Presence of β-Lactamase-producing Enterobacterales and Salmonella Isolates in Marine Mammals
Abstract
1. Introduction
2. Results
2.1. β-Lactamase Producing Isolates, Species Identification and Phenotypic Resistance
2.2. Characterization of Genotypic Resistance
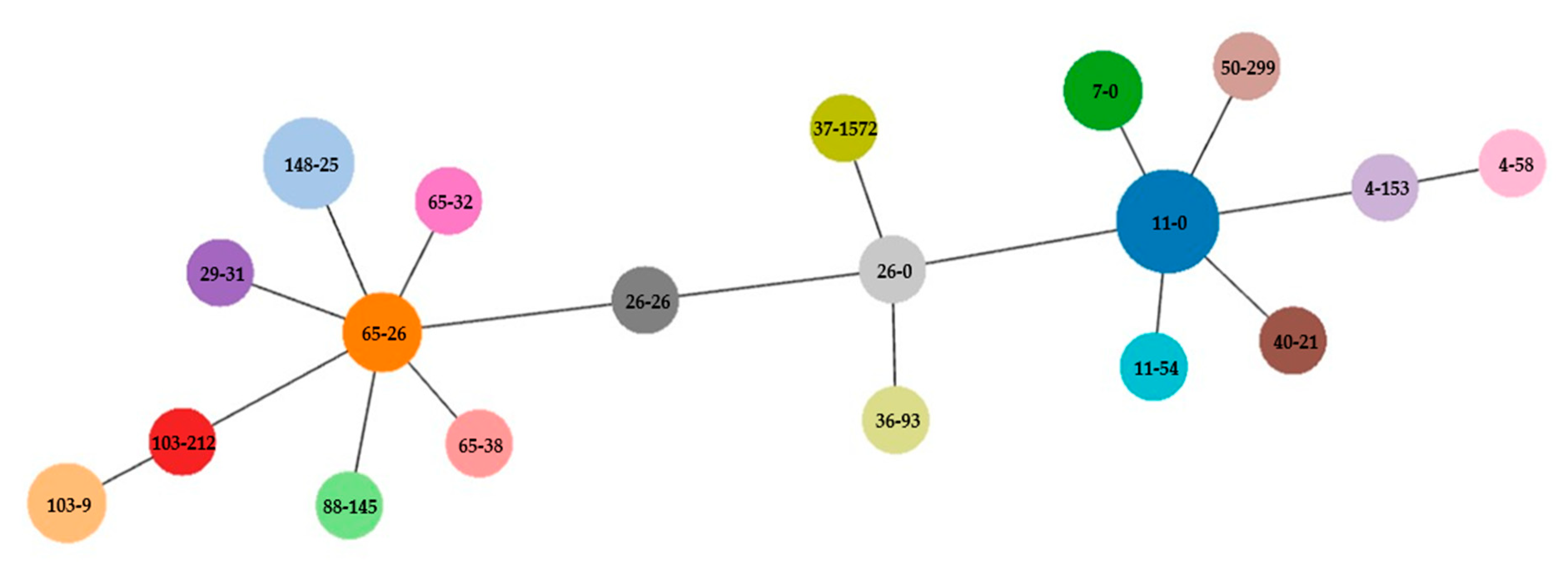
2.3. Molecular Typing Methods
2.4. Characterization of E. coli Virulence Genes
2.5. Whole-Genome Sequencing (WGS) of Selected E. coli Isolates
2.6. Characterization of Salmonella Isolates
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates
4.2. Antimicrobial and Biocide Susceptibility Testing
4.3. Characterization of Genotypic Resistance
4.4. Molecular Typing Methods
4.5. Virulence Genes
4.6. Whole-Genome Sequencing (WGS)
4.7. Characterization of Salmonella Isolates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bassetti, M.; Pecori, D.; Sibani, M.; Corcione, S.; Rosa, F.G.d. Epidemiology and Treatment of MDR Enterobacteriaceae. Curr. Treat. Options Infect. Dis. 2015, 7, 291–316. [Google Scholar] [CrossRef]
- Wilson, H.; Török, M.E. Extended-Spectrum Β-Lactamase-Producing and Carbapenemase-Producing Enterobacteriaceae. Microb. Genom. 2018, 4, e000197. [Google Scholar] [CrossRef]
- Gajdács, M.; Bátori, Z.; Ábrók, M.; Lázár, A.; Burián, K. Characterization of Resistance in Gram-Negative Urinary Isolates Using Existing and Novel Indicators of Clinical Relevance: A 10-Year Data Analysis. Life 2020, 10, 16. [Google Scholar] [CrossRef]
- Kadri, S.S.; Adjemian, J.; Lai, Y.L.; Spaulding, A.B.; Ricotta, E.; Prevots, D.R.; Palmore, T.N.; Rhee, C.; Klompas, M.; Dekker, J.P.; et al. Difficult-to-Treat Resistance in Gram-Negative Bacteremia at 173 US Hospitals: Retrospective Cohort Analysis of Prevalence, Predictors, and Outcome of Resistance to All First-line Agents. Clin. Infect. Dis. 2018, 67, 1803–1814. [Google Scholar] [CrossRef]
- Jacoby, G.A.; Munoz-Price, L.S. The New Beta-Lactamases. N. Engl. J. Med. 2005, 352, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Bush, K. Past and Present Perspectives on β-Lactamases. Antimicrob. Agents Chemother. 2018, 62, e01076-18. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, G.A. AmpC Beta-Lactamases. Clin. Microbiol. Rev. 2009, 22, 161–182. [Google Scholar] [CrossRef]
- Tamma, P.D.; Doi, Y.; Bonomo, R.A.; Johnson, J.K.; Simner, P.J. A Primer on AmpC β-Lactamases: Necessary Knowledge for an Increasingly Multidrug-Resistant World. Clin. Infect. Dis. 2019, 69, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Ewers, C.; Bethe, A.; Semmler, T.; Guenther, S.; Wieler, L.H. Extended-Spectrum β-Lactamase-Producing and AmpC-Producing Escherichia coli from Livestock and Companion Animals, and Their Putative Impact on Public Health: A Global Perspective. Clin. Microbiol. Infect. 2012, 18, 646–655. [Google Scholar] [CrossRef]
- Karl, H.A.; Chin, J.L.; Ueber, E.; Stauffer, P.H.; Hendley, J.W., II (Eds.) Beyond the Golden Gate—Oceanography, Geology, Biology, and Environmental Issues in the Gulf of the Farallones; U.S. Geological Survey: Reston, VA, USA; USGS Information Services: Denver, CO, USA, 2001.
- Hatosy, S.M.; Martiny, A.C. The Ocean as a Global Reservoir of Antibiotic Resistance Genes. Appl. Environ. Microbiol. 2015, 81, 7593–7599. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, G.; Song, W.; Ye, C.; Lin, H.; Li, Z.; Liu, W. Plastics in the Marine Environment Are Reservoirs for Antibiotic and Metal Resistance Genes. Environ. Int. 2019, 123, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Paikin, S.; Rokney, A.; Rubin-Blum, M.; Astrahan, P. Multidrug-Resistant Enterobacteriaceae in Coastal Water: An Emerging Threat. Antimicrob. Resist. Infect. Control 2020, 9, 169. [Google Scholar] [CrossRef]
- Nappier, S.P.; Liguori, K.; Ichida, A.M.; Stewart, J.R.; Jones, K.R. Antibiotic Resistance in Recreational Waters: State of the Science. Int. J. Environ. Res. Public Health 2020, 17, 8034. [Google Scholar] [CrossRef]
- Shallcross, L.J.; Howard, S.J.; Fowler, T.; Davies, S.C. Tackling the Threat of Antimicrobial Resistance: From Policy to Sustainable action. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140082. [Google Scholar] [CrossRef]
- Ramey, A.M.; Ahlstrom, C.A. Antibiotic Resistant Bacteria in Wildlife: Perspectives on Trends, Acquisition and Dissemination, Data Gaps, and Future Directions. J. Wildl. Dis. 2020, 56, 1–15. [Google Scholar] [CrossRef]
- Hocquet, D.; Muller, A.; Bertrand, X. What Happens IN Hospitals Does Not Stay in Hospitals: Antibiotic-Resistant Bacteria in Hospital Wastewater Systems. J. Hosp. Infect. 2016, 93, 395–402. [Google Scholar] [CrossRef]
- Muziasari, W.I.; Pitkänen, L.K.; Sørum, H.; Stedtfeld, R.D.; Tiedje, J.M.; Virta, M. The Resistome of Farmed Fish Feces Contributes to the Enrichment of Antibiotic Resistance Genes in Sediments below Baltic Sea Fish Farms. Front. Microbiol. 2017, 7, 2137. [Google Scholar] [CrossRef]
- Wallace, C.C.; Yund, P.O.; Ford, T.E.; Matassa, K.A.; Bass, A.L. Increase in antimicrobial resistance in bacteria isolated from stranded marine mammals of the Northwest Atlantic. EcoHealth 2013, 10, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Carroll, D.; Wang, J.; Fanning, S.; McMahon, B.J. Antimicrobial Resistance in Wildlife: Implications for Public Health. Zoonoses Public Health 2015, 62, 534–542. [Google Scholar] [CrossRef]
- Furness, L.E.; Campbell, A.; Zhang, L.; Gaze, W.H.; McDonald, R.A. Wild Small Mammals as Sentinels for the Environmental Transmission of Antimicrobial Resistance. Environ. Res. 2017, 154, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Rodríguez, C.; Alt, K.; Grobbel, M.; Hammerl, J.A.; Irrgang, A.; Szabo, I.; Stingl, K.; Schuh, E.; Wiehle, L.; Pfefferkorn, B.; et al. Wildlife as Sentinels of Antimicrobial Resistance in Germany? Front. Vet. Sci. 2021, 7, 627821. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.; Rhinehart, H.; Hansen, L.; Sweeney, J.; Townsend, F.; Stone, R.; Casper, D.R.; Scott, M.; Hohn, A.; Rowles, T. Bottlenose Dolphins as Marine Ecosystem Sentinels: Developing a Health Monitoring System. EcoHealth 2004, 1, 246–254. [Google Scholar] [CrossRef]
- Bossart, G.D. Marine Mammals as Sentinel Species for Oceans and Human Health. Vet. Pathol. 2011, 48, 676–690. [Google Scholar] [CrossRef]
- Reif, J.S. Animal Sentinels for Environmental and Public Health. Public Heal. Rep. 2011, 126, 50–57. [Google Scholar] [CrossRef]
- Johnson, S.P.; Nolan, S.; Gulland, F.M. Antimicrobial Susceptibility of Bacteria Isolated from Pinnipeds Stranded in Central and Northern California. J. Zoo Wildl. Med. 1998, 29, 288–294. [Google Scholar]
