PTX3 Effects on Osteogenic Differentiation in Osteoporosis: An In Vitro Study
Abstract
1. Introduction
2. Results
2.1. Histomorphometrical Analysis
2.2. Immunohistochemical Analysis
2.3. Human Recombinant Pentraxin 3 (hrPTX3) and Osteogenic Cocktail Treatment on Primary Osteoblasts Cultures
2.4. Effect of Treatment with hrPTX3 and Osteogenic Cocktail on the Calcification Process
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Bone Mineral Density Evaluation
4.3. Sampling
4.4. Histology
4.5. Histomorphometric Analysis
4.6. Immunohistochemistry
4.7. Human Osteoblast Primary Cell Cultures
4.8. Immunostaining of Primary Cell Cultures
4.9. Osteoblast Primary Cultures Conditioned with hrPTX3 and Osteogenic Cocktail
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarantino, U.; Greggi, C.; Cariati, I.; Visconti, V.V.; Gasparini, M.; Cateni, M.; Gasbarra, E.; Botta, A.; Salustri, A.; Scimeca, M. The Role of PTX3 in Mineralization Processes and Aging-Related Bone Diseases. Front. Immunol. 2021, 11, 622772. [Google Scholar] [CrossRef]
- Scimeca, M.; Salustri, A.; Bonanno, E.; Nardozi, D.; Rao, C.; Piccirilli, E.; Feola, M.; Tancredi, V.; Rinaldi, A.; Iolascon, G.; et al. Impairment of PTX3 expression in osteoblasts: A key element for osteoporosis. Cell Death Dis. 2017, 8, e3125. [Google Scholar] [CrossRef]
- Bottazzi, B.; Inforzato, A.; Messa, M.; Barbagallo, M.; Magrini, E.; Garlanda, C.; Mantovani, A. The pentraxins PTX3 and SAP in innate immunity, regulation of inflammation and tissue remodelling. J. Hepatol. 2016, 64, 1416–1427. [Google Scholar] [CrossRef]
