Abstract
Alzheimer’s disease (AD) is a neurodegenerative disease with a high incidence rate. The main pathological features of AD are β-amyloid plaques (APs), which are formed by β-amyloid protein (Aβ) deposition, and neurofibrillary tangles (NFTs), which are formed by the excessive phosphorylation of the tau protein. Although a series of studies have shown that the accumulation of metal ions, including calcium ions (Ca2+), can promote the formation of APs and NFTs, there is no systematic review of the mechanisms by which Ca2+ affects the development and progression of AD. In view of this, the current review summarizes the mechanisms by which Ca2+ is transported into and out of cells and organelles, such as the cell, endoplasmic reticulum, mitochondrial and lysosomal membranes to affect the balance of intracellular Ca2+ levels. In addition, dyshomeostasis of Ca2+ plays an important role in modulating the pathogenesis of AD by influencing the production and aggregation of Aβ peptides and tau protein phosphorylation and the ways that disrupting the metabolic balance of Ca2+ can affect the learning ability and memory of people with AD. In addition, the effects of these mechanisms on the synaptic plasticity are also discussed. Finally, the molecular network through which Ca2+ regulates the pathogenesis of AD is introduced, providing a theoretical basis for improving the clinical treatment of AD.
1. Introduction
Alzheimer’s disease (AD), commonly known as dementia, is a neurodegenerative disease with a high incidence rate. AD may share common biological pathways and is often associated with diabetes and other comorbidities [] Clinically, cognitive dysfunction is the main feature []. Although the pathogenesis of AD has not been definitely determined, it is generally believed that the pathogenesis of AD is related to the excessive production and deposition of β-amyloid protein (Aβ) and hyperphosphorylated tau protein []. On the one hand, Aβ is produced mainly through the amyloid metabolic pathway when the amyloid precursor protein (APP) is cleaved by β-secretase and γ-secretase to produce Aβ monomers []. On the other hand, the tau protein is hyperphosphorylated through the action of cyclin-dependent kinase 5 (Cdk5) and glycogen synthase kinase (GSK) 3β []. Both the Aβ and phosphorylated tau proteins have the ability to self-aggregate. Through this self-aggregation, they gradually form oligomers and fibers, which are deposited as β-amyloid plaques (APs) and neurofibrillary tangles (NFTs), respectively []. The formation of oligomers and fibers can mediate the pathological progress of AD by affecting the function of glial cells and neurons [].
A series of studies have shown that the onset of AD is related to aging; an unhealthy lifestyle, including smoking and drinking; health status, such as degree of heart disease, hypertension, obesity and diabetes; and genetic factors, such as APOE4 expression [,,,]. For the production of Aβ, mutations in APP and presenilin (PS), including PS1 and PS2, are the decisive factors [,,]. However, the phosphorylation of tau protein greatly affects the stability of microtubes in neurons, resulting in neuronal tangles []. In addition to the production and deposition of Aβ and phosphorylated tau protein, many metal ions contribute to metabolic disorders []. In PS-mutant AD brain tissue, a Ca2+ metabolic disorder was evident before the formation of APs or NFTs [], This observation was further corroborated by a series of evidence in different AD animal models [,,], which indicated that the metabolic disorder caused by Ca2+ located in the cytoplasm might be the cause of AD. Based on this hypothesis, previous studies have shown that Ca2+ influx can increase the production and aggregation of Aβ and phosphorylated tau protein and thus affect the learning and memory of patients with AD [,,].
Moreover, the imbalance of Ca2+ leads to dysregulated metabolism that affects many neurophysiological functions related to AD, including the regulation of neuroinflammation, response to neuronal injury, neuronal regeneration, neurotoxicity, autophagy and synaptic plasticity [,,,,]. The multifunctional AD-related neuropathological function of Ca2+ may be directly or indirectly mediated by Aβ and/or phosphorylated tau proteins. As the main pathological features of AD, monomeric or aggregate Aβ and phosphorylated tau proteins show regulatory effects on neuroinflammation, neuronal injury, neuronal regeneration, neurotoxicity, neuroprotection, autophagy and neural plasticity []. Either directly or indirectly, Ca2+ is involved in the regulation of these neuropathological functions through its specific transporters. Therefore, this review mainly explores the molecular mechanisms by which a Ca2+ imbalance in AD affects the regulation of Aβ, tau, and neural plasticity, specifically from the perspective of Ca2+ transporters in cell, mitochondrial, endoplasmic reticulum (ER) and lysosomal membranes.
2. APP Metabolic Products Including Aβ Facilitated the Influx of Ca2+ into the Neurons of AD Animals and Patients
The concentration of Ca2+ is strictly regulated under physiological conditions, whereas Ca2+ concentration is obviously elevated in the brains of AD patients and APP/PS1 Tg mice []. Kuchibhotla et al. found that Ca2+ is significantly increased in the dendrites and dendritic spines of neurons of APP/PS1 Tg mice []. In view of their observation, the natural question that arises is: What is the reason for Ca2+ elevation during the course of AD development and progression? It has been reported that Aβ1–40 has the ability to upregulate the influx of Ca2+ in rat cortical synaptosomes and cultured cortical neurons [,]. Moreover, the Aβ25–35 peptide has an effect similar to that of Aβ1–40, which can promote Ca2+ influx by activating L- and T-type Ca2+ channels in rat hippocampal slices []. Similar to the results in vivo, Aβ increased the Ca2+ influx in PC12 and SH-SY5Y cells in vitro [,]. In addition to activating ion channels, Aβ has the ability to activate PKA, which increases Ca2+ influx through L-VGCCs by activating calcium-binding proteins [].
Because of the self-aggregating characteristics of Aβ, the concentration of Ca2+ in the spines and dendrites of cortical pyramidal neurons around APs is higher than the normal value in adjacent resting neurons []. In addition to the effect of APs on Ca2+ in neurons, Bacskai and his colleagues quantitatively measured the resting-state Ca2+ concentration in astrocytes of APP/PS1 mice and observed the overall response of astrocytes to AP deposition. The results showed that the concentration of Ca2+ in the astrocytes of 6-month-old mutant mice was elevated compared to that of the WT controls []. It was confirmed that the resting level of Ca2+ reached 247 nmol/L in the cortical neurons of 3×Tg mice, which is twice that of the cortical neurons of non-Tg controls (110 nmol/L) []. Taking advantage of live cell imaging, the level of Ca2+ was found to be elevated in neurites, which were 20 μm from the central AP region, indicating the critical roles of APs in the homeostasis of Ca2+ in the spines and dendrites of neurons []. In astrocytes of 6-month-old APP/PS1 mice, Ca2+ was elevated in response to the deposition of APs []. In transient occlusion of the middle cerebral artery (MCAO) of hAPP695 transgenic (Tg) rats, Ca2+ colocalized with APs and was deposited in the thalamus []. Arispe et al. found that the aggregates of Aβ1–40 and Aβ1–42 can form a cation channel on the surface of an artificial lipid membrane that allows the passage of Ca2+ []. However, the channel showed low selectivity, and thus it also permitted the passage of Li+, K+ and Na+ []. In SH-SY5Y cells, oligomeric Aβ cannot selectively increase the Ca2+ permeability of cellular membranes, thereby increasing both Ca2+ influx from the extracellular space and Ca2+ leakage from intracellular Ca2+ stores []. The pore formation of Aβ was confirmed and corroborated by atomic force microscopy [], electron microscopy [,] and a theoretical model [,]. For example, high-resolution transmission electron microscopy revealed the presence of Aβ pores distributed in situ in the cell membranes of post-mortem AD patients []. In addition, the formation of Aβ pores is also considered a mechanism of neurotoxicity induction, which destroys cell homeostasis by inducing the leakage of Na+, K+ and Ca2+ through this highly conductive channel []. This observation reinforces the extreme toxicity of Aβ oligomers, which potentially disrupts the homeostasis of Ca2+ in neurons [,,]. The formation of Aβ pores is enhanced by the presence of phosphatidylserine, a cell surface marker of early apoptosis []. However, this kind of pore can be blocked by Zn2+, because Zn2+ can form a complex with Aβ to prevent the aggregation of Aβ, which inhibits the insertion of Aβ oligomers into the membrane, leading to the formation of pores [,,,]. In addition, the extent of the pore-forming activity of Aβ in the lipid bilayer is inversely proportional to the cholesterol level in the lipid mixture. Treatment with cyclodextrin significantly enhanced the toxicity of Aβ in PC12 cells by decreasing or inhibiting the increase in the cholesterol level of these cells []. In contrast, Kawahara and Kuroda found that increasing the cholesterol content on the surface of the cell membrane significantly reduced Aβ-induced Ca2+ influx [].
In addition to Aβ, sAPP is involved in regulating the homeostasis of Ca2+. For instance, sAPP mediates the effects of glutamate on the regulation of the homeostasis of Ca2+ by increasing the production of cyclic (c) GMP to activate K+ channels, which results in reduced Ca2+ levels in hippocampal neurons []. In addition, it has been reported that a PS1 mutation is a key factor for sAPP stabilization of the homeostasis of Ca2+ in hippocampal neurons []. A possible explanation for this effect may involve the reversed regulation of APP695 and InsP3R genes at the mRNA and protein levels during differentiation []. The APP intracellular domain (AICD), which is released after InsP3R cleavage of APP may act as a transcription factor to activate the Ca2+ signaling system [,]. As the cleavage fragments of APP are produced by different secretases, PSEN2 mutation has shown its effects on impairing the fusion between autophagosomes and lysosomes in PSEN2T122R mutated SH-SY5Y cells []. However, these effects are not caused by the activity of g-secretase but by decreasing the Ca2+ released from ER in an ER-dependent mechanism [].
3. Ca2+ Transporters on the Surface of the Nerve Cell Membrane Are Responsible for Promoting the Influx of Ca2+ during the Course of AD Development and Progression
In addition, there are many natural Ca2+ transporters on the surface of the nerve cell membrane (Figure 1). As an antagonist of N-methyl-D-aspartic acid receptor (NMDAR), memantine significantly inhibits Ca2+ influx and was the first Food and Drug Administration (FDA)-approved drug for the treatment of moderate to severe AD in patients []. This drug was designed because Aβ can interact with endogenous Ca2+ channels in the cell membrane to increase NMDAR-dependent Ca2+ influx []. On the basis of this drug, memantine nitrate-06 (MN-06) was developed to protect the neurotoxicity against glutamate via inhibiting the influx of Ca2+ and decreasing the activity of PI3-K/Akt/GSK-3β pathways in primary cultured rat cerebellar granule and hippocampal neurons []. Although Aβ oligomers can promote Ca2+ influx through NMDAR channels in a short period of time [], sustained exposure to Aβ oligomers decreases the expression of NMDAR, the extent of Ca2+ influx and the glutamate current in neurons [,,]. In addition to targeting NMDARs, the antagonists of amino-3-hydroxy-methylisoxazole-4-propionate receptor (AMPAR), such as LY451395, LY450108 and S18986, reverse Ca2+ influx in AD animal models [,,,].
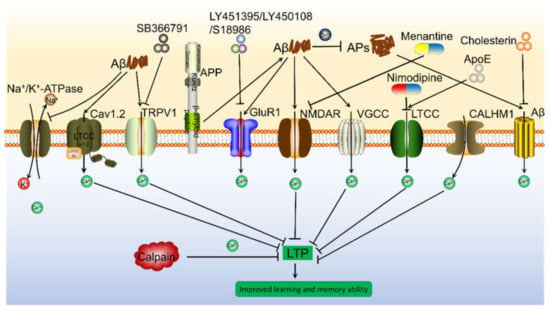
Figure 1.
Aβ is involved in regulating Ca2+ influx via modulating Ca2+ transporters on the neuronal membranes, which result in depressing LTP and inducing cognitive decline of AD animals. Aβ can activate Ca2+ transporters, including NMDAR, AMPAR, LTCC, Na+/K+-ATPase, CALHM1, TRPV1 and Cav1.2 etc., which result in promoting Ca2+ entry into the cytoplasm, leading to elevate the concentration of Ca2+ in the neuronal cells. In addition, oligomeric Aβ can not selectively increase Ca2+ permeability of cell membrane, leading to the influx of Ca2+ from the extracellular space. More importantly, these transporters of Ca2+ have the ability to mediate the effects of Ca2+ on the synaptic plasticity via different mechanisms.
In addition to glutamate receptors, there are a series of voltage gated Ca2+ channels (VGCCs) on the surface of the cell membrane that mediate the transportation of Ca2+. For example, Aβ blocked presynaptic P/Q-VGCC, which resulted in reduced Ca2+ influx into hippocampal neurons []. In contrast, Aβ1–40 concurrently enhanced the high threshold and low conductance of N- and T-VGCC and the high conductance of L-VGCC, which resulted in an increasing postsynaptic Ca2+ response in cortical neurons [,,]. In addition, Aβ impaired ion motive ATPases, which resulted in membrane depolarization and the opening of NMDAR pores and VGCCs, leading to an influx of Ca2+ and impaired Ca2+-ATPase, which resulted in inhibited Ca2+ efflux in primary cultured neurons and synaptosomes of an adult post-mortem hippocampus []. Although the mechanism by which CALHM1 serves as a cation channel in the brain is not completely clear, it has been reported as a pore-forming subunit whose activation can regulate Ca2+ influx, and it is regulated by the voltage and extracellular Ca2+ concentration of mouse cortical neurons []. As a potential Ca2+ transporter, it is further confirmed in CALHM1 knocking out mice []. As an important biomarker of AD, APOE does not directly regulate Ca2+ influx as a canonical cation channel, but it can promote the influx of Ca2+ by activating P/Q-VGCC in neurons [,]. In primary cultured astrocytes of APOE4−/− mice, APOE4 was found to be responsible for impairing neurons after brain injury [].
4. ER Is an Important Reservoir to Elevate the Levels of Ca2+ in the Neurons of AD
As an important reservoir of Ca2+ in neurons, endoplasmic Ca2+ can pass through InsP3Rs and ryanodine receptors (RyRs) to enter the cytosol (Figure 2). In the resting state, the intracellular level of Ca2+ remains at a relatively low level, between 50–300 nM. After activation, Ca2+ is mainly stored in the endoplasmic reticulum (ER), where the concentration of Ca2+ is approximately 100–500 nM and can be released into the cytoplasm through InsP3R and RyR [,]. Previous studies have shown that Aβ25–35 induces the transportation of Ca2+ in association with the activation of phospholipase C (PLC) and the production of inositol triphosphate (InsP3) []. In neurons, the addition of experimental Aβ significantly increased the Ca2+ response induced by InsP3R []. More specifically, exposing RyRs to Aβ1–42 increases the probability of channel opening, which results in an increased Ca2+ flux []. Similarly, Aβ aggregates have the ability to increase Ca2+ flux from the ER via InsP3R and RyR in human brain tissues and cells and in hippocampal CA1 pyramidal neurons [,,,].
