Proteomics and Post-Translational Modifications of Starch Biosynthesis-Related Proteins in Developing Seeds of Rice
Abstract
1. Introduction
2. Significant Proteins for Starch Biosynthesis in Rice Seeds
2.1. Amylose and Amylopectin Biosynthesis
2.2. Phosphorylation and Dephosphorylation of Glucan Chains
2.3. Disproportionation to Nonreducing End of Starch
2.4. Starch Granule Initiation
3. Proteomic Profiling of Starch Biosynthesis-Related Proteins
3.1. Specific Starch Biosynthesis-Related Proteins in Rice Seeds
3.2. Starch Biosynthesis-Related Proteins in Different Developmental Stages of Rice Seeds
3.3. Starch Biosynthesis-Related Proteins Respond to High Temperature (HT)
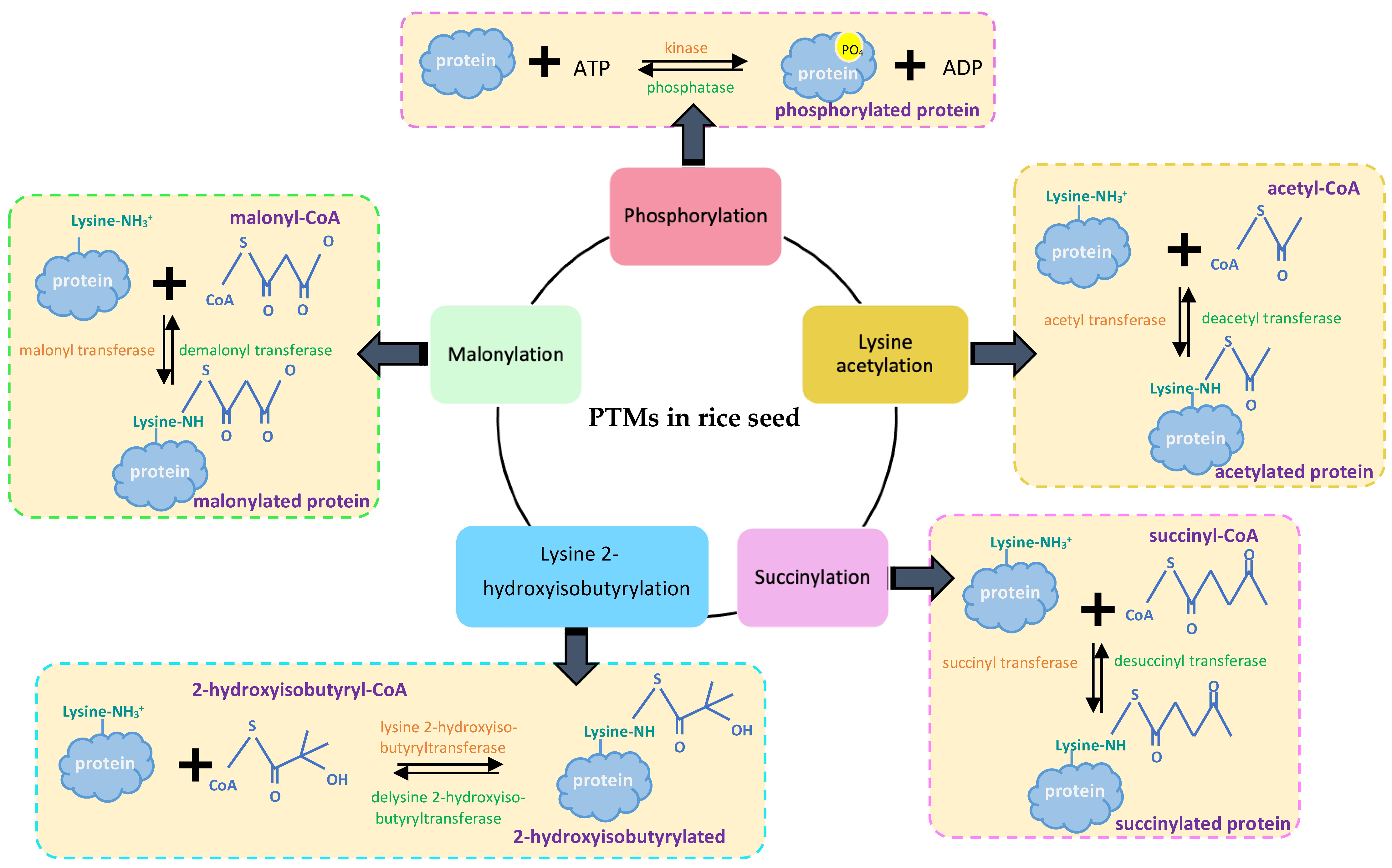
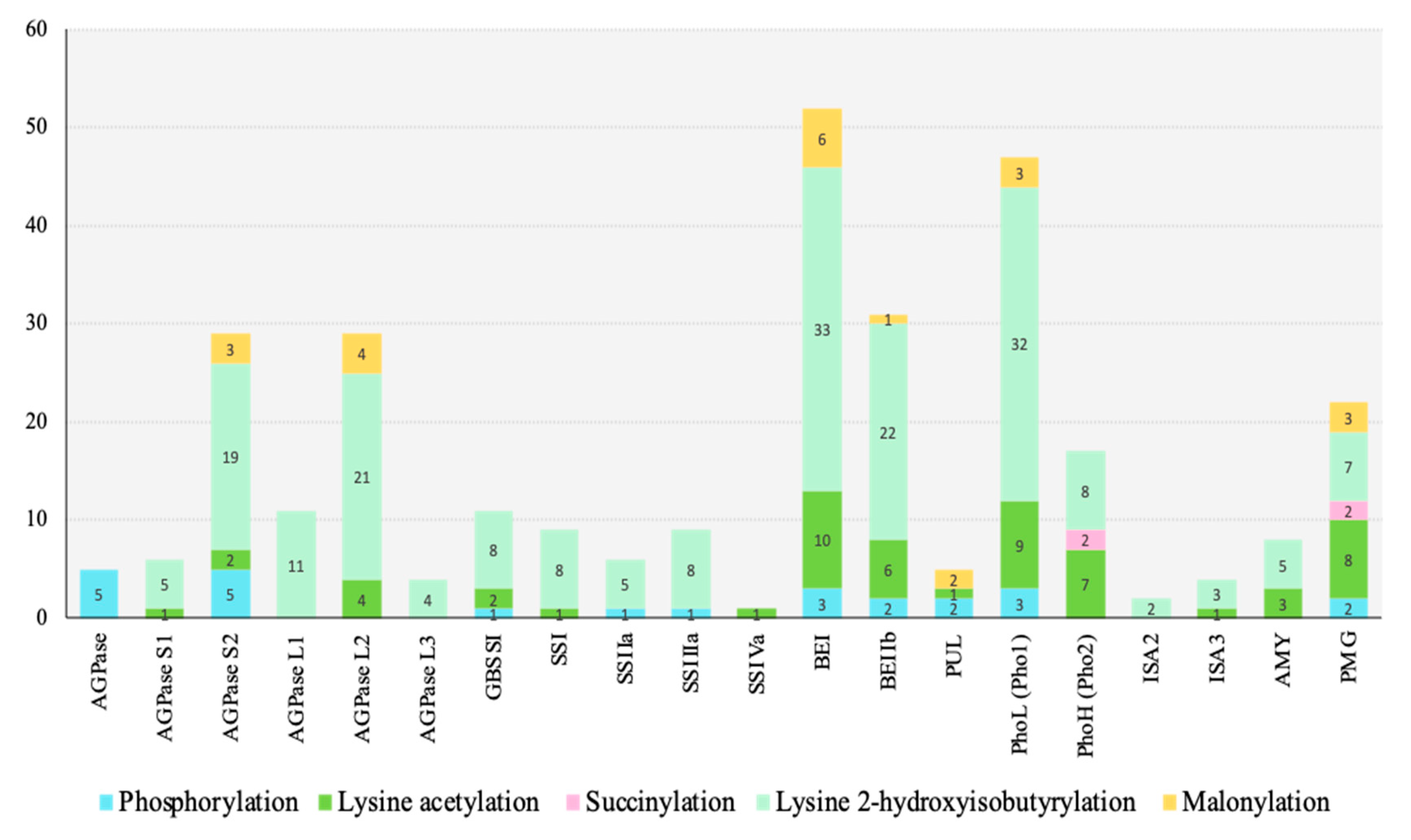
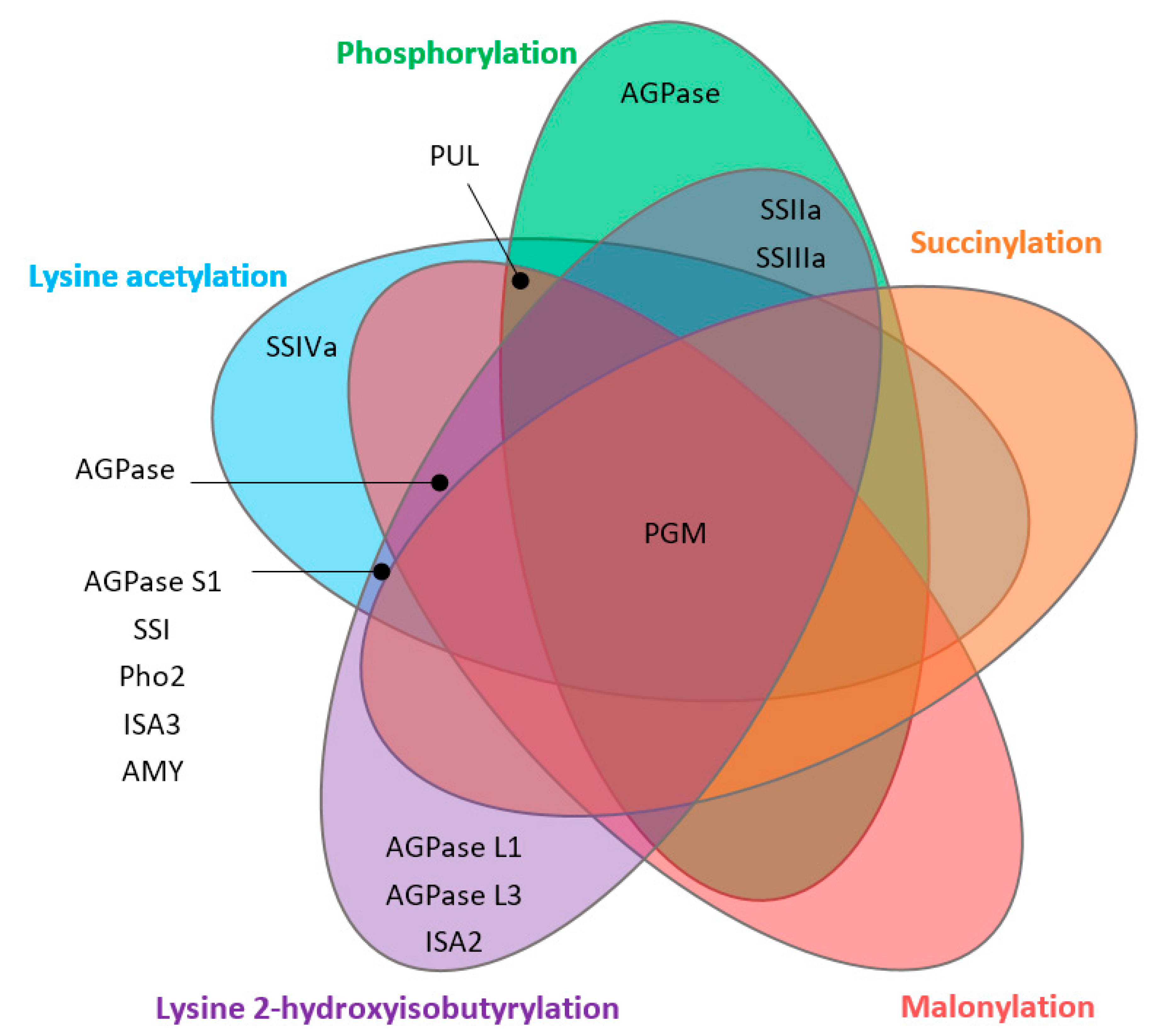
4. Starch Biosynthesis-Related Proteins Targeted by PTMs
4.1. Phosphorylation
4.1.1. Identification of Phosphorylated Protein in Rice Developing Seeds
4.1.2. Potential Role of Protein Phosphorylation in Starch Biosynthesis
4.2. Lysine Acetylation
4.3. Succinylation
4.4. Lysine 2-Hydroxyisobutyrylation (Khib) and Malonylation (Kmal)
5. Summary and Future Perspectives
- (1)
- Proteome alteration under climate change environment: Recently, the global population is facing challenging problems caused by global warming and climate change, which have a great impact on rice yield and quality. Further studies are needed to determine the consequences of climate change, e.g., high/low temperatures, carbon dioxide levels, drought stress, etc., on starch biosynthesis mechanism and regulation by using proteomic analysis.