- Thornton, S.M.; Nolan, S.; Gulland, F.M. Bacterial Isolates from California Sea Lions (Zalophus californianus), Harbor Seals (Phoca vitulina), and Northern Elephant Seals (Mirounga angustirostris) Admitted to a Rehabilitation Center Along the Central California Coast, 1994–1995. J. Zoo Wildl. Med. 1998, 29, 171–176. [Google Scholar]
- Stoddard, R.A.; Atwill, E.R.; Conrad, P.A.; Byrne, B.A.; Jang, S.; Lawrence, J.; McCowan, B.; Gulland, F.M.D. The Effect of Rehabilitation of Northern Elephant Seals (Mirounga angustirostris) on Antimicrobial Resistance of Commensal Escherichia coli. Vet. Microbiol. 2009, 133, 264–271. [Google Scholar] [CrossRef]
- Lockwood, S.K.; Chovan, J.L.; Gaydos, J.K. Aerobic Bacterial Isolations from Harbor Seals (Phoca vitulina) Stranded in Washington: 1992–2003. J. Zoo Wildl. Med. 2006, 37, 281–291. [Google Scholar] [CrossRef]
- Santestevan, N.A.; Angelis Zvoboda, D.d.; Prichula, J.; Pereira, R.I.; Wachholz, G.R.; Cardoso, L.A.; Moura, T.M.d.; Medeiros, A.W.; Amorin, D.B.d.; Tavares, M.; et al. Antimicrobial Resistance and Virulence Factor Gene Profiles of Enterococcus spp. Isolates from wild Arctocephalus australis (South American Fur Seal) and Arctocephalus tropicalis (Subantarctic Fur Seal). World J. Microbiol. Biotechnol. 2015, 31, 1935–1946. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.T.; Lubbers, B.V.; Schwarz, S.; Watts, J.L. Applying Definitions for Multidrug Resistance, Extensive Drug Resistance and Pandrug Resistance to Clinically Significant Livestock and Companion Animal Bacterial Pathogens. J. Antimicrob. Chemother. 2018, 73, 1460–1463. [Google Scholar] [CrossRef]
- Sarowska, J.; Futoma-Koloch, B.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Ksiazczyk, M.; Bugla-Ploskonska, G.; Choroszy-Krol, I. Virulence Factors, Prevalence and Potential Transmission of Extraintestinal Pathogenic Escherichia coli Isolated from Different Sources: Recent Reports. Gut Pathog. 2019, 11, 10. [Google Scholar] [CrossRef]
- Edgar, R.; Bibi, E. MdfA, an Escherichia coli Multidrug Resistance Protein with an Extraordinarily Broad Spectrum of Drug Recognition. J. Bacteriol. 1997, 179, 2274–2280. [Google Scholar] [CrossRef]
- Liebana, E.; Carattoli, A.; Coque, T.M.; Hasman, H.; Magiorakos, A.-P.; Mevius, D.; Peixe, L.; Poirel, L.; Schuepbach-Regula, G.; Torneke, K.; et al. Public Health Risks of Enterobacterial Isolates Producing Extended-Spectrum Β-Lactamases or AmpC β-Lactamases in Food and Food-Producing Animals: An EU Perspective of Epidemiology, Analytical Methods, Risk Factors, and Control Options. Clin. Infect. Dis. 2013, 56, 1030–1037. [Google Scholar] [CrossRef]
- Darwich, L.; Vidal, A.; Seminati, C.; Albamonte, A.; Casado, A.; López, F.; Molina-López, R.A.; Migura-Garcia, L. High Prevalence and Diversity of Extended-Spectrum β-Lactamase and Emergence of OXA-48 Producing Enterobacterales in Wildlife in Catalonia. PLoS ONE 2019, 14, e0210686. [Google Scholar] [CrossRef] [PubMed]
- Athanasakopoulou, Z.; Tsilipounidaki, K.; Sofia, M.; Chatzopoulos, D.C.; Giannakopoulos, A.; Karakousis, I.; Giannakis, V.; Spyrou, V.; Touloudi, A.; Satra, M.; et al. Poultry and Wild Birds as a Reservoir of CMY-2 Producing Escherichia coli: The First Large-Scale Study in Greece. Antibiotics 2021, 10, 235. [Google Scholar] [CrossRef]
- Poirel, L.; Potron, A.; La Cuesta, C.d.; Cleary, T.; Nordmann, P.; Munoz-Price, L.S. Wild Coastline Birds as Reservoirs of Broad-Spectrum-β-Lactamase-Producing Enterobacteriaceae in Miami Beach, Florida. Antimicrob. Agents Chemother. 2012, 56, 2756–2758. [Google Scholar] [CrossRef] [PubMed]
- Skarżyńska, M.; Zając, M.M.; Bomba, A.; Bocian, Ł.; Kozdruń, W.; Polak, M.; Wiącek, J.; Wasyl, D. Antimicrobial Resistance Glides in the Sky—Free-Living Birds as a Reservoir of Resistant Escherichia coli With Zoonotic Potential. Front. Microbiol. 2021, 12, 656223. [Google Scholar] [CrossRef]
- Fang, L.-X.; Sun, J.; Li, L.; Deng, H.; Huang, T.; Yang, Q.-E.; Li, X.; Chen, M.-Y.; Liao, X.-P.; Liu, Y.-H. Dissemination of the Chromosomally Encoded Cmy-2 Cephalosporinase Gene in Escherichia coli Isolated from Animals. Int. J. Antimicrob. Agents 2015, 46, 209–213. [Google Scholar] [CrossRef]
- Vale, A.P.; Shubin, L.; Cummins, J.; Leonard, F.C.; Barry, G. Detection of blaOXA-1, blaTEM-1, and Virulence Factors in E. coli Isolated From Seals. Front. Vet. Sci. 2021, 8, 583759. [Google Scholar] [CrossRef]
- Chaves, J.; Ladona, M.G.; Segura, C.; Coira, A.; Reig, R.; Ampurdanés, C. SHV-1 Beta-Lactamase Is Mainly a Chromosomally Encoded Species-Specific Enzyme in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2001, 45, 2856–2861. [Google Scholar] [CrossRef] [PubMed]
- Loncaric, I.; Beiglböck, C.; Feßler, A.T.; Posautz, A.; Rosengarten, R.; Walzer, C.; Ehricht, R.; Monecke, S.; Schwarz, S.; Spergser, J.; et al. Characterization of ESBL- and AmpC-Producing and Fluoroquinolone-Resistant Enterobacteriaceae Isolated from Mouflons (Ovis orientalis musimon) in Austria and Germany. PLoS ONE 2016, 11, e0155786. [Google Scholar] [CrossRef]
- Soto, E.; Abdelrazek, S.M.R.; Basbas, C.; Duignan, P.J.; Rios, C.; Byrne, B.A. Environmental Persistence and Disinfectant Susceptibility of Klebsiella pneumoniae Recovered from Pinnipeds Stranded on the California Coast. Vet. Microbiol. 2020, 241, 108554. [Google Scholar] [CrossRef]
- Kampf, G. Biocidal Agents Used for Disinfection Can Enhance Antibiotic Resistance in Gram-Negative Species. Antibiotics 2018, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, P.; Baráth, Z.; Gajdács, M. It’s Not Easy Being Green: A Narrative Review on the Microbiology, Virulence and Therapeutic Prospects of Multidrug-Resistant Pseudomonas aeruginosa. Antibiotics 2021, 10, 42. [Google Scholar] [CrossRef]
- Escobar-Páramo, P.; Le Menac’h, A.; Le Gall, T.; Amorin, C.; Gouriou, S.; Picard, B.; Skurnik, D.; Denamur, E. Identification of Forces Shaping the Commensal Escherichia coli Genetic Structure by Comparing Animal and Human Isolates. Environ. Microbiol. 2006, 8, 1975–1984. [Google Scholar] [CrossRef]
- Delport, T.C.; Harcourt, R.G.; Beaumont, L.J.; Webster, K.N.; Power, M.L. Molecular Detection of Antibiotic-Resistance Determinants in Escherichia coli Isolated from the Endangered Australian Sea Lion (Neophoca cinerea). J. Wildl. Dis. 2015, 51, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Fulham, M.; Power, M.; Gray, R. Comparative ecology of Escherichia coli in Endangered Australian Sea Lion (Neophoca cinerea) Pups. Infect. Genet. Evol. 2018, 62, 262–269. [Google Scholar] [CrossRef]
- Fulham, M.; Power, M.; Gray, R. Diversity and Distribution of Escherichia coli in Three Species of Free-Ranging Australian Pinniped Pups. Front. Mar. Sci. 2020, 7, 571171. [Google Scholar] [CrossRef]