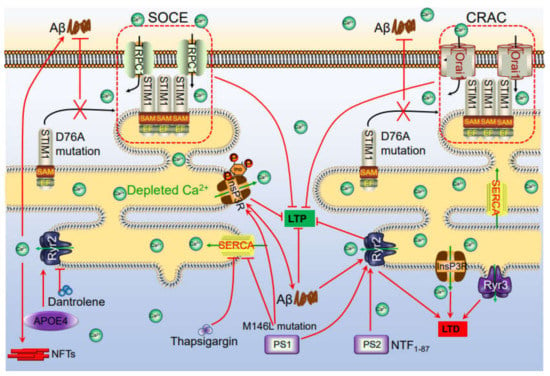
Figure 2.
Ca2+ channels in the ER involved in regulating phosphorylation of tau, production of Aβ, which deposited in APs and NFTs, leading to impair learning ability via influencing synaptic plasticity. The accumulation of Aβ in the neuronal cells induces the Ca2+ influx from the intracellular Ca2+ store, ER. In addition, Ca2+ depletion from ER triggers a sustained extracellular Ca2+ influx to the cytosol via a SOCE pathway, including TRPC1 and Orai1 by activating the STIM. During these processes, InsP3R and RyR2 played important roles in inducing Ca2+ influx from ER to cytosol, which results in regulating synaptic plasticity, phosphorylation of tau, deposition of Aβ, leading to cognitive impairment.
In addition to Aβ, PS1 exhibits the ability to interact with three key components of the Ca2+ signaling cascade, namely, InsP3R [,], RyR [,,] and sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA) []. Recently, Cheung et al., found that PS can physically interact with InsP3R to stimulate its gating activity, which results in an increase in Ca2+ even though there is no increase in Ca2+ in the lumens of the ER []. In SH-SY5Y cells, a PS1 mutation enhances the activity of PLC, leading to an increase in the level of IP3, which results in the release of Ca2+ from the ER []. Similarly, a PS mutation can stimulate Ca2+ release from the ER via InsP3R and RyR [,,]. In the ER membrane, there is a sarcoplasmic/endoplasmic reticulum ATPase (SERCA) pump in addition to InsP3R and RyR. In CHO cells, PS mutants bound to the SERCA pump, which disturbed the balance of Ca2+ []. In 3 × Tg mice, InsP3R and RyR mediated the release of Ca2+ from the ER, from which it entered the cytosol [,]. Interestingly, APOE4 may trigger the release of ER-Ca2+ via RyR, which promotes the formation of APs and NFTs [,,,]
However, the depletion of ER Ca2+ induces a continuous influx of extracellular Ca2+ into the cytoplasm by activating a classical store operated Ca2+ entry (SOCE) pathway. This process initially requires the sensor molecule of canonical systemic Ca2+ interactions in the ER (stromal interaction molecule, Stim) to sense ER Ca2+ depletion, which leads to activated Ca2+ channels on the surface of the cell membrane, such as Ca2+ release-activated Ca2+ (CRAC) channels, also known as calcium channel protein 1 (CRACM1, Orai1) channels [,]. Although Stim-related proteins, including Orai and TRPC, are located on the surface of the cell membrane, we prefer to discuss their roles in Ca2+ transportation because of their close relationship with the ER. As expected, SOCE disruption by the Stim1D76A mutation attenuated Ca2+ entry in primary neurons from AD mice with human mutant-PS1-knock-in skin fibroblasts from familial AD patients [,]. Other studies have shown that the expression level of Stim2 was downregulated by this PS1 mutant, which resulted in insufficient signals transmitted to the plasma membrane to activate SOCE, leading to reduced influx of Ca2+ when Ca2+ was depleted from the ER []. Moreover, PS1ΔE9 mutation induces the influx of Ca2+ via activating Stim1 in a SOCE-dependent mechanism in mouse hippocampal neurons []. Although there was no direct evidence showing their association with the activation of SOCE, TRPC3 and TRPC6 played roles in regulating the homeostasis of intracellular Ca2+ [,,].
5. Mitochondria and Lysosomes Also Act as Important Organelles for Regulating the Dyshomeostasis of Ca2+ during the Development and Progression of AD
In addition to the ER, mitochondria and lysosomes play important roles in the regulation of Ca2+ homeostasis, which has been reviewed in detail in a previous study [] (Figure 3 and Figure 4). In brief, there is evidence showing that the PS1L286V mutant can promote disorders in Ca2+ homeostasis in neurons by damaging mitochondria [,]. In PS1M146L mutant lymphoblasts, activation of InsP3R results in opening mPTP transporters in mitochondria []. In a series of AD-related mice and cell models, VDAC and MCU mediated the mitochondrial uptake of Ca2+ [,,]; the Na+/Ca2+ exchanger is critical for Ca2+ export across the inner mitochondrial membrane (IMM) [,,]; and the mitochondrial permeability transition pore (mPTP) is critical for the efflux of Ca2+ from neuronal mitochondria []. Although there is no direct evidence showing the involvement of Aβ in mitochondrial Ca2+ transportation, Aβ has the ability to open the mPTP, leading to the release of cytochrome C and caspases from mitochondria [,]. This evidence also indicates that the excessive accumulation of Aβ may be involved in the regulation of mitochondrial Ca2+ homeostasis. In contrast to that internalized by mitochondria, the Ca2+ uptake into lysosomes is mainly realized by the cooperation of a vacuolar type H+-ATPase (v-ATPase) and a putative Ca2+/H+ exchanger (CAX) [,]. The excretion of Ca2+ from lysosomes is mainly realized by TRPML and TPC []. When Ca2+ flows out of lysosomes through these VGCCs, defective autophagic lysosomes form, leading to autophagy []. Furthermore, the mutation or deletion of PS1 in AD leads to the disequilibrium of lysosomal Ca2+ by reducing the activity of the v-ATPase proton pumps on the lysosome, leading to AD pathogenesis []. In PS1 and 2 double knockout neurons, the number of lysosomal Ca2+ stores were significantly decreased, which resulted in a damaged autophagy process []. The imbalance of these processes (Table 1) affects the clearance of disease-related proteins in the pathogenesis of AD.
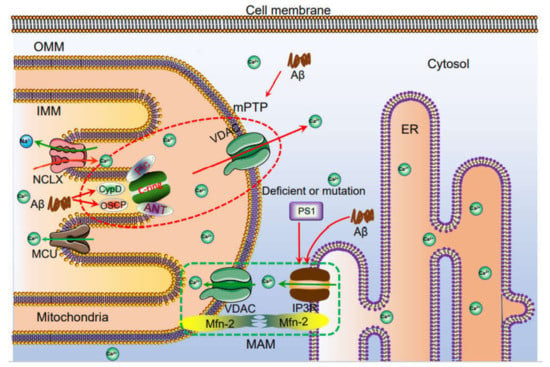
Figure 3.
The mechanisms of Ca2+ transportation between mitochondria and ER. Ca2+ is taken up to the mitochondria via MCU. Under physiological or pathological conditions, Ca2+ is continuously shuffled between ER and mitochondria via VDAC. Moreover, Ca2+ in mitochondria induces the formation of mPTP, which traversed Ca2+ and small molecules, such as ROS and cytochrome C from mitochondria to cytosol, leading to the potential apoptosis of neurons. The loss of neurons will cause the cognitive dysfunction. Deficient or mutation: Defective PS1 due to exon 9 deletion (ΔE9), as well as PS1M146V or PS1L286V mutations, lead to Ca2+ flow to mitochondria via mitochondria associated endoplasmic reticulum membrane, (MAM), which further promotes apoptosis.
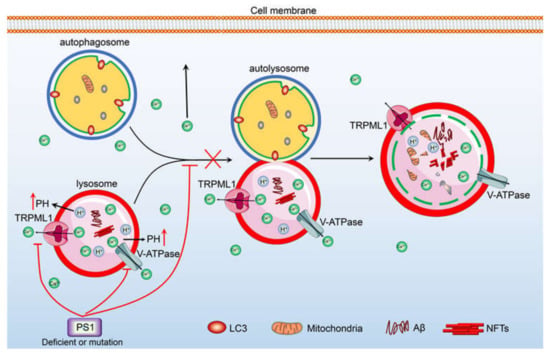
Figure 4.
Ca2+ potentially contribute to regulate the degradation of Aβ and the deposition of hyperphosphorylated tau via its transporters, including v-ATPase and TRPML1 etc., in the membrane of lysosome. TRPML1 and v-ATPase are responsible for inducing the efflux of Ca2+ from lysosome. The accumulation of Ca2+ in the cytosol can stimulate the phosphorylation of tau in the neurons, leading to the deposition of hyperphosphorylated tau in NFTs. In addition, the loss of PS1 induces the release of Ca2+ into the cytosol via TRPML1, which results in blocking the fusion between autophagosome and lysosome, leading to prevent the degradation of Aβ.

Table 1.
The levels of Ca2+ are elevated in the AD patients and animal models.
6. The Roles of Ca2+ in the Production and Deposition of Aβ during the Course of AD Development and Progression
An increase in Ca2+ levels is functionally related to most pathological features and pathogenic factors of AD, such as presenilin and APP mutations, APOE4 expression, CALHM1 mutation, Aβ plaque formation, tau hyperphosphorylation, apoptosis and synaptic dysfunction []. In the following discussion, we discuss these features individually. This section focuses on the regulation of Ca2+ metabolism during the production and deposition of Aβ and phosphorylation of tau protein (Table 2). In HEK293 cells overexpressing human APP, the Ca2+ ion carrier A23187 can increase Aβ production by increasing intracellular free Ca2+ [,]. In primary cultured neurons from 3 × Tg mice, Ca2+ chelator, BAPTA/AM and TRPV1 antagonist, capsazepine lowered the levels of Aβ and phosphorylated tau []. In SH-SY5Y neurons cultured in vitro, increased Ca2+ levels also led to an increase in the production of Aβ []. Other studies have shown that Ca2+ can promote the formation of the Aβ1–40 oligomer, which is also the main cause of AD neurotoxicity []. In addition, the increase in intracellular Ca2+ levels can also trigger the aggregation of Aβ, which forms fibrils, indicating that Ca2+ instability is a possible cause of sporadic AD []. The results of circular dichroism (CD) spectra demonstrated that 1–2 mM Ca2+ have the ability to alter the unfolded Aβ1–42 to β-sheet structure, which results in shortening the time of forming Aβ1–42 fibrils by thioflavin T staining []. During the formation of Aβ fibrils, Ca2+ seemed to accelerate the seeding effects of Aβ1–42 in AD [].

Table 2.
The roles of Ca2+ in the production and depostion of Aβ as well as the phosphorylation of tau.
7. Ca2+ Transporters on the Cell Membrane Are Potentially Contributed to the Role of Aβ in the Pathogenesis of AD
Since Ca2+ has been shown to play a role in the production and aggregation of Aβ, transporters on the surface of the cell membrane must have the potential to regulate the role of Aβ in the pathogenesis of AD. In SH-SY5Y cells and APP23 Tg mice, memantine, an antagonist of NMDAR, showed an inhibitory effect on the production of Aβ [,]. This result confirmed the theory that the activation of NMDAR can induce the production of Aβ []. In addition, a recent study with an AD Tg mouse model showed that Ca2+-permeable (CP) AMPAR was abnormally expressed in the brains of APP/PS1 Tg mice [,]. In line with this finding, recent studies have found that the direct injection of Aβ oligomers into hippocampal neurons in the CA1 region leads to the rapid insertion of CP AMPAR into synapses [,]. The activation of AMPAR can increase the α-secretase cleavage of APP, thereby inhibiting the production of Aβ []. In addition to these glutamate receptors, the CALMH1P86L polymorphic protein also increased the production of Aβ [,]. In rat cortical neurons, L-VGCC promoted Aβ production by increasing the Ca2+ influx [,] In this scenario, APOE, as a transmembrane protein, also participates in the regulation of Aβ production [,].
8. Ca2+ Leakage from ER Modulates the Production and Deposition of Aβ via Activating Ca2+ Transporters on ER
In addition to extracellular Ca2+ influx, the ER, as an intracellular reservoir, plays a regulatory role in the production of Aβ. For example, knocking out the expression of InsP3R in Sf9 and DT40 cells significantly reduced Aβ production []. In addition, previous studies have shown that RyR protein and mRNA expression levels were significantly increased in SH-SY5Y neuroblastoma cells and Tg2576 mice overexpressing wild-type βAPP or βAPPswe []. RyR, another important Ca2+ transporter on the surface of the ER membrane, also regulates Aβ production []. By inhibiting RyR activity, dantrolene decreased the activity levels of β- and γ-secretases and the formation of APs [,]. In AD patients with mild cognitive impairment, RyR2 expression is increased [,]. In mutant-APP-overexpressing SH-SY5Y neurons, the post-translational modification of RyR2 can affect Ca2+ leakage from the ER, leading to reduced production of Aβ from APP []. In addition, it has been reported that enhancing the binding of FKBP12.6 with RyR2 can stabilize the leakage of Ca2+ from the RyR2 channel, leading to the formation of fewer APs []. In addition to RyR2, the RyR3 level showed an upward trend in the hippocampus of several AD mouse models [,,]. In contrast to RyR2, some studies have shown that knocking out RyR3 reduces the formation of APs in the brains of AD mouse models []. By knocking out the expression of RyR3, RyR3 was found to exert a neuroprotective effect in the early stage of AD but promoted the development of AD in the late stage in a 3 × Tg mouse model [,]. Thapsigargin inhibition or siRNA knockout of SERCA, a Ca2+ channel in the ER, resulted in a decrease in Aβ production, while SERCA overexpression increased Aβ production []. Thapsigargin, a compound that inhibits Ca2+ uptake into the ER through SERCA, can increase the effects of caffeine on stimulating the release of Aβ by increasing the level of Ca2+ in the cytoplasm []. These conflicting reports are reconciled by previous reports showing that lower concentrations (10 nM) of thapsigargin stimulated the formation of Aβ, whereas higher concentrations (20 nM) of thapsigargin inhibited the production of Aβ in APP-overexpressing CHO cells [].
On the basis of SOCE, the overexpression of Stim1 and Orai1 can accelerate the production and deposition of Aβ []. In PS1M146V-overexpressing hippocampal neurons, SOCE is required for maintaining the morphology of mushroom spines, which results in modulating the production of Aβ and promoting memory functions [,]. In human neuroblastoma cells, the influx of Ca2+ mediated by SOCE can reduce the secretion of Aβ, suggesting that the loss of SOCE in the pathogenesis of AD leads to the production of Aβ and accelerates the onset of AD [,,]. Consistent with this hypothesis, inhibition of SOCE by overexpressing Orai2 results in the increased production of Aβ1–42 in SH-SY5Y and human neuroglioma H4 cells, suggesting a potential way to rescue the defects of AD and prevent the formation of APs by downregulating the expression of Orai2 [,].
