FLT3 Tyrosine Kinase Inhibitors for the Treatment of Fit and Unfit Patients with FLT3-Mutated AML: A Systematic Review
Abstract
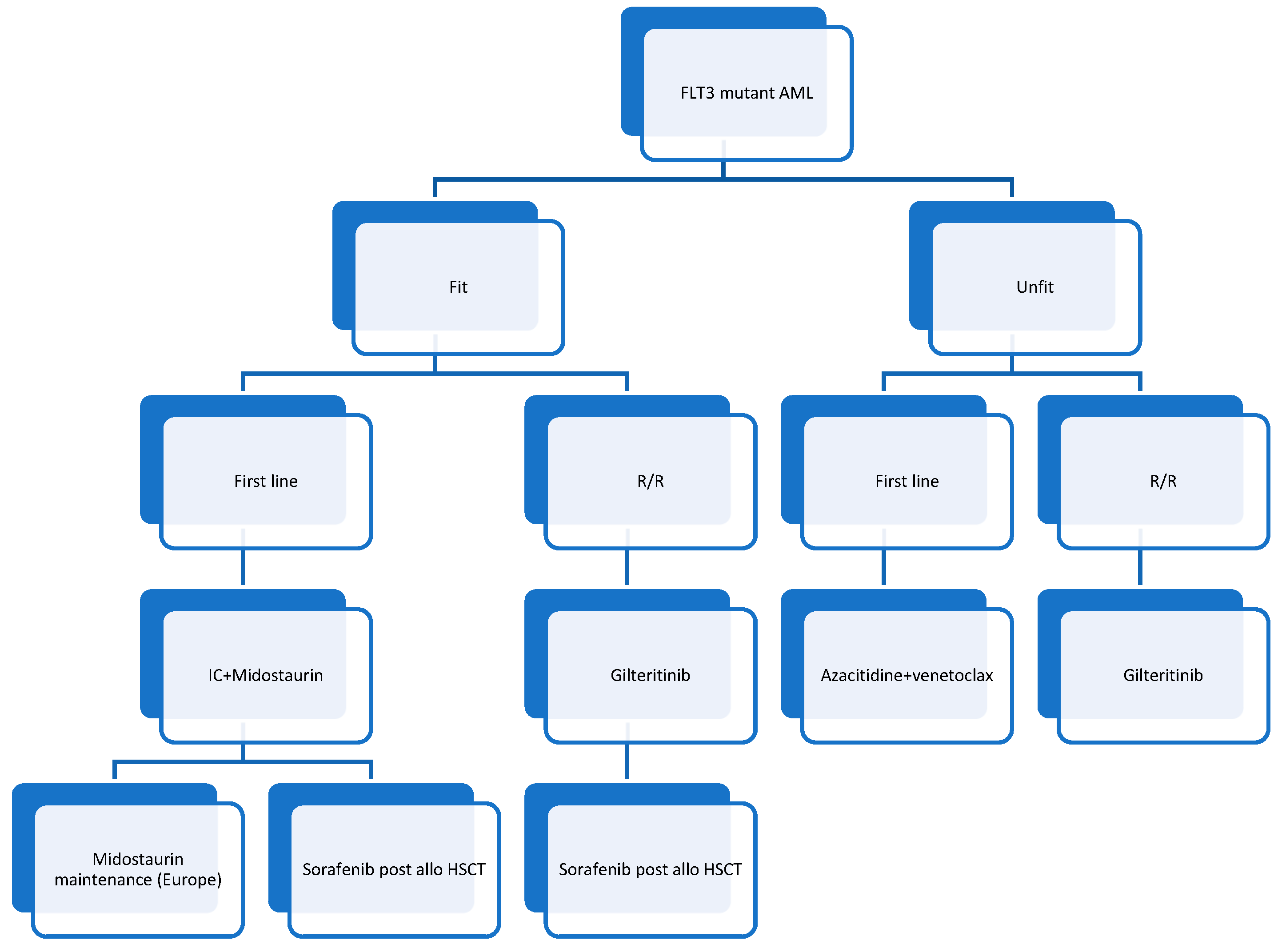
1. Introduction
2. Pharmacological Inhibition of FLT3 in AML
2.1. TKI in the Treatment of AML Patients
2.1.1. Midostaurin
2.1.2. Lestaurtinib
2.1.3. Quizartinib
2.1.4. Gilteritinib
2.1.5. Crenolanib
| Reference | Drug | Study Design | Number of Enrolled Patients | Treatment Phase | Type of FLT3 Mutant | Primary Endpoint | Survival Rates | Toxicities |
|---|---|---|---|---|---|---|---|---|
| Stone et al. [16] | Midostaurin | Phase 2 | 20 | R/R FLT3 mut AML | FLT3-ITD and TKD | Antileukemic activity | N/A | Nausea/vomiting |
| Fischer et al. [17] | Midostaurin | Phase 2 | 95 | R/R AML 35 FLT3 mut | FLT3-ITD and TKD | Safety/tolorability | N/A | |
| 60 FLT3 WT | ||||||||
| Stone et al. [19] | Midostaurin | Phase 3 | 717 | First line | FLT3-ITD and TKD | OS | OS midostauin > OS placebo (p = 0.009) | Anemia (more frequent in the Midostaurin group) |
| Cortes et al. [25] | Quizartinib | Phase 1 | 76 | R/R AML | Irrespective of FLT3-ITD status | Tolerability | N/A | Nausea, prolonged QT interval, vomiting, dysgeusia |
| Cortes et al. [26] | Quizartinib | Phase 3 | 367 | R/R AML | FLT3-ITD | OS | OS Quizartinib > OS salvage chemotherapy (p = 0.02) | Nausea, prolonged QT interval, vomiting, dysgueusi |
| Perl et al. [32] | Gilteritinib | Phase 1/2 | 252 | R/R AML | FLT3 mut and WT | Tolerability, safety, pharmacokinetics | N/A | Infections/Anemia/thrombopenia |
| Perl et al. [33] | Gilteritinib | Phase 3 | 371 | R/R AML | FLT3-ITD and TKD | OS and CR rates | OS Gilteritinib > OS salvage chemotherapy (p < 0.001). CI, 0.58 to 1.09) | Febrile neutropenia/anemia/thrombopenia |
| Aboudalle et al. [35] | Crenolanib | Phase I/II | 28 | R/R AML | FLT3-ITD and TKD | Tolerability/overall response rate (ORR) | N/A | No dose-limiting toxicity/no death related to Crenolanib/ORR: 46% |
2.2. FLT3 TKI and Demethylating Agents
2.3. TKI Maintenance Post Allogeneic Hematopoietic Stem Cell Transplantation
3. Perspectives
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Döhner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.; Wetzler, M.; Löwenberg, B. Therapeutic Advances in Acute Myeloid Leukemia. J Clin. Oncol. 2011, 29, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 Revision to the World Health Organization Classification of Myeloid Neoplasms and Acute Leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and Management of AML in Adults: 2017 ELN Recommendations from an International Expert Panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Meshinchi, S.; Appelbaum, F.R. Structural and Functional Alterations of FLT3 in Acute Myeloid Leukemia. Clin. Cancer Res. 2009, 15, 4263–4269. [Google Scholar] [CrossRef]
