Development of Real-Time and Conventional PCR Assays for Identifying a Newly Named Species of Root-Lesion Nematode (Pratylenchus dakotaensis) on Soybean
Abstract
1. Introduction
2. Results
2.1. Species-Specific PCR Primer Design
2.2. Primer Specificity
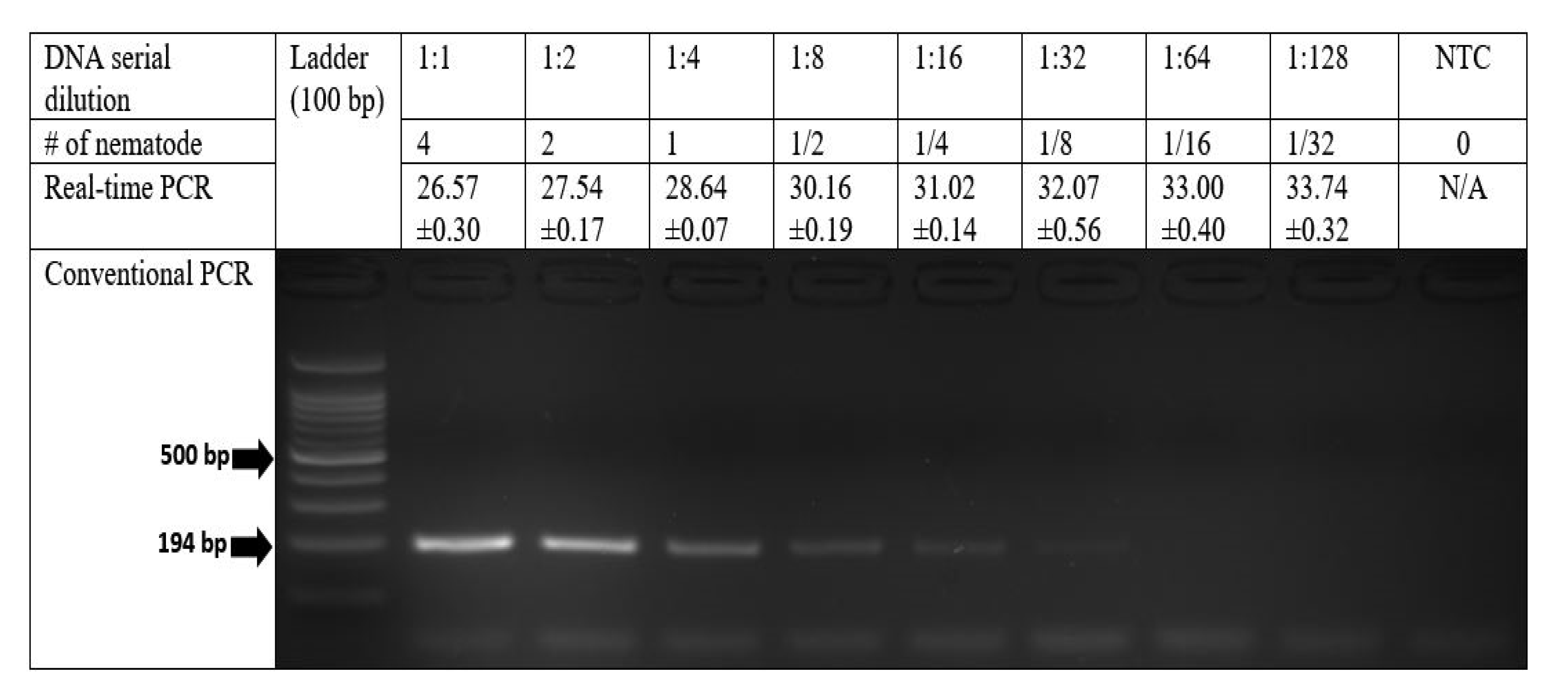
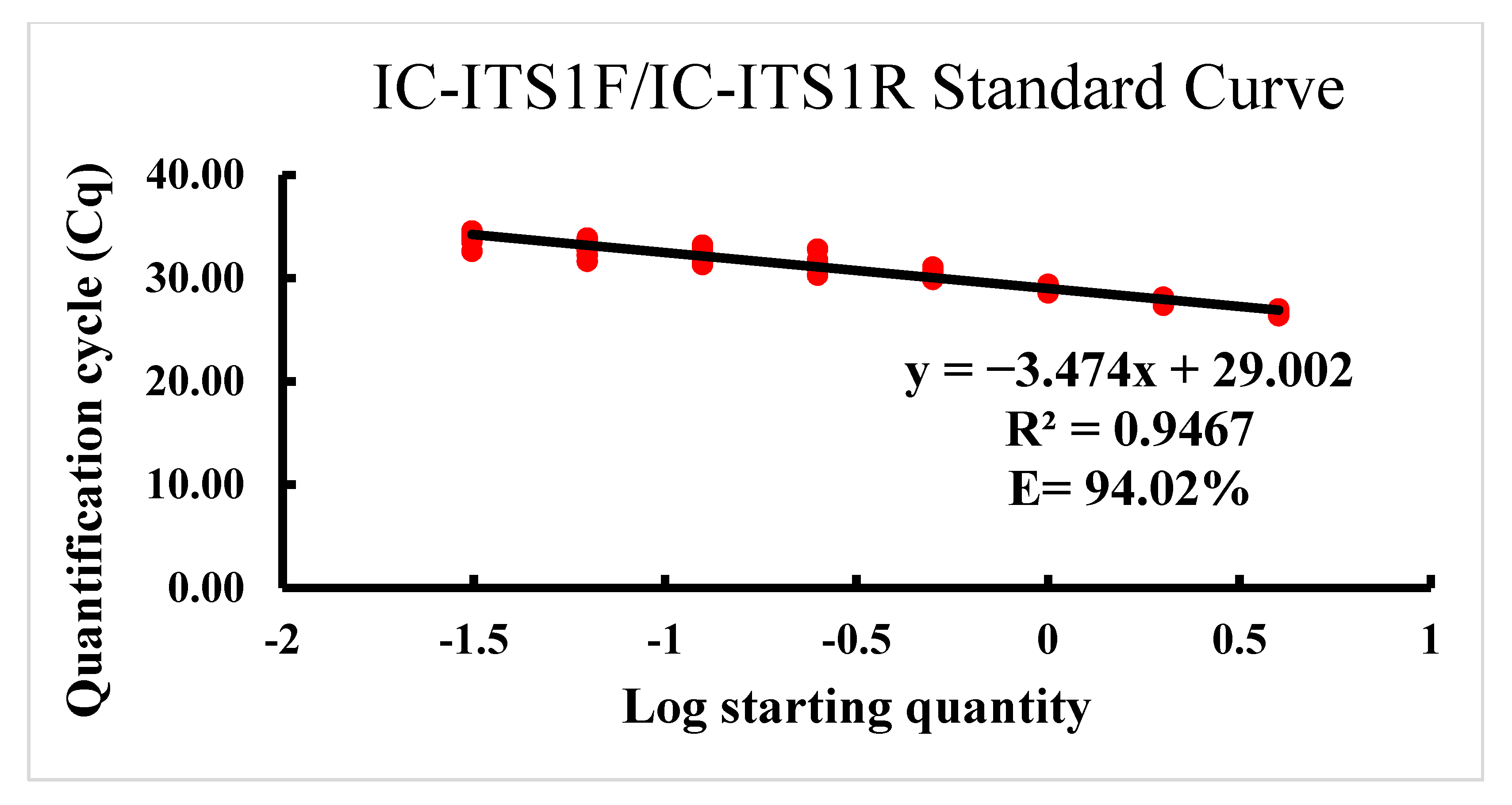
2.3. Detection Sensitivity of the PCR Assays
2.4. Identification of Pratylenchus spp. Collected from Soybean Fields
3. Discussion
4. Materials and Methods
4.1. Soil Sampling and Nematode Extraction from Soil
4.2. Root-Lesion Nematode Isolation and DNA Extraction
4.3. Direct Sequencing for Identity Confirmation
4.4. Species-Specific PCR Primer Design and Development
4.5. Detection Specificity of the Primers in PCR
4.6. Conventional PCR Assay
4.7. Real-Time PCR Assay
4.8. Detection Sensitivity of PCR Assays
4.9. Isolation, Identification, and Verification of Root-Lesion Nematode Species Collected from Soybean Fields
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sasser, J.N.; Freckman, D.W. A world perspective on nematology: The role of the society. In Vistas on Nematology: A Commemoration of the