9. Ca2+ Transporters on the Membranes of Mitochondria Are Also Involved in Regulating the Production and Deposition of Aβ during the Course of AD Development and Progression
In mitochondria, the abnormal interaction of voltage-dependent anion channel 1 (VDAC1) with Aβ and phosphorylated tau has the ability to induce the dysfunction of mitochondria during the course of AD development and progression []. In addition, Aβ can induce the opening of mPTP, which results in enhanced permeability of the brain mitochondria [,]. These observations indicated that Aβ might induce the efflux of Ca2+ from mitochondria, which enhances the pathogenesis of AD. In support of this hypothesis, a report suggested that reduced VDAC1 expression in VDAC1+/− mice decreased the mRNA expression levels of AD-related genes, including βAPP, Tau, PS1, PS2 and BACE1, compared with their expression levels in VDAC1+/+ mice []. Furthermore, in primary cultured neurons and APP/PS1 Tg mice carrying human APPKM670/671NL and PS1L166P mutants, treatment with dutasteride decreased the formation of APs by disrupting the function of the mPTP [].
10. The Roles of Ca2+ in Regulating the Phosphorylation of Tau
Apart from the production and deposition of Aβ, Ca2+ also induced the phosphorylation of tau via the GSK3β-activating pathway in SH-SY5Y cells [,]. In addition, a similar phenomenon was observed in primary cultured hippocampal neurons and immortalized GnRH neurons (GT1–7 cells) []. Similarly, we found that mPGES-1/PGE2/EPs/CDK5/p35/p25 signaling cascades mediated the effects of Ca2+ in stimulating the phosphorylation of tau in n2a and APP/PS1 Tg mice []. Furthermore, Ca2+ triggered Ca2+-activated kinases, which mediated the phosphorylation of tau, leading to the formation of NFTs in AD mouse models [,]. Although there are few reports showing the involvement of transporters in mediating the effects of Ca2+ on the phosphorylation of tau, there is evidence suggesting that AMPAR mediates the effects of Ca2+ on the phosphorylation of tau in PS1mut-knock-in mice [,]. Furthermore, alterations to the RyR Ca2+ release channel correlate with the formation of NFTs in AD patients []. On the basis of these observations, multiple transporters may mediate the effects of Ca2+ on the production and deposition of Aβ and hyperphosphorylated tau during the course of AD development and progression.
11. Ca2+ Accelerates the Cognitive Decline Associated with AD
As Ca2+ has been observed to be critical for the production and deposition of Aβ and hyperphosphorylated tau via its transporters, we also address its roles in the learning ability and memory of AD patients and experimental models (Table 3). In aging people, elevated levels of serum Ca2+ is thought to be associated with cognitive decline [,]. In AD patients, disorders of Ca2+ metabolism are also reported to be associated with dementia []. For this reason, Aβ oligomers were identified as critical for the influx of Ca2+ that results in impaired learning and memory through the inhibition of LTP, a form of synaptic plasticity [,,]. Because of the presence of Aβ, Ca2+-dependent enzymes located in the spine, such as calpain, are associated with synaptic dysfunction. Treatment with calpain inhibitors improved learning ability and memory by inducing LTP in Aβ-treated APP/PS1 Tg mice [].

Table 3.
Ca2+ accelerates the cognitive decline of AD.
12. Transporters on the Cell Membrane Mediated the Effects of Ca2+ on Inducing the Cognitive Decline of AD
Since the levels of Ca2+ are increased by activating calcineurin (CaN), the effects of Aβ in inducing deficits in learning and memory were blocked by inhibitors of CaN in APP/PS1 Tg mice []. Activation of the Ca2+-dependent protein phosphatase calcineurin (CaN) potentially impaired the cognition of AD by eliminating both NMDA and AMPA receptors through endocytosis []. In addition to CaN, NMDAR-specific antagonists showed beneficial effects on learning ability and memory in rats [,]. Consistent with this observation, blocking NMDAR attenuated cognitive decline by restoring the metabolic balance of Ca2+ in AD patients and AD mouse models [,]. The sustained expression of another glutamate receptor serving as a Ca2+ transporter, CP-AMPAR, in the early stage of AD accelerated the onset of neuronal network dysfunction and neuronal excitotoxicity, leading to successive cognitive decline by dysregulating the flux of Ca2+ []. These observations indicate that glutamate receptors, including NMDAR and AMPAR, are critical for mediating the effects of Ca2+ dysregulation on the learning ability of AD patients.
In addition, the increase in L-type Ca2+ currents in CA1 synapses leads to a decrease in cognitive function in 3 × Tg AD mice []. Furthermore, treatment with nifedipine, a calcium channel blocker, attenuated cognitive impairment in KK-A(y) mice, a type 2 diabetic mouse model []. These observations confirmed that nimodipine can enhance the learning ability of mild-to-moderate AD patients [,]. Similarly, ST101, an inhibitor of T-VGCC, can attenuate cognitive decline by enhancing LTP and the autophosphorylation of CaMKII in rats []. As an inhibitor of NMDAR, MK-801 attenuates cognitive decline by decreasing the concentration of Ca2+ in mice with traumatic brain injury (TBI) []. In Cav 2.1-knockout mice, ablation of Cav2.1 voltage-gated Ca2+ channels enhanced learning ability by reducing intracellular Ca2+ levels []. SB366791, a specific TRPV1 antagonist, ameliorated the poor cognitive performance of dopamine D3 receptor (D3R)-knockout mice []. Although it is not regarded as a canonical Ca2+ transporter, APOE4 shows the ability to worsen cognitive function by increasing serum Ca2+ levels in older people []. Moreover, the CALHM1P86L polymorphic protein has been found to be associated with AD in the ethnic Chinese Han population, even though no direct evidence has shown a relationship between Ca2+ and learning ability [].
13. Ca2+ Transporters on ER Are also Involved in Impairing the Memory of AD
For intracellular stores, the generation of InsP3 can enhance memory loss by activating the release of intracellular Ca2+ through a metabotropic glutamate receptor-activating mechanism []. As the natural ligand of InsP3R, InsP3 usually exerts its effects via its receptor to impair memory by triggering the release of Ca2+ from the ER in AD patients []. In addition to InsP3R, RyR was also shown to be critical for the cognitive decline of AD patients and mouse models []. For example, the expression of RyR2 was upregulated in patients with mild cognitive impairment and AD []. In addition, an inhibitor of RyRs, dantrolene, enhanced the learning ability of an AD mouse model via the rescue of lost synaptic plasticity []. To clarify the effect, the expression of RyR3 was knocked down, which resulted in impaired social behavior and memory in rats []. This result seemed to conflict with the outcomes induced by treatment of RyRs inhibitors. However, these conflicting results are reconciled by the fact that RyR3 knockdown induces the mRNA expression of RyR2 in the hippocampus of rats completing water maze tests compared with the swimming rat controls []. These observations demonstrate the key roles of RyR2 in affecting the learning ability of organisms affected by AD. In addition to its expression, the post-translational modification of RyR can induce cognitive deficits by stabilizing the leakage of Ca2+ from the ER []. Ca2+ depletion by InsP3R and RyR stimulates SOCE. Accordingly, the reduced expression of synaptic STIM2 and impaired SOCE destabilized mushroom spines, which resulted in reduced LTP-mediated memory formation in PSmut mice [,,]. Consistent with this observation, attenuation of SOCE in AD neurons might account for the cognitive decline associated with AD, suggesting possible roles for SOCE in regulating memory functions [].
14. Ca2+ Transporters on Mitochondria and Lysosomes Potentially Contribute to the Memory Loss of AD
Although there is no direct evidence to show the relationship between Ca2+ from mitochondria and lysosomes and the learning ability of AD patients, to the best of our knowledge, VDAC1 is a hub protein that interacts with more than 150 other proteins, including phosphorylated tau, Aβ, and γ-secretase, and it contributes to their toxic effects, triggering cell death and potentially leading to the dementia characteristic of AD []. In addition, DS16570511 and DS44170716 inhibit Ca2+ uptake in mitochondria by MCU, which resulted in the inhibition of Ca2+-induced mPTP opening and rescued cells from apoptotic death []. For lysosomes, tetrandrine and NED-19 inhibited TPCE2 to re-acidify the lysosome environment and reverse dysregulated autophagy [], which is important for the degradation of aggregated proteins during the course of AD development and progression [,]. On the basis of these observations, Ca2+ has the ability to modulate the learning ability of AD patients via the functions of its transporters.
15. The Roles of Ca2+ in Synaptic Plasticity
In neuroscience, synaptic plasticity refers to the connection between nerve cells, whose strength can be adjusted by cell-adhesion molecules, cytoskeletal proteins, ion channels and various receptor proteins [,]. Indeed, emerging evidence has revealed the central roles of Ca2+ in mediating the synaptic dysfunction in AD []. Given the roles of Ca2+ in producing Aβ, mutations of APP and PS1 have shown led to disruptions of synaptic processes by controlling the homeostasis of Ca2+ during the course of AD development and progression []. In addition, the C-terminus of APP has the ability to impair LTP in mice []. In fact, Aβ induces Ca2+ influx, which results in activating LTD, leading to erased memories in the early cognitive decline of AD patients []. Similarly, Aβ oligomers mediate the inhibitory effects of Ca2+ on LTP in hippocampal slices []. By knocking out the expression of PS1 in mice, LTP is reduced because of the disrupted function of the ER, Ca2+ leakage and reduction in the ER Ca2+ pool in AD []. In contrast, BAPTA-AM, as a chelator of Ca2+, induced LTP in aged rat hippocampal slices []. All this evidence demonstrated the effects of Ca2+ on synaptic plasticity.
16. Ca2+ Transporters on the Cell Membrane Are Involved in Regulating the Synaptic Plasticity
CaN is a member of the serine/threonine protein phosphatase family. It is a unique serine/threonine protein phosphatase that is regulated by Ca2+ and calmodulin. Currently, it is a multifunctional signaling enzyme, especially in regulating synaptic plasticity. For example, overexpressing CaN in young animals induces aging-like deficits of LTP, and deactivating CaN increases the synaptic strength in aged animals, which facilitates LTP []. Similarly, Ca2+-dependent CaN activation results in LTD by removing NMDAR and AMPAR via endocytosis in aged or APP Tg mice [,]. By inhibiting the activity of CaN, LTP is induced by inhibitors or Aβ in APP and Tg2576 mice [,].
With respect to Ca2+ transporters in the cell membrane, Aβ oligomers induce the dysfunction of Ca2+ and inhibit LTP in an NMDAR-dependent mechanism []. In addition, NMDAR mediated the entry of Ca2+ into spines and dendrites, which resulted in insufficient activation of LTP in the rat hippocampus []. Interestingly, NMDAR-dependent LTD requires transient incorporation of Ca2+-permeable (CP)-AMPAR into the synapse, which is mediated by AKAP150-anchored PKA and calcineurin []. Consistent with this observation, infusion of Aβ oligomers into the CA1 region of the hippocampus resulted in a rapid insertion of CP-AMPAR into synapses []. More directly, AMPAR mediated the effects of Ca2+, increasing not only LTP but also LTD. The mutation of GluR2, a subunit of AMPAR, obviously induced LTP in hippocampal slices []. CP-AMPAR insertion into synapses was required for the induction of LTP, which was induced by specific stimuli, leading to the assembly of heteromeric AMPARs containing both GluA1 and GluA2 subunits in CA1 hippocampal neurons []. In addition, CP-AMPAR mediated the effects of glycine on the induction of LTP-dependent spine enlargement via CaMKI-activating mechanisms in mature hippocampal neurons []. In cultured rat hippocampal neurons, Ca2+/calmodulin binding to PSD-95 induced the loss of synaptic PSD-95 and surface AMPARs, which resulted in activated LTD [].
In addition to NMDAR and AMPAR, the activation of VGCC induced LTP via CaMKII in hippocampal slides []. In addition, Cav1.2 expression is essential for LTP, synaptic plasticity, and memory in the hippocampus []. As Ca2+ transporters in the cell membrane, TRPs are involved in regulating synaptic plasticity. For example, TRPV1 activation by capsaicin and resiniferatoxin induces a switch from LTD to LTP by enhancing Ca2+ influx []. Treatment with the agonist of TRPV1 and 4-endocannabinoid anandamide (AEA) induced LTP in CB1−/− or TRPV1−/− mice [,]. In addition, the inhibition of TRPM2 enhanced LTP in traumatically injured brains of mice []. In contrast, TRPM4 reduction eliminated NMDAR-dependent LTP in CA1 hippocampal neurons [].
17. ER Transporters Are Responsible for Releasing Ca2+ from Internal Stores, Leading to Regulate the Synaptic Plasticity
With respect to intracellular Ca2+, LTD is induced via InsP3-mediated Ca2+ influx mechanisms []. Similarly, the activation of metabotropic glutamatergic receptors induced the production of InsP3 to release Ca2+ from internal stores, which resulted in promoting LTD in hippocampal slices []. Blocking InsP3R led to a switch of LTD to LTP and the elimination of heterosynaptic LTD, whereas blocking RyR eliminated both LTP and homosynaptic LTD at synapses that were activated, normally at low frequencies, in rat hippocampal slides and 3 × Tg mice [,]. In addition, knocking out the expression of RyR3 concurrently increases LTP and reduced LTD [,]. As critical genes for AD, presynaptic inactivation of PSs impairs LTP by controlling RyR-mediated Ca2+ release from the ER []. As Ca2+ depletion from the ER induces SOCE, it is reasonable to speculate that SOCE is involved in regulating synaptic plasticity. In FVB/NJ mice, reduction of SOCE-mediated Ca2+ entry reduced CaMKII activity, leading to destabilization of the mushroom spine and reducing LTP-mediated memory formation []. In the same experimental model, the overexpression of STIM1 in mouse brain neurons enhanced contextual learning and attenuated long-term depression []. With respect to mitochondrial Ca2+ stores, knocking out the expression of VDAC1 disrupts synaptic plasticity []. Similar to the effects of inhibiting mPTP by cyclosporine A, porin-deficient mice showed deficits in long- and short-term synaptic plasticity []. Based on these observations, these transporters mediated the regulatory effects of Ca2+ on synaptic plasticity (Table 4).

Table 4.
The roles of Ca2+ in synaptic plasticity.
18. Conclusions
During the development and progression of AD, Ca2+ is elevated in the cytosol of neuronal cells via its transportation from the extracellular space and intracellular stores through transporter-dependent mechanisms. Ca2+ accumulated in neuronal cells has the ability to induce the production and deposition of Aβ and hyperphosphorylated tau in APs and NFTs, leading to impaired learning ability in AD patients. Moreover, transporters in the cell membrane, endoplasmic reticulum, mitochondria and lysosomal membranes are critical for mediating the effects of Ca2+ on synaptic plasticity, which contribute to the cognitive decline associated with AD.
Author Contributions
L.-L.C. and P.-P.G. contributed to the conceptualization, writing, review and editing of the manuscript. P.W. contributed to the writing, review and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part or in whole by the National Natural Science Foundation of China (CN) (81771167 and 81870840).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no competing financial interests.