- Luskin, M.R.; Lee, J.-W.; Fernandez, H.F.; Lazarus, H.M.; Rowe, J.M.; Tallman, M.S.; Paietta, E.M.; Litzow, M.; Abdel-Wahab, O.; Patel, J.P.; et al. Results of the ECOG E1900 Trial in Younger Adults with AML Using an Event Free Survival Endpoint Are Concordant with Results Based on Overall Survival: Potential for a Surrogate Endpoint to Facilitate Rapid Approval of Therapies in AML. Blood 2014, 124, 2599. [Google Scholar] [CrossRef]
- Patel, J.P.; Gönen, M.; Figueroa, M.E.; Fernandez, H.; Sun, Z.; Racevskis, J.; Van Vlierberghe, P.; Dolgalev, I.; Thomas, S.; Aminova, O.; et al. Prognostic Relevance of Integrated Genetic Profiling in Acute Myeloid Leukemia. N. Engl. J. Med. 2012, 366, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Yanada, M.; Matsuo, K.; Suzuki, T.; Kiyoi, H.; Naoe, T. Prognostic Significance of FLT3 Internal Tandem Duplication and Tyrosine Kinase Domain Mutations for Acute Myeloid Leukemia: A Meta-Analysis. Leukemia 2005, 19, 1345–1349. [Google Scholar] [CrossRef]
- Brunet, S.; Labopin, M.; Esteve, J.; Cornelissen, J.; Socié, G.; Iori, A.P.; Verdonck, L.F.; Volin, L.; Gratwohl, A.; Sierra, J.; et al. Impact of FLT3 Internal Tandem Duplication on the Outcome of Related and Unrelated Hematopoietic Transplantation for Adult Acute Myeloid Leukemia in First Remission: A Retrospective Analysis. J. Clin. Oncol. 2012, 30, 735–741. [Google Scholar] [CrossRef]
- Schlenk, R.F.; Kayser, S.; Bullinger, L.; Kobbe, G.; Casper, J.; Ringhoffer, M.; Held, G.; Brossart, P.; Lübbert, M.; Salih, H.R.; et al. Differential Impact of Allelic Ratio and Insertion Site in FLT3-ITD–Positive AML with Respect to Allogeneic Transplantation. Blood 2014, 124, 3441–3449. [Google Scholar] [CrossRef]
- Leick, M.B.; Levis, M.J. The Future of Targeting FLT3 Activation in AML. Curr. Hematol. Malig. Rep. 2017, 12, 153–167. [Google Scholar] [CrossRef]
- Weisberg, E.; Roesel, J.; Furet, P.; Bold, G.; Imbach, P.; Flörsheimer, A.; Caravatti, G.; Jiang, J.; Manley, P.; Ray, A.; et al. Antileukemic Effects of Novel First- and Second-Generation FLT3 Inhibitors. Genes Cancer 2010, 1, 1021–1032. [Google Scholar] [CrossRef]
- Antar, A.I.; Otrock, Z.K.; Jabbour, E.; Mohty, M.; Bazarbachi, A. FLT3 Inhibitors in Acute Myeloid Leukemia: Ten Frequently Asked Questions. Leukemia 2020, 34, 682–696. [Google Scholar] [CrossRef]
- Stone, R.M.; DeAngelo, D.J.; Klimek, V.; Galinsky, I.; Estey, E.; Nimer, S.D.; Grandin, W.; Lebwohl, D.; Wang, Y.; Cohen, P.; et al. Patients with Acute Myeloid Leukemia and an Activating Mutation in FLT3 Respond to a Small-Molecule FLT3 Tyrosine Kinase Inhibitor, PKC. Blood 2005, 105, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.; Stone, R.M.; Deangelo, D.J.; Galinsky, I.; Estey, E.; Lanza, C.; Fox, E.; Ehninger, G.; Feldman, E.J.; Schiller, G.J.; et al. Phase IIB Trial of Oral Midostaurin (PKC412), the FMS-like Tyrosine Kinase 3 Receptor (FLT3) and Multi-Targeted Kinase Inhibitor, in Patients with Acute Myeloid Leukemia and High-Risk Myelodysplastic Syndrome with Either Wild-Type or Mutated FLT3. J. Clin. Oncol. 