- Doni, A.; D’Amico, G.; Morone, D.; Mantovani, A.; Garlanda, C. Humoral innate immunity at the crossroad between microbe and matrix recognition: The role of PTX3 in tissue damage. Semin. Cell Dev. Biol. 2017, 61, 31–40. [Google Scholar] [CrossRef]
- Garlanda, C.; Bottazzi, B.; Bastone, A.; Mantovani, A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu. Rev. Immunol. 2005, 23, 337–366. [Google Scholar] [CrossRef] [PubMed]
- Garlanda, C.; Bottazzi, B.; Magrini, E.; Inforzato, A.; Mantovani, A. PTX3, a Humoral Pattern Recognition Molecule, in Innate Immunity, Tissue Repair, and Cancer. Physiol. Rev. 2018, 98, 623–639. [Google Scholar] [CrossRef]
- Bottazzi, B.; Doni, A.; Garlanda, C.; Mantovani, A. An integrated view of humoral innate immunity: Pentraxins as a paradigm. Annu. Rev. Immunol. 2010, 28, 157–183. [Google Scholar] [CrossRef] [PubMed]
- Deban, L.; Russo, R.C.; Sironi, M.; Moalli, F.; Scanziani, M.; Zambelli, V.; Cuccovillo, I.; Bastone, A.; Gobbi, M.; Valentino, S.; et al. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat. Immunol. 2010, 11, 328–334. [Google Scholar] [CrossRef]
- Garlanda, C.; Hirsch, E.; Bozza, S.; Salustri, A.; De Acetis, M.; Nota, R.; Maccagno, A.; Riva, F.; Bottazzi, B.; Peri, G.; et al. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature 2002, 420, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Reading, P.C.; Bozza, S.; Gilbertson, B.; Tate, M.; Moretti, S.; Job, E.R.; Crouch, E.C.; Brooks, A.G.; Brown, L.E.; Bottazzi, B.; et al. Antiviral activity of the long chain pentraxin PTX3 against influenza viruses. J. Immunol. 2008, 180, 3391–3398. [Google Scholar] [CrossRef]