- (2)
- The number and new types of PTMs in rice seeds: Although five types of PTMs were identified from rice seeds, whether there are other PTMs in rice seed has not been fully addressed. For the number of PTMs sites, Khib showed the highest number of targeted starch biosynthesis proteins (17 proteins), while the lowest number was observed in succinylation (2 proteins). Whether more PTMs would be found under the specific genotype or under the specific abiotic conditions such as heat stress, high carbon dioxide levels, etc., is unknown.
- (3)
- The roles and regulation mechanisms of PTMs on starch biosynthesis: Little is known about the roles of individual PTM on the starch biosynthesis proteins and the impact of PTMs on enzymes’ activities, protein–protein interaction (protein complex formation), and starch functionality. The phosphorylation is well reported in protein complex formation during the starch biosynthesis process in the endosperm of cereal crops. In-depth regulatory studies on protein–protein interactions are necessary to understand the role of protein complex formation in starch biosynthesis in different crops.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Available Statement
Conflicts of Interest
References
- FAO. FAOSTAT. Available online: http://www.fao.org/home/en (accessed on 8 February 2020).
- Jiang, C.; Cheng, Z.; Zhang, C.; Yu, T.; Zhong, Q.; Shen, J.Q.; Huang, X. Proteomic analysis of seed storage proteins in wild rice species of the Oryza genus. Proteome Sci. 2014, 12, 51. [Google Scholar] [CrossRef]
- Wang, W.; Mauleon, R.; Hu, Z.; Chebotarov, D.; Tai, S.; Wu, Z.; Li, M.; Zheng, T.; Fuentes, R.R.; Zhang, F.; et al. Genomic variation in 3010 diverse accessions of Asian cultivated rice. Nature 2018, 557, 43–49. [Google Scholar] [CrossRef]
- Gutaker, R.M.; Groen, S.C.; Bellis, E.S.; Choi, J.Y.; Pires, I.S.; Bocinsky, R.K.; Slayton, E.R.; Wilkins, O.; Castillo, C.C.; Negrão, S.; et al. Genomic history and ecology of the geographic spread of rice. Nat. Plants 2020, 6, 492–502. [Google Scholar] [CrossRef]
- Li, X.; Wu, L.; Wang, J.; Sun, J.; Xia, X.; Geng, X.; Wang, X.; Xu, Z.; Xu, Q. Genome sequencing of rice subspecies and genetic analysis of recombinant lines reveals regional yield- and quality-associated loci. BMC Biol. 2018, 16, 102. [Google Scholar] [CrossRef]
- Khush, G.S. Origin, dispersal, cultivation and variation of rice. Plant Mol. Biol. 1997, 35, 25–34. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Yang, P. Proteomics of rice seed germination. Front. Plant Sci. 2013, 4, 246. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Choi, S.H.; Park, B.S.; Song, J.T.; Kim, M.C.; Koh, H.J.; Seo, H.S. Proteomic analysis of the rice seed for quality improvement. Plant Breed. 2009, 128, 541–550. [Google Scholar] [CrossRef]
- Goren, A.; Ashlock, D.; Tetlow, I.J. Starch formation inside plastids of higher plants. Protoplasma 2018, 255, 1855–1876. [Google Scholar] [CrossRef]
- Zeeman, S.C.; Kossmann, J.; Smith, A.M. Starch: Its metabolism, evolution, and biotechnological modification in plants. Annu. Rev. Plant Biol. 2010, 61, 209–234. [Google Scholar] [CrossRef]
- Ball, S.G.; Morell, M.K. From bacterial glycogen to starch: Understanding the biogenesis of the plant starch granule. Annu. Rev. Plant Biol. 2003, 54, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Jobling, S. Improving starch for food and industrial applications. Curr. Opin. Plant Biol. 2004, 7, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.K.; Chang, M.C.; Tsai, Y.G.; Lur, H.S. Proteomic analysis of the expression of proteins related to rice quality during caryopsis development and the effect of high temperature on expression. Proteomics 2005, 5, 2140–2156. [Google Scholar] [CrossRef]
- Kawagoe, Y.; Kubo, A.; Satoh, H.; Takaiwa, F.; Nakamura, Y. Roles of isoamylase and ADP-glucose pyrophosphorylase in starch granule synthesis in rice endosperm. Plant J. 2005, 42, 164–174. [Google Scholar] [CrossRef]
- Fujita, N.; Yoshida, M.; Asakura, N.; Ohdan, T.; Miyao, A.; Hirochika, H.; Nakamura, Y. Function and characterization of starch synthase I using mutants in rice. Plant Physiol. 2006, 140, 1070–1084. [Google Scholar] [CrossRef]
- Thurston, G.; Regan, S.; Rampitsch, C.; Xing, T. Proteomic and phosphoproteomic approaches to understand plant–pathogen interactions. Physiol. Mol. Plant. Pathol. 2005, 66, 3–11. [Google Scholar] [CrossRef]
- Cánovas, F.M.; Dumas-Gaudot, E.; Recorbet, G.; Jorrin, J.; Mock, H.-P.; Rossignol, M. Plant proteome analysis. Proteomics 2004, 4, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.P.; Brenton, A.G.; Smith, C.J.; Dudley, E. Plant proteome analysis by mass spectrometry: Principles, problems, pitfalls and recent developments. Phytochemistry 2004, 65, 1449–1485. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, X.; Yang, X.; Li, G.; Tang, S.; Wang, S.; Ding, Y.; Liu, Z. Proteomic analysis of proteins related to rice grain chalkiness using iTRAQ and a novel comparison system based on a notched-belly mutant with white-belly. BMC Plant Biol. 2014, 14, 163. [Google Scholar] [CrossRef]
- Wittmann-Liebold, B.; Graack, H.-R.; Pohl, T. Two-dimensional gel electrophoresis as tool for proteomics studies in combination with protein identification by mass spectrometry. Proteomics 2006, 6, 4688–4703. [Google Scholar] [CrossRef]
- Yang, P.; Li, X.; Wang, X.; Chen, H.; Chen, F.; Shen, S. Proteomic analysis of rice (Oryza sativa) seeds during germination. Proteomics 2007, 7, 3358–3368. [Google Scholar] [CrossRef]
- Xu, S.B.; Li, T.; Deng, Z.Y.; Chong, K.; Xue, Y.; Wang, T. Dynamic proteomic analysis reveals a switch between central carbon metabolism and alcoholic fermentation in rice filling grains. Plant. Physiol. 2008, 148, 908–925. [Google Scholar] [CrossRef] [PubMed]
- Koller, A.; Washburn, M.P.; Lange, B.M.; Andon, N.L.; Deciu, C.; Haynes, P.A.; Hays, L.; Schieltz, D.; Ulaszek, R.; Wei, J.; et al. Proteomic survey of metabolic pathways in rice. Proc. Natl. Acad. Sci. USA 2002, 99, 11969. [Google Scholar] [CrossRef]
- Xu, S.B.; Yu, H.T.; Yan, L.F.; Wang, T. Integrated proteomic and cytological study of rice endosperms at the storage phase. J. Proteome Res. 2010, 9, 4906–4918. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Koh, H.-J. A label-free quantitative shotgun proteomics analysis of rice grain development. Proteome Sci. 2011, 9, 61. [Google Scholar] [CrossRef]
- Yu, H.; Wang, T. Proteomic dissection of endosperm starch granule associated proteins reveals a network coordinating starch biosynthesis and amino acid metabolism and glycolysis in rice endosperms. Front. Plant Sci. 2016, 7, 707. [Google Scholar] [CrossRef]
- Han, C.; Wang, K.; Yang, P. Gel-based comparative phosphoproteomic analysis on rice embryo during germination. Plant. Cell Physiol. 2014, 55, 1376–1394. [Google Scholar] [CrossRef]
- Pang, Y.; Zhou, X.; Chen, Y.; Bao, J.S. Comparative phosphoproteomic analysis of the developing seeds in two Indica rice (Oryza sativa L.) cultivars with different starch quality. J. Agric. Food Chem. 2018, 66, 3030–3037. [Google Scholar] [CrossRef]
- Huang, K.-Y.; Su, M.-G.; Kao, H.-J.; Hsieh, Y.-C.; Jhong, J.-H.; Cheng, K.-H.; Huang, H.-D.; Lee, T.-Y. dbPTM 2016: 10-year anniversary of a resource for post-translational modification of proteins. Nucleic Acids Res. 2016, 44, D435–D446. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, Y.; Qiu, J.; Li, Z.; Zhao, J.; Tong, X.; Zhang, J. A quantitative acetylomic analysis of early seed development in rice (Oryza sativa L.). Int. J. Mol. Sci. 2017, 18, 1376. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Hou, Y.; Tong, X.; Wang, Y.; Lin, H.; Liu, Q.; Zhang, W.; Li, Z.; Nallamilli, B.R.; Zhang, J. Quantitative phosphoproteomic analysis of early seed development in rice (Oryza sativa L.). Plant Mol. Biol. 2016, 90, 249–265. [Google Scholar] [CrossRef]