- Schaufler, K.; Semmler, T.; Wieler, L.H.; Wöhrmann, M.; Baddam, R.; Ahmed, N.; Müller, K.; Kola, A.; Fruth, A.; Ewers, C.; et al. Clonal Spread and Interspecies Transmission of Clinically Relevant ESBL-Producing Escherichia coli of ST410—Another Successful Pandemic Clone? FEMS Microbiol. Ecol. 2016, 92, fiv155. [Google Scholar] [CrossRef] [PubMed]
- Giufrè, M.; Errico, G.; Accogli, M.; Monaco, M.; Villa, L.; Distasi, M.A.; Del Gaudio, T.; Pantosti, A.; Carattoli, A.; Cerquetti, M. Emergence of NDM-5-Producing Escherichia coli Sequence Type 167 Clone in Italy. Int. J. Antimicrob. Agents 2018, 52, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yu, X.; Xie, M.; Wang, X.; Liao, K.; Xue, W.; Chan, E.W.-C.; Zhang, R.; Chen, S. Widespread Dissemination of Carbapenem-Resistant Escherichia coli Sequence Type 167 Strains Harboring blaNDM-5 in Clinical Settings in China. Antimicrob. Agents Chemother. 2016, 60, 4364–4368. [Google Scholar] [CrossRef]
- Dadashi, M.; Yaslianifard, S.; Hajikhani, B.; Kabir, K.; Owlia, P.; Goudarzi, M.; Hakemivala, M.; Darban-Sarokhalil, D. Frequency Distribution, Genotypes and Prevalent Sequence Types of New Delhi Metallo-β-Lactamase-Producing Escherichia coli among Clinical Isolates around the World: A Review. J. Glob. Antimicrob. Resist. 2019, 19, 284–293. [Google Scholar] [CrossRef]
- Guenther, S.; Aschenbrenner, K.; Stamm, I.; Bethe, A.; Semmler, T.; Stubbe, A.; Stubbe, M.; Batsajkhan, N.; Glupczynski, Y.; Wieler, L.H.; et al. Comparable High Rates of Extended-Spectrum-Beta-Lactamase-Producing Escherichia coli in Birds of Prey from Germany and Mongolia. PLoS ONE 2012, 7, e53039. [Google Scholar] [CrossRef][Green Version]
- Dolejska, M.; Masarikova, M.; Dobiasova, H.; Jamborova, I.; Karpiskova, R.; Havlicek, M.; Carlile, N.; Priddel, D.; Cizek, A.; Literak, I. High Prevalence of Salmonella and IMP-4-Producing Enterobacteriaceae in the Silver Gull on Five Islands, Australia. J. Antimicrob. Chemother. 2016, 71, 63–70. [Google Scholar] [CrossRef][Green Version]
- Schaufler, K.; Semmler, T.; Wieler, L.H.; Trott, D.J.; Pitout, J.; Peirano, G.; Bonnedahl, J.; Dolejska, M.; Literak, I.; Fuchs, S.; et al. Genomic and Functional Analysis of Emerging Virulent and Multidrug-Resistant Escherichia coli Lineage Sequence Type 648. Antimicrob. Agents Chemother. 2019, 63, e00243-19. [Google Scholar] [CrossRef]
- Guenther, S.; Grobbel, M.; Beutlich, J.; Bethe, A.; Friedrich, N.D.; Goedecke, A.; Lübke-Becker, A.; Guerra, B.; Wieler, L.H.; Ewers, C. CTX-M-15-Type Extended-Spectrum Beta-Lactamases-Producing Escherichia coli from Wild Birds in Germany. Environ. Microbiol. Rep. 2010, 2, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Shnaiderman-Torban, A.; Steinman, A.; Meidan, G.; Paitan, Y.; Abu Ahmad, W.; Navon-Venezia, S. Petting Zoo Animals as an Emerging Reservoir of Extended-Spectrum β-Lactamase and AmpC-Producing Enterobacteriaceae. Front. Microbiol. 2019, 10, 2488. [Google Scholar] [CrossRef]
- Guenther, S.; Semmler, T.; Stubbe, A.; Stubbe, M.; Wieler, L.H.; Schaufler, K. Chromosomally Encoded Esbl Genes in Escherichia coli of ST38 from Mongolian Wild Birds. J. Antimicrob. Chemother. 2017, 72, 1310–1313. [Google Scholar] [CrossRef]
- Schaufler, K.; Nowak, K.; Düx, A.; Semmler, T.; Villa, L.; Kourouma, L.; Bangoura, K.; Wieler, L.H.; Leendertz, F.H.; Guenther, S. Clinically Relevant ESBL-Producing K. pneumoniae ST307 and E. coli ST38 in an Urban West African Rat Population. Front. Microbiol. 2018, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Mukerji, S.; Stegger, M.; Truswell, A.V.; Laird, T.; Jordan, D.; Abraham, R.J.; Harb, A.; Barton, M.; O’Dea, M.; Abraham, S. Resistance to critically important antimicrobials in Australian silver gulls (Chroicocephalus novaehollandiae) and evidence of anthropogenic origins. J. Antimicrob. Chemother. 2019, 74, 2566–2574. [Google Scholar] [CrossRef]
- Zhou, Z.; Alikhan, N.F.; Mohamed, K.; Achtman, M.; the Agama Study Group. The EnteroBase User’s Guide, with Case Studies on Salmonella Transmissions, Yersinia pestis Phylogeny and Escherichia core Genomic Diversity. Genome Res. 2020, 30, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Pitout, J.D.D. Extraintestinal Pathogenic Escherichia coli: A Combination of Virulence with Antibiotic Resistance. Front. Microbiol. 2012, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Chattaway, M.A.; Jenkins, C.; Ciesielczuk, H.; Day, M.; DoNascimento, V.; Day, M.; Rodríguez, I.; van Essen-Zandbergen, A.; Schink, A.-K.; Wu, G.; et al. Evidence of Evolving Extraintestinal Enteroaggregative Escherichia coli ST38 Clone. Emerg. Infect. Dis. 2014, 20, 1935–1937. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Z.-B.; Zeng, Z.-L.; Yang, X.-W.; Huang, Y.; Liu, J.-H. The Role of Wildlife (Wild Birds) in the Global Transmission of Antimicrobial Resistance Genes. Zool. Res. 2017, 38, 55–80. [Google Scholar] [CrossRef]
- Nowak, K.; Fahr, J.; Weber, N.; Lübke-Becker, A.; Semmler, T.; Weiss, S.; Mombouli, J.-V.; Wieler, L.H.; Guenther, S.; Leendertz, F.H.; et al. Highly Diverse and Antimicrobial Susceptible Escherichia coli Display a Naïve Bacterial Population in Fruit Bats from the Republic of Congo. PLoS ONE 2017, 12, e0178146. [Google Scholar] [CrossRef]
- Johnson, T.J.; Wannemuehler, Y.; Johnson, S.J.; Stell, A.L.; Doetkott, C.; Johnson, J.R.; Kim, K.S.; Spanjaard, L.; Nolan, L.K. Comparison of Extraintestinal Pathogenic Escherichia coli Strains from Human and Avian Sources Reveals a Mixed Subset Representing Potential Zoonotic Pathogens. Appl. Environ. Microbiol. 2008, 74, 7043–7050. [Google Scholar] [CrossRef]
- Singer, R.S. Urinary Tract Infections Attributed to Diverse ExPEC Strains in Food Animals: Evidence and Data Gaps. Front. Microbiol. 2015, 6, 28. [Google Scholar] [CrossRef]
- Luna, G.M.; Vignaroli, C.; Rinaldi, C.; Pusceddu, A.; Nicoletti, L.; Gabellini, M.; Danovaro, R.; Biavasco, F. Extraintestinal Escherichia coli Carrying Virulence Genes in Coastal Marine Sediments. Appl. Environ. Microbiol. 2010, 76, 5659–5668. [Google Scholar] [CrossRef]
- Navon-Venezia, S.; Kondratyeva, K.; Carattoli, A. Klebsiella Pneumoniae: A Major Worldwide Source and Shuttle for Antibiotic Resistance. FEMS Microbiol. Rev. 2017, 41, 252–275. [Google Scholar] [CrossRef] [PubMed]
- Loncaric, I.; Cabal Rosel, A.; Szostak, M.P.; Licka, T.; Allerberger, F.; Ruppitsch, W.; Spergser, J. Broad-Spectrum Cephalosporin-Resistant Klebsiella spp. Isolated from Diseased Horses in Austria. Animals 2020, 10, 332. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Belas, A.; Aboim, C.; Cavaco-Silva, P.; Trigueiro, G.; Gama, L.T.; Pomba, C. Evidence of Sharing of Klebsiella pneumoniae Strains between Healthy Companion Animals and Cohabiting Humans. J. Clin. Microbiol. 2019, 57, e01537-18. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Li, Z.; Lan, S.; Liu, W.; Li, X.; Zhou, Z.; Song, Z.; Wu, J.; Zhang, M.; Shan, W. Characterization of Klebsiella pneumoniae Associated with Cattle Infections in Southwest China Using Multi-Locus Sequence Typing (MLST), Antibiotic Resistance and Virulence-Associated Gene Profile Analysis. Braz. J. Microbiol. 2018, 49, 93–100. [Google Scholar] [CrossRef]