- Porte, R.; Davoudian, S.; Asgari, F.; Parente, R.; Mantovani, A.; Garlanda, C.; Bottazzi, B. The Long Pentraxin PTX3 as a Humoral Innate Immunity Functional Player and Biomarker of Infections and Sepsis. Front. Immunol. 2019, 10, 794. [Google Scholar] [CrossRef]
- Cieślik, P.; Hrycek, A. Long pentraxin 3 (PTX3) in the light of its structure, mechanism of action and clinical implications. Autoimmunity 2012, 45, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Camozzi, M.; Zacchigna, S.; Rusnati, M.; Coltrini, D.; Ramirez-Correa, G.; Bottazzi, B.; Mantovani, A.; Giacca, M.; Presta, M. Pentraxin 3 inhibits fibroblast growth factor 2-dependent activation of smooth muscle cells in vitro and neointima formation in vivo. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1837–1842. [Google Scholar] [CrossRef]
- Leali, D.; Inforzato, A.; Ronca, R.; Bianchi, R.; Belleri, M.; Coltrini, D.; Di Salle, E.; Sironi, M.; Norata, G.D.; Bottazzi, B.; et al. Long pentraxin 3/tumor necrosis factor-stimulated gene-6 interaction: A biological rheostat for fibroblast growth factor 2-mediated angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Bonacina, F.; Barbieri, S.S.; Cutuli, L.; Amadio, P.; Doni, A.; Sironi, M.; Tartari, S.; Mantovani, A.; Bottazzi, B.; Garlanda, C.; et al. Vascular pentraxin 3 controls arterial thrombosis by targeting collagen and fibrinogen induced platelets aggregation. Biochim. Biophys. Acta 2016, 1862, 1182–1190. [Google Scholar] [CrossRef]
- Bonfiglio, R.; Scimeca, M.; Toschi, N.; Pistolese, C.A.; Giannini, E.; Antonacci, C.; Ciuffa, S.; Tancredi, V.; Tarantino, U.; Albonici, L.; et al. Radiological, Histological and Chemical Analysis of Breast Microcalcifications: Diagnostic Value and Biological