- Xing, S.; Meng, X.; Zhou, L.; Mujahid, H.; Zhao, C.; Zhang, Y.; Wang, C.; Peng, Z. Proteome profile of starch granules purified from rice (Oryza sativa) endosperm. PLoS ONE 2016, 11, e0168467. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Lv, Y.; Mujahid, H.; Edelmann, M.J.; Zhao, H.; Peng, X.; Peng, Z. Proteome-wide lysine acetylation identification in developing rice (Oryza sativa) seeds and protein co-modification by acetylation, succinylation, ubiquitination, and phosphorylation. Biochim. Biophys. Acta (BBA)–Proteins Proteom. 2018, 1866, 451–463. [Google Scholar] [CrossRef]
- He, D.; Wang, Q.; Li, M.; Damaris, R.N.; Yi, X.; Cheng, Z.; Yang, P. Global proteome analyses of lysine acetylation and succinylation reveal the widespread involvement of both modification in metabolism in the embryo of germinating rice seed. J. Proteome Res. 2016, 15, 879–890. [Google Scholar] [CrossRef]
- Mujahid, H.; Meng, X.; Xing, S.; Peng, X.; Wang, C.; Peng, Z. Malonylome analysis in developing rice (Oryza sativa) seeds suggesting that protein lysine malonylation is well-conserved and overlaps with acetylation and succinylation substantially. J. Proteom. 2018, 170, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Xing, S.; Perez, L.M.; Peng, X.; Zhao, Q.; Redoña, E.D.; Wang, C.; Peng, Z. Proteome-wide analysis of lysine 2-hydroxyisobutyrylation in developing rice (Oryza sativa) seeds. Sci. Rep. 2017, 7, 17486. [Google Scholar] [CrossRef]
- Seck, P.A.; Diagne, A.; Mohanty, S.; Wopereis, M.C.S. Crops that feed the world 7: Rice. Food Secur. 2012, 4, 7–24. [Google Scholar] [CrossRef]
- Chang, T.-S.; Liu, C.-W.; Lin, Y.-L.; Li, C.-Y.; Wang, A.Z.; Chien, M.-W.; Wang, C.-S.; Lai, C.-C. Mapping and comparative proteomic analysis of the starch biosynthetic pathway in rice by 2D PAGE/MS. Plant Mol. Biol. 2017, 95, 333–343. [Google Scholar] [CrossRef]
- Bao, J.S. Biotechnology for rice grain quality improvement. In Rice Chemistry and Technology, 4th ed.; Bao, J.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 443–471. [Google Scholar] [CrossRef]
- Akihiro, T.; Mizuno, K.; Fujimura, T. Gene expression of ADP-glucose pyrophosphorylase and starch contents in rice cultured cells are cooperatively regulated by sucrose and ABA. Plant Cell Physiol. 2005, 46, 937–946. [Google Scholar] [CrossRef]
- Hannah, L.C.; James, M. The complexities of starch biosynthesis in cereal endosperms. Curr. Opin. Biotechnol. 2008, 19, 160–165. [Google Scholar] [CrossRef]
- Nakamura, Y.; Francisco, P.B.; Hosaka, Y.; Sato, A.; Sawada, T.; Kubo, A.; Fujita, N. Essential amino acids of starch synthase IIa differentiate amylopectin structure and starch quality between japonica and indica rice varieties. Plant Mol. Biol. 2005, 58, 213–227. [Google Scholar] [CrossRef]
- Fujita, N.; Yoshida, M.; Kondo, T.; Saito, K.; Utsumi, Y.; Tokunaga, T.; Nishi, A.; Satoh, H.; Park, J.-H.; Jane, J.-L.; et al. Characterization of SSIIIa-deficient mutants of rice: The function of SSIIIa and pleiotropic effects by SSIIIa deficiency in the rice endosperm. Plant Physiol. 2007, 144, 2009–2023. [Google Scholar] [CrossRef]
- Ohdan, T.; Francisco, P.B., Jr.; Sawada, T.; Hirose, T.; Terao, T.; Satoh, H.; Nakamura, Y. Expression profiling of genes involved in starch synthesis in sink and source organs of rice. J. Exp. Bot. 2005, 56, 3229–3244. [Google Scholar] [CrossRef]
- Hirose, T.; Terao, T. A comprehensive expression analysis of the starch synthase gene family in rice (Oryza sativa L.). Planta 2004, 220, 9–16. [Google Scholar] [CrossRef]
- Pfister, B.; Zeeman, S.C. Formation of starch in plant cells. Cell Mol. Life Sci. 2016, 73, 2781–2807. [Google Scholar] [CrossRef]
- Nakamura, Y. Towards a better understanding of the metabolic system for amylopectin biosynthesis in plants: Rice endosperm as a model tissue. Plant Cell Physiol. 2002, 43, 718–725. [Google Scholar] [CrossRef]
- Zhu, L.; Gu, M.; Meng, X.; Cheung, S.C.K.; Yu, H.; Huang, J.; Sun, Y.; Shi, Y.; Liu, Q. High-amylose rice improves indices of animal health in normal and diabetic rats. Plant. Biotechnol. J. 2012, 10, 353–362. [Google Scholar] [CrossRef]
- Nakamura, Y.; Utsumi, Y.; Sawada, T.; Aihara, S.; Utsumi, C.; Yoshida, M.; Kitamura, S. Characterization of the reactions of starch branching enzymes from rice endosperm. Plant. Cell Physiol. 2010, 51, 776–794. [Google Scholar] [CrossRef]
- Fujita, N.; Toyosawa, Y.; Utsumi, Y.; Higuchi, T.; Hanashiro, I.; Ikegami, A.; Akuzawa, S.; Yoshida, M.; Mori, A.; Inomata, K.; et al. Characterization of pullulanase (PUL)-deficient mutants of rice (Oryza sativa L.) and the function of PUL on starch biosynthesis in the developing rice endosperm. J. Exp. Bot. 2009, 60, 1009–1023. [Google Scholar] [CrossRef]
- Hwang, S.-K.; Koper, K.; Okita, T.W. The plastid phosphorylase as a multiple-role player in plant metabolism. Plant Sci. 2020, 290, 110303. [Google Scholar] [CrossRef]
- Van Berkel, J.; Conrads-Strauch, J.; Steup, M. Glucan-phosphorylase forms in cotyledons of Pisum sativum L.: Localization, developmental change, in-vitro translation, and processing. Planta 1991, 185, 432–439. [Google Scholar] [CrossRef]
- Satoh, H.; Shibahara, K.; Tokunaga, T.; Nishi, A.; Tasaki, M.; Hwang, S.-K.; Okita, T.W.; Kaneko, N.; Fujita, N.; Yoshida, M.; et al. Mutation of the plastidial alpha-glucan phosphorylase gene in rice affects the synthesis and structure of starch in the endosperm. Plant Cell 2008, 20, 1833–1849. [Google Scholar] [CrossRef]
- Hwang, S.-K.; Singh, S.; Cakir, B.; Satoh, H.; Okita, T.W. The plastidial starch phosphorylase from rice endosperm: Catalytic properties at low temperature. Planta 2016, 243, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-K.; Eom, J.-S.; Hwang, S.-K.; Shin, D.; An, G.; Okita, T.W.; Jeon, J.-S. Plastidic phosphoglucomutase and ADP-glucose pyrophosphorylase mutants impair starch synthesis in rice pollen grains and cause male sterility. J. Exp. Bot. 2016, 67, 5557–5569. [Google Scholar] [CrossRef]
- Ritte, G.; Lloyd, J.R.; Eckermann, N.; Rottmann, A.; Kossmann, J.; Steup, M. The starch-related R1 protein is an α-glucan, water dikinase. Proc. Natl. Acad. Sci. USA 2002, 99, 7166. [Google Scholar] [CrossRef]
- Mikkelsen, R.; Baunsgaard, L.; Blennow, A. Functional characterization of alpha-glucan, water dikinase, the starch phosphorylating enzyme. Biochem. J. 2004, 377, 525–532. [Google Scholar] [CrossRef]
- Kötting, O.; Pusch, K.; Tiessen, A.; Geigenberger, P.; Steup, M.; Ritte, G. Identification of a novel enzyme required for starch metabolism in Arabidopsis leaves. The phosphoglucan, Water Dikinase. Plant Physiol. 2005, 137, 242. [Google Scholar] [CrossRef]
- Baunsgaard, L.; Lütken, H.; Mikkelsen, R.; Glaring, M.A.; Pham, T.T.; Blennow, A. A novel isoform of glucan, water dikinase phosphorylates pre-phosphorylated α-glucans and is involved in starch degradation in Arabidopsis. Plant J. 2005, 41, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Blennow, A.; Engelsen, S.B. Helix-breaking news: Fighting crystalline starch energy deposits in the cell. Trends Plant Sci. 2010, 15, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Tagliabracci, V.S.; Roach, P.J. Insights into the mechanism of polysaccharide dephosphorylation by a glucan phosphatase. Proc. Natl. Acad. Sci. USA 2010, 107, 15312–15313. [Google Scholar] [CrossRef]
- Reimann, R.; Ritte, G.; Steup, M.; Appenroth, K.-J. Association of α-amylase and the R1 protein with starch granules precedes the initiation of net starch degradation in turions of Spirodela polyrhiza. Physiol. Plant 2002, 114, 2–12. [Google Scholar] [CrossRef]
- Hirose, T.; Aoki, N.; Harada, Y.; Okamura, M.; Hashida, Y.; Ohsugi, R.; Miyao, A.; Hirochika, H.; Terao, T. Disruption of a rice gene for α-glucan water dikinase, OsGWD1, leads to hyperaccumulation of starch in leaves but exhibits limited effects on growth. Front. Plant Sci. 2013, 4, 147. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, X.; Zhou, X.; Hebelstrup, K.H.; Blennow, A.; Bao, J. Highly phosphorylated functionalized rice starch produced by transgenic rice expressing the potato GWD1 gene. Sci. Rep. 2017, 7, 3339. [Google Scholar] [CrossRef]