References
- Surguchov, A. Caveolin: A New Link Between Diabetes and AD. Cell. Mol. Neurobiol. 2020, 40, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Elgh, E.; Åstot, A.L.; Fagerlund, M.; Eriksson, S.; Olsson, T.; Näsman, B. Cognitive Dysfunction, Hippocampal Atrophy and Glucocorticoid Feedback in Alzheimer’s Disease. Biol. Psychiatry 2006, 59, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Tarasoff-Conway, J.M.; Carare, R.O.; Osorio, R.S.; Glodzik, L.; Butler, T.; Fieremans, E.; Axel, L.; Rusinek, H.; Nicholson, C.; Zlokovic, B.V.; et al. Clearance systems in the brain—implications for Alzheimer disease. Nat. Rev. Neurol. 2015, 11, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Almenar-Queralt, A.; Leblanc, J.F.; Roberts, E.A.; Goldstein, L.S.B. Kinesin-mediated axonal transport of a membrane compartment containing β-secretase and presenilin-1 requires APP. Nat. Cell Biol. 2001, 414, 643–648. [Google Scholar] [CrossRef]
- Mazanetz, M.P.; Fischer, P.M. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat. Rev. Drug Discov. 2007, 6, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Tomiyama, T.; Matsuyama, S.; Iso, H.; Umeda, T.; Takuma, H.; Ohnishi, K.; Ishibashi, K.; Teraoka, R.; Sakama, N.; Yamashita, T.; et al. A Mouse Model of Amyloid Oligomers: Their Contribution to Synaptic Alteration, Abnormal Tau Phosphorylation, Glial Activation, and Neuronal Loss In Vivo. J. Neurosci. 2010, 30, 4845–4856. [Google Scholar] [CrossRef]
- Heyman, A.; Wilkinson, W.E.; Stafford, J.A.; Helms, M.J.; Sigmon, A.H.; Weinberg, T. Alzheimer’s disease: A study of epidemiological aspects. Ann. Neurol. 1984, 15, 335–341. [Google Scholar] [CrossRef]
- Patterson, C.; Feightner, J.W.; Garcia, A.; Hsiung, G.-Y.R.; Macknight, C.; Sadovnick, A.D. Diagnosis and treatment of dementia: 1. Risk assessment and primary prevention of Alzheimer disease. Can. Med. Assoc. J. 2008, 178, 548–556. [Google Scholar] [CrossRef]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef]
- O’Donoghue, M.C.; Murphy, S.E.; Zamboni, G.; Nobre, A.C.; Mackay, C.E. APOE genotype and cognition in healthy individuals at risk of Alzheimer’s disease: A review. Cortex 2018, 104, 103–123. [Google Scholar] [CrossRef]
- Haass, C.; Lemere, C.A.; Capell, A.; Citron, M.; Seubert, P.; Schenk, D.; Lannfelt, L.; Selkoe, D.J. The Swedish mutation causes early-onset Alzheimer’s disease by β-secretase cleavage within the secretory pathway. Nat. Med. 1995, 1, 1291–1296. [Google Scholar] [CrossRef]
- Sinha, S.; Lieberburg, I. Cellular mechanisms of beta -amyloid production and secretion. Proc. Natl. Acad. Sci. USA 1999, 96, 11049–11053. [Google Scholar] [CrossRef]
- Citron, M.; Westaway, D.; Xia, W.; Carlson, G.; Diehl, T.; Levesque, G.; Johnson-Wood, K.; Lee, M.; Seubert, P.; Davis, A.; et al. Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid β-protein in both transfected cells and transgenic mice. Nat. Med. 1997, 3, 67–72. [Google Scholar] [CrossRef]
- Alonso, A.D.C.; Grundke-Iqbal, I.; Iqbal, K. Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat. Med. 1996, 2, 783–787. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Z.-Y. Metal ions influx is a double edged sword for the pathogenesis of Alzheimer’s disease. Ageing Res. Rev. 2017, 35, 265–290. [Google Scholar] [CrossRef]
- Etcheberrigaray, R.; Hirashima, N.; Neec, L.; Prince, J.; Govonid, S.; Racchie, M.; Tanzi, R.E.; Alkon, D.L. Calcium Responses in Fibroblasts from Asymptomatic Members of Alzheimer’s Disease Families. Neurobiol. Dis. 1998, 5, 37–45. [Google Scholar] [CrossRef]
- Yu, J.-T.; Chang, R.C.-C.; Tan, L. Calcium dysregulation in Alzheimer’s disease: From mechanisms to therapeutic opportunities. Prog. Neurobiol. 2009, 89, 240–255. [Google Scholar] [CrossRef]
- Cao, L.-L.; Guan, P.-P.; Liang, Y.-Y.; Huang, X.-S.; Wang, P. Calcium Ions Stimulate the Hyperphosphorylation of Tau by Activating Microsomal Prostaglandin E Synthase 1. Front. Aging Neurosci. 2019, 11, 108. [Google Scholar] [CrossRef]
- Cao, L.-L.; Guan, P.-P.; Liang, Y.-Y.; Huang, X.-S.; Wang, P. Cyclooxygenase-2 is Essential for Mediating the Effects of Calcium Ions on Stimulating Phosphorylation of Tau at the Sites of Ser 396 and Ser 404. J. Alzheimer’s Dis. 2019, 68, 1095–1111. [Google Scholar] [CrossRef]
- Zempel, H.; Thies, E.; Mandelkow, E.-M. A Oligomers Cause Localized Ca2+ Elevation, Missorting of Endogenous Tau into Dendrites, Tau Phosphorylation, and Destruction of Microtubules and Spines. J. Neurosci. 2010, 30, 11938–11950. [Google Scholar] [CrossRef] [PubMed]
- Tong, B.C.-K.; Wu, A.J.; Li, M.; Cheung, K.-H. Calcium signaling in Alzheimer’s disease & therapies. Biochim. Biophys. Acta BBA Bioenerg. 2018, 1865, 1745–1760. [Google Scholar] [CrossRef]
- Sama, D.M.; Norris, C.M. Calcium dysregulation and neuroinflammation: Discrete and integrated mechanisms for age-related synaptic dysfunction. Ageing Res. Rev. 2013, 12, 982–995. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, D.; Farrelly, O.; Miles, L.; Li, F.; Kim, S.E.; Lo, T.Y.; Wang, F.; Li, T.; Thompson-Peer, K.L.; et al. The Mechanosensitive Ion Channel Piezo Inhibits Axon Regeneration. Neuron 2019, 102, 373–389.e6. [Google Scholar] [CrossRef] [PubMed]
- Wahlestedt, C.; Golanov, E.; Yamamoto, S.; Yee, F.; Ericson, H.; Yoo, H.; Inturrisi, C.E.; Reis, D.J. Antisense oligodeoxynucleotides to NMDA-R1 receptor channel protect cortical neurons from excitotoxicity and reduce focal ischaemic infarctions. Nat. Cell Biol. 1993, 363, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Decuypere, J.-P.; Bultynck, G.; Parys, J.B. A dual role for Ca2+ in autophagy regulation. Cell Calcium 2011, 50, 242–250. [Google Scholar] [CrossRef]
- Liu, S.J.; Zukin, R.S. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007, 30, 126–134. [Google Scholar] [CrossRef]
- Kuchibhotla, K.V.; Goldman, S.T.; Lattarulo, C.R.; Wu, H.-Y.; Hyman, B.T.; Bacskai, B.J. Aβ Plaques Lead to Aberrant Regulation of Calcium Homeostasis In Vivo Resulting in Structural and Functional Disruption of Neuronal Networks. Neuron 2008, 59, 214–225. [Google Scholar] [CrossRef]
- MacManus, A.; Ramsden, M.; Murray, M.; Henderson, Z.; Pearson, H.A.; Campbell, V.A. Enhancement of 45Ca2+ Influx and Voltage-dependent Ca2+ Channel Activity by β-Amyloid-(1–40) in Rat Cortical Synaptosomes and Cultured Cortical Neurons. J. Biol. Chem. 2000, 275, 4713–4718. [Google Scholar] [CrossRef]
- Calvo-Rodriguez, M.; Hou, S.S.; Snyder, A.C.; Kharitonova, E.K.; Russ, A.N.; Das, S.; Fan, Z.; Muzikansky, A.; Garcia-Alloza, M.; Serrano-Pozo, A.; et al. Increased mitochondrial calcium levels associated with neuronal death in a mouse model of Alzheimer’s disease. Nat. Commun. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Li, L.; Tsai, H.-J.; Li, L.; Wang, X.-M. Icariin Inhibits the Increased Inward Calcium Currents Induced by Amyloid-β25-35 Peptide in CA1 Pyramidal Neurons of Neonatal Rat Hippocampal Slice. Am. J. Chin. Med. 2010, 38, 113–125. [Google Scholar] [CrossRef]
- Yallampalli, S.; Micci, M.-A.; Taglialatela, G. Ascorbic acid prevents β-amyloid-induced intracellular calcium increase and cell death in PC12 cells. Neurosci. Lett. 1998, 251, 105–108. [Google Scholar] [CrossRef]
- Ekinci, F.J.; Linsley, M.-D.; Shea, T.B. β-Amyloid-induced calcium influx induces apoptosis in culture by oxidative stress rather than tau phosphorylation. Mol. Brain Res. 2000, 76, 389–395. [Google Scholar] [CrossRef]
- Anekonda, T.S.; Quinn, J.F. Calcium channel blocking as a therapeutic strategy for Alzheimer’s disease: The case for isradipine. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2011, 1812, 1584–1590. [Google Scholar] [CrossRef]
- Hermes, M.; Eichhoff, G.; Garaschuk, O. Intracellular calcium signalling in Alzheimer’s disease. J. Cell. Mol. Med. 2009, 14, 30–41. [Google Scholar] [CrossRef]
- Demuro, A.; Parker, I.; Stutzmann, G.E. Calcium Signaling and Amyloid Toxicity in Alzheimer Disease. J. Biol. Chem. 2010, 285, 12463–12468. [Google Scholar] [CrossRef]
- Mäkinen, S.; Van Groen, T.; Clarke, J.; Thornell, A.; Corbett, D.; Hiltunen, M.; Soininen, H.; Jolkkonen, J. Coaccumulation of Calcium and β-Amyloid in the Thalamus after Transient Middle Cerebral Artery Occlusion in Rats. Br. J. Pharmacol. 2007, 28, 263–268. [Google Scholar] [CrossRef]
- Arispe, N.; Diaz, J.; Durell, S.R.; Shafrir, Y.; Guy, H.R. Polyhistidine Peptide Inhibitor of the Aβ Calcium Channel Potently Blocks the Aβ-Induced Calcium Response in Cells. Theoretical Modeling Suggests a Cooperative Binding Process. Biochemistry 2010, 49, 7847–7853. [Google Scholar] [CrossRef]
- Arispe, N.; Rojas, E.; Pollard, H.B. Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: Blockade by tromethamine and aluminum. Proc. Natl. Acad. Sci. USA 1993, 90, 567–571. [Google Scholar] [CrossRef]
- Lin, H.; Bhatia, R.; Lal, R. Amyloid β protein forms ion channels: Implications for Alzheimer’s disease pathophysiology. FASEB J. 2001, 15, 2433–2444. [Google Scholar] [CrossRef]
- Lashuel, H.A.; Hartley, D.; Petre, B.M.; Walz, T.; Lansbury, P.T., Jr. Amyloid pores from pathogenic mutations. Nat. Cell Biol. 2002, 418, 291. [Google Scholar] [CrossRef] [PubMed]
- Lashuel, H.A.; Hartley, D.M.; Petre, B.M.; Wall, J.S.; Simon, M.N.; Walz, T.; Lansbury, P.T. Mixtures of Wild-type and a Pathogenic (E22G) Form of Aβ40 in Vitro Accumulate Protofibrils, Including Amyloid Pores. J. Mol. Biol. 2003, 332, 795–808. [Google Scholar] [CrossRef]
- Durell, S.; Guy, H.; Arispe, N.; Rojas, E.; Pollard, H. Theoretical models of the ion channel structure of amyloid beta-protein. Biophys. J. 1994, 67, 2137–2145. [Google Scholar] [CrossRef]
- Jang, H.; Ma, B.; Lal, R.; Nussinov, R. Models of Toxic β-Sheet Channels of Protegrin-1 Suggest a Common Subunit Organization Motif Shared with Toxic Alzheimer β-Amyloid Ion Channels. Biophys. J. 2008, 95, 4631–4642. [Google Scholar] [CrossRef]
- Pollard, H.B.; Rojas, E.; Arispe, N. A New Hypothesis for the Mechanism of Amyloid Toxicity, Based on the Calcium Channel Activity of Amyloid β Protein (AβP) in Phospholipid Bilayer Membranes. Ann. N. Y. Acad. Sci. 1993, 695, 165–168. [Google Scholar] [CrossRef]
- Kayed, R.; Head, E.; Thompson, J.L.; McIntire, T.M.; Milton, S.C.; Cotman, C.W.; Glabe, C.G. Common Structure of Soluble Amyloid Oligomers Implies Common Mechanism of Pathogenesis. Science 2003, 300, 486–489. [Google Scholar] [CrossRef]
- Demuro, A.; Mina, E.; Kayed, R.; Milton, S.C.; Parker, I.; Glabe, C.G. Calcium Dysregulation and Membrane Disruption as a Ubiquitous Neurotoxic Mechanism of Soluble Amyloid Oligomers. J. Biol. Chem. 2005, 280, 17294–17300. [Google Scholar] [CrossRef]
- Deshpande, A.; Mina, E.; Glabe, C.; Busciglio, J. Different Conformations of Amyloid beta Induce Neurotoxicity by Distinct Mechanisms in Human Cortical Neurons. J. Neurosci. 2006, 26, 6011–6018. [Google Scholar] [CrossRef]
- Lee, G.; Pollard, H.B.; Arispe, N. Annexin 5 and apolipoprotein E2 protect against Alzheimer’s amyloid-β-peptide cytotoxicity by competitive inhibition at a common phosphatidylserine interaction site. Peptides 2002, 23, 1249–1263. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Canevari, L.; Duchen, M.R. Changes in Intracellular Calcium and Glutathione in Astrocytes as the Primary Mechanism of Amyloid Neurotoxicity. J. Neurosci. 2003, 23, 5088–5095. [Google Scholar] [CrossRef]
- Arispe, N.; Pollard, H.B.; Rojas, E. Zn2+ interaction with Alzheimer amyloid beta protein calcium channels. Proc. Natl. Acad. Sci. USA 1996, 93, 1710–1715. [Google Scholar] [CrossRef]
- Bush, A.I. The metallobiology of Alzheimer’s disease. Trends Neurosci. 2003, 26, 207–214. [Google Scholar] [CrossRef]
- Rhee, S.K.; Quist, A.P.; Lal, R. Amyloid β Protein-(1–42) Forms Calcium-permeable, Zn2+-sensitive Channel. J. Biol. Chem. 1998, 273, 13379–13382. [Google Scholar] [CrossRef]