2010, 28, 4339–4345. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.M.; Fischer, T.; Paquette, R.; Schiller, G.; Schiffer, C.A.; Ehninger, G.; Cortes, J.; Kantarjian, H.M.; DeAngelo, D.J.; Huntsman-Labed, A.; et al. Phase IB Study of the FLT3 Kinase Inhibitor Midostaurin with Chemotherapy in Younger Newly Diagnosed Adult Patients with Acute Myeloid Leukemia. Leukemia 2012, 26, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef]
- Levis, M. Midostaurin Approved for FLT3-Mutated AML. Blood 2017, 129, 3403–3406. [Google Scholar] [CrossRef]
- Sierra, J. Phase 3b Study Assessing the Safety and Efficacy of Midostaurin in Younger and Older Patients with Newly Diagnosed, FLT3-Mutated Acute Myeloid Leukemia (AML) Who Are Eligible for 7+3 or 5+2 Chemotherapy. Proceedings of 62nd ASH Annual Meeting and Exposition, 5–8 December 2020; American Society of Hematology: Washington, DC, USA. [Google Scholar]
- Knapper, S.; Russell, N.; Gilkes, A.; Hills, R.K.; Gale, R.E.; Cavenagh, J.D.; Jones, G.; Kjeldsen, L.; Grunwald, M.R.; Thomas, I.; et al. A Randomized Assessment of Adding the Kinase Inhibitor Lestaurtinib to First-Line Chemotherapy for FLT3-Mutated AML. Blood 2017, 129, 1143–1154. [Google Scholar] [CrossRef]
- Daver, N.; Cortes, J.; Ravandi, F.; Patel, K.P.; Burger, J.A.; Konopleva, M.; Kantarjian, H. Secondary Mutations as Mediators of Resistance to Targeted Therapy in Leukemia. Blood 2015, 125, 3236–3245. [Google Scholar] [CrossRef] [PubMed]
- Zarrinkar, P.P.; Gunawardane, R.N.; Cramer, M.D.; Gardner, M.F.; Brigham, D.; Belli, B.; Karaman, M.W.; Pratz, K.W.; Pallares, G.; Chao, Q.; et al. AC220 Is a Uniquely Potent and Selective Inhibitor of FLT3 for the Treatment of Acute Myeloid Leukemia (AML). Blood 2009, 114, 2984–2992. [Google Scholar] [CrossRef]
- Cortes, J.E.; Kantarjian, H.; Foran, J.M.; Ghirdaladze, D.; Zodelava, M.; Borthakur, G.; Gammon, G.; Trone, D.; Armstrong, R.C.; James, J.; et al. Phase I Study of Quizartinib Administered Daily to Patients With Relapsed or Refractory Acute Myeloid Leukemia Irrespective of FMS-Like Tyrosine Kinase 3–Internal Tandem Duplication Status. J. Clin. Oncol. 2013, 31, 3681. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Khaled, S.; Martinelli, G.; Perl, A.E.; Ganguly, S.; Russell, N.; Krämer, A.; Dombret, H.; Hogge, D.; Jonas, B.A.; et al. Quizartinib versus Salvage Chemotherapy in Relapsed or Refractory FLT3-ITD Acute Myeloid Leukaemia (QuANTUM-R): A Multicentre, Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol. 2019, 20, 984–997. [Google Scholar] [CrossRef]