- Huang, L.-F.; Liu, Y.-K.; Su, S.-C.; Lai, C.-C.; Wu, C.-R.; Chao, T.-J.; Yang, Y.-H. Genetic engineering of transitory starch accumulation by knockdown of OsSEX4 in rice plants for enhanced bioethanol production. Biotechnol. Bioeng. 2020, 117, 933–944. [Google Scholar] [CrossRef]
- Critchley, J.H.; Zeeman, S.C.; Takaha, T.; Smith, A.M.; Smith, S.M. A critical role for disproportionating enzyme in starch breakdown is revealed by a knock-out mutation in Arabidopsis. Plant. J. 2001, 26, 89–100. [Google Scholar] [CrossRef]
- Blennow, A.; Nielsen, T.H.; Baunsgaard, L.; Mikkelsen, R.; Engelsen, S.B. Starch phosphorylation: A new front line in starch research. Trends Plant Sci. 2002, 7, 445–450. [Google Scholar] [CrossRef]
- Kötting, O.; Santelia, D.; Edner, C.; Eicke, S.; Marthaler, T.; Gentry, M.S.; Comparot-Moss, S.; Chen, J.; Smith, A.M.; Steup, M.; et al. Starch-Excess4 is a laforin-like phosphoglucan phosphatase required for starch degradation in Arabidopsis thaliana. Plant Cell 2009, 21, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Santelia, D.; Kötting, O.; Seung, D.; Schubert, M.; Thalmann, M.; Bischof, S.; Meekins, D.A.; Lutz, A.; Patron, N.; Gentry, M.S.; et al. The phosphoglucan phosphatase like sex four2 dephosphorylates starch at the C3-position in Arabidopsis. Plant Cell 2011, 23, 4096–4111. [Google Scholar] [CrossRef] [PubMed]
- Zeeman, S.C.; Rees, T.A. Changes in carbohydrate metabolism and assimilate export in starch-excess mutants of Arabidopsis. Plant Cell Environ. 1999, 22, 1445–1453. [Google Scholar] [CrossRef]
- Ma, J.; Jiang, Q.-T.; Wei, L.; Yang, Q.; Zhang, X.-W.; Peng, Y.-Y.; Chen, G.-Y.; Wei, Y.-M.; Liu, C.; Zheng, Y.-L. Conserved structure and varied expression reveal key roles of phosphoglucan phosphatase gene starch excess 4 in barley. Planta 2014, 240, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Akdogan, G.; Kubota, J.; Kubo, A.; Takaha, T.; Kitamura, S. Expression and characterization of rice disproportionating enzymes. J. Appl. Glycosci. 2011, 58, 99–105. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, D.; Liu, J.; Liu, Q.Q.; Liu, H.; Tian, L.; Jiang, L.; Qu, L.Q. Plastidial disproportionating enzyme participates in starch synthesis in rice endosperm by transferring maltooligosyl groups from amylose and amylopectin to amylopectin. Plant Physiol. 2015, 169, 2496. [Google Scholar] [CrossRef] [PubMed]
- Chia, T.; Thorneycroft, D.; Chapple, A.; Messerli, G.; Chen, J.; Zeeman, S.C.; Smith, S.M.; Smith, A.M. A cytosolic glucosyltransferase is required for conversion of starch to sucrose in Arabidopsis leaves at night. Plant J. 2004, 37, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Seung, D.; Smith, A.M. Starch granule initiation and morphogenesis—progress in Arabidopsis and cereals. J. Exp. Bot. 2019, 70, 771–784. [Google Scholar] [CrossRef]
- Seung, D. Amylose in starch: Towards an understanding of biosynthesis, structure and function. New Phytol. 2020, 228, 1490–1504. [Google Scholar] [CrossRef]
- Lohmeier-Vogel, E.M.; Kerk, D.; Nimick, M.; Wrobel, S.; Vickerman, L.; Muench, D.G.; Moorhead, G.B.G. Arabidopsis At5g39790 encodes a chloroplast-localized, carbohydrate-binding, coiled-coil domain-containing putative scaffold protein. BMC Plant Biol. 2008, 8, 120. [Google Scholar] [CrossRef]
- Seung, D.; Soyk, S.; Coiro, M.; Maier, B.A.; Eicke, S.; Zeeman, S.C. Protein targeting to starch is required for localising granule-bound starch synthase to starch granules and for normal amylose synthesis in Arabidopsis. PLoS Biol. 2015, 13, e1002080. [Google Scholar] [CrossRef]
- Seung, D.; Echevarría-Poza, A.; Steuernagel, B.; Smith, A.M. Natural polymorphisms in Arabidopsis result in wide variation or loss of the amylose component of starch. Plant Physiol. 2020, 182, 870. [Google Scholar] [CrossRef] [PubMed]
- Seung, D.; Boudet, J.; Monroe, J.; Schreier, T.B.; David, L.C.; Abt, M.; Lu, K.-J.; Zanella, M.; Zeeman, S.C. Homologs of Protein Targeting to Starch control starch granule initiation in Arabidopsis leaves. Plant Cell 2017, 29, 1657. [Google Scholar] [CrossRef]
- Seung, D.; Schreier, T.B.; Bürgy, L.; Eicke, S.; Zeeman, S.C. Two plastidial coiled-coil proteins are essential for normal starch granule initiation in Arabidopsis. Plant Cell 2018, 30, 1523. [Google Scholar] [CrossRef]
- Wang, W.; Wei, X.; Jiao, G.; Chen, W.; Wu, Y.; Sheng, Z.; Hu, S.; Xie, L.; Wang, J.; Tang, S.; et al. Gbss-Binding Protein, encoding a CBM48 domain-containing protein, affects rice quality and yield. J. Integr. Plant Biol. 2020, 62, 948–966. [Google Scholar] [CrossRef]
- Peng, C.; Wang, Y.; Liu, F.; Ren, Y.; Zhou, K.; Lv, J.; Zheng, M.; Zhao, S.; Zhang, L.; Wang, C.; et al. Floury Endosperm6 encodes a CBM48 domain-containing protein involved in compound granule formation and starch synthesis in rice endosperm. Plant J. 2014, 77, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.T.; Garneau-Tsodikova, S.; Gatto Jr, G.J. Protein posttranslational modifications: The chemistry of proteome diversifications. Angew. Chem. Int. Ed. 2005, 44, 7342–7372. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, A.; Wiese, E.K.; Hitosugi, T. Enzymatic and metabolic regulation of lysine succinylation. Genes Dis. 2020, 7, 166–171. [Google Scholar] [CrossRef]
- Ishimaru, T.; Matsuda, T.; Ohsugi, R.; Yamagishi, T. Morphological development of rice caryopses located at the different positions in a panicle from early to middle stage of grain filling. Funct Plant Biol. 2003, 30, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, H.; Tang, J.; Li, Z.; Li, Z.; Chen, D.; Lin, W. A proteomic study on molecular mechanism of poor grain-filling of rice (Oryza sativa L.) inferior spikelets. PLoS ONE 2014, 9, e89140. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, J.; Du, T.; Zhao, H.; Li, Z.; Li, Z.; Lin, W. Mechanism of developmental stagnancy of rice inferior spikelets at early grain-filling stage as revealed by proteomic analysis. Plant Mol. Biol. Report. 2015, 33, 1844–1863. [Google Scholar] [CrossRef]
- Liao, J.-L.; Zhou, H.-W.; Zhang, H.-Y.; Zhong, P.-A.; Huang, Y.-J. Comparative proteomic analysis of differentially expressed proteins in the early milky stage of rice grains during high temperature stress. J. Exp. Bot. 2014, 65, 655–671. [Google Scholar] [CrossRef]
- Timabud, T.; Yin, X.; Pongdontri, P.; Komatsu, S. Gel-free/label-free proteomic analysis of developing rice grains under heat stress. J. Proteom. 2016, 133, 1–19. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Hu, M.; Wang, Z.; Hua, H.; Yin, C.; Zeng, H. Different effects of night versus day high temperature on rice quality and accumulation profiling of rice grain proteins during grain filling. Plant Cell Rep. 2011, 30, 1641–1659. [Google Scholar] [CrossRef]
- Kaneko, K.; Sasaki, M.; Kuribayashi, N.; Suzuki, H.; Sasuga, Y.; Shiraya, T.; Inomata, T.; Itoh, K.; Baslam, M.; Mitsui, T. Proteomic and glycomic characterization of rice chalky grains produced under moderate and high-temperature conditions in field system. Rice 2016, 9, 26. [Google Scholar] [CrossRef]
- Ishimaru, T.; Horigane, A.K.; Ida, M.; Iwasawa, N.; San-oh, Y.A.; Nakazono, M.; Nishizawa, N.K.; Masumura, T.; Kondo, M.; Yoshida, M. Formation of grain chalkiness and changes in water distribution in developing rice caryopses grown under high-temperature stress. J. Cereal Sci. 2009, 50, 166–174. [Google Scholar] [CrossRef]
- Lemeer, S.; Heck, A.J.R. The phosphoproteomics data explosion. Curr. Opin. Chem. Biol. 2009, 13, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Yang, P. Phosphoproteomics in Cereals. In Plant Phosphoproteomics: Methods and Protocols; Schulze, W.X., Ed.; Springer: New York, NY, USA, 2015; pp. 47–57. [Google Scholar]
- Reinders, J.; Sickmann, A. State-of-the-art in phosphoproteomics. Proteomics 2005, 5, 4052–4061. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, N.; Nakagami, H.; Mochida, K.; Daudi, A.; Tomita, M.; Shirasu, K.; Ishihama, Y. Large-scale phosphorylation mapping reveals the extent of tyrosine phosphorylation in Arabidopsis. Mol. Syst. Biol. 2008, 4, 193. [Google Scholar] [CrossRef]
- Adams, J.A. Kinetic and catalytic mechanisms of protein kinases. Chem. Rev. 2001, 101, 2271–2290. [Google Scholar] [CrossRef]