Significance. J. Mammary Gland Biol. Neoplasia 2018, 23, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Antonacci, C.; Colombo, D.; Bonfiglio, R.; Buonomo, O.C.; Bonanno, E. Emerging prognostic markers related to mesenchymal characteristics of poorly differentiated breast cancers. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2016, 37, 5427–5435. [Google Scholar] [CrossRef] [PubMed]
- Visconti, V.V.; Greggi, C.; Fittipaldi, S.; Casamassima, D.; Tallarico, M.G.; Romano, F.; Botta, A.; Tarantino, U. The long pentraxin PTX3: A novel serum marker to improve the prediction of osteoporosis and osteoarthritis bone-related phenotypes. J. Orthop. Surg. Res. 2021, 16, 288. [Google Scholar] [CrossRef]
- Kim, Y.; Park, J.-Y.; Park, H.-J.; Kim, M.-K.; Kim, Y.-I.; Kim, H.J.; Bae, S.-K.; Bae, M.-K. Pentraxin-3 Modulates Osteogenic/Odontogenic Differentiation and Migration of Human Dental Pulp Stem Cells. Int. J. Mol. Sci. 2019, 20, 5778. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Zhou, X.-Z.; Li, N.; Guo, Y.-C.; Chen, T.-P. Pentraxin 3 promotes the osteoblastic differentiation of MC3T3-E1 cells through the PI3K/Akt signaling pathway. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef]
- Moalli, F.; Jaillon, S.; Inforzato, A.; Sironi, M.; Bottazzi, B.; Mantovani, A.; Garlanda, C. Pathogen recognition by the long pentraxin PTX3. J. Biomed. Biotechnol. 2011, 2011, 830421. [Google Scholar] [CrossRef] [PubMed]
- Diniz, S.N.; Nomizo, R.; Cisalpino, P.S.; Teixeira, M.M.; Brown, G.D.; Mantovani, A.; Gordon, S.; Reis, L.F.L.; Dias, A.A.M. PTX3 function as an opsonin for the dectin-1-dependent internalization of zymosan by macrophages. J. Leukoc. Biol. 2004, 75, 649–656. [Google Scholar] [CrossRef]