- Yang, F.; Deng, B.; Liao, W.; Wang, P.; Chen, P.; Wei, J. High Rate of Multiresistant Klebsiella pneumoniae from Human and Animal Origin. Infect. Drug Resist. 2019, 12, 2729–2737. [Google Scholar] [CrossRef]
- Bonnedahl, J.; Hernandez, J.; Stedt, J.; Waldenström, J.; Olsen, B.; Drobni, M. Extended-Spectrum β-Lactamases in Escherichia coli and Klebsiella pneumoniae in Gulls, Alaska, USA. Emerg. Infect. Dis. 2014, 20, 897–899. [Google Scholar] [CrossRef]
- Janatova, M.; Albrechtova, K.; Petrzelkova, K.J.; Dolejska, M.; Papousek, I.; Masarikova, M.; Cizek, A.; Todd, A.; Shutt, K.; Kalousova, B.; et al. Antimicrobial-Resistant Enterobacteriaceae from Humans and Wildlife in Dzanga-Sangha Protected Area, Central African Republic. Vet. Microbiol. 2014, 171, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Luo, J.; Wang, C.; Wen, Q.; Duan, M.; Zhang, H.; He, H. Detection of Drug-Resistant Klebsiella pneumoniae in Chinese Hares (Lepus sinensis). J. Wildl. Dis. 2014, 50, 109–112. [Google Scholar] [CrossRef]
- Bachiri, T.; Bakour, S.; Lalaoui, R.; Belkebla, N.; Allouache, M.; Rolain, J.M.; Touati, A. Occurrence of Carbapenemase-Producing Enterobacteriaceae Isolates in the Wildlife: First Report of OXA-48 in Wild Boars in Algeria. Microb. Drug Resist. 2018, 24, 337–345. [Google Scholar] [CrossRef]
- Seguel, M.; Gottdenker, N.L.; Colegrove, K.; Johnson, S.; Struve, C.; Howerth, E.W. Hypervirulent Klebsiella pneumoniae in California Sea Lions (Zalophus californianus): Pathologic Findings in Natural Infections. Vet. Pathol. 2017, 54, 846–850. [Google Scholar] [CrossRef]
- Whitaker, D.M.; Reichley, S.R.; Griffin, M.J.; Prager, K.; Richey, C.A.; Kenelty, K.V.; Stevens, B.N.; Lloyd-Smith, J.O.; Johnson, C.K.; Duignan, P.; et al. Hypermucoviscous Klebsiella pneumoniae Isolates from Stranded and Wild-Caught Marine Mammals of the US Pacific Coast: Prevalence, Phenotype, and Genotype. J. Wildl. Dis. 2018, 54, 659–670. [Google Scholar] [CrossRef]
- Jang, S.; Wheeler, L.; Carey, R.B.; Jensen, B.; Crandall, C.M.; Schrader, K.N.; Jessup, D.; Colegrove, K.; Gulland, F.M.D. Pleuritis and Suppurative Pneumonia Associated with a Hypermucoviscosity Phenotype of Klebsiella pneumoniae in California Sea Lions (Zalophus californianus). Vet. Microbiol. 2010, 141, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Lepuschitz, S.; Schill, S.; Stoeger, A.; Pekard-Amenitsch, S.; Huhulescu, S.; Inreiter, N.; Hartl, R.; Kerschner, H.; Sorschag, S.; Springer, B.; et al. Whole Genome Sequencing Reveals Resemblance between ESBL-Producing and Carbapenem Resistant Klebsiella pneumoniae Isolates from Austrian Rivers and Clinical Isolates from Hospitals. Sci. Total Environ. 2019, 662, 227–235. [Google Scholar] [CrossRef]
- Liapis, E.; Pantel, A.; Robert, J.; Nicolas-Chanoine, M.-H.; Cavalié, L.; van der Mee-Marquet, N.; Champs, C.d.; Aissa, N.; Eloy, C.; Blanc, V.; et al. Molecular Epidemiology of OXA-48-Producing Klebsiella pneumoniae in France. Clin. Microbiol. Infect. 2014, 20, O1121–O1123. [Google Scholar] [CrossRef] [PubMed]
- Gharout-Sait, A.; Alsharapy, S.-A.; Brasme, L.; Touati, A.; Kermas, R.; Bakour, S.; Guillard, T.; de Champs, C. Enterobacteriaceae Isolates Carrying the New Delhi Metallo-β-Lactamase Gene in Yemen. J. Med. Microbiol. 2014, 63, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- López-Camacho, E.; Paño-Pardo, J.R.; Ruiz-Carrascoso, G.; Wesselink, J.-J.; Lusa-Bernal, S.; Ramos-Ruiz, R.; Ovalle, S.; Gómez-Gil, R.; Pérez-Blanco, V.; Pérez-Vázquez, M.; et al. Population Structure of OXA-48-Producing Klebsiella pneumoniae ST405 Isolates During a Hospital Outbreak Characterised by Genomic Typing. J. Glob. Antimicrob. Resist. 2018, 15, 48–54. [Google Scholar] [CrossRef]
- Ballén, V.; Sáez, E.; Benmessaoud, R.; Houssain, T.; Alami, H.; Barkat, A.; Kabiri, M.; Moraleda, C.; Bezad, R.; Vila, J.; et al. First Report of a Klebsiella pneumoniae ST466 Strain Causing Neonatal Sepsis Harbouring the blaCTX-M-15 Gene in Rabat, Morocco. FEMS Microbiol. Lett. 2015, 362, 1–4. [Google Scholar] [CrossRef]
- Stoddard, R.A.; Gulland, M.D.F.; Atwill, E.R.; Lawrence, J.; Jang, S.; Conrad, P.A. Salmonella and Campylobacter spp. in Northern Elephant Seals, California. Emerg. Infect. Dis. 2005, 11, 1967–1969. [Google Scholar] [CrossRef]
- Stoddard, R.A.; DeLong, R.L.; Byrne, B.A.; Jang, S.; Gulland, F.M.D. Prevalence and Characterization of Salmonella spp. among Marine Animals in the Channel Islands, California. Dis. Aquat. Organ. 2008, 81, 5–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berardi, T.; Shapiro, K.; Byrne, B.A.; Miller, W. Prevalence and Characterization of Salmonella Shed by Captive and Free-Range California Sea Lions (Zalophus californianus) from a Rehabilitation Center and Three State Reserves along the California Coast. J. Zoo Wildl. Med. 2014, 45, 527–533. [Google Scholar] [CrossRef]
- Stoddard, R.A.; Atwill, E.R.; Gulland, F.M.D.; Miller, M.A.; Dabritz, H.A.; Paradies, D.M.; Worcester, K.R.; Jang, S.; Lawrence, J.; Byrne, B.A.; et al. Risk Factors for Infection with Pathogenic and Antimicrobial-Resistant Fecal Bacteria in Northern Elephant Seals in California. Public Heal. Rep. 2008, 123, 360–370. [Google Scholar] [CrossRef]
- Aguirre, A.A.; Tabor, G. Introduction: Marine Vertebrates as Sentinels of Marine Ecosystem Health. EcoHealth 2004, 1, 236–238. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, CLSI Supplement M100, 30th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Kohlmann, R.; Bähr, T.; Gatermann, S.G. Species-Specific Mutation Rates for AmpC Derepression in Enterobacterales with Chromosomally Encoded Inducible AmpC β-Lactamase. J. Antimicrob. Chemother. 2018, 73, 1530–1536. [Google Scholar] [CrossRef]
- Schug, A.R.; Bartel, A.; Scholtzek, A.D.; Meurer, M.; Brombach, J.; Hensel, V.; Fanning, S.; Schwarz, S.; Feßler, A.T. Biocide Susceptibility Testing of Bacteria: Development of a Broth Microdilution Method. Vet. Microbiol. 2020, 248, 108791. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.D.; Jamil, B.; Syed, M.A.; Abbasi, S.A.; Weiß, D.; Slickers, P.; Monecke, S.; Engelmann, I.; Ehricht, R. Prevalence of Carbapenemase-Producing Organisms at the Kidney Center of Rawalpindi (Pakistan) and Evaluation of an Advanced Molecular Microarray-Based Carbapenemase Assay. Future Microbiol. 2018, 13, 1225–1246. [Google Scholar] [CrossRef] [PubMed]