- Fang, Y.; Deng, X.; Lu, X.; Zheng, J.; Jiang, H.; Rao, Y.; Zeng, D.; Hu, J.; Zhang, X.; Xue, D. Differential phosphoproteome study of the response to cadmium stress in rice. Ecotoxicol. Environ. Saf. 2019, 180, 780–788. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, Z.; Long, H.; Zhang, Z.; Hong, Y.; Zhang, X.; You, C.; Liang, W.; Ma, H.; Lu, P. Proteomic and phosphoproteomic analyses reveal extensive phosphorylation of regulatory proteins in developing rice anthers. Plant. J. 2015, 84, 527–544. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, X.; Qiu, J.; Li, Z.; Zhao, J.; Hou, Y.; Tang, L.; Zhang, J. A phosphoproteomic landscape of rice (Oryza sativa) tissues. Physiol. Plant. 2017, 160, 458–475. [Google Scholar] [CrossRef]
- Sun, R.; Qin, S.; Zhang, T.; Wang, Z.; Li, H.; Li, Y.; Nie, Y. Comparative phosphoproteomic analysis of blast resistant and susceptible rice cultivars in response to salicylic acid. BMC Plant Biol. 2019, 19, 454. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Yang, P.; Sakata, K.; Komatsu, S. Quantitative proteomics reveals the role of protein phosphorylation in rice embryos during early stages of germination. J. Proteome Res. 2014, 13, 1766–1782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, C.-Y.; Lv, D.-W.; Zhen, S.-M.; Li, X.-H.; Yan, Y.-M. Comparative phosphoproteome analysis of the developing grains in bread wheat (Triticum aestivum L.) under well-watered and water-deficit conditions. J. Proteome Res. 2014, 13, 4281–4297. [Google Scholar] [CrossRef]
- Vu, L.D.; Zhu, T.; Verstraeten, I.; van de Cotte, B.; The International Wheat Genome Sequencing, C.; Gevaert, K.; De Smet, I. Temperature-induced changes in the wheat phosphoproteome reveal temperature-regulated interconversion of phosphoforms. J. Exp. Bot. 2018, 69, 4609–4624. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Deng, X.; Zhang, M.; Zhu, G.; Lv, D.; Wang, Y.; Zhu, D.; Yan, Y. Comparative phosphoproteomic analysis under high-nitrogen fertilizer reveals central phosphoproteins promoting wheat grain starch and protein synthesis. Front. Plant. Sci. 2017, 8, 67. [Google Scholar] [CrossRef][Green Version]
- Chen, G.-X.; Zhou, J.-W.; Liu, Y.-L.; Lu, X.-B.; Han, C.-X.; Zhang, W.-Y.; Xu, Y.-H.; Yan, Y.-M. Biosynthesis and regulation of wheat amylose and amylopectin from proteomic and phosphoproteomic characterization of granule-binding proteins. Sci. Rep. 2016, 6, 33111. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.-W.; Zhu, G.-R.; Zhu, D.; Bian, Y.-W.; Liang, X.-N.; Cheng, Z.-W.; Deng, X.; Yan, Y.-M. Proteomic and phosphoproteomic analysis reveals the response and defense mechanism in leaves of diploid wheat T. monococcum under salt stress and recovery. J. Proteom. 2016, 143, 93–105. [Google Scholar] [CrossRef]
- Ishikawa, S.; Barrero, J.M.; Takahashi, F.; Nakagami, H.; Peck, S.C.; Gubler, F.; Shinozaki, K.; Umezawa, T. Comparative phosphoproteomic analysis reveals a decay of ABA signaling in barley embryos during after-ripening. Plant Cell Physiol. 2019, 60, 2758–2768. [Google Scholar] [CrossRef]
- Ishikawa, S.; Barrero, J.; Takahashi, F.; Peck, S.; Gubler, F.; Shinozaki, K.; Umezawa, T. Comparative phosphoproteomic analysis of Barley Embryos with different dormancy during imbibition. Int. J. Mol. Sci. 2019, 20, 451. [Google Scholar] [CrossRef]
- Cao, H.; Zhou, Y.; Chang, Y.; Zhang, X.; Li, C.; Ren, D. Comparative phosphoproteomic analysis of developing maize seeds suggests a pivotal role for enolase in promoting starch synthesis. Plant Sci. 2019, 289, 110243. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Bai, X.; Jiang, C.; Li, Z. Phosphoproteomic analysis of two contrasting maize inbred lines provides insights into the mechanism of salt-stress tolerance. Int. J. Mol. Sci. 2019, 20, 1886. [Google Scholar] [CrossRef]
- Hu, X.; Wu, L.; Zhao, F.; Zhang, D.; Li, N.; Zhu, G.; Li, C.; Wang, W. Phosphoproteomic analysis of the response of maize leaves to drought, heat and their combination stress. Front. Plant Sci. 2015, 6, 298. [Google Scholar] [CrossRef]
- Wu, L.; Wang, S.; Wu, J.; Han, Z.; Wang, R.; Wu, L.; Zhang, H.; Chen, Y.; Hu, X. Phosphoproteomic analysis of the resistant and susceptible genotypes of maize infected with sugarcane mosaic virus. Amino Acids 2015, 47, 483–496. [Google Scholar] [CrossRef]
- Lu, T.C.; Meng, L.B.; Yang, C.P.; Liu, G.F.; Liu, G.J.; Ma, W.; Wang, B.C. A shotgun phosphoproteomics analysis of embryos in germinated maize seeds. Planta 2008, 228, 1029–1041. [Google Scholar] [CrossRef]
- Nakagami, H.; Sugiyama, N.; Mochida, K.; Daudi, A.; Yoshida, Y.; Toyoda, T.; Tomita, M.; Ishihama, Y.; Shirasu, K. Large-scale comparative phosphoproteomics identifies conserved phosphorylation sites in plants. Plant Physiol. 2010, 153, 1161–1174. [Google Scholar] [CrossRef]
- Tetlow, I.J.; Wait, R.; Lu, Z.; Akkasaeng, R.; Bowsher, C.G.; Esposito, S.; Kosar-Hashemi, B.; Morell, M.K.; Emes, M.J. Protein phosphorylation in amyloplasts regulates starch branching enzyme activity and protein-protein interactions. Plant Cell 2004, 16, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Makhmoudova, A.; Lee, E.A.; Wait, R.; Emes, M.J.; Tetlow, I.J. The amylose extender mutant of maize conditions novel protein–protein interactions between starch biosynthetic enzymes in amyloplasts. J. Exp. Bot. 2009, 60, 4423–4440. [Google Scholar] [CrossRef]
- Tetlow, I.J.; Beisel, K.G.; Cameron, S.; Makhmoudova, A.; Liu, F.; Bresolin, N.S.; Wait, R.; Morell, M.K.; Emes, M.J. Analysis of protein complexes in wheat amyloplasts reveals functional interactions among starch biosynthetic enzymes. Plant Physiol. 2008, 146, 1878. [Google Scholar] [CrossRef] [PubMed]
- Mehrpouyan, S.; Menon, U.; Tetlow, I.J.; Emes, M.J. Protein phosphorylation regulates maize endosperm starch synthase IIa activity and protein−protein interactions. Plant J. 2021, 105, 1098–1112. [Google Scholar] [CrossRef]
- Liu, F.; Ahmed, Z.; Lee, E.A.; Donner, E.; Liu, Q.; Ahmed, R.; Morell, M.K.; Emes, M.J.; Tetlow, I.J. Allelic variants of the amylose extender mutation of maize demonstrate phenotypic variation in starch structure resulting from modified protein–protein interactions. J. Exp. Bot. 2012, 63, 1167–1183. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Tetlow, I.J.; Ahmed, R.; Morell, M.K.; Emes, M.J. Protein–protein interactions among enzymes of starch biosynthesis in high-amylose barley genotypes reveal differential roles of heteromeric enzyme complexes in the synthesis of A and B granules. Plant Sci. 2015, 233, 95–106. [Google Scholar] [CrossRef]
- Liu, D.-R.; Huang, W.-X.; Cai, X.-L. Oligomerization of rice granule-bound starch synthase 1 modulates its activity regulation. Plant Sci. 2013, 210, 141–150. [Google Scholar] [CrossRef]
- Wang, S.J.; Liu, L.F.; Chen, C.K.; Chen, L.W. Regulations of granule-bound starch synthase I gene expression in rice leaves by temperature and drought stress. Biol. Plant. 2006, 50, 537–541. [Google Scholar] [CrossRef]
- Crofts, N.; Abe, N.; Oitome, N.F.; Matsushima, R.; Hayashi, M.; Tetlow, I.J.; Emes, M.J.; Nakamura, Y.; Fujita, N. Amylopectin biosynthetic enzymes from developing rice seed form enzymatically active protein complexes. J. Exp. Bot. 2015, 66, 4469–4482. [Google Scholar] [CrossRef]
- Crofts, N.; Iizuka, Y.; Abe, N.; Miura, S.; Kikuchi, K.; Matsushima, R.; Fujita, N. Rice mutants lacking Starch Synthase I or Branching Enzyme IIb activity altered starch biosynthetic protein complexes. Front. Plant Sci. 2018, 9, 1817. [Google Scholar] [CrossRef]
- Chen, Y.; Pang, Y.; Bao, J.S. Expression profiles and protein complexes of starch biosynthetic enzymes from white-core and waxy mutants induced from high amylose Indica rice. Rice Sci. 2020, 27, 152–161. [Google Scholar] [CrossRef]
- Hwang, S.-K.; Koper, K.; Satoh, H.; Okita, T.W. Rice endosperm starch phosphorylase (Pho1) assembles with disproportionating enzyme (Dpe1) to form a protein complex that enhances synthesis of malto-oligosaccharides. J. Biol. Chem. 2016, 291, 19994–20007. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, J.; Gao, X.; Zhou, Y.; Wen, L.; Yang, X.; Yao, X.; Ren, J.; Xue, Y. CPLA 1.0: An integrated database of protein lysine acetylation. Nucleic Acids Res. 2011, 39, D1029–D1034. [Google Scholar] [CrossRef]