- Altman, J.K.; Foran, J.M.; Pratz, K.W.; Trone, D.; Cortes, J.E.; Tallman, M.S. Phase 1 Study of Quizartinib in Combination with Induction and Consolidation Chemotherapy in Patients with Newly Diagnosed Acute Myeloid Leukemia. Am. J. Hematol. 2018, 93, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Fodil, S.; Raffoux, E.; Dumas, P.Y.; Desbrosses, Y.; Larosa, F.; Chantepie, S.; Larcher, M.V.; Mear, J.B.; Peterlin, P.; Hunault-Berger, M.; et al. Data from French Named Patient Program of Quizartinib in Relapsed/Refractory Acute Myeloid Leukemia. Leuk. Lymphoma 2021, 0, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Daiichi Sankyo, Inc. A Phase 3, Double-Blind., Placebo-Controlled Study of Quizartinib Administered in Combination With Induction and Consolidation Chemotherapy, and Administered as Continuation Therapy in Subjects 18 to 75 Years Old With Newly Diagnosed FLT3-ITD (+) Acute Myeloid Leukemia (QuANTUM First); ClinicalTrials.gov: Bethesda, MD, USA, 2020. [Google Scholar]
- Lee, L.Y.; Hernandez, D.; Rajkhowa, T.; Smith, S.C.; Raman, J.R.; Nguyen, B.; Small, D.; Levis, M. Preclinical Studies of Gilteritinib, a next-Generation FLT3 Inhibitor. Blood 2017, 129, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Park, I.-K.; Mishra, A.; Chandler, J.; Whitman, S.P.; Marcucci, G.; Caligiuri, M.A. Inhibition of the Receptor Tyrosine Kinase Axl Impedes Activation of the FLT3 Internal Tandem Duplication in Human Acute Myeloid Leukemia: Implications for Axl as a Potential Therapeutic Target. Blood 2013, 121, 2064–2073. [Google Scholar] [CrossRef]
- Perl, A.E.; Altman, J.K.; Cortes, J.; Smith, C.; Litzow, M.; Baer, M.R.; Claxton, D.; Erba, H.P.; Gill, S.; Goldberg, S.; et al. Selective Inhibition of FLT3 by Gilteritinib in Relapsed or Refractory Acute Myeloid Leukaemia: A Multicentre, First-in-Human, Open-Label, Phase 1-2 Study. Lancet Oncol. 2017, 18, 1061–1075. [Google Scholar] [CrossRef]
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolini, S.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Ustun, C.; et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N. Engl. J. Med. 2019, 381, 1728–1740. [Google Scholar] [CrossRef]
- Galanis, A.; Ma, H.; Rajkhowa, T.; Ramachandran, A.; Small, D.; Cortes, J.; Levis, M. Crenolanib Is a Potent Inhibitor of FLT3 with Activity against Resistance-Conferring Point Mutants. Blood 2014, 123, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Arog Pharmaceuticals, Inc. Phase I-II Study of Crenolanib Combined With Standard Salvage Chemotherapy, and Crenolanib Combined With 5-Azacitidine in Acute Myeloid Leukemia Patients With FLT3 Activating Mutations; ClinicalTrials.gov: Bethesda, MD, USA, 2020. [Google Scholar]
- Collins, R.; Iyer, S.; Jethava, Y.; Karanes, C.; Messahel, B. Results of a Pilot Study Combining Crenolanib with Standard Salvage Chemotherapy in Relapsed/Refractory AML. Available online: https://library.ehaweb.org/eha/2020/eha25th/294557/robert.collins.results.of.a.pilot.study.combining.Crenolanib.with.standard.html (accessed on 10 April 2021).
- Arog Pharmaceuticals Inc. Phase III Randomized, Double-Blind., Placebo-Controlled Study Investigating the Efficacy of the Addition of Crenolanib to Salvage Chemotherapy Versus Salvage Chemotherapy Alone in Subjects ≤ 75 Years of Age With Relapsed/Refractory FLT3 Mutated Acute Myeloid Leukemia; ClinicalTrials.gov: Bethesda, MD, USA, 2020. [Google Scholar]