- Sandvang, D.; Aarestrup, F.M. Characterization of Aminoglycoside Resistance Genes and Class 1 Integrons in Porcine and Bovine Gentamicin-Resistant Escherichia coli. Microb. Drug Resist. 2000, 6, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Boerlin, P.; Travis, R.; Gyles, C.L.; Reid-Smith, R.; Janecko, N.; Lim, H.; Nicholson, V.; McEwen, S.A.; Friendship, R.; Archambault, M. Antimicrobial Resistance and Virulence Genes of Escherichia coli Isolates from Swine in Ontario. Appl. Environ. Microbiol. 2005, 71, 6753–6761. [Google Scholar] [CrossRef]
- Gay, K.; Robicsek, A.; Strahilevitz, J.; Park, C.H.; Jacoby, G.; Barrett, T.J.; Medalla, F.; Chiller, T.M.; Hooper, D.C. Plasmid-Mediated Quinolone Resistance in Non-Typhi Serotypes of Salmonella enterica. Clin. Infect. Dis. 2006, 43, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Robicsek, A.; Jacoby, G.A.; Sahm, D.; Hooper, D.C. Prevalence in the United States of Aac(6’)-Ib-cr Encoding a Ciprofloxacin-Modifying Enzyme. Antimicrob. Agents Chemother. 2006, 50, 3953–3955. [Google Scholar] [CrossRef]
- Yamane, K.; Wachino, J.; Suzuki, S.; Arakawa, Y. Plasmid-Mediated qepA Gene among Escherichia coli Clinical Isolates from Japan. Antimicrob. Agents Chemother. 2008, 52, 1564–1566. [Google Scholar] [CrossRef]
- Cavaco, L.M.; Hasman, H.; Xia, S.; Aarestrup, F.M. qnrD, a Novel Gene Conferring Transferable Quinolone Resistance in Salmonella enterica Serovar Kentucky and Bovismorbificans Strains of Human Origin. Antimicrob. Agents Chemother. 2009, 53, 603–608. [Google Scholar] [CrossRef]
- Szczepanowski, R.; Linke, B.; Krahn, I.; Gartemann, K.-H.; Gützkow, T.; Eichler, W.; Pühler, A.; Schlüter, A. Detection of 140 Clinically Relevant Antibiotic-Resistance Genes in the Plasmid Metagenome of Wastewater Treatment Plant Bacteria Showing Reduced Susceptibility to Selected Antibiotics. Microbiology 2009, 155, 2306–2319. [Google Scholar] [CrossRef]
- Šeputienė, V.; Povilonis, J.; Ružauskas, M.; Pavilonis, A.; Sužiedėlienė, E. Prevalence of Trimethoprim Resistance Genes in Escherichia coli Isolates of Human and Animal Origin in Lithuania. J. Med. Microbiol. 2010, 59, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Dolejska, M.; Frolkova, P.; Florek, M.; Jamborova, I.; Purgertova, M.; Kutilova, I.; Cizek, A.; Guenther, S.; Literak, I. CTX-M-15-Producing Escherichia coli Clone B2-O25b-ST131 and Klebsiella spp. Isolates in Municipal Wastewater Treatment Plant Effluents. J. Antimicrob. Chemother. 2011, 66, 2784–2790. [Google Scholar] [CrossRef]
- Miranda, A.; Ávila, B.; Díaz, P.; Rivas, L.; Bravo, K.; Astudillo, J.; Bueno, C.; Ulloa, M.T.; Hermosilla, G.; Del Canto, F.; et al. Emergence of Plasmid-Borne dfrA14 Trimethoprim Resistance Gene in Shigella sonnei. Front. Cell. Infect. Microbiol. 2016, 6, 77. [Google Scholar] [CrossRef]
- Wang, M.; Guo, Q.; Xu, X.; Wang, X.; Ye, X.; Wu, S.; Hooper, D.C.; Wang, M. New Plasmid-Mediated Quinolone Resistance Gene, qnrC, Found in a Clinical Isolate of Proteus mirabilis. Antimicrob. Agents Chemother. 2009, 53, 1892–1897. [Google Scholar] [CrossRef]
- Dierikx, C.M.; van Duijkeren, E.; Schoormans, A.H.W.; van Essen-Zandbergen, A.; Veldman, K.; Kant, A.; Huijsdens, X.W.; van der Zwaluw, K.; Wagenaar, J.A.; Mevius, D.J. Occurrence and Characteristics of Extended-Spectrum-β-Lactamase- and AmpC-Producing Clinical Isolates Derived from Companion Animals and Horses. J. Antimicrob. Chemother. 2012, 67, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Kehrenberg, C.; Schwarz, S. Distribution of Florfenicol Resistance Genes fexA and cfr among Chloramphenicol-Resistant Staphylococcus isolates. Antimicrob. Agents Chemother. 2006, 50, 1156–1163. [Google Scholar] [CrossRef]
- Dallenne, C.; Da Costa, A.; Decré, D.; Favier, C.; Arlet, G. Development of a Set of Multiplex PCR Assays for the Detection of Genes Encoding Important Beta-Lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010, 65, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, F.J.; Hanson, N.D. Detection of Plasmid-Mediated AmpC Beta-Lactamase Genes in Clinical Isolates by Using Multiplex PCR. J. Clin. Microbiol. 2002, 40, 2153–2162. [Google Scholar] [CrossRef] [PubMed]
- Everett, M.J.; Jin, Y.F.; Ricci, V.; Piddock, L.J. Contributions of Individual Mechanisms to Fluoroquinolone Resistance in 36 Escherichia coli Strains Isolated from Humans and Animals. Antimicrob. Agents Chemother. 1996, 40, 2380–2386. [Google Scholar] [CrossRef]
- Caroff, N.; Espaze, E.; Bérard, I.; Richet, H.; Reynaud, A. Mutations in the AmpC Promoter of Escherichia coli Isolates Resistant to Oxyiminocephalosporins without Extended Spectrum Beta-Lactamase Production. FEMS Microbiol. Lett. 1999, 173, 459–465. [Google Scholar] [CrossRef][Green Version]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichia coli Phylo-Typing Method Revisited: Improvement of Specificity and Detection of New Phylo-Groups. Environ. Microbiol. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Weissman, S.J.; Johnson, J.R.; Tchesnokova, V.; Billig, M.; Dykhuizen, D.; Riddell, K.; Rogers, P.; Qin, X.; Butler-Wu, S.; Cookson, B.T.; et al. High-Resolution Two-Locus Clonal Typing of Extraintestinal Pathogenic Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 1353–1360. [Google Scholar] [CrossRef]
- Roer, L.; Johannesen, T.B.; Hansen, F.; Stegger, M.; Tchesnokova, V.; Sokurenko, E.; Garibay, N.; Allesøe, R.; Thomsen, M.C.F.; Lund, O.; et al. CHTyper, a Web Tool for Subtyping of Extraintestinal Pathogenic Escherichia coli Based on the fumC and fimH Alleles. J. Clin. Microbiol. 2018, 56, e00063-18. [Google Scholar] [CrossRef] [PubMed]
- Francisco, A.P.; Vaz, C.; Monteiro, P.T.; Melo-Cristino, J.; Ramirez, M.; Carriço, J.A. PHYLOViZ: Phylogenetic Inference and Data Visualization for Sequence Based Typing Methods. BMC Bioinform. 2012, 13, 1–10. [Google Scholar] [CrossRef]
- Brisse, S.; Fevre, C.; Passet, V.; Issenhuth-Jeanjean, S.; Tournebize, R.; Diancourt, L.; Grimont, P. Virulent Clones of Klebsiella pneumoniae: Identification and Evolutionary Scenario Based on Genomic and Phenotypic Characterization. PLoS ONE 2009, 4, e4982. [Google Scholar] [CrossRef]
- Diancourt, L.; Passet, V.; Verhoef, J.; Grimont, P.A.D.; Brisse, S. Multilocus Sequence Typing of Klebsiella pneumoniae Nosocomial Isolates. J. Clin. Microbiol. 2005, 43, 4178–4182. [Google Scholar] [CrossRef]
- Braun, S.D.; Ziegler, A.; Methner, U.; Slickers, P.; Keiling, S.; Monecke, S.; Ehricht, R. Fast DNA Serotyping and Antimicrobial Resistance Gene Determination of Salmonella enterica with an Oligonucleotide Microarray-Based Assay. PLoS ONE 2012, 7, e46489. [Google Scholar] [CrossRef]