- Choudhary, C.; Kumar, C.; Gnad, F.; Nielsen, M.L.; Rehman, M.; Walther, T.C.; Olsen, J.V.; Mann, M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 2009, 325, 834. [Google Scholar] [CrossRef]
- Nallamilli, B.R.R.; Edelmann, M.J.; Zhong, X.; Tan, F.; Mujahid, H.; Zhang, J.; Nanduri, B.; Peng, Z. Global analysis of lysine acetylation suggests the involvement of protein acetylation in diverse biological processes in rice (Oryza sativa). PLoS ONE 2014, 9, e89283. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Yang, C.; Xiong, H.; Lin, Y.; Yao, J.; Li, H.; Xie, L.; Zhao, W.; Yao, Y.; et al. Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science 2010, 327, 1004–1007. [Google Scholar] [CrossRef]
- Glozak, M.A.; Sengupta, N.; Zhang, X.; Seto, E. Acetylation and deacetylation of non-histone proteins. Gene 2005, 363, 15–23. [Google Scholar] [CrossRef]
- Yan, Z.; Shen, Z.; Gao, Z.-F.; Chao, Q.; Qian, C.-R.; Zheng, H.; Wang, B.-C. A comprehensive analysis of the lysine acetylome reveals diverse functions of acetylated proteins during de-etiolation in Zea mays. J. Plant Physiol. 2020, 248, 153158. [Google Scholar] [CrossRef]
- Zhen, S.; Deng, X.; Wang, J.; Zhu, G.; Cao, H.; Yuan, L.; Yan, Y. First comprehensive proteome analyses of lysine acetylation and succinylation in seedling leaves of Brachypodium distachyon L. Sci. Rep. 2016, 6, 31576. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, M.; Xie, Z.; Dai, L.; Chen, Y.; Zhao, Y. Identification of lysine succinylation as a new post-translational modification. Nat. Chem. Biol. 2011, 7, 58–63. [Google Scholar] [CrossRef]
- Xie, Z.; Dai, J.; Dai, L.; Tan, M.; Cheng, Z.; Wu, Y.; Boeke, J.D.; Zhao, Y. Lysine succinylation and lysine malonylation in histones. Mol. Cell. Proteom.: MCP 2012, 11, 100–107. [Google Scholar] [CrossRef]
- Xu, H.; Chen, X.; Xu, X.; Shi, R.; Suo, S.; Cheng, K.; Zheng, Z.; Wang, M.; Wang, L.; Zhao, Y.; et al. Lysine acetylation and succinylation in HeLa Cells and their essential roles in response to UV-induced Stress. Sci. Rep. 2016, 6, 30212. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Li, X.; Shen, J.; Xu, Y.; Shi, H.; Mu, X.; Pan, J.; Zhao, T.; Li, M.; et al. CPT1A-mediated succinylation of S100A10 increases human gastric cancer invasion. J. Cell Mol. Med. 2019, 23, 293–305. [Google Scholar] [CrossRef]
- Kidwai, S.A.; Ansari, A.A.; Salahuddin, A. Effect of succinylation (3-carboxypropionylation) on the conformation and immunological activity of ovalbumin. Biochem. J. 1976, 155, 171–180. [Google Scholar] [CrossRef]
- Kurmi, K.; Hitosugi, S.; Wiese, E.K.; Boakye-Agyeman, F.; Gonsalves, W.I.; Lou, Z.; Karnitz, L.M.; Goetz, M.P.; Hitosugi, T. Carnitine Palmitoyltransferase 1A has a lysine succinyltransferase activity. Cell Rep. 2018, 22, 1365–1373. [Google Scholar] [CrossRef]
- Jin, W.; Wu, F. Proteome-wide identification of lysine succinylation in the proteins of tomato (Solanum lycopersicum). PLoS ONE 2016, 11, e0147586. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, X.; Li, J.; Zhou, X.; Liu, Y.; Zhong, L.; Tang, Y.; Zheng, H.; Liu, J.; Zhan, R.; et al. Global analysis of lysine succinylation in patchouli plant leaves. Hortic. Res. 2019, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Wang, Y.; Sun, J.H.; Qian, W.J.; Xie, H.; Ding, Y.Q.; Ding, Z.T. A qualitative proteome-wide lysine succinylation profiling of tea revealed its involvement in primary metabolism. Mol. Biol. 2020, 54, 144–155. [Google Scholar] [CrossRef]
- Dai, L.; Peng, C.; Montellier, E.; Lu, Z.; Chen, Y.; Ishii, H.; Debernardi, A.; Buchou, T.; Rousseaux, S.; Jin, F.; et al. Lysine 2-hydroxyisobutyrylation is a widely distributed active histone mark. Nat. Chem. Biol. 2014, 10, 365–370. [Google Scholar] [CrossRef]
- Hirschey, M.D.; Zhao, Y. Metabolic regulation by lysine malonylation, succinylation, and glutarylation. Mol. Cell. Proteom. MCP 2015, 14, 2308–2315. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Lu, Z.; Xie, Z.; Cheng, Z.; Chen, Y.; Tan, M.; Luo, H.; Zhang, Y.; He, W.; Yang, K.; et al. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol. Cell Proteom. 2011, 10, M111.012658. [Google Scholar] [CrossRef]
- Huang, H.; Tang, S.; Ji, M.; Tang, Z.; Shimada, M.; Liu, X.; Qi, S.; Locasale, J.W.; Roeder, R.G.; Zhao, Y.; et al. p300-dediated lysine 2-hydroxyisobutyrylation regulates glycolysis. Mol. Cell 2018, 70, 663–678.e666. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Li, P.; Chen, X.; Liu, H.; Huang, J.; Luo, C.; Hsiang, T.; Zheng, L. Comprehensive identification of lysine 2-hydroxyisobutyrylated proteins in Ustilaginoidea virens reveals the involvement of lysine 2-hydroxyisobutyrylation in fungal virulence. J. Integr. Plant. Biol. 2021, 63, 409–425. [Google Scholar] [CrossRef]
- Du, Y.; Cai, T.; Li, T.; Xue, P.; Zhou, B.; He, X.; Wei, P.; Liu, P.; Yang, F.; Wei, T. Lysine malonylation is elevated in type 2 diabetic mouse models and enriched in metabolic associated proteins. Mol. Cell. Proteom. MCP 2015, 14, 227–236. [Google Scholar] [CrossRef]
- Galván-Peña, S.; Carroll, R.G.; Newman, C.; Hinchy, E.C.; Palsson-McDermott, E.; Robinson, E.K.; Covarrubias, S.; Nadin, A.; James, A.M.; Haneklaus, M.; et al. Malonylation of GAPDH is an inflammatory signal in macrophages. Nat. Commun. 2019, 10, 338. [Google Scholar] [CrossRef]
- Colak, G.; Pougovkina, O.; Dai, L.; Tan, M.; Te Brinke, H.; Huang, H.; Cheng, Z.; Park, J.; Wan, X.; Liu, X.; et al. Proteomic and biochemical studies of lysine malonylation suggest its malonic aciduria-associated regulatory role in mitochondrial function and fatty acid oxidation. Mol. Cell. Proteom. MCP 2015, 14, 3056–3071. [Google Scholar] [CrossRef] [PubMed]


| Sample | Aim of Study | Technique | Identified Proteins | Details of Results |
|---|---|---|---|---|
| leaf, root, and seed of Nipponbare (Japonica) [23] | To identify protein expression in leaf, root (49 DAG), and seed (14 DAG). | 2-DE HPLC–MS/MS MudPIT | AGPase (id: 7670) leaf, seed AGPase small subunit (id: 44074) leaf, seed AGPase (id: 9904, 34550) seed AGPase (id: 50182) leaf GBSS (id: 31122) leaf, seed GBSS (id: 31130) seed SS precursor (id: 99443, 52528) seed SS (id: 26269) seed SBE isoform rbe3 (id: 36892) seed SBE (id: 20648, 27094, 53238, 55740) seed DBE (id: 14376) PhoH isoenzyme (id: 12500) seed Pho1 (id: 32714) seed AMY precursor (id: 21708) seed AMY (id: 24707) seed ISA (id: 23091) seed ISA (id: 23496) seed PGM, chloroplast precursor (id: 34039) seed PGM, cytoplasmic (id: 38302) leaf, root, seed | Proteins involving in starch biosynthesis were observed in both leaf and seed tissues. Starch degradation-related proteins were observed only in seed tissue. Two isoforms of small AGPase subunit were detected in both leaf and seed whereas another two isoforms of large AGPase subunit were identified only in seed tissue. The third isoform of large AGPase subunit was observed in leaf. |
| DY1102 (Wuyujing3 (Japonica) treated with 0.5% ethyl methanesulfonate (EMS)) (notched-belly mutant with white belly) [19] | To identify the differentially expressed proteins between the chalky and the translucent parts of DY1102 grains. | iTRAQ LC-MS/MS | AGPase, SSII, SSIII, SBE, Pho1, PGM, AMY, and putative starch synthase DULL1 (SSIIIa) | Downregulation of AMY was observed in chalky part. Downregulation of AMY contributes to starch hydrolysis and the formation of chalkiness. |
| SSIIIa was one of the differentially expressed proteins and increased in chalky part. The increase in SSIIIa expression did not result in the increased proportion of long amylopectin chains (DP > 30). | ||||