- Arog Pharmaceuticals Inc. Phase III Randomized Study of Crenolanib Versus Midostaurin Administered Following Induction Chemotherapy and Consolidation Therapy in Newly Diagnosed Subjects With FLT3 Mutated Acute Myeloid Leukemia; ClinicalTrials.gov: Bethesda, MD, USA, 2020. [Google Scholar]
- Ravandi, F.; Alattar, M.L.; Grunwald, M.R.; Rudek, M.A.; Rajkhowa, T.; Richie, M.A.; Pierce, S.; Daver, N.; Garcia-Manero, G.; Faderl, S.; et al. Phase 2 Study of Azacitidine plus Sorafenib in Patients with Acute Myeloid Leukemia and FLT-3 Internal Tandem Duplication Mutation. Blood 2013, 121, 4655–4662. [Google Scholar] [CrossRef] [PubMed]
- Ohanian, M.; Garcia-Manero, G.; Levis, M.; Jabbour, E.; Daver, N.; Borthakur, G.; Kadia, T.; Pierce, S.; Burger, J.; Richie, M.A.; et al. Sorafenib Combined with 5-Azacitidine in Older Patients with Untreated FLT3-ITD Mutated Acute Myeloid Leukemia. Am. J. Hematol. 2018, 93, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.B.; Kambhampati, S.; Fiskus, W.; Wick, J.; Dutreix, C.; Ganguly, S.; Aljitawi, O.; Reyes, R.; Fleming, A.; Abhyankar, S.; et al. Preclinical and Phase I Results of Decitabine in Combination with Midostaurin (PKC412) for Newly Diagnosed Elderly or Relapsed/Refractory Adult Patients with Acute Myeloid Leukemia. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2013, 33, 1341–1352. [Google Scholar] [CrossRef]
- Strati, P.; Kantarjian, H.; Ravandi, F.; Nazha, A.; Borthakur, G.; Daver, N.; Kadia, T.; Estrov, Z.; Garcia-Manero, G.; Konopleva, M.; et al. Phase I/II Trial of the Combination of Midostaurin (PKC412) and 5-Azacitidine for Patients with Acute Myeloid Leukemia and Myelodysplastic Syndrome. Am. J. Hematol. 2015, 90, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, M.; Kantarjian, H.M.; Levis, M.; Guerra, V.; Borthakur, G.; Alvarado, Y.; DiNardo, C.D.; Kadia, T.; Garcia-Manero, G.; Ohanian, M.; et al. A Phase I/II Study of the Combination of Quizartinib with Azacitidine or Low-Dose Cytarabine for the Treatment of Patients with Acute Myeloid Leukemia and Myelodysplastic Syndrome. Haematologica 2020. [Google Scholar] [CrossRef]
- Wang, E. Phase 3, Multicenter, Open-Label Study of Gilteritinib, Gilteritinib Plus Azacitidine, or Azacitidine Alone in Newly Diagnosed FLT3 mutated (FLT3mut+) Acute Myeloid Leukemia (AML) Patients Ineligible for Intensive Induction Chemotherapy. Blood 2020, 136, 27, ASH, December 5 2020. [Google Scholar]
- Astellas Reports XOSPATA® (Gilteritinib) in Combination with Azacitidine Did Not Meet Endpoint of Overall Survival in Newly Diagnosed FLT3 Mutation-Positive Acute Myeloid Leukemia Patients Ineligible for Intensive Induction Chemotherapy | Astellas Pharma Inc. GLOBAL WEBSITE. Available online: https://www.astellas.com/en/news/16296 (accessed on 3 April 2021).