- Geue, L.; Schares, S.; Mintel, B.; Conraths, F.J.; Müller, E.; Ehricht, R. Rapid Microarray-Based Genotyping of Enterohemorrhagic Escherichia coli Serotype O156:H25/H-/Hnt Isolates from Cattle and Clonal Relationship Analysis. Appl. Environ. Microbiol. 2010, 76, 5510–5519. [Google Scholar] [CrossRef]
- Lepuschitz, S.; Huhulescu, S.; Hyden, P.; Springer, B.; Rattei, T.; Allerberger, F.; Mach, R.L.; Ruppitsch, W. Characterization of a Community-Acquired-MRSA USA300 Isolate from a River Sample in Austria and Whole Genome Sequence Based Comparison to a Diverse Collection of USA300 Isolates. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Wirth, T.; Falush, D.; Lan, R.; Colles, F.; Mensa, P.; Wieler, L.H.; Karch, H.; Reeves, P.R.; Maiden, M.C.J.; Ochman, H.; et al. Sex and Virulence in Escherichia coli: An Evolutionary Perspective. Mol. Microbiol. 2006, 60, 1136–1151. [Google Scholar] [CrossRef] [PubMed]
- Beghain, J.; Bridier-Nahmias, A.; Le Nagard, H.; Denamur, E.; Clermont, O. ClermonTyping: An Easy-to-Use and Accurate in Silico Method for Escherichia Genus Strain Phylotyping. Microb. Genom. 2018, 4, e000192. [Google Scholar] [CrossRef]
- Clermont, O.; Dixit, O.V.A.; Vangchhia, B.; Condamine, B.; Dion, S.; Bridier-Nahmias, A.; Denamur, E.; Gordon, D. Characterization and Rapid Identification of Phylogroup G in Escherichia coli, a Lineage with High Virulence and Antibiotic Resistance Potential. Environ. Microbiol. 2019, 21, 3107–3117. [Google Scholar] [CrossRef]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.-L.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic Resistome Surveillance with the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef] [PubMed]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for Predictions of Phenotypes from Genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Zankari, E.; Allesøe, R.; Joensen, K.G.; Cavaco, L.M.; Lund, O.; Aarestrup, F.M. PointFinder: A Novel Web Tool for WGS-Based Detection of Antimicrobial Resistance Associated with Chromosomal Point Mutations in Bacterial Pathogens. J. Antimicrob. Chemother. 2017, 72, 2764–2768. [Google Scholar] [CrossRef]
- Pal, C.; Bengtsson-Palme, J.; Rensing, C.; Kristiansson, E.; Larsson, D.G.J. BacMet: Antibacterial Biocide and Metal Resistance Genes Database. Nucleic Acids Res. 2014, 42, D737–D743. [Google Scholar] [CrossRef] [PubMed]
- Joensen, K.G.; Scheutz, F.; Lund, O.; Hasman, H.; Kaas, R.S.; Nielsen, E.M.; Aarestrup, F.M. Real-Time Whole-Genome Sequencing for Routine Typing, Surveillance, and Outbreak Detection of Verotoxigenic Escherichia coli. J. Clin. Microbiol. 2014, 52, 1501–1510. [Google Scholar] [CrossRef]
- Tetzschner, A.M.M.; Johnson, J.R.; Johnston, B.D.; Lund, O.; Scheutz, F. In Silico Genotyping of Escherichia coli Isolates for Extraintestinal Virulence Genes by Use of Whole-Genome Sequencing Data. J. Clin. Microbiol. 2020, 58, e01269-20. [Google Scholar] [CrossRef]
- Joensen, K.G.; Tetzschner, A.M.M.; Iguchi, A.; Aarestrup, F.M.; Scheutz, F. Rapid and Easy In Silico Serotyping of Escherichia coli Isolates by Use of Whole-Genome Sequencing Data. J. Clin. Microbiol. 2015, 53, 2410–2426. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In Silico Detection and Typing of Plasmids Using PlasmidFinder and Plasmid Multilocus Sequence Typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef] [PubMed]
- Arredondo-Alonso, S.; Rogers, M.R.C.; Braat, J.C.; Verschuuren, T.D.; Top, J.; Corander, J.; Willems, R.J.L.; Schürch, A.C. Mlplasmids: A User-Friendly Tool to Predict Plasmid- and Chromosome-Derived Sequences for Single Species. Microb. Genom. 2018, 4, e000224. [Google Scholar] [CrossRef] [PubMed]
- Grimont, P.; Weill, F.X. Antigenic Formulae of the Salmonella Serovars; WHO: Paris, France, 1997. [Google Scholar]
- Ziemer, C.J.; Steadham, S.R. Evaluation of the Specificity of Salmonella PCR Primers Using Various Intestinal Bacterial Species. Lett. Appl. Microbiol. 2003, 37, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Itho, Y.; Fujinaga, Y.; Khan, A.Q.; Sultana, F.; Miyake, M.; Hirose, K.; Yamamoto, H.; Ezaki, T. Development of Nested PCR Based on the ViaB Sequence to Detect Salmonella typhi. J. Clin. Microbiol. 1995, 33, 775–777. [Google Scholar] [CrossRef]
| Isolate | Animal 1 | Species | Phylo- Group | CH- Clonotype | ESBL/ AmpC | Phenotype 2 | Genotype | Virulence Genes | ampC-Promoter 3 | QRDR GyrA 4 | QRDR ParC 4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | Zc | E. coli | B1 | CH65-26 | AmpC | AMP, AMC, PIP, CFZ | fimH | 39 | n.d. | n.d. | |
| 8/2 | Zc | E. coli | A | CH65-26 | AmpC | AMP, AMC, PIP, CFZ, CTX, CAZ | blaCMY-2 | fimH | 39 | n.d. | n.d. |
| 11/1 | Ma | E. coli | F | CH88-145 | AmpC | AMP, AMC, PIP, CFZ | blaTEM-1, blaCMY-2, sul2, dfrA1, aadA1, strA, strB | fimH, astA | w.t. | n.d. | n.d. |
| 17 | Pv | E. coli | B2 | CH103-212 | AmpC | AMP, AMC, PIP, CFZ, FOF | blaCMY-2, dfrA14, dfrA19 | fimH, cnf1, hylA | 58 | n.d. | n.d. |
| 21 | Zc | E. coli | B1 | CH65-38 | AmpC and ESBL | AMC, PIP, CFZ, CTX, CAZ, ATM, SXT, TET, CHL, GEN, TOB, CIP | blaCTX-M-15, blaOXA-1, sul1, dfrA17, tet(B), cmlA, aadA4, aadA5, aac(6’)-Ib | fimH, iucD, papC | 39 | Ser83Leu, Asp87Asn | Ser80Ile |
| 41 | Zc | E. coli | B1 | CH29-31 | ESBL | AMC, PIP, CFZ, CTX, CAZ, SXT | blaCTX-M-15, dfrA17, aadA4 | fimH, astA | n.a. | n.d. | n.d. |
| 53/1 | Zc | E. coli | E | CH148-25 | AmpC | AMC, PIP, CFZ, CTX, CAZ, SXT, TET, CHL | blaTEM-1, blaCMY-2, dfrA17, tet(B), catA, strA, strB | fimH, iucD, papC, bfpA, cdtA, tcdAp2 | n.a. | n.d. | n.d. |
| 90 | Zc | E. coli | F | CH4-153 | AmpC and ESBL | AMC, PIP, CFZ, CTX, CAZ, ATM, SXT | blaCTX-M-15, blaCMY-2, sul2, dfrA14, strA, strB, qnrS | fimH | n.a. | n.d. | n.d. |
| 92 | Zc | E. coli | E | CH148-25 | AmpC | AMC, PIP, CFZ, CTX, CAZ, SXT, TET | blaTEM-1, blaCMY-2, sul2, dfrA17, tet(B), aadA4, strA, strB | fimH, iucD, papC | w.t. | n.d. | n.d. |
| 117 | Zc | E. coli | B2 | CH103-9 | AmpC | AMC, PIP, CFZ, CTX, CAZ | blaCMY-2 | fimH, cnf1 | n.a. | n.d. | n.d. |
| 147a | Zc | E. coli | A | CH7-0 | AmpC | AMC, PIP, CFZ, CTX, CAZ, ATM, TET | blaTEM-1, blaCMY-2, dfrA5, tet(A) | fimH | w.t. | n.d. | n.d. |
| 147b | Zc | E. coli | B2 | CH40-21 | AmpC | AMC, PIP, CFZ, CTX, CAZ, SXT, TET, CHL, GEN, TOB | blaTEM-1, blaCMY-2, sul1, sul2, dfrA1, tet(A), catA, cmlA, floR, aadB, strA, strB | fimH, astA, cnf1 | w.t. | n.d. | n.d. |
| 149a | Ma | E. coli | A | CH7-0 | AmpC | AMC, PIP, CFZ, CTX, CAZ, ATM, TET | blaTEM-1, blaCMY-2, tet(A) | fimH | n.a. | n.d. | n.d. |
| 156 | Zc | E. coli | A | CH11-0 | AmpC | AMC, CFZ, CTX, CAZ, SXT, TET, CIP | blaCMY-2, sul1, dfrA17, tet(B), aadA4 | fimH, astA, iucD, papC | w.t. | Ser83Leu, Asp87Asn | Ser80Ile |
| 158 | Zc | E. coli | E | CH148-25 | AmpC | AMC, PIP, CFZ, CTX, CAZ, ATM, SXT, TET, CHL | blaTEM-1, blaCMY-2, sul2, dfrA17, tet(B), catA, aadA4, strA, strB | fimH, iucD, papC | w.t. | n.d. | n.d. |