- van Rossum, A.P.; Fazzini, F.; Limburg, P.C.; Manfredi, A.A.; Rovere-Querini, P.; Mantovani, A.; Kallenberg, C.G.M. The prototypic tissue pentraxin PTX3, in contrast to the short pentraxin serum amyloid P, inhibits phagocytosis of late apoptotic neutrophils by macrophages. Arthritis Rheum. 2004, 50, 2667–2674. [Google Scholar] [CrossRef]
- Cappuzzello, C.; Doni, A.; Dander, E.; Pasqualini, F.; Nebuloni, M.; Bottazzi, B.; Mantovani, A.; Biondi, A.; Garlanda, C.; D’Amico, G. Mesenchymal Stromal Cell-Derived PTX3 Promotes Wound Healing via Fibrin Remodeling. J. Investig. Dermatol. 2016, 136, 293–300. [Google Scholar] [CrossRef]
- Scarchilli, L.; Camaioni, A.; Bottazzi, B.; Negri, V.; Doni, A.; Deban, L.; Bastone, A.; Salvatori, G.; Mantovani, A.; Siracusa, G.; et al. PTX3 interacts with inter-alpha-trypsin inhibitor: Implications for hyaluronan organization and cumulus oophorus expansion. J. Biol. Chem. 2007, 282, 30161–30170. [Google Scholar] [CrossRef]
- Wisniewski, H.-G.; Vilcek, J. Cytokine-induced gene expression at the crossroads of innate immunity, inflammation and fertility: TSG-6 and PTX3/TSG-14. Cytokine Growth Factor Rev. 2004, 15, 129–146. [Google Scholar] [CrossRef]
- Lee, E.-J.; Song, D.-H.; Kim, Y.-J.; Choi, B.; Chung, Y.-H.; Kim, S.-M.; Koh, J.-M.; Yoon, S.-Y.; Song, Y.; Kang, S.-W.; et al. PTX3 stimulates osteoclastogenesis by increasing osteoblast RANKL production. J. Cell. Physiol. 2014, 229, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Grčević, D.; Sironi, M.; Valentino, S.; Deban, L.; Cvija, H.; Inforzato, A.; Kovačić, N.; Katavić, V.; Kelava, T.; Kalajzić, I.; et al. The Long Pentraxin 3 Plays a Role in Bone Turnover and Repair. Front. Immunol. 