–997. [Google Scholar] [CrossRef]
- Phyo, A.P.; Nkhoma, S.; Stepniewska, K.; Ashley, E.A.; Nair, S.; McGready, R.; ler Moo, C.; Al-Saai, S.; Dondorp, A.M.; Lwin, K.M.; et al. Emergence of artemisinin-resistant malaria on the western border of Thailand: A longitudinal study. Lancet 2012, 379, 1960–1966. [Google Scholar] [CrossRef]
- Gadalla, N.B.; Adam, I.; Elzaki, S.E.; Bashir, S.; Mukhtar, I.; Oguike, M.; Gadalla, A.; Mansour, F.; Warhurst, D.; El-Sayed, B.B.; et al. Increased pfmdr1 copy number and sequence polymorphisms in Plasmodium falciparum isolates from Sudanese malaria patients treated with artemether-lumefantrine. Antimicrob. Agents Chemother. 2011, 55, 5408–5411. [Google Scholar] [CrossRef]
- Ngasala, B.E.; Malmberg, M.; Carlsson, A.M.; Ferreira, P.E.; Petzold, M.G.; Blessborn, D.; Bergqvist, Y.; Gil, J.P.; Premji, Z.; Bjorkman, A.; et al. Efficacy and effectiveness of artemether-lumefantrine after initial and repeated treatment in children <5 years of age with acute uncomplicated Plasmodium falciparum malaria in rural Tanzania: A randomized trial. Clin. Infect. Dis. 2011, 52, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Ngasala, B.E.; Malmberg, M.; Carlsson, A.M.; Ferreira, P.E.; Petzold, M.G.; Blessborn, D.; Bergqvist, Y.; Gil, J.P.; Premji, Z.; Martensson, A. Effectiveness of artemether-lumefantrine provided by community health workers in under-five children with uncomplicated malaria in rural Tanzania: An open label prospective study. Malar. J. 2011, 10, 64. [Google Scholar] [CrossRef]
- Kamugisha, E.; Jing, S.; Minde, M.; Kataraihya, J.; Kongola, G.; Kironde, F.; Swedberg, G. Efficacy of artemether-lumefantrine in treatment of malaria among under-fives and prevalence of drug resistance markers in Igombe-Mwanza, north-western Tanzania. Malar. J. 2012, 11, 58. [Google Scholar] [CrossRef]
- Ursing, J.; Kofoed, P.E.; Rodrigues, A.; Blessborn, D.; Thoft-Nielsen, R.; Bjorkman, A.; Rombo, L. Similar efficacy and tolerability of double-dose chloroquine and artemether-lumefantrine for treatment of Plasmodium falciparum infection in Guinea-Bissau: A randomized trial. J. Infect. Dis. 2011, 203, 109–116. [Google Scholar] [CrossRef]
- Ali, I.M.; Netongo, P.M.; Atogho-Tiedeu, B.; Ngongang, E.O.; Ajua, A.; Achidi, E.A.; Mbacham, W.F. Amodiaquine-Artesunate versus Artemether-Lumefantrine against Uncomplicated Malaria in Children Less Than 14 Years in Ngaoundere, North Cameroon: Efficacy, Safety, and Baseline Drug Resistant Mutations in pfcrt, pfmdr1, and pfdhfr Genes. Malar. Res. Treat. 2013, 2013, 234683. [Google Scholar] [CrossRef]
- Some, A.F.; Sere, Y.Y.; Dokomajilar, C.; Zongo, I.; Rouamba, N.; Greenhouse, B.; Ouedraogo, J.B.; Rosenthal, P.J. Selection of known Plasmodium falciparum resistance-mediating polymorphisms by artemether-lumefantrine and amodiaquine-sulfadoxine-pyrimethamine but not dihydroartemisinin-piperaquine in Burkina Faso. Antimicrob. Agents Chemother. 2010, 54, 1949–1954. [Google Scholar] [CrossRef][Green Version]