| Isolate | Animal 1 | Species 2 | ST (Klebsiella) 3 | ESBL/AmpC | Phenotype 4 | Genotype | QRDR GyrA 5 | QRDR ParC 5 |
|---|---|---|---|---|---|---|---|---|
| 8/1 | Zc | K. pneumoniae | 466 | AmpC | AMC, PIP, CFZ, CTX, CAZ, SXT, TET, CHL, GEN, TOB, | blaSHV-33, blaTEM-1, blaCMY-2, sul1, sul2, dfrA1, tet(A), catA, cmlA, floR, aadB, strA, strB | n.d. | n.d. |
| 10 | Zc | K. pneumoniae | 466 | AmpC | AMC, PIP, CFZ, CTX, CAZ, SXT, TET, CHL, GEN, TOB, FOF, NIT | blaSHV-33, blaTEM-1, blaCMY-2, sul1, sul2, dfrA1, tet(A), catA, cmlA, floR, aadB, strA, strB | n.d. | n.d. |
| 11/2 | Ma | K. pneumoniae | 466 | AmpC | AMC, PIP, CFZ, CTX, CAZ, SXT, TET, CHL, GEN, TOB, FOF, NIT | blaSHV-33, blaTEM-1, blaCMY-2, sul1, sul2, dfrA1, tet(A), catA, cmlA, floR aadB, strA, strB | n.d. | n.d. |
| 14 | Pv | K. pneumoniae | 466 | AmpC | AMC, PIP, CFZ, CTX, CAZ, SXT, TET, CHL, GEN, TOB, FOF, NIT | blaSHV-33, blaTEM-1, blaCMY-2, sul1, sul2, dfrA1, tet(A), catA, cmlA, floR, aadB, strA, strB | n.d. | n.d. |
| 16 | Pv | K. pneumoniae | 466 | AmpC | AMC, PIP, CFZ, CTX, CAZ, SXT, TET, CHL, GEN, TOB, FOF, NIT | blaSHV-33, blaTEM-1, blaCMY-2, sul1, dfrA1, tet(A), catA, cmlA, floR, aadB, strA, strB | n.d. | n.d. |
| 19 | Pv | K. pneumoniae | 466 | AmpC | AMC, PIP, CFZ, CTX, CAZ, SXT, TET, CHL, GEN, TOB, FOF, NIT | blaSHV-33, blaTEM-1, blaCMY-2, sul1, sul2, dfrA1, tet(A), catA, cmlA, floR, aadB, strA, strB | n.d. | n.d. |
| 154a | Zc | K. pneumoniae | 466 | AmpC | AMC, PIP, CFZ, CTX, CAZ, SXT, TET, CHL, GEN, TOB | blaSHV-33, blaTEM-1, blaCMY-2, sul1, sul2, dfrA1, tet(A), catA, cmlA, floR, aadB, strA, strB | n.d. | n.d. |
| 160 | Zc | K. pneumoniae | 466 | AmpC | AMC, PIP, CTX, CAZ, SXT, TET, CHL, GEN, TOB | blaTEM-1, blaCMY-2, sul1, sul2, dfrA1, tet(A), catA, cmlA, floR, aadB, strA, strB | n.d. | n.d. |
| 161 | Pv | K. pneumoniae | 405 | AmpC | AMC, PIP, SXT, TET, CHL, GEN, TOB, CIP | blaSHV-11, blaTEM-1, blaOXA-1, blaCMY-2, sul1, sul2, dfrA1, dfrA14, tet(A), catA, cmlA, floR, aac(6´)-Ib, aadB, strA, strB, qnrB | w.t. | w.t. |
| 164 | Zc | K. pneumoniae | 466 | AmpC | AMC, PIP, CFZ, CTX, CAZ, SXT, TET, CHL, GEN, TOB | blaSHV-33, blaTEM-1, blaCMY-2, sul2, dfrA1, tet(A), catA, cmlA, floR, aadB, strA, strB | n.d. | n.d. |
| 173 | Ma | K. pneumoniae | 405 | AmpC | AMC, PIP, CFZ, CTX, CAZ, SXT, TET, CHL, GEN, TOB, CIP | blaSHV-11, blaTEM-1, blaOXA-1, blaCMY-2, sul1, sul2, dfrA1, dfrA14, tet(A), catA, cmlA, floR, aac(6′)-Ib, aadB, strA, strB, qnrB | w.t. | n.a. |
| 175 | Zc | K. pneumoniae | 405 | AmpC | AMC, PIP, CFZ, CTX, CAZ, SXT, TET, CHL, GEN, TOB, CIP | blaSHV-11, blaTEM-1, blaOXA-1, blaCMY-2, sul1, sul2, dfrA1, dfrA14, tet(A), catA, cmlA, floR, aac(6′)-Ib, aadB, strA, strB, qnrB | w.t. | n.a. |
| 9 | Zc | C. koseri | n.a. | AmpC de-repression | intrinsic + CTX, CAZ, TET, CHL | blaCMY, sul2, tet(A), cmlA, floR, strA, strB | n.d. | n.d. |
| 28 | Zc | C. koseri | n.a. | AmpC de-repression | intrinsic + CTX, CAZ, SXT, TET, CHL, GEN, TOB, FOF, CIP | blaTEM-1, blaOXA-1, blaCMY, sul1, sul2, dfrA1, dfrA14, tet(A), cmlA, aadB, aac(6′)-Ib, strA, strB, qnrB | w.t. | w.t. |
| 30 | Zc | C. koseri | n.a. | AmpC de-repression | intrinsic + CTX, CAZ, SXT | blaCMY, sul2 | w.t. | n.a. |
| 35 | Zc | C. koseri | n.a. | AmpC de-repression | intrinsic + CTX, CAZ, SXT, TET, CHL, GEN, TOB, FOF, CIP | blaTEM-1, blaOXA-1, blaCMY, sul1, sul2, dfrA1, dfrA14, tet(A), cmlA, aadB, aac(6´)-Ib, strA, strB, qnrB | w.t. | w.t. |
| 53/2 | Zc | C. koseri | n.a. | AmpC de-repression | intrinsic + CTX, CAZ, SXT, TET, CHL | blaTEM-1, blaCMY, sul2, dfrA14, tet(A), tet(B), catA, cmlA, floR, strA, strB, qnrB | n.d. | n.d. |
| 149b | Ma | C. freundii complex | n.a. | AmpC de-repression | AMC, PIP, CFZ, CTX, CAZ, SXT, TET, CHL, GEN, TOB | blaTEM-1, blaCMY, sul1, dfrA12, dfrA19, tet(A), aadA2, aac(6´)-IIc, strA, strB | n.d. | n.d. |
| 151 | Zc | C. freundii complex | n.a. | AmpC de-repression | AMC, PIP, CFZ, CTX, CAZ, SXT, TET, CHL, GEN | blaTEM-1, blaCMY, sul1, dfrA12, dfrA19, tet(A), aadA2, aac(6´)-IIc, strA, strB | n.d. | n.d. |
| 154b | Zc | C. freundii complex | n.a. | AmpC de-repression | AMC, PIP, CFZ, CTX, CAZ, SXT, TET, CHL, GEN | blaTEM-1, blaCMY, sul1, sul2, dfrA12, dfrA19, tet(A), aadA2, aac(6´)-IIc, strA, strB | n.d. | n.d. |
| 163 | Zc | C. freundii complex | n.a. | AmpC de-repression | AMC, PIP, CFZ, CTX, CAZ, SXT, TET, CHL, GEN, TOB, CIP | blaTEM-1, blaCMY, sul1, sul2, dfrA12, dfrA19, tet(A), aadA2, strA, strB, qnrB | n.a. | n.a. |
| 165 | Zc | C. freundii complex | n.a. | AmpC de-repression | AMC, PIP, CFZ, CTX, CAZ, SXT, TET, CHL, GEN, TOB | blaTEM-1, blaCMY, sul1, sul2, dfrA12, dfrA19, tet(A), aadA2, strA, strB | n.d. | n.d. |
| 169 | Zc | C. koseri | n.a. | AmpC de-repression | AMC, PIP, CFZ, CTX, CAZ, SXT, TET, GEN | blaTEM-1, blaCMY, sul2, dfrA14, tet(A), strA, strB | n.d. | n.d. |
| 179 | Zc | C. koseri | n.a. | AmpC de-repression | AMC, PIP, CFZ, CTX, CAZ, SXT, TET, GEN | blaTEM-1, blaCMY, sul2, dfrA14, tet(A), strA, strB | n.d. | n.d. |
| 192/1 | Zc | C. freundii complex | n.a. | AmpC de-repression | AMC, PIP, FOX, CFZ, CAZ, CTX, SXT, TET | blaTEM-1, blaCMY, sul1, sul2, dfrA12, tet(A) | n.d. | n.d. |
| 213 | Kb | C. freundii complex | n.a. | AmpC de-repression | AMC, PIP, FOX, CFZ, CTX, CAZ, SXT, TET | blaCMY, sul1, dfrA12, tet(A) | n.d. | n.d. |
| 215 | Kb | C. freundii complex | n.a. | AmpC de-repression | AMC, PIP, FOX, CFZ, CTX, CAZ, SXT | blaCMY, sul1, dfrA12 | n.d. | n.d. |
| 224b | Ma | C. freundii complex | n.a. | AmpC de-repression | AMC, PIP, FOX, CFZ, CTX, CAZ, SXT, TET, CHL, GEN, TOB | blaTEM-1, blaCMY, sul1, sul2, dfrA1, cmlA, floR, aadB, strA, strB | n.d. | n.d. |
| 226 | Zc | C. koseri | n.a. | AmpC de-repression | AMC, PIP, FOX, CFZ, CTX, CAZ, SXT, TET, CHL, GEN, TOB | blaCMY, sul2, tet(A), tet(D), floR, strA, strB | n.d. | n.d. |
| 230c | Zc | C. koseri | n.a. | AmpC de-repression | AMC, PIP, FOX, CFZ, CTX, CAZ, SXT, TET, GEN | blaTEM-1, blaCMY, sul2, dfrA14, tet(D), strA, strB | n.d. | n.d. |
| 43 | Zc | En. cloacae complex | n.a. | AmpC de-repression | intrinsic + CTX, CAZ, TET, SXT | blaTEM-1, sul2, dfrA14, tet(D), strA, strB | n.d. | n.d. |
| 66 | Zc | En. cancerogenus | n.a. | AmpC de-repression | intrinsic + CTX, CAZ | n.d. | n.d. | |
| 130 | Ma | L. amnigena | n.a. | AmpC and ESBL | AMC, PIP, CFZ, CTX, CAZ, SXT, CHL | blaDHA-1, blaSHV-12, sul1, sul2, dfrA14, cmlA, floR, aac(6´)-Ib, qnrB | n.d. | n.d. |
| 132 | Ma | L. amnigena | n.a. | AmpC and ESBL | AMC, PIP, CFZ, CTX, CAZ, SXT, CHL | blaDHA-1, blaSHV-12, sul1, sul2, dfrA14, cmlA, floR, aac(6´)-Ib, qnrB | n.d. | n.d. |