- Chen, Y.-B.; Li, S.; Lane, A.A.; Connolly, C.; Del Rio, C.; Valles, B.; Curtis, M.; Ballen, K.; Cutler, C.; Dey, B.R.; et al. Phase I Trial of Maintenance Sorafenib after Allogeneic Hematopoietic Stem Cell Transplantation for Fms-like Tyrosine Kinase 3 Internal Tandem Duplication Acute Myeloid Leukemia. Biol. Blood Marrow Transpl. 2014, 20, 2042–2048. [Google Scholar] [CrossRef]
- Burchert, A.; Bug, G.; Fritz, L.V.; Finke, J.; Stelljes, M.; Röllig, C.; Wollmer, E.; Wäsch, R.; Bornhäuser, M.; Berg, T.; et al. Sorafenib Maintenance After Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia With FLT3–Internal Tandem Duplication Mutation (SORMAIN). J. Clin. Oncol. 2020, 38, 2993–3002. [Google Scholar] [CrossRef]
- Xuan, L.; Wang, Y.; Huang, F.; Fan, Z.; Xu, Y.; Sun, J.; Xu, N.; Deng, L.; Li, X.; Liang, X.; et al. Sorafenib Maintenance in Patients with FLT3-ITD Acute Myeloid Leukaemia Undergoing Allogeneic Haematopoietic Stem-Cell Transplantation: An Open-Label, Multicentre, Randomised Phase 3 Trial. Lancet Oncol. 2020, 21, 1201–1212. [Google Scholar] [CrossRef]
- Maziarz, R.T.; Levis, M.; Patnaik, M.M.; Scott, B.L.; Mohan, S.R.; Deol, A.; Rowley, S.D.; Kim, D.D.H.; Hernandez, D.; Rajkhowa, T.; et al. Midostaurin after Allogeneic Stem Cell Transplant in Patients with FLT3 -Internal Tandem Duplication-Positive Acute Myeloid Leukemia. Bone Marrow Transplant. 2020, 1–10. [Google Scholar] [CrossRef]
- Astellas Pharma Global Development, Inc. A Multi-Center, Randomized, Double-Blind., Placebo-Controlled Phase III Trial of the FLT3 Inhibitor Gilteritinib Administered as Maintenance Therapy Following Allogeneic Transplant. for Patients With FLT3/ITD AML; ClinicalTrials.gov: Bethesda, MD, USA, 2021. [Google Scholar]
- Gordon, M.J.; Tardi, P.; Loriaux, M.M.; Spurgeon, S.E.; Traer, E.; Kovacsovics, T.; Mayer, L.D.; Tyner, J.W. CPX-351 Exhibits Potent and Direct Ex Vivo Cytotoxicity against AML Blasts with Enhanced Efficacy for Cells Harboring the FLT3-ITD Mutation. Leuk. Res. 2017, 53, 39–49. [Google Scholar] [CrossRef]
- Lancet, J.E.; Uy, G.L.; Cortes, J.E.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; et al. CPX-351 (Cytarabine and Daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J. Clin. Oncol. 2018, 36, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- Chiche, E.; Rahmé, R.; Bertoli, S.; Dumas, P.-Y.; Micol, J.-B.; Hicheri, Y.; Pasquier, F.; Peterlin, P.; Chevallier, P.; Thomas, X.; et al. Real-Life Experience with CPX-351 and Impact on the Outcome of High-Risk AML Patients: A Multicentric French Cohort. Blood Adv. 2021, 5, 176–184. [Google Scholar] [CrossRef]