2018, 9, 417. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, U.; Iolascon, G.; Cianferotti, L.; Masi, L.; Marcucci, G.; Giusti, F.; Marini, F.; Parri, S.; Feola, M.; Rao, C.; et al. Clinical guidelines for the prevention and treatment of osteoporosis: Summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J. Orthop. Traumatol. Off. J. Ital. Soc. Orthop. Traumatol. 2017, 18, 3–36. [Google Scholar] [CrossRef]
- Celi, M.; Rao, C.; Scialdoni, A.; Tempesta, V.; Gasbarra, E.; Pistillo, P.; Tarantino, U. Bone mineral density evaluation in osteoporosis: Why yes and why not? Aging Clin. Exp. Res. 2013, 25 (Suppl. 1), S47–S49. [Google Scholar] [CrossRef]
- Piccirilli, E.; Gasbarra, E.; Baldi, J.; Pistillo, P.; Tarantino, U. Gerontology & Geriatric Research Can Muscular Impairment be the “Key ” for Femoral Fracture? J. Gerontol. Geriatr. Res. 2014, 3, 3–6. [Google Scholar] [CrossRef]
- Fox, C.H.; Johnson, F.B.; Whiting, J.; Roller, P.P. Formaldehyde fixation. J. Histochem. Cytochem. Off. J. Histochem. Soc. 1985, 33, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, U.; Celi, M.; Rao, C.; Feola, M.; Cerocchi, I.; Gasbarra, E.; Ferlosio, A.; Orlandi, A. Hip osteoarthritis and osteoporosis: Clinical and histomorphometric considerations. Int. J. Endocrinol. 2014, 2014, 372021. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef] [PubMed]
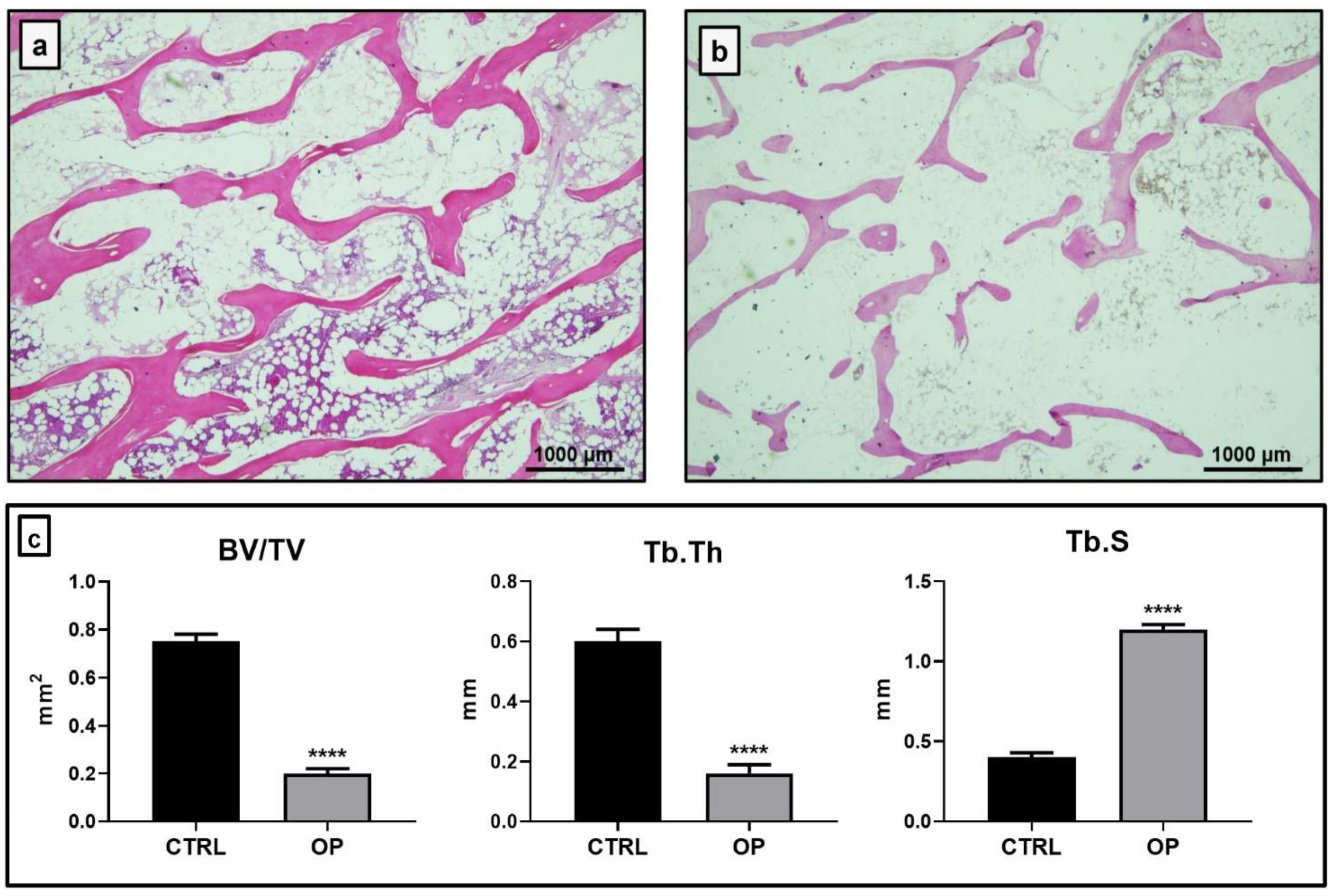
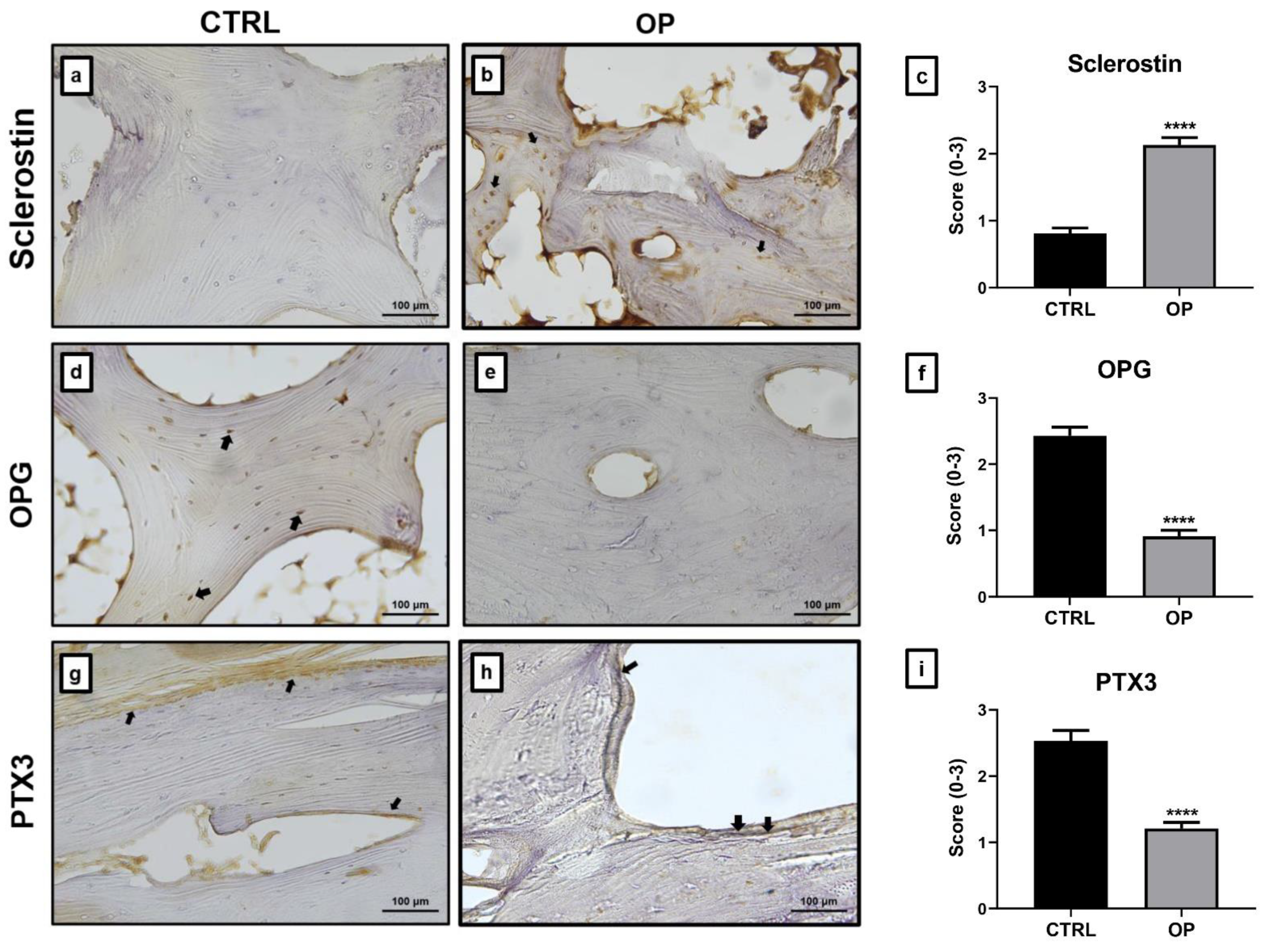
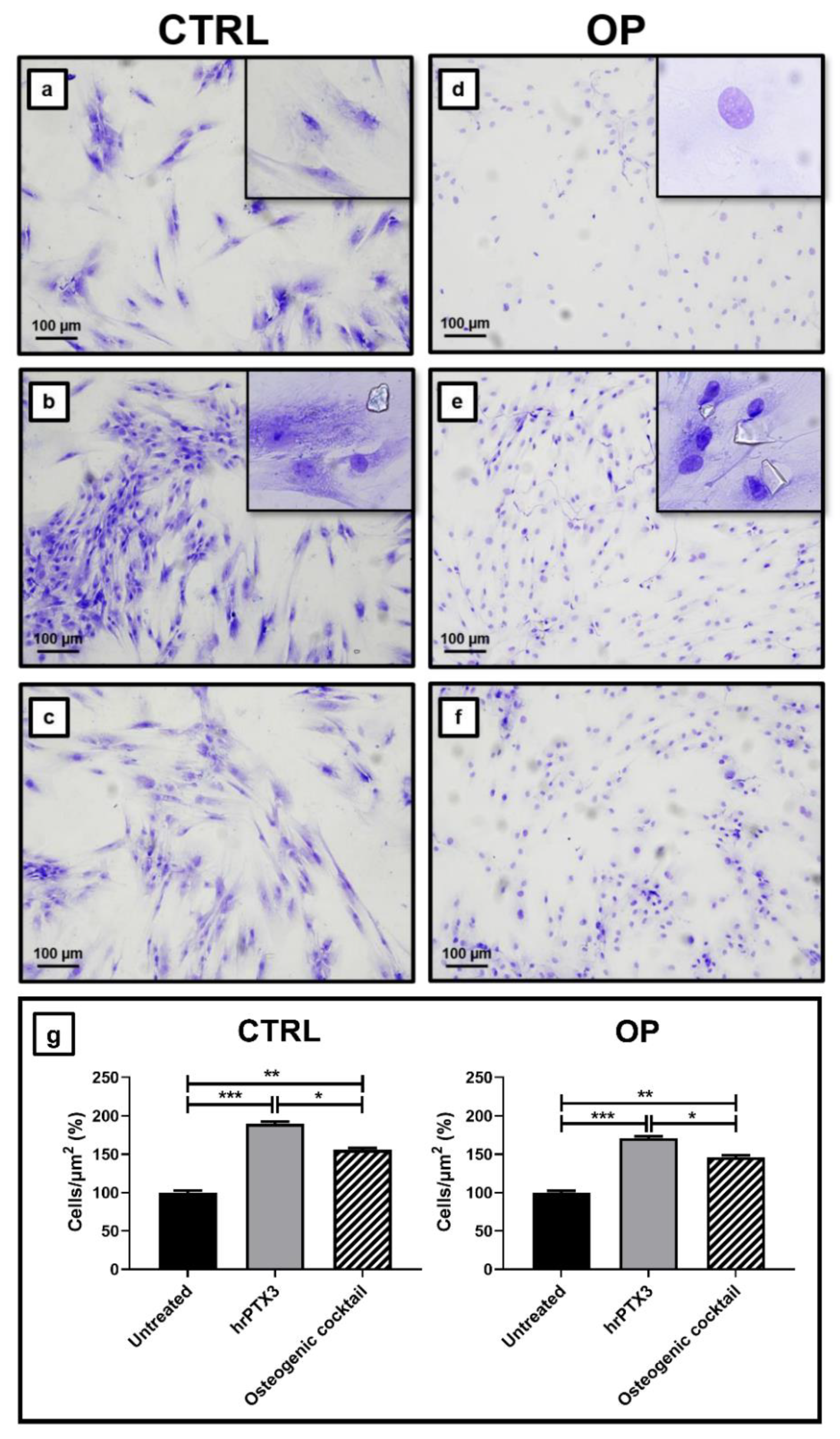
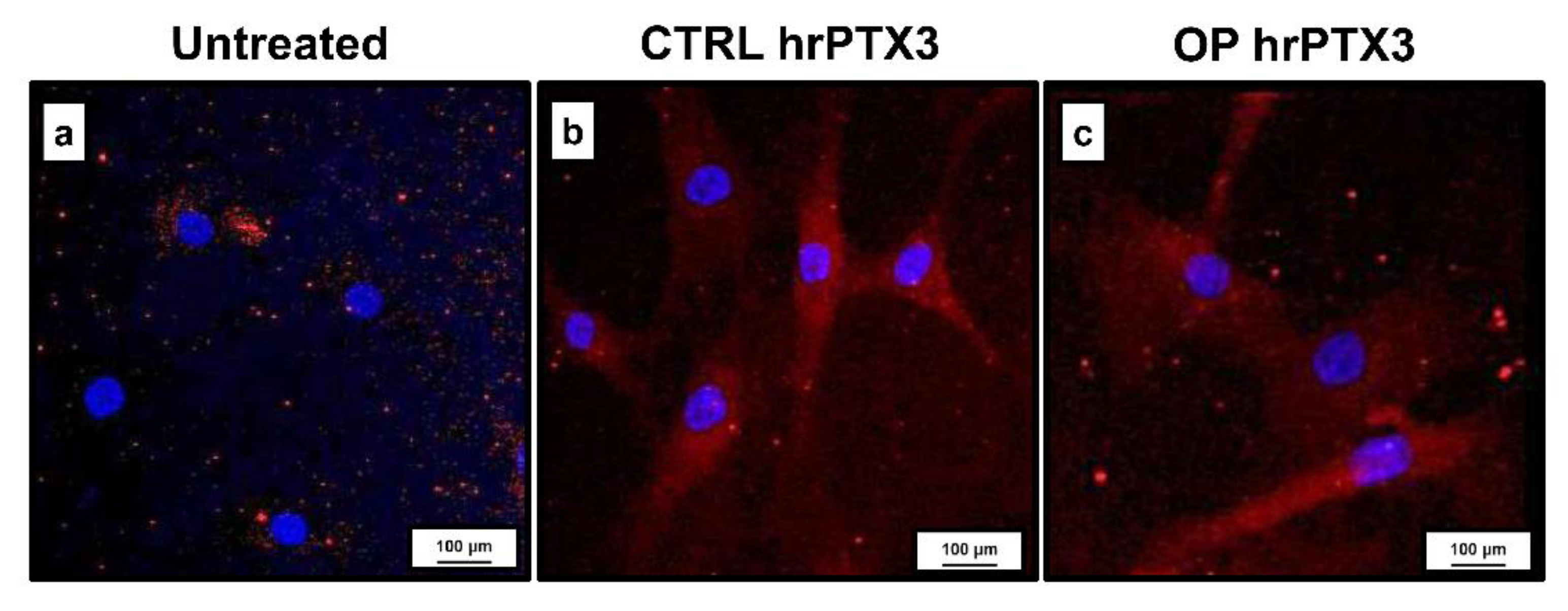