| Nipponbare (Japonica) [32] | To develop a method for rice starch granule purification from mature endosperm and identify starch granule-associated proteins. | LC-MS/MS | AGPase S2, AGPase L1, AGPase L2 GBSSI, GBSSII SBE 1, SBE3 SSI, SSII-1, SSII-3, SSIIIa PUL Pho1 ISA2 | Besides 14 identified starch biosynthesis proteins, the other candidate starch granule-associated proteins involving in starch biosynthesis were also identified by LC-MS/MS including Hsp70, putative Brittle-1 protein, and PPDK. Compared with Tris-HCl buffer extraction method, the proteome extracted by the phenol buffer had more proteins and displayed almost all identified proteins extracted by Tris-HCl buffer. |
| Sample | Aim of Study | Technique | Identified Proteins | Details of Results |
|---|---|---|---|---|
| Taichung Native 1 (TN 1, Indica) and Tainung 67, (TNG 67, Japonica) [13] | To investigate the changes in protein expression patterns during rice caryopsis development (6, 9, 12, 15, and 32 DAF). | 2-DE LC-MS/MS | GBSS (Waxy) | The expression of GBSS increased after 6 DAF was coincident with the increase in amylose content. GBSS protein was highly expressed in kernels of rice with high amylose content (TN1). |
| Nipponbare (Japonica) [22] | To study the protein expression profiles related to grain filling during 6–20 DAF. | 2-DE MALDI-TOF/TOF | ISA I, AMY, Pho, PGM, AGPaseL2, AGPaseL3, AGPaseS2a/b | All identified proteins were continuously increased from 6 to 20 DAF. Some AGPase isoforms had the highest peak of protein expression at 16 DAF and decrease thereafter. |
| ISA3 | ISA3 increased at 6 DAF, showed the highest expression at 10 DAF, and decreased thereafter. | |||
| SSI | No result of expression pattern. | |||
| Zhonghua 10 (Japonica) [24] | To study the cellular features and proteomics of rice endosperm from 12, 15, and 18 DAF. | 2D-DIGE MALDI-TOF/ TOF-MS | PUL Pho1 AGPase L AGPase S2 | Most of the protein expression patterns showed increase in abundance from 12 to 18 DAF. Some isoforms of PUL and AGPase S2 had the highest peak of expression at 15 DAF. Pho1 decreased the expression level form 12–18 DAF. AGPase L showed the highest variation of expression patterns including the expression levels continuously decreased and increased from 12–18 DAF, showed the highest peak and lowest peak at 15 DAF. The completion of starch granule packing was firstly observed in the inner part of endosperm at 15 DAF and showed entire endosperm at 18 DAF. AGPase L and Pho1 were significantly coexpressed with proteins in redox regulation (SOD and APX, respectively) |
| Ilpumbyeo (Japonica) [25] | To identify the differentially expressed proteins of rice grains at 10, 20, 30 DAF and the fully mature grain (45 DAF). | MudPIT | Pho1 PUL AMY SS 2–3 GWD SBE | All identified 6 starch biosynthesis proteins were reproducibly identified and differentially expressed during four stages (10, 20, 30, and 45 DAF). All of these proteins had the highest expression levels at the fully mature grain except SS 2–3 in which its abundance increased until 20 DAF after that decreased at 30 DAF and increased at fully mature grain. The authors suggested that the expression profile of starch biosynthesis proteins was similar to previous research of Xu et al. [22] |
| Jinhui No. 809 (Indica) [87] | To identify the differentially expressed proteins between superior (SS) and inferior spikelet (IS) at the early (EGS), mid (MGS), and late (LGS) grain-filling stages. | 2-DE MALDI-TOF/MS LC-ESI-MS/MS | AGPase GBSS PUL | AGPase, GBSS, and PUL isoforms were downregulated in inferior spikelets at EGS. |
| AGPase S | AGPase S showed downregulation in both MGS and LGS. | |||
| Jinhui No. 809 (Indica) [88] | To identify the differentially expressed proteins of 10 DAF superior spikelet (SS) and 10 and 20 DAF inferior spikelet (IS). | 2-DE MALDI-TOF/MS LC-ESI-MS/MS | AGPase GBSS SBE 1 SBE 3 PUL | AGPase had lower expression level in 10 DAF IS compared with both 10 DAF SS and 20 DAF IS. SBE 3, AGPase, PUL, and SBE 1 were detected as the 14-3-3 interacting proteins. AGPase and GBSS might be involved in the developmental stagnancy stage (DSS) of IS especially at the early grain-filling stage. |
| Zhonghua 10 (Japonica) [26] | To identify the SGAPs of rice at 10, 15, and 20 DAF. | 2D-DIGE MALDI-TOF/ TOF-MS | Pho1 PUL SSI AGPase L2 GBSSI AGPase S2a | Protein abundance of Pho1, PUL, SSI, and AGPase S2a slowly increased from 10 to 15 DAF and then drastically increased from 15 to 20 DAF. GBSSI showed linearly decreased abundance levels from 10 to 20 DAF. GBSSI and SSI were found only in starch granule-associated (SGA) form. AGPase, Pho1, and PUL were observed in both soluble and SGA forms. |
| Sample | Aim of Study | Technique | Identified Proteins | Details of Results |
|---|---|---|---|---|
| Taichung Native 1 (TN 1, Indica) and Tainung 67, (TNG 67, Japonica) [13] | To determine the candidate proteins associated with grain quality under HT, 35/30 °C (day/night). | 2-DE LC-MS/MS | GBSS (Waxy) | HT caused the reduction of GBSS in TGN67 and decreased the levels of amylose content of TNG67 at 15 DAF (12.3 ± 0.5%) compared with those (15.6 ± 0.4%) under the control temperature (30/25 °C). Protein expression of TN1 showed relatively stable in both HT and control conditions. TNG67 showed more sensitivity to HT than TN1. |
| 9311 (Indica) [91] | To identify the differentially accumulated proteins of rice at 5, 10, 15, and 20 DAF under day HT (DHT, 35/27 °C) and night HT (NHT, 27/35 °C). | 2-DE MALDI-TOF MS/MS | PGM PUL | One and five isoforms of PUL and PGM were differentially accumulated in response to DHT and NHT and detected in all 5, 10, 15, and 20 DAF with different accumulation patterns. Three PUL isoforms (spot 34, 35, and 36) were increased in parallel abundance from 5 to 20 DAF, while another (spot 37 and 38) showed slowly increase at 5–10 DAF and highly increase in abundance at 15 and 20 DAF. |
| XN0437T (heat-tolerant) XN0437S (heat-sensitive) [89] | To identify the differentially expressed proteins during rice grain development at 1, 3, and 5 day after HT treatment (38.0 ± 0.5 °C) compared with control (25.0 ± 0.5 °C) | 2-DE MALDI-TOF/TOF MS | PUL DBE GBSS AGPase L | All 4 proteins involving in starch biosynthesis showed downregulation in both rice lines under HT stress compared with the control treatment. AGPase L was higher accumulated in the heat-tolerant rice at 1 day after HT and showed lower accumulation at 3 and 5 days after HT compared to heat-sensitive rice. PUL, DBE, and GBSS had lower expression levels in heat-tolerant rice at all three-time points. |
| Perfect and chalky rice grains (Koshihikari (Japonica)) [92] | To study the proteomic profile of the translucent and opaque grains under moderate (in 2009, 24.4 °C) and HT (in 2010, 28.0 °C) conditions. | iTRAQ MS/MS | SSI SSII PUL GBSSI BEIIb | All identified proteins showed downregulation in chalky rice compared to perfect grain. Protein expression of SSII, PUL, and BEIIb under moderate temperature was lower than HT condition, while the others, SSI and GBSSI showed higher abundance under HT. |
| AMY (AmyII-3) | AMY showed upregulation in chalky rice in both conditions and chalky rice under HT stress had higher AMY abundance than moderate temperature. | |||
| DBE BEI | Both DBE and BEI were increased in HT but downregulated in moderate temperature. | |||
| KDML105 (Indica) [90] | To identify the differentially changed proteins of rice grains under heat stress (40/26 °C) at the milky, dough, and mature stages. | nanoLC-MS/MS | AMY | AMY showed the highest in abundance at milky then decreased in dough and disappeared at mature stage. |
| AGPase | AGPase had the un-change expression in both milky and dough stages and double increased in mature stage. | |||
| SBEI GBSSI | Protein abundance of both SBEI and GBSSI was increased almost three times from milky to dough stages. Both SBEI and GBSSI were not found at mature stage. | |||
| AGPase L2 | AGPase L2 was detected only at dough stage. | |||
| SBE3 AGPase L2 SSIIa SSI | All proteins were detected only in milky stage in which the AGPase L2 showed the highest abundance followed by SSI, SBE3, and SS IIa. | |||
| ISA | ISA was detected in both milky and mature stages and showed the highest abundance at mature stage. |
| Phosphorylated Protein | Uniprot ACCN | Identified Phosphosite(s) a | Subspecies | Reference |