- Lekana-Douki, J.B.; Dinzouna Boutamba, S.D.; Zatra, R.; Zang Edou, S.E.; Ekomy, H.; Bisvigou, U.; Toure-Ndouo, F.S. Increased prevalence of the Plasmodium falciparum Pfmdr1 86N genotype among field isolates from Franceville, Gabon after replacement of chloroquine by artemether-lumefantrine and artesunate-mefloquine. Infect. Genet. Evol. 2011, 11, 512–517. [Google Scholar] [CrossRef]
- Lobo, E.; de Sousa, B.; Rosa, S.; Figueiredo, P.; Lobo, L.; Pateira, S.; Fernandes, N.; Nogueira, F. Prevalence of pfmdr1 alleles associated with artemether-lumefantrine tolerance/resistance in Maputo before and after the implementation of artemisinin-based combination therapy. Malar. J. 2014, 13, 300. [Google Scholar] [CrossRef]
- Mungthin, M.; Khositnithikul, R.; Sitthichot, N.; Suwandittakul, N.; Wattanaveeradej, V.; Ward, S.A.; Na-Bangchang, K. Association between the pfmdr1 gene and in vitro artemether and lumefantrine sensitivity in Thai isolates of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 2010, 83, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Duah, N.O.; Matrevi, S.A.; de Souza, D.K.; Binnah, D.D.; Tamakloe, M.M.; Opoku, V.S.; Onwona, C.O.; Narh, C.A.; Quashie, N.B.; Abuaku, B.; et al. Increased pfmdr1 gene copy number and the decline in pfcrt and pfmdr1 resistance alleles in Ghanaian Plasmodium falciparum isolates after the change of anti-malarial drug treatment policy. Malar. J. 2013, 12, 377. [Google Scholar] [CrossRef] [PubMed]
- Nkhoma, S.; Nair, S.; Mukaka, M.; Molyneux, M.E.; Ward, S.A.; Anderson, T.J. Parasites bearing a single copy of the multi-drug resistance gene (pfmdr-1) with wild-type SNPs predominate amongst Plasmodium falciparum isolates from Malawi. Acta Trop. 2009, 111, 78–81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Happi, C.T.; Gbotosho, G.O.; Folarin, O.A.; Sowunmi, A.; Hudson, T.; O’Neil, M.; Milhous, W.; Wirth, D.F.; Oduola, A.M. Selection of Plasmodium falciparum multidrug resistance gene 1 alleles in asexual stages and gametocytes by artemether-lumefantrine in Nigerian children with uncomplicated falciparum malaria. Antimicrob. Agents Chemother. 2009, 53, 888–895. [Google Scholar] [CrossRef]
- Kavishe, R.A.; Paulo, P.; Kaaya, R.D.; Kalinga, A.; van Zwetselaar, M.; Chilongola, J.; Roper, C.; Alifrangis, M. Surveillance of artemether-lumefantrine associated Plasmodium falciparum multidrug resistance protein-1 gene polymorphisms in Tanzania. Malar. J. 2014, 13, 264. [Google Scholar] [CrossRef] [PubMed]
- Baliraine, F.N.; Rosenthal, P.J. Prolonged selection of pfmdr1 polymorphisms after treatment of falciparum malaria with artemether-lumefantrine in Uganda. J. Infect. Dis. 2011, 204, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- Dahlstrom, S.; Ferreira, P.E.; Veiga, M.I.; Sedighi, N.; Wiklund, L.; Martensson, A.; Farnert, A.; Sisowath, C.; Osorio, L.; Darban, H.; et al. Plasmodium falciparum multidrug resistance protein 1 and artemisinin-based combination therapy in Africa. J. Infect. Dis. 2009, 200, 1456–1464. [Google Scholar] [CrossRef]