| 197/2 | Zc | P. mirabilis | n.a. | AmpC | AMC, PIP, CAZ, CTX, SXT, CHL, GEN | blaTEM-1, blaCMY-2, sul1, sul2, dfrA1, catA, cmlA, floR, aadB, strA, strB | n.d. | n.d. |
| Isolate | Animal 1 | Phylo-Group | CH-Clono-Typing | SEROGENOTYPE 2 | ST | ESBL/AmpC | Phenotype 3 | Genotype | Virulence Genes | ampC Promoter 4 | QRDR 5 GyrA 4 | QRDR ParC 4 | ParE 4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 68 | Zc | A | CH11-0 | ONT:H9 | 167 | ESBL | AMC, PIP, CFZ, CTX, CAZ, ATM, SXT, TET, GEN, TOB, CIP | blaCTX-M-15, blaOXA-1, sul1, dfrA17, tet(A), tet(B), catB3, aadA4, aadA5, aac(3)-IIa, aac(6′)-Ib-cr, mdfA | astA, capU, fyuA, irp2, iss, iucC, iutA, sitA, terC | w.t. | Ser83Leu, Asp87Asn | Ser80Ile | S458A |
| 171 | Pv | D | CH50-299 | ONT:H1 | 5748 | AmpC | AMC, PIP, CFZ, CTX, CAZ | blaCMY-4, mdfA | air, chuA, cia, eilA, lpfA, terC, traT | w.t. | w.t. | w.t. | w.t. |
| 183 | Zc | A | CH11-0 | ONT:H9 | 167 | ESBL | AMC, PIP, CFZ, CTX, CAZ, ATM, SXT, TET, TOB, CIP | blaCTX-M-15, sul1, sul2, dfrA17, tet(A), tet(B), catB3, aadA5, aph(3″)-Ib, aph(6)-Id, aac(6′)-Ib-cr, mdfA | astA, capU, fyuA, irp2, iss, iucC, iutA, sitA, terC, traT | w.t. | Ser83Leu, Asp87Asn | Ser80Ile | S458A |
| 192/2 | Zc | B1 | CH65-32 | O9:H10 | 1431 | AmpC | AMC, PIP, FOX, CFZ, CAZ, CTX, SXT, CIP | blaTEM-1B, blaCMY-2, sul2, dfrA5, aph(3″)-Ib, aph(6)-Id, mdfA | capU, cba, cia, cma, cvaC, etsC, fyuA, hlyF, iroN, irp2, iss, iucC, iutA, lpfA, mchF, ompT, sitA, terC, traT | -19 | Ser83Leu, Asp87Asn | Ser80Ile | S458A |
| 197/1 | Zc | F | CH4-58 | ONT:H42 | 648 | AmpC | AMC, PIP, FOX, CFZ, CAZ, CTX, ATM, SXT, TET, CHL, GEN, TOB | blaTEM-1B, blaCMY-2, sul1, sul2, dfrA1, tet(A), cmlA1, floR, aph(3″)-Ib, aph(6)-Id, ant(2″)-Ia, mdfA | air, cea, celb, chuA, focCsfaE, focG, fyuA, iroN, irp2, kpsE, kpsMII, lpfA, mchB, mchC, mchF, mcmA, sfaD, sitA, terC, yfcV | -28 | w.t. | w.t. | w.t. |
| 202 | Zc | B2 | CH103-9 | ONT:H31 | 372 | AmpC | AMC, PIP, FOX, CFZ | mdfA | cea, chuA, cnf1, focCsfaE, focG, focI, fyuA, hra, ibeA, iroN, irp2, iss, mchB, mchC, mchF, mcmA, ompT, papA, papC, sitA, terC, usp, vat, yfcV | -32 | w.t. | w.t. | w.t. |
| 206 | Zc | D | CH26-26 | ONT:H18 | 963 | AmpC | AMC, PIP, FOX, CFZ, CTX, CAZ | blaCMY-2, mdfA | air, astA, chuA, eilA, fyuA, irp2, kpsE, senB, sitA, terC, traT | w.t. | w.t. | w.t. | w.t. |
| 209 | Zc | D | CH26-0 | ONT:H15 | 38 | ESBL | AMC, PIP, FOX, CFZ, CTX, CAZ | blaCTX-M-15, blaTEM-1B, qnrS1, mdfA | air, chuA, eilA, iss, kpsE, kpsMII, terC | w.t. | w.t. | w.t. | w.t. |
| 224a | Ma | F | CH37-1572 * | ONT:H28 | 4957 | AmpC | AMC, PIP, FOX, CFZ, CTX, CAZ, SXT, TET, CHL, GEN, TOB | blaTEM-1B, blaCMY-2, sul1, sul2, dfrA1, tet(A), cmlA1, floR, aph(3″)-Ib, aph(6)-Id, ant(2″)-Ia, mdfA | air, chuA, cia, cvaC, etsC, hlyF, iroN, iss, lpfA, mchF, ompT, sitA, terC, traT, usp, yfcV | -28 | w.t. | w.t. | w.t. |
| 230a | Zc | D | CH36-93 | ONT:H15 | 349 | AmpC | AMC, FOX, CFZ, CAZ | blaCMY-2, mdfA | cba, chuA, cia, cma, eilA, fyuA, hra, irp2, kpsE, terC, traT | w.t. | w.t. | w.t. | S458A |
| 230b | Zc | A | CH11-0 | ONT:H9 | 167 | AmpC | AMC, FOX, CFZ, CTX, CAZ, SXT, TET, FOF, CIP | blaCMY-2, sul1, dfrA17, tet(A), tet(B), aadA5, mdfA | astA, capU, cba, cia, cma, fyuA, irp2, iss, iucC, iutA, sitA, terC, traT | w.t. | w.t. | Ser80Ile | w.t. |
| 234 | Zc | A | CH11-54 | ONT:H4 | 484 | ESBL | AMP, CFZ, CTX | blaCTX-M-15, qnrS1, mdfA | aap, astA, iss, kpsE, kpsMII, ompT, terC, traT | w.t. | w.t. | w.t. | I355T |
| Isolate | Animal 1 | Serotype 2 | Phenotype 3 | Genotype | Virulence Genes |
|---|---|---|---|---|---|
| 8. S. | Zc | S. Saintpaul (antigenic formula: 1,4,5,12 : e,h : 1,2) | n.r. | hilA, stn | |
| 27 S. | Zc | S. Newport (antigenic formula 6,8 : e,h : 1,2) | n.r. | hilA, stn | |
| 42 S. | Zc | AmpC S. Saintpaul (antigenic formula 1,4,5,12 : e,h : 1,2) | AMC, PIP, CFZ, CTX, CAZ | blaCMY-2 | hilA, stn |
| 56 S. | Zc | S. Havana (antigenic formula 1,13,23 : f,g : -) | n.r. | hilA, stn | |
| 62 S. | Zc | S. Havana (antigenic formula 1,13,23 : f,g : -) | n.r. | hilA, stn | |
| 91 S. | Zc | S. Havana (antigenic formula 1,13,23 : f,g : -) | n.r. | hilA, stn | |
| 113 S. | Zc | S. Havana (antigenic formula 1,13,23 : f,g : -) | n.r. | hilA, stn | |
| 115 S. | Ma | S. Havana (antigenic formula 1,13,23 : f,g : -) | n.r. | hilA, stn | |
| 125 S. | Ma | S. Newport (antigenic formula 6,8 : e,h : 1,2) | n.r. | hilA, stn | |
| 127 S. | Ma | S. Havana (antigenic formula 1,13,23 : f,g : -) | n.r. | hilA, stn | |
| 135 S. | Ma | S. Reading (antigenic formula 1,4,5,12 : e,h : 1,5) | n.r. | hilA, stn | |
| 226 S. | Zc | S. Braenderup (antigenic formula 6,7 : e,h : e,n,z15) | n.r. | hilA, stn | |
| 245 S. | Zc | S. Reading (antigenic formula 1,4,5,12 : e,h : 1,5) | n.r. | hilA, stn | |
| 255 S. | Zc | S. Reading (antigenic formula 1,4,5,12 : e,h . 1,5) | n.r. | hilA, stn | |
| 273 S. | Zc | S. Albany (antigenic formula 8,20 : z4,z24 : -) | n.r. | hilA, stn | |
| 277 S. | Ma | S. Albany (antigenic formula 8,20 : z4,z24 : -) | n.r. | hilA, stn | |
| 279 S. | Ma | S. Havana (antigenic formula 1,13,23 : f,g : -) | n.r. | hilA, stn | |
| 281 S. | Zc | S. Havana (antigenic formula 1,13,23 : f,g : -) | n.r. | hilA, stn |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grünzweil, O.M.; Palmer, L.; Cabal, A.; Szostak, M.P.; Ruppitsch, W.; Kornschober, C.; Korus, M.; Misic, D.; Bernreiter-Hofer, T.; Korath, A.D.J.; et al. Presence of β-Lactamase-producing Enterobacterales and Salmonella Isolates in Marine Mammals. Int. J. Mol. Sci. 2021, 22, 5905. https://doi.org/10.3390/ijms22115905
Grünzweil OM, Palmer L, Cabal A, Szostak MP, Ruppitsch W, Kornschober C, Korus M, Misic D, Bernreiter-Hofer T, Korath ADJ, et al. Presence of β-Lactamase-producing Enterobacterales and Salmonella Isolates in Marine Mammals. International Journal of Molecular Sciences. 2021; 22(11):5905. https://doi.org/10.3390/ijms22115905
Chicago/Turabian StyleGrünzweil, Olivia M., Lauren Palmer, Adriana Cabal, Michael P. Szostak, Werner Ruppitsch, Christian Kornschober, Maciej Korus, Dusan Misic, Tanja Bernreiter-Hofer, Anna D. J. Korath, and et al. 2021. "Presence of β-Lactamase-producing Enterobacterales and Salmonella Isolates in Marine Mammals" International Journal of Molecular Sciences 22, no. 11: 5905. https://doi.org/10.3390/ijms22115905
APA StyleGrünzweil, O. M., Palmer, L., Cabal, A., Szostak, M. P., Ruppitsch, W., Kornschober, C., Korus, M., Misic, D., Bernreiter-Hofer, T., Korath, A. D. J., Feßler, A. T., Allerberger, F., Schwarz, S., Spergser, J., Müller, E., Braun, S. D., Monecke, S., Ehricht, R., Walzer, C., ... Loncaric, I. (2021). Presence of β-Lactamase-producing Enterobacterales and Salmonella Isolates in Marine Mammals. International Journal of Molecular Sciences, 22(11), 5905. https://doi.org/10.3390/ijms22115905