|---|---|---|---|---|
| AGPase | B8XEC3 | S62, S381 | indica | [28] |
| T68 | japonica and indica | [28,31] | ||
| A2Y7W1 | S491 | japonica | [27] | |
| - | - | indica | [87,88] | |
| AGPS2 | D4AIA3 | S13 | japonica and indica | [28,31] |
| S17, S22, S35, S36 | Indica | [28] | ||
| GBSSI | - | - | indica | [87] |
| SSIIa | P0C586 | S126 | indica | [28] |
| SSIIIa | Q6Z1D6 | S96 | japonica and indica | [28,31] |
| BEI | D0TZI4 | S562, S620, S814, S815 | indica | [28] |
| BEIIb | A2X5K0 | S685, S715 | indica | [28] |
| PUL | D0TZH1 | S154, S155, S869 | indica | [28] |
| Pho1 | Q9AUV8 | S494, S645 | indica | [28] |
| PGM | Q9AUQ4 | S124 | japonica | [31] |
| Q33AE4 | S167 | japonica | [31] | |
| - | - | indica | [88] |
| Acetylated Protein | Uniprot ACCN | Acetylation Position | Modified Peptide a | Lysine Motif b | Tissue-Specific b | Reference |
|---|---|---|---|---|---|---|
| AGPase S1 | Q69T99 | 203 | MDYQK(ac)FIQAHR | - | - | [30] |
| AGPase S2 | P15280 | 217 | MDYEK(ac)FIQAHR | - | starch granule/seeds (7 DAP)/seeds (15 DAF) | [32]/[30]/[33] |
| 261 | IVEFAEK(ac)PK | KF | starch granule/seeds (unpollinated pistil and 7 DAP)/seeds (15 DAF) | [32]/[30]/[33] | ||
| AGPase L2 | Q5VNT5 | 250 | ASDYGLVK(ac)FDDSGR | KF | starch granule/seeds (3 and 7 DAP)/seeds (15 DAF) | [32]/[30]/[33] |
| 260 | VIAFSEK(ac)PK | - | starch granule | [32] | ||
| 310 | DVLLDILK(ac)SK | - | Seeds (7 DAP) | [30] | ||
| 312 | SK(ac)YAHLQDFGSEILPR | - | Seeds (7 DAP) | [30] | ||
| GBSSI | Q0DEV5 | 444 | KFEK(ac)LLK | - | starch granule/seeds (15 DAF) | [32]/[33] |
| 452 | SMEEK(ac)YPGK | KY | starch granule/seeds (15 DAF) | [32]/[33] | ||
| SSI | Q0DEC8 | 193 | NFANAFYTEK(ac)HIK | - | seeds (3 and 7 DAP) | [30] |
| SSIVa | Q5JMA0 | 589 | AQYYGEHDDFK(ac)R | - | seeds (15 DAF) | [33] |
| SBEI | Q0D9D0 | 89 | LEEFK(ac)DHFNYR | - | starch granule/seeds (15 DAF) | [32]/[33] |
| 103 | YLDQK(ac)CLIEK | - | starch granule/seeds (15 DAF) | [32]/[33] | ||
| 118 | HEGGLEEFSK(ac)GYLK | KXXXK | starch granule/seeds (15 DAF) | [32]/[33] | ||
| 164 | DK(ac)FGIWSIK | KF | starch granule/seeds (15 DAF) | [32]/[33] | ||
| 236 | YVFK(ac)HPR | KH | starch granule/seeds (15 DAF) | [32]/[33] | ||
| 372 | GYHK(ac)LWDSR | KXXXXR | starch granule/seeds (15 DAF) | [32]/[33] | ||
| 614 | EGNNWSYDK(ac)CR | - | starch granule/seeds (15 DAF) | [32]/[33] | ||
| 662 | QIVSDMNEK(ac)DK | - | starch granule/seeds (15 DAF) | [32]/[33] | ||
| 697 | VGCDLPGK(ac)YR | KY | starch granule/seeds (15 DAF) | [32]/[33] | ||
| 809 | GM(ox)K(ac)FVFR | KXXXR | starch granule/seeds (15 DAF) | [32]/[33] | ||
| SBEIIb | Q6H6P8 | 134 | VVEELAAEQK(ac)PR | - | seeds (15 DAF) | [33] |
| 303 | YIFK(ac)HPQPK | KH | Seed (7 DAP)/seeds (15 DAF) | [30]/[33] | ||
| 587 | WSEK(ac)CVTYAESHDQALVGDK | - | seeds (unpollinated pistil and 7 DAP) | [30] | ||
| 688 | FIPGNNNSYDK(ac)CR | - | seeds (7 DAP) | [30] | ||
| 738 | KHEEDK(ac)MIIFEK | - | starch granule/seeds (15 DAF) | [32]/[33] | ||
| 771 | VGCLKPGK(ac)YK | KY | starch granule/seeds (15 DAF) | [32]/[33] | ||
| ISA3 | Q6K4A4 | 130 | K(ac)YFGVAEEK | KY | seeds (15 DAF) | [33] |
| PUL | Q7X834 | 805 | NEENWHLIK(ac)PR | - | seeds (15 DAF) | [33] |
| PMG | Q9AUQ4 | 8 | VLFSVTK(su)K | - | embryos (24 HAI) | [34] |
| 18 | ATTPFDGQK(ac)PGTSGLR | - | embryos (24 HAI)/seeds (15 DAF) | [34]/[33] | ||
| ATTPFDGQK(su)PGTSGLR | embryos (24 HAI) | [34] | ||||
| 69 | YFSK(ac)DAVQIITK | - | embryos (24 HAI) | [34] | ||
| 206 | LMK(ac)TIFDFESIK | - | embryos (24 HAI) | [34] | ||
| 215 | TIFDFESIK(ac)K | - | seeds (15 DAF) | [33] | ||
| 275 | EDFGGGHPDPNLTYAK(ac)ELVDR | - | embryos (24 HAI) | [34] | ||
| 361 | NLNLK(ac)FFEVPTGWK | - | embryos (24 HAI) | [34] | ||
| 506 | DPVDGSVSK(ac)HQGVR | KH | embryos (24 HAI)/seeds (15 DAF) | [34]/[33] | ||
| 543 | VYIEQYEK(ac)DSSK | KXXXK | seeds (15 DAF) | [33] | ||
| PhoH | Q8LQ33 | 169 | YGLFK(ac)QCITK | - | embryos (24 HAI) | [34] |
| 409 | HMEIIEEIDK(ac)R | - | embryos (24 HAI) | [34] | ||
| 412 | FK(su)EMVISTR | - | embryos (24 HAI) | [34] | ||
| 439 | ILDNSNPQK(su)PVVR | - | embryos (24 HAI) | [34] | ||
| 645 | LVNDVGAVVNNDPDVNK(ac)YLK | - | embryos (24 HAI) | [34] | ||
| 747 | FEEAK(ac)QLIR | KXXXR | seeds (15 DAF) | [33] | ||
| 818 | MSILNTAGSGK(ac)FSSDR | - | embryos (24 HAI) | [34] | ||
| PhoL | Q9AUV8 | 216 | YK(ac)HGLFK | KH | starch granule/seeds (unpollinated pistil, 3 DAP and 7 DAP)/seeds (15 DAF) | [32]/[30]/[33] |
| 255 | TDVSYPVK(ac)FYGK | KXXXK | starch granule/seeds (15 DAF) | [32]/[33] | ||
| 451 | YGTEDTSLLK(ac)K | - | starch granule/seeds (15 DAF) | [32]/[33] | ||
| 504 | SLEPSVVVEEK(ac)TVSK | KXXXK | starch granule/seeds (15 DAF) | [32]/[33] | ||
| 594 | FQNK(ac)TNGVTPR | - | starch granule/seeds (15 DAF) | [32]/[33] | ||
| 734 | AFATYVQAK(ac)R | - | seeds (7 DAP) | [30] | ||
| 846 | AQGK(ac)FVPDPR | KF | starch granule/seeds (15 DAF) | [32]/[33] | ||
| 913 | DQK(ac)LWTR | KXXXR | starch granule/seeds (15 DAF) | [32]/[33] | ||
| 928 | MSILNTASSSK(ac)FNSDR | KF | starch granule/seeds (15 DAF) | [32] | ||
| AMY | Q0J528 | 88 | LYDLDASK(ac)YGTEAELK | - | embryos (24 HAI)/- | [34] |
| 123 | CADYK(ac)DSR | - | - | [30] | ||
| P27933 | 88 | LYDLDASK(ac)YGTAAELK | - | - | [30] | |
| 215 | GYSTDIAK(ac)MYVESCK | - | - | [30] |
| Protein | Uniprot ACCN | No. of Khib and Kmal * | Position |
|---|---|---|---|
| AGPase S1 | Q69T99 | 5 | 203, 234, 249, 442, 462 |
| AGPase S2 | P15280 | 19 | 102, 132, 217, 239, 248, 261, 263, 268, 285, 360, 385, 403, 406, 441, 447, 456, 467, 476, 496 |
| 3 * | 106, 360, 403 | ||
| AGPase L1 | Q6AVT2 | 11 | 100, 194, 196, 247, 299, 331, 326, 369, 446, 456, 470, |
| AGPase L2 | Q5VNT5 | 21 | 37, 74, 187, 223, 250, 263, 273, 286, 301, 302, 310, 312, 334, 364, 371, 392, 425, 443, 449, 459, 504, |
| 4 * | 250, 312, 371, 449 | ||
| AGPase L3 | Q688T8 | 4 | 202, 228, 315, 376 |
| GBSSI | Q0DEV5 | 8 | 181, 192, 309, 381, 385, 530, 538, 549 |
| SSI | Q0DEC8 | 8 | 193, 196, 349, 357, 429, 461, 467, 570 |
| SSII-3 | Q0DDE3 | 5 | 151, 244, 346, 378, 532 |
| SSIIIa | Q6Z1D6 | 8 | 228, 649, 761, 794, 808, 961, 1203, 1604 |
| SBEI | Q0D9D0 | 33 | 62, 64, 84, 89, 103, 108, 118, 122, 157, 164, 171, 186, 215, 236, 319, 324, 372, 423, 500, 506, 524, 540, 549, 614, 662, 664, 683, 689, 697, 744, 775, 796, 809 |
| 6 * | 108, 118, 506, 524, 689, 809 | ||
| SBEIIb | Q6H6P8 | 22 | 134, 146, 158, 191, 231, 268, 299, 328, 386, 466, 558, 564, 571, 587, 603, 612, 636, 677, 688, 719, 738, 773, |
| 1 * | 719 | ||
| AMY | Q0J528 | 5 | 39, 88, 105, 207, 262 |
| Q0JJV2 | 1 | 88 | |
| ISA2 | Q6AU80 | 2 | 319, 369 |
| ISA3 | Q6K4A4 | 2 | 266, 269 |
| PhoH | Q8LQ33 | 8 | 115, 409, 425, 533, 542, 595, 721, 818, |
| PhoL | Q9AUV8 | 32 | 134, 255, 259, 277, 289, 356, 381, 410, 418, 429, 441, 451, 471, 493, 504, 590, 617, 630, 636, 657, 665, 681, 725, 734, 738, 846, 893, 904, 913, 928, 940, 946, |
| 3 * | 259, 493, 657 | ||
| PUL | Q7X834 | 2 * | 274, 871 |
| PGM | Q33AE4 | 7 | 61, 67, 118, 413, 492, 584, 595 |
| Q9AUQ4 | 3 * | 54, 458, 568 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tappiban, P.; Ying, Y.; Xu, F.; Bao, J. Proteomics and Post-Translational Modifications of Starch Biosynthesis-Related Proteins in Developing Seeds of Rice. Int. J. Mol. Sci. 2021, 22, 5901. https://doi.org/10.3390/ijms22115901
Tappiban P, Ying Y, Xu F, Bao J. Proteomics and Post-Translational Modifications of Starch Biosynthesis-Related Proteins in Developing Seeds of Rice. International Journal of Molecular Sciences. 2021; 22(11):5901. https://doi.org/10.3390/ijms22115901
Chicago/Turabian StyleTappiban, Piengtawan, Yining Ying, Feifei Xu, and Jinsong Bao. 2021. "Proteomics and Post-Translational Modifications of Starch Biosynthesis-Related Proteins in Developing Seeds of Rice" International Journal of Molecular Sciences 22, no. 11: 5901. https://doi.org/10.3390/ijms22115901
APA StyleTappiban, P., Ying, Y., Xu, F., & Bao, J. (2021). Proteomics and Post-Translational Modifications of Starch Biosynthesis-Related Proteins in Developing Seeds of Rice. International Journal of Molecular Sciences, 22(11), 5901. https://doi.org/10.3390/ijms22115901