- Arispe, N.; Doh, M. Plasma membrane cholesterol controls the cytotoxicity of Alzheimer’s disease AβP (1–40) and (1–42) peptides. FASEB J. 2002, 16, 1526–1536. [Google Scholar] [CrossRef]
- Kawahara, M.; Kuroda, Y. Intracellular Calcium Changes in Neuronal Cells Induced by Alzheimer’s β-Amyloid Protein Are Blocked by Estradiol and Cholesterol. Cell. Mol. Neurobiol. 2001, 21, 1–13. [Google Scholar] [CrossRef]
- Barger, S.W.; Fiscus, R.R.; Ruth, P.; Hofmann, F.; Mattson, M.P. Role of Cyclic GMP in the Regulation of Neuronal Calcium and Survival by Secreted Forms of β-Amyloid Precursor. J. Neurochem. 2002, 64, 2087–2096. [Google Scholar] [CrossRef]
- Guo, Q.; Robinson, N.; Mattson, M.P. Secreted β-Amyloid Precursor Protein Counteracts the Proapoptotic Action of Mutant Presenilin-1 by Activation of NF-κB and Stabilization of Calcium Homeostasis. J. Biol. Chem. 1998, 273, 12341–12351. [Google Scholar] [CrossRef]
- Murray, J.N.; Igwe, O.J. Regulation of ?-amyloid precursor protein and inositol 1,4,5-trisphosphate receptor gene expression during differentiation of a human neuronal cell line. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2003, 27, 351–363. [Google Scholar] [CrossRef]
- Cao, X.; Südhof, T.C. A Transcriptively Active Complex of APP with Fe65 and Histone Acetyltransferase Tip60. Science 2001, 293, 115–120. [Google Scholar] [CrossRef]
- Leissring, M.A.; Murphy, M.P.; Mead, T.R.; Akbari, Y.; Sugarman, M.C.; Jannatipour, M.; Anliker, B.; Müller, U.; Saftig, P.; De Strooper, B.; et al. A physiologic signaling role for the -secretase-derived intracellular fragment of APP. Proc. Natl. Acad. Sci. USA 2002, 99, 4697–4702. [Google Scholar] [CrossRef]
- Fedeli, C.; Filadi, R.; Rossi, A.; Mammucari, C.; Pizzo, P. PSEN2 (presenilin 2) mutants linked to familial Alzheimer disease impair autophagy by altering Ca2+ homeostasis. Autophagy 2019, 15, 2044–2062. [Google Scholar] [CrossRef] [PubMed]
- Bullock, R. Efficacy and Safety of Memantine in Moderate-to-Severe Alzheimer Disease: The Evidence to Date. Alzheimer Dis. Assoc. Disord. 2006, 20, 23–29. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.G.; Velasco, P.T.; Lambert, M.P.; Viola, K.; Fernandez, S.J.; Ferreira, S.T.; Klein, W.L. Aβ Oligomers Induce Neuronal Oxidative Stress through an N-Methyl-D-aspartate Receptor-dependent Mechanism That Is Blocked by the Alzheimer Drug Memantine. J. Biol. Chem. 2007, 282, 11590–11601. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qiu, X.; Mak, S.; Guo, B.; Hu, S.; Wang, J.; Luo, F.; Xu, D.; Sun, Y.; Zhang, G.; et al. Multifunctional memantine nitrate significantly protects against glutamate-induced excitotoxicity via inhibiting calcium influx and attenuating PI3K/Akt/GSK3beta pathway. Chem. Interact. 2020, 325, 109020. [Google Scholar] [CrossRef]
- Kelly, B.L.; Ferreira, A. β-Amyloid-induced Dynamin 1 Degradation Is Mediated by N-Methyl-D-Aspartate Receptors in Hippocampal Neurons. J. Biol. Chem. 2006, 281, 28079–28089. [Google Scholar] [CrossRef]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; E Shepardson, N.; Smith, I.; Brett, F.M.; A Farrell, M.; Rowan, M.J.; A Lemere, C.; et al. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef]
- Snyder, E.M.; Nong, Y.; Almeida, C.G.; Paul, S.; Moran, T.; Choi, E.Y.; Nairn, A.C.; Salter, M.W.; Lombroso, P.J.; Gouras, G.K.; et al. Regulation of NMDA receptor trafficking by amyloid-β. Nat. Neurosci. 2005, 8, 1051–1058. [Google Scholar] [CrossRef]
- Dewachter, I.; Filipkowski, R.; Priller, C.; Ris, L.; Neyton, J.; Croes, S.; Terwel, D.; Gysemans, M.; Devijver, H.; Borghgraef, P.; et al. Deregulation of NMDA-receptor function and down-stream signaling in APP[V717I] transgenic mice. Neurobiol. Aging 2009, 30, 241–256. [Google Scholar] [CrossRef]
- Chappell, A.S.; Gonzales, C.; Williams, J.; Witte, M.M.; Mohs, R.C.; Sperling, R. AMPA potentiator treatment of cognitive deficits in Alzheimer disease. Neurology 2007, 68, 1008–1012. [Google Scholar] [CrossRef]
- Trzepacz, P.T.; Cummings, J.; Konechnik, T.; Forrester, T.D.; Chang, C.; Dennehy, E.B.; Willis, B.A.; Shuler, C.; Tabas, L.B.; Lyketsos, C. Mibampator (LY451395) randomized clinical trial for agitation/aggression in Alzheimer’s disease. Int. Psychogeriatrics 2013, 25, 707–719. [Google Scholar] [CrossRef]
- Bloss, E.B.; Hunter, R.G.; Waters, E.M.; Muñoz, C.; Bernard, K.; McEwen, B.S. Behavioral and biological effects of chronic S18986, a positive AMPA receptor modulator, during aging. Exp. Neurol. 2008, 210, 109–117. [Google Scholar] [CrossRef]
- Jhee, S.S.; Chappell, A.S.; Zarotsky, V.; Moran, S.V.; Rosenthal, M.; Kim, E.; Chalon, S.; Toublanc, N.; Brandt, J.; Coutant, D.E.; et al. Multiple-Dose Plasma Pharmacokinetic and Safety Study of LY450108 and LY451395 (AMPA Receptor Potentiators) and Their Concentration in Cerebrospinal Fluid in Healthy Human Subjects. J. Clin. Pharmacol. 2006, 46, 424–432. [Google Scholar] [CrossRef]
- Nimmrich, V.; Grimm, C.; Draguhn, A.; Barghorn, S.; Lehmann, A.; Schoemaker, H.; Hillen, H.; Gross, G.; Ebert, U.; Bruehl, C. Amyloid Oligomers (A 1-42 Globulomer) Suppress Spontaneous Synaptic Activity by Inhibition of P/Q-Type Calcium Currents. J. Neurosci. 2008, 28, 788–797. [Google Scholar] [CrossRef]
- Rovira, C.; Arbez, N.; Mariani, J. Aβ(25–35) and Aβ(1–40) act on different calcium channels in CA1 hippocampal neurons. Biochem. Biophys. Res. Commun. 2002, 296, 1317–1321. [Google Scholar] [CrossRef]
- Hermann, D.; Mezler, M.; Müller, M.K.; Wicke, K.; Gross, G.; Draguhn, A.; Bruehl, C.; Nimmrich, V. Synthetic Aβ oligomers (Aβ1–42 globulomer) modulate presynaptic calcium currents: Prevention of Aβ-induced synaptic deficits by calcium channel blockers. Eur. J. Pharmacol. 2013, 702, 44–55. [Google Scholar] [CrossRef]
- Mark, R.J.; Hensley, K.; A Butterfield, D.; Mattson, M.P. Amyloid beta-peptide impairs ion-motive ATPase activities: Evidence for a role in loss of neuronal Ca2+ homeostasis and cell death. J. Neurosci. 1995, 15, 6239–6249. [Google Scholar] [CrossRef]
- Malenka, R.C. Synaptic plasticity in the hippocampus: LTP and LTD. Cell 1994, 78, 535–538. [Google Scholar] [CrossRef]
- Tanis, J.E.; Ma, Z.; Krajacic, P.; He, L.; Foskett, J.K.; Lamitina, T. CLHM-1 is a Functionally Conserved and Conditionally Toxic Ca2+-Permeable Ion Channel in Caenorhabditis elegans. J. Neurosci. 2013, 33, 12275–12286. [Google Scholar] [CrossRef]
- Wang, X.S.; Gruenstein, E. Rapid elevation of neuronal cytoplasmic calcium by apolipoprotein E peptide. J. Cell Physiol. 1997, 173, 73–83. [Google Scholar] [CrossRef]
- Müller, W.; Meske, V.; Berlin, K.; Scharnagl, H.; Marz, W.; Ohm, T. Apolipoprotein E Isoforms Increase Intracellular Ca2+Differentially Through a ω-Agatoxin IVa-Sensitive Ca2+-Channel. Brain Pathol. 1998, 8, 641–653. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, S.; Zhao, H.; Wang, Y.; Chen, X.; Sun, X. Effects of apolipoprotein E gene polymorphism on the intracellular Ca2+ concentration of astrocytes in the early stages post injury. Exp. Ther. Med. 2017, 15, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro, E.; Oliveira, C.R. Involvement of endoplasmic reticulum Ca2+ release through ryanodine and inositol 1,4,5-triphosphate receptors in the neurotoxic effects induced by the amyloid-? peptide. J. Neurosci. Res. 2004, 76, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Supnet, C.; Grant, J.; Kong, H.; Westaway, D.; Mayne, M. Amyloid-β-(1-42) Increases Ryanodine Receptor-3 Expression and Function in Neurons of TgCRND8 Mice. J. Biol. Chem. 2006, 281, 38440–38447. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Ozawa, H.; Saito, T.; Takahata, N.; Takemura, H. Calcium mobilization evoked by amyloid β-protein involves inositol 1,4,5-trisphosphate production in human platelets. Life Sci. 1998, 62, 705–713. [Google Scholar] [CrossRef]
- Schapansky, J.; Olson, K.; Van Der Ploeg, R.; Glazner, G. NF-κB activated by ER calcium release inhibits Aβ-mediated expression of CHOP protein: Enhancement by AD-linked mutant presenilin 1. Exp. Neurol. 2007, 208, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Shtifman, A.; Ward, C.W.; Laver, D.R.; Bannister, M.L.; Lopez, J.R.; Kitazawa, M.; LaFerla, F.M.; Ikemoto, N.; Querfurth, H.W. Amyloid-β protein impairs Ca2+ release and contractility in skeletal muscle. Neurobiol. Aging 2010, 31, 2080–2090. [Google Scholar] [CrossRef]
- Müller, M.; Cárdenas, C.; Mei, L.; Cheung, K.-H.; Foskett, J.K. Constitutive cAMP response element binding protein (CREB) activation by Alzheimer’s disease presenilin-driven inositol trisphosphate receptor (InsP3R) Ca2+signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 13293–13298. [Google Scholar] [CrossRef] [PubMed]
- Marcantoni, A.; Cerullo, M.S.; Buxeda, P.; Tomagra, G.; Giustetto, M.; Chiantia, G.; Carabelli, V.; Carbone, E. Amyloid Beta42 oligomers up-regulate the excitatory synapses by potentiating presynaptic release while impairing postsynaptic NMDA receptors. J. Physiol. 2020, 598, 2183–2197. [Google Scholar] [CrossRef]
- Stutzmann, G.E. Calcium Dysregulation, IP3 Signaling, and Alzheimer’s Disease. Neuroscience 2005, 11, 110–115. [Google Scholar] [CrossRef]
- Cheung, K.-H.; Shineman, D.; Müller, M.; Cárdenas, C.; Mei, L.; Yang, J.; Tomita, T.; Iwatsubo, T.; Lee, V.M.-Y.; Foskett, J.K. Mechanism of Ca2+ Disruption in Alzheimer’s Disease by Presenilin Regulation of InsP3 Receptor Channel Gating. Neuron 2008, 58, 871–883. [Google Scholar] [CrossRef]
- Stutzmann, G.E.; Smith, I.; Caccamo, A.; Oddo, S.; LaFerla, F.M.; Parker, I. Enhanced Ryanodine Receptor Recruitment Contributes to Ca2+ Disruptions in Young, Adult, and Aged Alzheimer’s Disease Mice. J. Neurosci. 2006, 26, 5180–5189. [Google Scholar] [CrossRef] [PubMed]
- Rybalchenko, V.; Hwang, S.-Y.; Rybalchenko, N.; Koulen, P. The cytosolic N-terminus of presenilin-1 potentiates mouse ryanodine receptor single channel activity. Int. J. Biochem. Cell Biol. 2008, 40, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Hayrapetyan, V.; Rybalchenko, V.; Rybalchenko, N.; Koulen, P. The N-terminus of presenilin-2 increases single channel activity of brain ryanodine receptors through direct protein–protein interaction. Cell Calcium 2008, 44, 507–518. [Google Scholar] [CrossRef]
- Green, K.N.; DeMuro, A.; Akbari, Y.; Hitt, B.D.; Smith, I.F.; Parker, I.; LaFerla, F.M. SERCA pump activity is physiologically regulated by presenilin and regulates amyloid β production. J. Cell Biol. 2008, 181, 1107–1116. [Google Scholar] [CrossRef]
- Cedazo-Mínguez, A.; Popescu, B.O.; Ankarcrona, M.; Nishimura, T.; Cowburn, R.F. The Presenilin 1 ΔE9 Mutation Gives Enhanced Basal Phospholipase C Activity and a Resultant Increase in Intracellular Calcium Concentrations. J. Biol. Chem. 2002, 277, 36646–36655. [Google Scholar] [CrossRef]
- Mattson, M.P.; LaFerla, F.M.; Chan, S.L.; A Leissring, M.; Shepel, P.; Geiger, J.D. Calcium signaling in the ER: Its role in neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2000, 23, 222–229. [Google Scholar] [CrossRef]
- Cheung, K.-H.; Mei, L.; Mak, D.-O.D.; Hayashi, I.; Iwatsubo, T.; Kang, D.E.; Foskett, J.K. Gain-of-Function Enhancement of IP3 Receptor Modal Gating by Familial Alzheimer’s Disease-Linked Presenilin Mutants in Human Cells and Mouse Neurons. Sci. Signal. 2010, 3, ra22. [Google Scholar] [CrossRef]
- Ohkubo, N.; Mitsuda, N.; Tamatani, M.; Yamaguchi, A.; Lee, Y.-D.; Ogihara, T.; Vitek, M.P.; Tohyama, M. Apolipoprotein E4 Stimulates cAMP Response Element-binding Protein Transcriptional Activity through the Extracellular Signal-regulated Kinase Pathway. J. Biol. Chem. 2001, 276, 3046–3053. [Google Scholar] [CrossRef]
- Namba, Y.; Tomonaga, M.; Kawasaki, H.; Otomo, E.; Ikeda, K. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer’s disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 1991, 541, 163–166. [Google Scholar] [CrossRef]