- Children’s Oncology Group. A Phase 3 Randomized Trial for Patients With De Novo AML Comparing Standard Therapy Including Gemtuzumab Ozogamicin (GO) to CPX-351 With GO, and the Addition of the FLT3 Inhibitor Gilteritinib for Patients With FLT3 Mutations; ClinicalTrials.gov: Bethesda, MD, USA, 2021. [Google Scholar]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Mali, R.S.; Zhang, Q.; DeFilippis, R.; Cavazos, A.; Kuruvilla, V.M.; Raman, J.; Mody, V.; Choo, E.F.; Dail, M.; Shah, N.P.; et al. Venetoclax Combines Synergistically with FLT3 Inhibition to Effectively Target Leukemic Cells in FLT3-ITD+ Acute Myeloid Leukemia Models. Haematologica 2021, 106, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Maiti, A.; DiNardo, C.D.; Daver, N.G.; Rausch, C.R.; Ravandi, F.; Kadia, T.M.; Pemmaraju, N.; Borthakur, G.; Bose, P.; Issa, G.C.; et al. Triplet Therapy with Venetoclax, FLT3 Inhibitor and Decitabine for FLT3 -Mutated Acute Myeloid Leukemia. Blood Cancer J. 2021, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.C.; Wang, Q.; Chin, C.-S.; Salerno, S.; Damon, L.E.; Levis, M.J.; Perl, A.E.; Travers, K.J.; Wang, S.; Hunt, J.P.; et al. Validation of ITD Mutations in FLT3 as a Therapeutic Target in Human Acute Myeloid Leukaemia. Nature 2012, 485, 260–263. [Google Scholar] [CrossRef]
- Moore, A.S.; Faisal, A.; Gonzalez de Castro, D.; Bavetsias, V.; Sun, C.; Atrash, B.; Valenti, M.; de Haven Brandon, A.; Avery, S.; Mair, D.; et al. Selective FLT3 Inhibition of FLT3-ITD+ Acute Myeloid Leukaemia Resulting in Secondary D835Y Mutation: A Model for Emerging Clinical Resistance Patterns. Leukemia 2012, 26, 1462–1470. [Google Scholar] [CrossRef]
- Lam, S.S.Y.; Leung, A.Y.H. Overcoming Resistance to FLT3 Inhibitors in the Treatment of FLT3-Mutated AML. Int. J. Mol. Sci. 2020, 21, 1537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Savage, S.; Schultz, A.R.; Bottomly, D.; White, L.; Segerdell, E.; Wilmot, B.; McWeeney, S.K.; Eide, C.A.; Nechiporuk, T.; et al. Clinical Resistance to Crenolanib in Acute Myeloid Leukemia Due to Diverse Molecular Mechanisms. Nat. Commun. 2019, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- McMahon, C.M.; Ferng, T.; Canaani, J.; Wang, E.S.; Morrissette, J.J.D.; Eastburn, D.J.; Pellegrino, M.; Durruthy-Durruthy, R.; Watt, C.D.; Asthana, S.; et al. Clonal Selection with RAS Pathway Activation Mediates Secondary Clinical Resistance to Selective FLT3 Inhibition in Acute Myeloid Leukemia. Cancer Discov. 2019, 9, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Alfayez, M.; DiNardo, C.D.; Borthakur, G.; Kadia, T.M.; Konopleva, M.Y.; Loghavi, S.; Kanagal-Shamanna, R.; Patel, K.P.; Jabbour, E.J.; et al. Outcomes with Sequential FLT3-Inhibitor-Based Therapies in Patients with AML. J. Hematol. Oncol. 2020, 13, 132. [Google Scholar] [CrossRef] [PubMed]
| Agent | Type | Dose | Target |
|---|---|---|---|
| Sorafenib | First generation, type I | 400 mg BID | FLT3-ITD, RAF, VEGFR1/2/3, PDGFRβ, KIT, RET |
| Midostaurin | First generation, type I | 50 mg BID | FLT3-ITD, FLT3-TKD, PKC, SYK, FLK-1, AKT, PKA, KIT, FGR, SRC, PDGFRα/β, VEGFR1/2 |
| Quizartinib | Second generation, type II | 60 mg once a day | FLT3-ITD, KIT, PDGFR |
| Gilteritinib | Second generation, type I | 120 mg once a day | FLT3-ITD, FLT3-TKD, LTK, ALK, AXL |
| Crenolanib | Second generation, type I | 100 mg TID | FLT3-ITD, FLT3-TKD, PDGFRβ |