- Venkatesan, M.; Gadalla, N.B.; Stepniewska, K.; Dahal, P.; Nsanzabana, C.; Moriera, C.; Price, R.N.; Martensson, A.; Rosenthal, P.J.; Dorsey, G.; et al. Polymorphisms in Plasmodium falciparum chloroquine resistance transporter and multidrug resistance 1 genes: Parasite risk factors that affect treatment outcomes for P. falciparum malaria after artemether-lumefantrine and artesunate-amodiaquine. Am. J. Trop. Med. Hyg. 2014, 91, 833–843. [Google Scholar] [CrossRef]
- Ariey, F.; Witkowski, B.; Amaratunga, C.; Beghain, J.; Langlois, A.C.; Khim, N.; Kim, S.; Duru, V.; Bouchier, C.; Ma, L.; et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014, 505, 50–55. [Google Scholar] [CrossRef]
- Gupta, B.; Xu, S.; Wang, Z.; Sun, L.; Miao, J.; Cui, L.; Yang, Z. Plasmodium falciparum multidrug resistance protein 1 (pfmrp1) gene and its association with in vitro drug susceptibility of parasite isolates from north-east Myanmar. J. Antimicrob. Chemother. 2014, 69, 2110–2117. [Google Scholar] [CrossRef][Green Version]
- Djimde, A.A.; Makanga, M.; Kuhen, K.; Hamed, K. The emerging threat of artemisinin resistance in malaria: Focus on artemether-lumefantrine. Expert Rev. Anti. Infect. Ther. 2015, 13, 1031–1045. [Google Scholar] [CrossRef] [PubMed]
- Dama, S.; Niangaly, H.; Ouattara, A.; Sagara, I.; Sissoko, S.; Traore, O.B.; Bamadio, A.; Dara, N.; Djimde, M.; Alhousseini, M.L.; et al. Reduced ex vivo susceptibility of Plasmodium falciparum after oral artemether-lumefantrine treatment in Mali. Malar. J. 2017, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Dokomajilar, C.; Nsobya, S.L.; Greenhouse, B.; Rosenthal, P.J.; Dorsey, G. Selection of Plasmodium falciparum pfmdr1 alleles following therapy with artemether-lumefantrine in an area of Uganda where malaria is highly endemic. Antimicrob. Agents Chemother. 2006, 50, 1893–1895. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lefevre, G.; Carpenter, P.; Souppart, C.; Schmidli, H.; McClean, M.; Stypinski, D. Pharmacokinetics and electrocardiographic pharmacodynamics of artemether-lumefantrine (Riamet) with concomitant administration of ketoconazole in healthy subjects. Br. J. Clin. Pharmacol. 2002, 54, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Mwai, L.; Kiara, S.M.; Abdirahman, A.; Pole, L.; Rippert, A.; Diriye, A.; Bull, P.; Marsh, K.; Borrmann, S.; Nzila, A. In vitro activities of piperaquine, lumefantrine, and dihydroartemisinin in Kenyan Plasmodium falciparum isolates and polymorphisms in pfcrt and pfmdr1. Antimicrob. Agents Chemother. 2009, 53, 5069–5073. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, M.; Ferreira, P.E.; Tarning, J.; Ursing, J.; Ngasala, B.; Bjorkman, A.; Martensson, A.; Gil, J.P. Plasmodium falciparum drug resistance phenotype as assessed by patient antimalarial drug levels and its association with pfmdr1 polymorphisms. J. Infect. Dis. 2013, 207, 842–847. [Google Scholar] [CrossRef]
- Zeile, I.; Gahutu, J.B.; Shyirambere, C.; Steininger, C.; Musemakweri, A.; Sebahungu, F.; Karema, C.; Harms, G.; Eggelte, T.A.; Mockenhaupt, F.P. Molecular markers of Plasmodium falciparum drug resistance in southern highland Rwanda. Acta Trop. 2012, 121, 50–54. [Google Scholar] [CrossRef]