- Stutzmann, G.E. The Pathogenesis of Alzheimers Disease—Is It a Lifelong “Calciumopathy”? Neuroscientist 2007, 13, 546–559. [Google Scholar] [CrossRef]
- Resende, R.; Ferreiro, E.; Pereira, C.; Oliveira, C.R. ER stress is involved in Aβ-induced GSK-3β activation and tau phosphorylation. J. Neurosci. Res. 2008, 86, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Putney, J.W. Capacitative calcium entry in the nervous system. Cell Calcium 2003, 34, 339–344. [Google Scholar] [CrossRef]
- Park, C.Y.; Hoover, P.J.; Mullins, F.M.; Bachhawat, P.; Covington, E.D.; Raunser, S.; Walz, T.; Garcia, K.C.; Dolmetsch, R.E.; Lewis, R.S. STIM1 Clusters and Activates CRAC Channels via Direct Binding of a Cytosolic Domain to Orai 1. Cell 2009, 136, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Bojarski, L.; Herms, J.; Kuznicki, J. Calcium dysregulation in Alzheimer’s disease. Neurochem. Int. 2008, 52, 621–633. [Google Scholar] [CrossRef]
- Zeiger, W.; Vetrivel, K.S.; Buggia-Prévot, V.; Nguyen, P.D.; Wagner, S.L.; Villereal, M.L.; Thinakaran, G. Ca2+ Influx through Store-operated Ca2+ Channels Reduces Alzheimer Disease β-Amyloid Peptide Secretion. J. Biol. Chem. 2013, 288, 26955–26966. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, H.; Liu, J.; Popugaeva, E.; Xu, N.-J.; Feske, S.; White, C.L.; Bezprozvanny, I. Reduced Synaptic STIM2 Expression and Impaired Store-Operated Calcium Entry Cause Destabilization of Mature Spines in Mutant Presenilin Mice. Neuron 2014, 82, 79–93. [Google Scholar] [CrossRef]
- Ryazantseva, M.; Goncharova, A.; Skobeleva, K.; Erokhin, M.; Methner, A.; Georgiev, P.; Kaznacheyeva, E. Presenilin-1 Delta E9 Mutant Induces STIM1-Driven Store-Operated Calcium Channel Hyperactivation in Hippocampal Neurons. Mol. Neurobiol. 2018, 55, 4667–4680. [Google Scholar] [CrossRef]
- Li, H.-S.; Xu, X.-Z.S.; Montell, C. Activation of a TRPC3-Dependent Cation Current through the Neurotrophin BDNF. Neuron 1999, 24, 261–273. [Google Scholar] [CrossRef]
- Lessard, C.B.; Lussier, M.P.; Cayouette, S.; Bourque, G.; Boulay, G. The overexpression of presenilin2 and Alzheimer’s-disease-linked presenilin2 variants influences TRPC6-enhanced Ca2+ entry into HEK293 cells. Cell. Signal. 2005, 17, 437–445. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, Q.; Zhou, P.; Li, S.; Zhu, F. HERV-W env regulates calcium influx via activating TRPC3 channel together with depressing DISC1 in human neuroblastoma cells. J. Neuro Virol. 2019, 25, 101–113. [Google Scholar] [CrossRef]
- Keller, J.N.; Guo, Q.; Holtsberg, F.W.; Bruce-Keller, A.J.; Mattson, M.P. Increased Sensitivity to Mitochondrial Toxin-Induced Apoptosis in Neural Cells Expressing Mutant Presenilin-1 Is Linked to Perturbed Calcium Homeostasis and Enhanced Oxyradical Production. J. Neurosci. 1998, 18, 4439–4450. [Google Scholar] [CrossRef]
- Kruman, I.; Guo, Q.; Mattson, M.P. Calcium and reactive oxygen species mediate staurosporine-induced mitochondrial dysfunction and apoptosis in PC12 cells. J. Neurosci. Res. 1998, 51, 293–308. [Google Scholar] [CrossRef]
- Toglia, P.; Ullah, G. The gain-of-function enhancement of IP3-receptor channel gating by familial Alzheimer’s disease-linked presenilin mutants increases the open probability of mitochondrial permeability transition pore. Cell Calcium 2016, 60, 13–24. [Google Scholar] [CrossRef]
- Cuadrado-Tejedor, M.; Vilariño, M.; Cabodevilla, F.; Del Río, J.; Frechilla, D.; Pérez-Mediavilla, A. Enhanced Expression of the Voltage-Dependent Anion Channel 1 (VDAC1) in Alzheimer’s Disease Transgenic Mice: An Insight into the Pathogenic Effects of Amyloid-β. J. Alzheimer’s Dis. 2011, 23, 195–206. [Google Scholar] [CrossRef]
- Williams, G.S.B.; Boyman, L.; Chikando, A.C.; Khairallah, R.J.; Lederer, W.J. Mitochondrial calcium uptake. Proc. Natl. Acad. Sci. USA 2013, 110, 10479–10486. [Google Scholar] [CrossRef]
- Kirichok, Y.; Krapivinsky, G.; Clapham, D.E. The mitochondrial calcium uniporter is a highly selective ion channel. Nat. Cell Biol. 2004, 427, 360–364. [Google Scholar] [CrossRef]
- Gunter, T.; Buntinas, L.; Sparagna, G.; Eliseev, R.; Gunter, K. Mitochondrial calcium transport: Mechanisms and functions. Cell Calcium 2000, 28, 285–296. [Google Scholar] [CrossRef]
- Palty, R.; Ohana, E.; Hershfinkel, M.; Volokita, M.; Elgazar, V.; Beharier, O.; Silverman, W.F.; Argaman, M.; Sekler, I. Lithium-Calcium Exchange Is Mediated by a Distinct Potassium-independent Sodium-Calcium Exchanger. J. Biol. Chem. 2004, 279, 25234–25240. [Google Scholar] [CrossRef]
- Lytton, J. Na+/Ca2+ exchangers: Three mammalian gene families control Ca2+ transport. Biochem. J. 2007, 406, 365–382. [Google Scholar] [CrossRef]
- Baumgartner, H.K.; Gerasimenko, J.V.; Thorne, C.; Ferdek, P.; Pozzan, T.; Tepikin, A.V.; Petersen, O.H.; Sutton, R.; Watson, A.J.; Gerasimenko, O.V. Calcium elevation in mitochondria is the main Ca2+ requirement for mitochondrial permeability transition pore (mPTP) opening. J. Biol. Chem. 2009, 284, 20796–20803. [Google Scholar] [CrossRef]
- Du, H.; Yan, S.S. Mitochondrial permeability transition pore in Alzheimer’s disease: Cyclophilin D and amyloid beta. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2010, 1802, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Ohsumi, Y.; Anraku, Y. Calcium transport driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J. Biol. Chem. 1983, 258, 5614–5617. [Google Scholar] [CrossRef]
- Patel, S.; Docampo, R. Acidic calcium stores open for business: Expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol. 2010, 20, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Garrity, A.G.; Wang, W.; Collier, C.M.; Levey, S.A.; Gao, Q.; Xu, H. The endoplasmic reticulum, not the pH gradient, drives calcium refilling of lysosomes. eLife 2016, 5, e15887. [Google Scholar] [CrossRef]
- Tian, X.; Gala, U.; Zhang, Y.; Shang, W.; Jaiswal, S.N.; Di Ronza, A.; Jaiswal, M.; Yamamoto, S.; Sandoval, H.; DuRaine, L.; et al. A voltage-gated calcium channel regulates lysosomal fusion with endosomes and autophagosomes and is required for neuronal homeostasis. PLoS Biol. 2015, 13, e1002103. [Google Scholar] [CrossRef]
- McBrayer, M.; Nixon, R.A. Lysosome and calcium dysregulation in Alzheimer’s disease: Partners in crime. Biochem. Soc. Trans. 2013, 41, 1495–1502. [Google Scholar] [CrossRef]
- Coen, K.; Flannagan, R.S.; Baron, S.; Carraro-Lacroix, L.R.; Wang, D.; Vermeire, W.; Michiels, C.; Munck, S.; Baert, V.; Sugita, S.; et al. Lysosomal calcium homeostasis defects, not proton pump defects, cause endo-lysosomal dysfunction in PSEN-deficient cells. J. Cell Biol. 2012, 198, 23–35. [Google Scholar] [CrossRef]
- Fox, A.P.; Nowycky, M.C.; Tsien, R.W. Single-channel recordings of three types of calcium channels in chick sensory neurones. J. Physiol. 1987, 394, 173–200. [Google Scholar] [CrossRef]
- Sun, L.; Wei, H. Ryanodine Receptors: A Potential Treatment Target in Various Neurodegenerative Disease. Cell. Mol. Neurobiol. 2020, 1–12. [Google Scholar] [CrossRef]
- Chan, S.L.; Mayne, M.; Holden, C.P.; Geiger, J.D.; Mattson, M.P. Presenilin-1 Mutations Increase Levels of Ryanodine Receptors and Calcium Release in PC12 Cells and Cortical Neurons. J. Biol. Chem. 2000, 275, 18195–18200. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Y.; Liang, G.; Xu, Z.; Chu, C.T.; Wei, H. Alzheimer’s Disease Presenilin-1 Mutation Sensitizes Neurons to Impaired Autophagy Flux and Propofol Neurotoxicity: Role of Calcium Dysregulation. J. Alzheimer’s Dis. 2019, 67, 137–147. [Google Scholar] [CrossRef]
- Greotti, E.; Capitanio, P.; Wong, A.; Pozzan, T.; Pizzo, P.; Pendin, D. Familial Alzheimer’s disease-linked presenilin mutants and intracellular Ca2+ handling: A single-organelle, FRET-based analysis. Cell Calcium 2019, 79, 44–56. [Google Scholar] [CrossRef]
- Churchill, G.C.; Okada, Y.; Thomas, J.M.; Genazzani, A.A.; Patel, S.; Galione, A. NAADP Mobilizes Ca2+ from Reserve Granules, Lysosome-Related Organelles, in Sea Urchin Eggs. Cell 2002, 111, 703–708. [Google Scholar] [CrossRef]
- Querfurth, H.W.; Selkoe, D.J. Calcium Ionophore Increases Amyloid.beta. Peptide Production by Cultured Cells. Biochemistry 1994, 33, 4550–4561. [Google Scholar] [CrossRef]
- Querfurth, H.W.; Jiang, J.; Geiger, J.D.; Selkoe, D.J. Caffeine Stimulates Amyloid β-Peptide Release from β-Amyloid Precursor Protein-Transfected HEK293 Cells. J. Neurochem. 1997, 69, 1580–1591. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Kim, J.; Ham, S.; Park, J.H.Y.; Han, S.; Jung, Y.-K.; Shim, I.; Han, J.-S.; Lee, K.W.; et al. Ca2+-permeable TRPV1 pain receptor knockout rescues memory deficits and reduces amyloid-β and tau in a mouse model of Alzheimer’s disease. Hum. Mol. Genet. 2019, 29, 228–237. [Google Scholar] [CrossRef]
- Itkin, A.; Dupres, V.; Dufrêne, Y.F.; Bechinger, B.; Ruysschaert, J.-M.; Raussens, V. Calcium Ions Promote Formation of Amyloid β-Peptide (1–40) Oligomers Causally Implicated in Neuronal Toxicity of Alzheimer’s Disease. PLoS ONE 2011, 6, e18250. [Google Scholar] [CrossRef]
- Green, K.N.; LaFerla, F.M. Linking Calcium to Aβ and Alzheimer’s Disease. Neuron 2008, 59, 190–194. [Google Scholar] [CrossRef]
- Ahmad, A.; Muzaffar, M.; Ingram, V.M. Ca2+, within the physiological concentrations, selectively accelerates Aβ42 fibril formation and not Aβ40 in vitro. Biochim. Biophys. Acta BBA Proteins Proteom. 2009, 1794, 1537–1548. [Google Scholar] [CrossRef]
- Guo, Q.; Fu, W.; Sopher, B.L.; Miller, M.W.; Ware, C.B.; Martin, G.M.; Mattson, M.P. Increased vulnerability of hippocampal neurons to excitotoxic necrosis in presenilin-1 mutant knock-in mice. Nat. Med. 1999, 5, 101–106. [Google Scholar] [CrossRef]
- Leissring, M.A.; Akbari, Y.; Fanger, C.M.; Cahalan, M.D.; Mattson, M.P.; LaFerla, F.M. Capacitative Calcium Entry Deficits and Elevated Luminal Calcium Content in Mutant Presenilin-1 Knockin Mice. J. Cell Biol. 2000, 149, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Ueda, M.; Masuda, T.; Misumi, Y.; Yamashita, T.; Ando, Y. Memantine, a Noncompetitive N-Methyl-d-Aspartate Receptor Antagonist, Attenuates Cerebral Amyloid Angiopathy by Increasing Insulin-Degrading Enzyme Expression. Mol. Neurobiol. 2019, 56, 8573–8588. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.-Z.; Li, B.; Li, Y.-C.; Yang, X.-L.; Zhang, W.; Zhong, L.; Tang, S.-J. Activation of NMDA Receptors Upregulates A Disintegrin and Metalloproteinase 10 via a Wnt/MAPK Signaling Pathway. J. Neurosci. 2012, 32, 3910–3916. [Google Scholar] [CrossRef] [PubMed]
- Hoey, S.E.; Buonocore, F.; Cox, C.J.; Hammond, V.J.; Perkinton, M.S.; Williams, R.J. AMPA Receptor Activation Promotes Non-Amyloidogenic Amyloid Precursor Protein Processing and Suppresses Neuronal Amyloid-β Production. PLoS ONE 2013, 8, e78155. [Google Scholar] [CrossRef] [PubMed]
- Dreses-Werringloer, U.; Lambert, J.-C.; Vingtdeux, V.; Zhao, H.; Vais, H.; Siebert, A.; Jain, A.; Koppel, J.; Rovelet-Lecrux, A.; Hannequin, D.; et al. A Polymorphism in CALHM1 Influences Ca2+ Homeostasis, Aβ Levels, and Alzheimer’s Disease Risk. Cell 2008, 133, 1149–1161. [Google Scholar] [CrossRef]
- Castellano, J.M.; Kim, J.; Stewart, F.R.; Jiang, H.; DeMattos, R.B.; Patterson, B.W.; Fagan, A.M.; Morris, J.C.; Mawuenyega, K.G.; Cruchaga, C.; et al. Human apoE Isoforms Differentially Regulate Brain Amyloid- Peptide Clearance. Sci. Transl. Med. 2011, 3, 89ra57. [Google Scholar] [CrossRef]
- Kelliher, M.; Fastbom, J.; Cowburn, R.; Bonkale, W.; Ohm, T.; Ravid, R.; Sorrentino, V.; O’Neill, C. Alterations in the ryanodine receptor calcium release channel correlate with Alzheimer’s disease neurofibrillary and β-amyloid pathologies. Neuroscience 1999, 92, 499–513. [Google Scholar] [CrossRef]
- Chakroborty, S.; Briggs, C.; Miller, M.B.; Goussakov, I.; Schneider, C.; Kim, J.; Wicks, J.; Richardson, J.C.; Conklin, V.; Cameransi, B.G.; et al. Stabilizing ER Ca2+ Channel Function as an Early Preventative Strategy for Alzheimer’s Disease. PLoS ONE 2012, 7, e52056. [Google Scholar] [CrossRef]