| Indication | Agent | Trial |
|---|---|---|
| Salvage monotherapy | Midostaurin | Phase I, phase II |
| Quizartinib | Phase II, phase III | |
| Gilteritinib | Phase II, phase III | |
| Crenolanib | Phase II | |
| Combination with intensive chemotherapy (newly diagnosed) | Midostaurin | Phase III |
| Quizartinib | Phase I, Phase III (ongoing) | |
| Gilteritinib | Phase III (ongoing) | |
| Crenolanib | Phase III (ongoing) | |
| Combination with demethylating agents | Sorafenib | Retrospective study |
| Midostaurin | Phase II study | |
| Quizartinib | Phase I/II | |
| Gilteritinib | Phase III (failed) | |
| Crenolanib | Preclinical | |
| Posttransplantation maintenance | Sorafenib | Phase II, phase III |
| Midostaurin | Phase III (failed) | |
| Quizartinib | Phase III (ongoing) | |
| Gilteritinib | Phase III (ongoing) | |
| Crenolanib | Phase III (ongoing) |
| Reference | Drug | Study Design | N | Type of FLT3 Mut | Response | Survival |
|---|---|---|---|---|---|---|
| Ravandi et al. [38] | Sorafenib + Azacitidine | Phase II | 43 | FLT3-ITD (93%) | CR 16% | Median OS 6.2 months |
| Ohanian et al. [39] | Sorafenib + Azacitidine | Phase I/II | 27 | FLT3-ITD (100%) | CR 26% | Median OS 8.3 months |
| Williams et al. [40] | Midostaurin + Decitabine | Phase I | 16 | FLT3-ITD (13%) | CHR 26% | |
| Strati et al. [41] | Midostaurin + Azacitidine | Phase I/II | 54 | FLT3-ITD (68%), TKD (6%) | ORR 26% | |
| Swaminathan et al. [42] | Quizartinib + Azacitidine/LDAC | Phase I/II | 73 | Phase I: FLT3-ITD and WT, Phase II: FLT3-ITD only | Q + A: CR 22% Q + LDAC: CR 8% | Q + A: median OS 13.4 monthsQ + LDAC Median OS: 6.7 months |
| Wang et al. [43] | Gilteritinib + Azacitidine vs. Azacitidine alone | Phase III | 136 | FLT3-mutated AML | Trial halted (did not meet endpoint) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loschi, M.; Sammut, R.; Chiche, E.; Cluzeau, T. FLT3 Tyrosine Kinase Inhibitors for the Treatment of Fit and Unfit Patients with FLT3-Mutated AML: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 5873. https://doi.org/10.3390/ijms22115873
Loschi M, Sammut R, Chiche E, Cluzeau T. FLT3 Tyrosine Kinase Inhibitors for the Treatment of Fit and Unfit Patients with FLT3-Mutated AML: A Systematic Review. International Journal of Molecular Sciences. 2021; 22(11):5873. https://doi.org/10.3390/ijms22115873
Chicago/Turabian StyleLoschi, Michael, Rinzine Sammut, Edmond Chiche, and Thomas Cluzeau. 2021. "FLT3 Tyrosine Kinase Inhibitors for the Treatment of Fit and Unfit Patients with FLT3-Mutated AML: A Systematic Review" International Journal of Molecular Sciences 22, no. 11: 5873. https://doi.org/10.3390/ijms22115873
APA StyleLoschi, M., Sammut, R., Chiche, E., & Cluzeau, T. (2021). FLT3 Tyrosine Kinase Inhibitors for the Treatment of Fit and Unfit Patients with FLT3-Mutated AML: A Systematic Review. International Journal of Molecular Sciences, 22(11), 5873. https://doi.org/10.3390/ijms22115873