- Ochong, E.O.; van den Broek, I.V.; Keus, K.; Nzila, A. Short report: Association between chloroquine and amodiaquine resistance and allelic variation in the Plasmodium falciparum multiple drug resistance 1 gene and the chloroquine resistance transporter gene in isolates from the upper Nile in southern Sudan. Am. J. Trop. Med. Hyg. 2003, 69, 184–187. [Google Scholar] [CrossRef]
- Tinto, H.; Guekoun, L.; Zongo, I.; Guiguemde, R.T.; D’Alessandro, U.; Ouedraogo, J.B. Chloroquine-resistance molecular markers (Pfcrt T76 and Pfmdr-1 Y86) and amodiaquine resistance in Burkina Faso. Trop. Med. Int. Health 2008, 13, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Plowe, C.V.; Djimde, A.; Wellems, T.E.; Diop, S.; Kouriba, B.; Doumbo, O.K. Community pyrimethamine-sulfadoxine use and prevalence of resistant Plasmodium falciparum genotypes in Mali: A model for deterring resistance. Am. J. Trop. Med. Hyg. 1996, 55, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Djimde, A.A.; Barger, B.; Kone, A.; Beavogui, A.H.; Tekete, M.; Fofana, B.; Dara, A.; Maiga, H.; Dembele, D.; Toure, S.; et al. A molecular map of chloroquine resistance in Mali. FEMS Immunol. Med. Microbiol. 2010, 58, 113–118. [Google Scholar] [CrossRef]
- Djimde, A.A.; Doumbo, O.K.; Traore, O.; Guindo, A.B.; Kayentao, K.; Diourte, Y.; Niare-Doumbo, S.; Coulibaly, D.; Kone, A.K.; Cissoko, Y.; et al. Clearance of drug-resistant parasites as a model for protective immunity in Plasmodium falciparum malaria. Am. J. Trop. Med. Hyg. 2003, 69, 558–563. [Google Scholar] [CrossRef] [PubMed]
- PNLP: PNDLclP. Manuel du Participant. BG VF: Formation Pour la Prise en Charge du Paludisme Dans les Structures Sanitaires; PNLP: Bamako, Mali, 2009. [Google Scholar]
- Plowe, C.V.; Djimde, A.; Bouare, M.; Doumbo, O.; Wellems, T.E. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: Polymerase chain reaction methods for surveillance in Africa. Am. J. Trop. Med. Hyg. 1995, 52, 565–568. [Google Scholar] [CrossRef]
- Snounou, G.; Zhu, X.; Siripoon, N.; Jarra, W.; Thaithong, S.; Brown, K.N.; Viriyakosol, S. Biased distribution of msp1 and msp2 allelic variants in Plasmodium falciparum populations in Thailand. Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 369–374. [Google Scholar] [CrossRef]
- Ranford-Cartwright, L.C.; Taylor, J.; Umasunthar, T.; Taylor, L.H.; Babiker, H.A.; Lell, B.; Schmidt-Ott, J.R.; Lehman, L.G.; Walliker, D.; Kremsner, P.G. Molecular analysis of recrudescent parasites in a Plasmodium falciparum drug efficacy trial in Gabon. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 719–724. [Google Scholar] [CrossRef]
- Su, X.; Ferdig, M.T.; Huang, Y.; Huynh, C.Q.; Liu, A.; You, J.; Wootton, J.C.; Wellems, T.E. A genetic map and recombination parameters of the human malaria parasite Plasmodium falciparum. Science 1999, 286, 1351–1353. [Google Scholar] [CrossRef]
- Duraisingh, M.T.; Jones, P.; Sambou, I.; von Seidlein, L.; Pinder, M.; Warhurst, D.C. The tyrosine-86 allele of the pfmdr1 gene of Plasmodium falciparum is associated with increased sensitivity to the anti-malarials mefloquine and artemisinin. Mol. Biochem. Parasitol. 2000, 108, 13–23. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).