- Oulès, B.; Del Prete, D.; Greco, B.; Zhang, X.; Lauritzen, I.; Sevalle, J.; Moreno, S.; Paterlini-Bréchot, P.; Trebak, M.; Checler, F.; et al. Ryanodine Receptor Blockade Reduces Amyloid- Load and Memory Impairments in Tg2576 Mouse Model of Alzheimer Disease. J. Neurosci. 2012, 32, 11820–11834. [Google Scholar] [CrossRef]
- Lacampagne, A.; Liu, X.; Reiken, S.; Bussiere, R.; Meli, A.; Lauritzen, I.; Teich, A.F.; Zalk, R.; Saint, N.; Arancio, O.; et al. Post-translational remodeling of ryanodine receptor induces calcium leak leading to Alzheimer’s disease-like pathologies and cognitive deficits. Acta Neuropathol. 2017, 134, 749–767. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Supnet, C.; Sun, S.; Zhang, H.; Good, L.; Popugaeva, E.; Bezprozvanny, I. The role of ryanodine receptor type 3 in a mouse model of Alzheimer disease. Channels 2014, 8, 230–242. [Google Scholar] [CrossRef]
- Buxbaum, J.D.; Ruefli, A.A.; Parker, C.A.; Cypess, A.M.; Greengard, P. Calcium regulates processing of the Alzheimer amyloid protein precursor in a protein kinase C-independent manner. Proc. Natl. Acad. Sci. USA 1994, 91, 4489–4493. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, L.; Pchitskaya, E.; Zakharova, O.D.; Saito, T.; Saido, T.C.; Bezprozvanny, I. Neuronal Store-Operated Calcium Entry and Mushroom Spine Loss in Amyloid Precursor Protein Knock-In Mouse Model of Alzheimer’s Disease. J. Neurosci. 2015, 35, 13275–13286. [Google Scholar] [CrossRef]
- Kyung, T.; Lee, S.; Kim, J.E.; Cho, T.; Park, H.; Jeong, Y.-M.; Kim, D.; Shin, A.; Kim, S.; Baek, J.; et al. Optogenetic control of endogenous Ca2+ channels in vivo. Nat. Biotechnol. 2015, 33, 1092–1096. [Google Scholar] [CrossRef]
- Yoo, A.S.; Cheng, I.; Chung, S.; Grenfell, T.Z.; Lee, H.; Pack-Chung, E.; Handler, M.; Shen, J.; Xia, W.; Tesco, G.; et al. Presenilin-Mediated Modulation of Capacitative Calcium Entry. Neuron 2000, 27, 561–572. [Google Scholar] [CrossRef]
- Calvo-Rodriguez, M.; Hernando-Perez, E.; Nuñez, L.; Villalobos, C. Amyloid β Oligomers Increase ER-Mitochondria Ca2+ Cross Talk in Young Hippocampal Neurons and Exacerbate Aging-Induced Intracellular Ca2+ Remodeling. Front. Cell. Neurosci. 2019, 13. [Google Scholar] [CrossRef]
- Mattson, M.P.; Cheng, B.; Culwell, A.R.; Esch, F.S.; Lieberburg, I.; Rydel, R.E. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the β-amyloid precursor protein. Neuron 1993, 10, 243–254. [Google Scholar] [CrossRef]
- Scremin, E.; Agostini, M.; Leparulo, A.; Pozzan, T.; Greotti, E.; Fasolato, C. ORAI2 Down-Regulation Potentiates SOCE and Decreases Aβ42 Accumulation in Human Neuroglioma Cells. Int. J. Mol. Sci. 2020, 21, 5288. [Google Scholar] [CrossRef]
- Manczak, M.; Sheiko, T.; Craigen, W.J.; Reddy, P.H. Reduced VDAC1 Protects Against Alzheimer’s Disease, Mitochondria, and Synaptic Deficiencies. J. Alzheimer’s Dis. 2013, 37, 679–690. [Google Scholar] [CrossRef]
- Šoškić, V.; Klemm, M.; Proikas-Cezanne, T.; Schwall, G.P.; Poznanović, S.; Stegmann, W.; Groebe, K.; Zengerling, H.; Schoepf, R.; Burnet, M.; et al. A Connection between the Mitochondrial Permeability Transition Pore, Autophagy, and Cerebral Amyloidogenesis. J. Proteome Res. 2008, 7, 2262–2269. [Google Scholar] [CrossRef]
- Hartigan, J.A.; Johnson, G.V. Transient Increases in Intracellular Calcium Result in Prolonged Site-selective Increases in Tau Phosphorylation through a Glycogen Synthase Kinase 3β-dependent Pathway. J. Biol. Chem. 1999, 274, 21395–21401. [Google Scholar] [CrossRef]
- Yamamoto, H.; Hiragami, Y.; Murayama, M.; Ishizuka, K.; Kawahara, M.; Takashima, A. Phosphorylation of tau at serine 416 by Ca2+/calmodulin-dependent protein kinase II in neuronal soma in brain. J. Neurochem. 2005, 94, 1438–1447. [Google Scholar] [CrossRef]
- Avila, J.; Pérez, M.; Lim, F.; Gómez-Ramos, A.; Hernández, F.; Lucas, J.J. Tau in neurodegenerative diseases: Tau phosphorylation and assembly. Neurotox. Res. 2004, 6, 477–482. [Google Scholar] [CrossRef]
- LaFerla, F.M.; Green, K.N.; Oddo, S. Intracellular amyloid-β in Alzheimer’s disease. Nat. Rev. Neurosci. 2007, 8, 499–509. [Google Scholar] [CrossRef]
- Whitcomb, D.J.; Hogg, E.L.; Regan, P.; Piers, T.; Narayan, P.; Whitehead, G.; Winters, B.L.; Kim, D.-H.; Kim, E.; George-Hyslop, P.S.; et al. Intracellular oligomeric amyloid-beta rapidly regulates GluA1 subunit of AMPA receptor in the hippocampus. Sci. Rep. 2015, 5, 10934. [Google Scholar] [CrossRef]
- Pierrot, N.; Ghisdal, P.; Caumont, A.-S.; Octave, J.-N. Intraneuronal amyloid-β1-42 production triggered by sustained increase of cytosolic calcium concentration induces neuronal death. J. Neurochem. 2004, 88, 1140–1150. [Google Scholar] [CrossRef]
- Bruno, A.M.; Huang, J.Y.; Bennett, D.A.; Marr, R.A.; Hastings, M.L.; Stutzmann, G.E. Altered ryanodine receptor expression in mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2012, 33, 1001.e1–1001.e6. [Google Scholar] [CrossRef]
- Antonell, A.; Lladó, A.; Altirriba, J.; Botta-Orfila, T.; Balasa, M.; Fernández, M.; Ferrer, I.; Sánchez-Valle, R.; Molinuevo, J.L. A preliminary study of the whole-genome expression profile of sporadic and monogenic early-onset Alzheimer’s disease. Neurobiol. Aging 2013, 34, 1772–1778. [Google Scholar] [CrossRef]
- Chami, M.; Checler, F. Ryanodine receptors. Channels 2014, 8, 168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manczak, M.; Reddy, P.H. Abnormal interaction of VDAC1 with amyloid beta and phosphorylated tau causes mitochondrial dysfunction in Alzheimer’s disease. Hum. Mol. Genet. 2012, 21, 5131–5146. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Zhang, Y.; Li, B.; Wu, Y.; Li, B. Effect of β-amyloid (25-35) on mitochondrial function and expression of mitochondrial permeability transition pore proteins in rat hippocampal neurons. J. Cell. Biochem. 2011, 112, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.I.; Santos, M.S.; Moreno, A.; Oliveira, C. Amyloid β-Peptide Promotes Permeability Transition Pore in Brain Mitochondria. Biosci. Rep. 2001, 21, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Toescu, E.C.; Verkhratsky, A. The importance of being subtle: Small changes in calcium homeostasis control cognitive decline in normal aging. Aging Cell 2007, 6, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Reijo, T.; Mikko, B.; Antti, S.; Timo, S.; Mikko, B. Serum Calcium And Prediction Of Cognitive Decline In Old Age. J. Am. Geriatr. Soc. 2008, 56, 1573–1574. [Google Scholar] [CrossRef]
- Heck, A.; Fastenrath, M.; Coynel, D.; Auschra, B.; Bickel, H.; Freytag, V.; Gschwind, L.; Hartmann, F.; Jessen, F.; Kaduszkiewicz, H.; et al. Genetic Analysis of Association Between Calcium Signaling and Hippocampal Activation, Memory Performance in the Young and Old, and Risk for Sporadic Alzheimer Disease. JAMA Psychiatry 2015, 72, 1029–1036. [Google Scholar] [CrossRef]
- Walsh, D.M.; Townsend, M.; Podlisny, M.B.; Shankar, G.M.; Fadeeva, J.V.; El Agnaf, O.; Hartley, D.M.; Selkoe, D.J. Certain Inhibitors of Synthetic Amyloid -Peptide (A ) Fibrillogenesis Block Oligomerization of Natural A and Thereby Rescue Long-Term Potentiation. J. Neurosci. 2005, 25, 2455–2462. [Google Scholar] [CrossRef]
- Bliss, T.V.P.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nat. Cell Biol. 1993, 361, 31–39. [Google Scholar] [CrossRef]
- Hu, W.-Y.; He, Z.-Y.; Yang, L.-J.; Zhang, M.; Xing, D.; Xiao, Z.-C. The Ca2+channel inhibitor 2-APB reverses β-amyloid-induced LTP deficit in hippocampus by blocking BAX and caspase-3 hyperactivation. Br. J. Pharmacol. 2015, 172, 2273–2285. [Google Scholar] [CrossRef]
- Trinchese, F.; Fa’, M.; Liu, S.; Zhang, H.; Hidalgo, A.; Schmidt, S.D.; Yamaguchi, H.; Yoshii, N.; Mathews, P.M.; Nixon, R.A.; et al. Inhibition of calpains improves memory and synaptic transmission in a mouse model of Alzheimer disease. J. Clin. Investig. 2008, 118, 2796–2807. [Google Scholar] [CrossRef]
- Dineley, K.T.; Hogan, D.; Zhang, W.-R.; Taglialatela, G. Acute inhibition of calcineurin restores associative learning and memory in Tg2576 APP transgenic mice. Neurobiol. Learn. Mem. 2007, 88, 217–224. [Google Scholar] [CrossRef]
- Berridge, M.J. Calcium hypothesis of Alzheimer’s disease. Pflügers Arch. Eur. J. Physiol. 2010, 459, 441–449. [Google Scholar] [CrossRef]
- Dudek, S.M.; Bear, M.F. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc. Natl. Acad. Sci. USA 1992, 89, 4363–4367. [Google Scholar] [CrossRef]
- Morris, R.G.M.; Anderson, E.; Lynch, G.S.; Baudry, M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nat. Cell Biol. 1986, 319, 774–776. [Google Scholar] [CrossRef]
- Hsiung, G.-Y.R.; Feldman, H.H. Pharmacological treatment in moderate-to-severe Alzheimer’s disease. Expert Opin. Pharmacother. 2008, 9, 2575–2582. [Google Scholar] [CrossRef]
- Minkeviciene, R.; Banerjee, P.; Tanila, H. Memantine Improves Spatial Learning in a Transgenic Mouse Model of Alzheimer’s Disease. J. Pharmacol. Exp. Ther. 2004, 311, 677–682. [Google Scholar] [CrossRef]
- Palop, J.J. Epilepsy and Cognitive Impairments in Alzheimer Disease. Arch. Neurol. 2009, 66, 435–440. [Google Scholar] [CrossRef]
- Wang, Y.; Mattson, M.P. L-type Ca2+ currents at CA1 synapses, but not CA3 or dentate granule neuron synapses, are increased in 3xTgAD mice in an age-dependent manner. Neurobiol. Aging 2014, 35, 88–95. [Google Scholar] [CrossRef]
- Tsukuda, K.; Mogi, M.; Li, J.-M.; Iwanami, J.; Min, L.-J.; Sakata, A.; Fujita, T.; Iwai, M.; Horiuchi, M. Diabetes-Associated Cognitive Impairment Is Improved by a Calcium Channel Blocker, Nifedipine. Hypertension 2008, 51, 528–533. [Google Scholar] [CrossRef]
- Doody, R.S.; I Gavrilova, S.; Sano, M.; Thomas, R.G.; Aisen, P.S.; O Bachurin, S.; Seely, L.; Hung, D. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer’s disease: A randomised, double-blind, placebo-controlled study. Lancet 2008, 372, 207–215. [Google Scholar] [CrossRef]
- Moriguchi, S.; Shioda, N.; Yamamoto, Y.; Tagashira, H.; Fukunaga, K. The T-type voltage-gated calcium channel as a molecular target of the novel cognitive enhancer ST101: Enhancement of long-term potentiation and CaMKII autophosphorylation in rat cortical slices. J. Neurochem. 2012, 121, 44–53. [Google Scholar] [CrossRef]
- Deshpande, L.S.; Sun, D.A.; Sombati, S.; Baranova, A.; Wilson, M.S.; Attkisson, E.; Hamm, R.J.; DeLorenzo, R.J. Alterations in neuronal calcium levels are associated with cognitive deficits after traumatic brain injury. Neurosci. Lett. 2008, 441, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Mallmann, R.T.; Elgueta, C.; Sleman, F.; Castonguay, J.; Wilmes, T.; Maagdenberg, A.V.D.; Klugbauer, N. Ablation of CaV2.1 Voltage-Gated Ca2+ Channels in Mouse Forebrain Generates Multiple Cognitive Impairments. PLoS ONE 2013, 8, e78598. [Google Scholar] [CrossRef]
- Micale, V.; Cristino, L.; Tamburella, A.; Petrosino, S.; Leggio, G.M.; Di Marzo, V.; Drago, F. Enhanced cognitive performance of dopamine D3 receptor “knock-out” mice in the step-through passive-avoidance test: Assessing the role of the endocannabinoid/endovanilloid systems. Pharmacol. Res. 2010, 61, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, P.; Oleksik, A.M.; Mooijaart, S.P.; De Craen, A.; Westendorp, R.G. APOE genotype modulates the effect of serum calcium levels on cognitive function in old age. Neurology 2009, 72, 821–828. [Google Scholar] [CrossRef]
- Cui, P.-J.; Zheng, L.; Cao, L.; Wang, Y.; Deng, Y.-L.; Wang, G.; Xu, W.; Tang, H.-D.; Ma, J.-F.; Zhang, T.; et al. CALHM1 P86L Polymorphism is a Risk Factor for Alzheimer’s Disease in the Chinese Population. J. Alzheimer’s Dis. 2010, 19, 31–35. [Google Scholar] [CrossRef]
- Shilling, D.; Müller, M.; Takano, H.; Mak, D.-O.D.; Abel, T.; Coulter, D.A.; Foskett, J.K. Suppression of InsP3 Receptor-Mediated Ca2+ Signaling Alleviates Mutant Presenilin-Linked Familial Alzheimer’s Disease Pathogenesis. J. Neurosci. 2014, 34, 6910–6923. [Google Scholar] [CrossRef]