| Parameters | CTRL | OP | t-Test (Mann–Whitney Test) |
|---|---|---|---|
| Age (years) | 49.1 ± 1.3 | 78.5 ± 2.1 | *** p < 0.001 |
| T-score (L1–L4) | 1.1 ± 0.03 | −2.5 ± 0.5 | ** p < 0.01 |
| T-score (femoral neck) | 1.5 ± 0.2 | −2.7 ± 0.14 | ** p < 0.01 |
| T-score (total femur) | 1.3 ± 0.3 | −2.1 ± 0.3 | ** p < 0.01 |
| Phosphorous (mg/dl) | 3.8 ± 0.4 | 2.6 ± 0.2 | * p < 0.05 |
| Calcium (mg/dl) | 6.99 ± 0.12 | 9.5 ± 0.2 | * p < 0.05 |
| SCORE | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Positive Cells | ≤2 | 3 ≤ x ≤ 12 | 13 ≤ x ≤ 22 | ≥23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greggi, C.; Cariati, I.; Onorato, F.; Iundusi, R.; Scimeca, M.; Tarantino, U. PTX3 Effects on Osteogenic Differentiation in Osteoporosis: An In Vitro Study. Int. J. Mol. Sci. 2021, 22, 5944. https://doi.org/10.3390/ijms22115944
Greggi C, Cariati I, Onorato F, Iundusi R, Scimeca M, Tarantino U. PTX3 Effects on Osteogenic Differentiation in Osteoporosis: An In Vitro Study. International Journal of Molecular Sciences. 2021; 22(11):5944. https://doi.org/10.3390/ijms22115944
Chicago/Turabian StyleGreggi, Chiara, Ida Cariati, Federica Onorato, Riccardo Iundusi, Manuel Scimeca, and Umberto Tarantino. 2021. "PTX3 Effects on Osteogenic Differentiation in Osteoporosis: An In Vitro Study" International Journal of Molecular Sciences 22, no. 11: 5944. https://doi.org/10.3390/ijms22115944
APA StyleGreggi, C., Cariati, I., Onorato, F., Iundusi, R., Scimeca, M., & Tarantino, U. (2021). PTX3 Effects on Osteogenic Differentiation in Osteoporosis: An In Vitro Study. International Journal of Molecular Sciences, 22(11), 5944. https://doi.org/10.3390/ijms22115944