- Jaworska, A.; Dzbek, J.; Styczynska, M.; Kuznicki, J. Analysis of calcium homeostasis in fresh lymphocytes from patients with sporadic Alzheimer’s disease or mild cognitive impairment. Biochim. Biophys. Acta BBA Bioenerg. 2013, 1833, 1692–1699. [Google Scholar] [CrossRef][Green Version]
- Lü, N.; Lü, B.-C.; Cheng, L.-Z.; Zhang, Y.-Q.; Li, Y.-Q.; Zhao, Z.-Q. Involvement of ryanodine receptors in tetanic sciatic stimulation-induced long-term potentiation of spinal dorsal horn and persistent pain in rats. J. Neurosci. Res. 2012, 90, 1096–1104. [Google Scholar] [CrossRef]
- Matsuo, N.; Tanda, K.; Nakanishi, K.; Yamasaki, N.; Toyama, K.; Takao, K.; Takeshima, H.; Miyakawa, T. Comprehensive behavioral phenotyping of ryanodine receptor type3 (RyR3) knockout mice: Decreased social contact duration in two social interaction tests. Front. Behav. Neurosci. 2009, 3, 3. [Google Scholar] [CrossRef]
- Alkon, D.L.; Nelson, T.J.; Zhao, W.; Cavallaro, S. Time domains of neuronal Ca2+ signaling and associative memory: Steps through a calexcitin, ryanodine receptor, K+ channel cascade. Trends Neurosci. 1998, 21, 529–537. [Google Scholar] [CrossRef]
- Tong, B.C.-K.; Lee, C.S.-K.; Cheng, W.-H.; Lai, K.-O.; Foskett, J.K.; Cheung, K.-H. Familial Alzheimer’s disease–associated presenilin 1 mutants promote γ-secretase cleavage of STIM1 to impair store-operated Ca2+entry. Sci. Signal. 2016, 9, ra89. [Google Scholar] [CrossRef]
- Emptage, N.J.; Reid, C.; Fine, A. Calcium Stores in Hippocampal Synaptic Boutons Mediate Short-Term Plasticity, Store-Operated Ca2+ Entry, and Spontaneous Transmitter Release. Neuron 2001, 29, 197–208. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Nahon-Crystal, E.; Shteinfer-Kuzmine, A.; Gupta, R. VDAC1, mitochondrial dysfunction, and Alzheimer’s disease. Pharmacol. Res. 2018, 131, 87–101. [Google Scholar] [CrossRef]
- Kon, N.; Murakoshi, M.; Isobe, A.; Kagechika, K.; Miyoshi, N.; Nagayama, T. DS16570511 is a small-molecule inhibitor of the mitochondrial calcium uniporter. Cell Death Discov. 2017, 3, 17045. [Google Scholar] [CrossRef]
- Kon, N.; Satoh, A.; Miyoshi, N. A small-molecule DS44170716 inhibits Ca2+-induced mitochondrial permeability transition. Sci. Rep. 2017, 7, 3864. [Google Scholar] [CrossRef]
- Sakurai, Y.; Kolokoltsov, A.A.; Chen, C.-C.; Tidwell, M.W.; Bauta, W.E.; Klugbauer, N.; Grimm, C.; Wahl-Schott, C.; Biel, M.; Davey, R.A. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science 2015, 347, 995–998. [Google Scholar] [CrossRef]
- Pickford, F.; Masliah, E.; Britschgi, M.; Lucin, K.; Narasimhan, R.; Jaeger, P.A.; Small, S.; Spencer, B.; Rockenstein, E.; Levine, B.; et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid β accumulation in mice. J. Clin. Investig. 2008, 118, 2190–2199. [Google Scholar] [CrossRef]
- Birks, J.; López-Arrieta, J.; López-Arrieta, J.M. Nimodipine for primary degenerative, mixed and vascular dementia. Cochrane Database Syst. Rev. 2002, 2002, CD000147. [Google Scholar] [CrossRef]
- Hopp, S.C.; D’Angelo, H.M.; E Royer, S.; Kaercher, R.M.; Crockett, A.M.; Adzovic, L.; Wenk, G.L. Calcium dysregulation via L-type voltage-dependent calcium channels and ryanodine receptors underlies memory deficits and synaptic dysfunction during chronic neuroinflammation. J. Neuroinflamm. 2015, 12, 56. [Google Scholar] [CrossRef]
- Luengo, E.; Buendia, I.; Fernández-Mendívil, C.; Trigo-Alonso, P.; Negredo, P.; Michalska, P.; Hernández-García, B.; Sánchez-Ramos, C.; Bernal, J.A.; Ikezu, T.; et al. Pharmacological doses of melatonin impede cognitive decline in tau-related Alzheimer models, once tauopathy is initiated, by restoring the autophagic flux. J. Pineal Res. 2019, 67, e12578. [Google Scholar] [CrossRef]
- Mattson, M.P.; Partin, J.; Begley, J. Amyloid β-peptide induces apoptosis-related events in synapses and dendrites. Brain Res. 1998, 807, 167–176. [Google Scholar] [CrossRef]
- Jin, Y. Synaptogenesis: Insights from worm and fly. Curr. Opin. Neurobiol. 2002, 12, 71–79. [Google Scholar] [CrossRef]
- Mattson, M.P.; Chan, S.L. Dysregulation of Cellular Calcium Homeostasis in Alzheimer’s Disease: Bad Genes and Bad Habits. J. Mol. Neurosci. 2001, 17, 205–224. [Google Scholar] [CrossRef]
- Chan, S.L.; Furukawa, K.; Mattson, M.P. Presenilins and APP in Neuritic and Synaptic Plasticity: Implications for the Pathogenesis of Alzheimer’s Disease. NeuroMol. Med. 2002, 2, 167–196. [Google Scholar] [CrossRef]
- Nalbantoglu, J.; Tirado-Santiago, G.; Lahsaïni, A.; Poirier, J.; Goncalves, O.; Verge, G.; Momoli, F.; Welner, S.A.; Massicotte, G.; Julien, J.-P.; et al. Impaired learning and LTP in mice expressing the carboxy terminus of the Alzheimer amyloid precursor protein. Nat. Cell Biol. 1997, 387, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Nelson, O.; Supnet, C.; Liu, H.; Bezprozvanny, I. Familial Alzheimer’s Disease Mutations in Presenilins: Effects on Endoplasmic Reticulum Calcium Homeostasis and Correlation with Clinical Phenotypes. J. Alzheimer’s Dis. 2010, 21, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Tonkikh, A.; Janus, C.; El-Beheiry, H.; Pennefather, P.S.; Samoilova, M.; McDonald, P.; Ouanounou, A.; Carlen, P.L. Calcium chelation improves spatial learning and synaptic plasticity in aged rats. Exp. Neurol. 2006, 197, 291–300. [Google Scholar] [CrossRef]
- Winder, D.G.; Mansuy, I.M.; Osman, M.; Moallem, T.M.; Kandel, E.R. Genetic and Pharmacological Evidence for a Novel, Intermediate Phase of Long-Term Potentiation Suppressed by Calcineurin. Cell 1998, 92, 25–37. [Google Scholar] [CrossRef]
- Foster, T.C.; Sharrow, K.M.; Masse, J.R.; Norris, C.M.; Kumar, A. Calcineurin links Ca2+ dysregulation with brain aging. J. Neurosci. 2001, 21, 4066–4073. [Google Scholar] [CrossRef]
- Yamin, G. NMDA receptor-dependent signaling pathways that underlie amyloid β-protein disruption of LTP in the hippocampus. J. Neurosci. Res. 2009, 87, 1729–1736. [Google Scholar] [CrossRef]
- Raymond, C.R.; Redman, S.J. Spatial segregation of neuronal calcium signals encodes different forms of LTP in rat hippocampus. J. Physiol. 2006, 570, 97–111. [Google Scholar] [CrossRef]
- Sanderson, J.L.; Gorski, J.A.; Dell’Acqua, M.L. NMDA Receptor-Dependent LTD Requires Transient Synaptic Incorporation of Ca 2+ -Permeable AMPARs Mediated by AKAP150-Anchored PKA and Calcineurin. Neuron 2016, 89, 1000–1015. [Google Scholar] [CrossRef]
- Jia, Z.; Agopyan, N.; Miu, P.; Xiong, Z.; Henderson, J.; Gerlai, R.; A Taverna, F.; Velumian, A.; MacDonald, J.; Carlen, P.; et al. Enhanced LTP in Mice Deficient in the AMPA Receptor GluR2. Neuron 1996, 17, 945–956. [Google Scholar] [CrossRef]
- Plant, K.; A Pelkey, K.; A Bortolotto, Z.; Morita, D.; Terashima, A.; McBain, C.J.; Collingridge, G.L.; Isaac, J.T.R. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat. Neurosci. 2006, 9, 602–604. [Google Scholar] [CrossRef]
- Fortin, D.A.; Davare, M.A.; Srivastava, T.; Brady, J.D.; Nygaard, S.; Derkach, V.A.; Soderling, T.R. Long-Term Potentiation-Dependent Spine Enlargement Requires Synaptic Ca2+-Permeable AMPA Receptors Recruited by CaM-Kinase I. J. Neurosci. 2010, 30, 11565–11575. [Google Scholar] [CrossRef]
- Chowdhury, D.; Hell, J.W. Ca2+/Calmodulin Binding to PSD-95 Downregulates Its Palmitoylation and AMPARs in Long-Term Depression. Front. Synaptic Neurosci. 2019, 11. [Google Scholar] [CrossRef]
- Huber, K.M.; Mauk, M.D.; Kelly, P.T. LTP induced by activation of voltage-dependent Ca2+ channels requires protein kinase activity. NeuroReport 1995, 6, 1281–1284. [Google Scholar] [CrossRef][Green Version]
- White, J.A.; McKinney, B.C.; John, M.C.; Powers, P.A.; Kamp, T.J.; Murphy, G.G. Conditional forebrain deletion of the L-type calcium channel CaV1.2 disrupts remote spatial memories in mice. Learn. Mem. 2008, 15, 1–5. [Google Scholar] [CrossRef]
- Li, H.-B.; Mao, R.-R.; Zhang, J.-C.; Yang, Y.; Cao, J.; Xu, L. Antistress Effect of TRPV1 Channel on Synaptic Plasticity and Spatial Memory. Biol. Psychiatry 2008, 64, 286–292. [Google Scholar] [CrossRef]
- Gerdeman, G.L.; Ronesi, J.; Lovinger, D.M. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat. Neurosci. 2002, 5, 446–451. [Google Scholar] [CrossRef]
- Chávez, A.E.; Chiu, C.Q.; Castillo, P.E. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat. Neurosci. 2010, 13, 1511–1518. [Google Scholar] [CrossRef]
- James, E.O.; Clevenger, A.C.; Dietz, R.M.; Patsos, O.P. A Novel Peptide Inhibitor Of Trpm2 Channels Reduces Memory Deficits And Improves Ltp Following Traumatic Brain Injury In Mice. J. Neurotrauma 2018. Available online: https://www.google.com/search?tbm=bks&q=104.+232.+Orfila%2C+James%2C+E.%2C+Clevenger%2C+Amy%2C+C.%2C+Dietz%2C+Robert%2C+M.%2C+Patsos+%282018%29+A+NOVEL+PEPTIDE+INHIBITOR+OF+TRPM2+CHANNELS+REDUCES+MEMORY+DEFICITS+AND+IMPROVES+LTP+FOLLOWING+TRAUMATIC+BRAIN+INJURY+IN+MICE.+Journal+of+Neurotrauma (accessed on 13 March 2021).
- Menigoz, A.; Ahmed, T.; Sabanov, V.; Philippaert, K.; Pinto, S.; Kerselaers, S.; Segal, A.; Freichel, M.; Voets, T.; Nilius, B.; et al. TRPM4-dependent post-synaptic depolarization is essential for the induction of NMDA receptor-dependent LTP in CA1 hippocampal neurons. Pflügers Arch. Eur. J. Physiol. 2016, 468, 593–607. [Google Scholar] [CrossRef]
- Miyata, M.; Finch, E.A.; Khiroug, L.; Hashimoto, K.; Hayasaka, S.; Oda, S.-I.; Inouye, M.; Takagishi, Y.; Augustine, G.J.; Kano, M. Local Calcium Release in Dendritic Spines Required for Long-Term Synaptic Depression. Neuron 2000, 28, 233–244. [Google Scholar] [CrossRef]
- A Cummings, J.; Mulkey, R.M.; A Nicoll, R.; Malenka, R.C. Ca2+ Signaling Requirements for Long-Term Depression in the Hippocampus. Neuron 1996, 16, 825–833. [Google Scholar] [CrossRef]
- Kato, H.K.; Kassai, H.; Watabe, A.M.; Aiba, A.; Manabe, T. Functional coupling of the metabotropic glutamate receptor, InsP3receptor and L-type Ca2+channel in mouse CA1 pyramidal cells. J. Physiol. 2012, 590, 3019–3034. [Google Scholar] [CrossRef] [PubMed]
- Shimuta, M.; Yoshikawa, M.; Fukaya, M.; Watanabe, M.; Takeshima, H.; Manabe, T. Postsynaptic Modulation of AMPA Receptor-Mediated Synaptic Responses and LTP by the Type 3 Ryanodine Receptor. Mol. Cell. Neurosci. 2001, 17, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Chakroborty, S.; Kim, J.; Schneider, C.; Jacobson, C.; Molgó, J.; Stutzmann, G.E. Early Presynaptic and Postsynaptic Calcium Signaling Abnormalities Mask Underlying Synaptic Depression in Presymptomatic Alzheimer’s Disease Mice. J. Neurosci. 2012, 32, 8341–8353. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, B.; Beglopoulos, V.; Wines-Samuelson, M.; Zhang, D.; Dragatsis, I.; Südhof, T.C.; Shen, J. Presenilins are essential for regulating neurotransmitter release. Nat. Cell Biol. 2009, 460, 632–636. [Google Scholar] [CrossRef]
- Majewski, Ł.; Maciąg, F.; Boguszewski, P.M.; Wasilewska, I.; Wiera, G.; Wójtowicz, T.; Mozrzymas, J.; Kuznicki, J. Overexpression of STIM1 in neurons in mouse brain improves contextual learning and impairs long-term depression. Biochim. Biophys. Acta BBA Bioenerg. 2017, 1864, 1071–1087. [Google Scholar] [CrossRef] [PubMed]
- Weeber, E.J.; Levy, M.; Sampson, M.J.; Anflous, K.; Armstrong, D.L.; Brown, S.E.; Sweatt, J.D.; Craigen, W.J. The Role of Mitochondrial Porins and the Permeability Transition Pore in Learning and Synaptic Plasticity. J. Biol. Chem. 2002, 277, 18891–18897. [Google Scholar] [CrossRef]
- Mulkey, R.M.; Malenka, R.C. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron 1992, 9, 967–975. [Google Scholar] [CrossRef]
- Obenaus, A.; Mody, I.; Baimbridge, K.G. Dantrolene-Na (Dantrium) blocks induction of long-term potentiation in hippocampal slices. Neurosci. Lett. 1989, 98, 172–178. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).