Twenty-Fifth Anniversary of the Society of Nematologists, 1st ed.; Veech, J.A., Dickerson, D.W., Eds.; Society of Nematologists: Lakeland, FL, USA, 1987; pp. 7–14. [Google Scholar]
- Castillo, P.; Vovlas, N. Pratylenchus (Nematoda: Pratylenchidae): Diagnosis, biology, pathogenicity and management. Nematol. Monogr. Perspect. 2007, 6, 1–543. [Google Scholar] [CrossRef]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Davis, E.L.; MacGuidwin, A.E. Lesion nematode disease. Plant Health Instr. 2000. [Google Scholar] [CrossRef]
- Taylor, S.P.; Vanstone, V.A.; Ware, A.H.; McKay, A.C.; Szot, D.; Russ, M.H. Measuring yield loss in cereals caused by root-lesion nematodes (Pratylenchus Neglectus and P. Thornei) with and without nematicide. Aust. J. Agric. Res. 1999, 50, 617–622. [Google Scholar] [CrossRef]
- Smiley, R.W.; Whittaker, R.G.; Gourlie, J.A.; Easley, S.A. Suppression of wheat growth and yield by Pratylenchus neglectus in the Pacific Northwest. Plant Dis. 2005, 89, 958–968. [Google Scholar] [CrossRef]
- Holgado, R.; Skau, K.A.O.; Magnusson, C. Field damage in potato by lesion nematode Pratylenchus penetrans, its association with tuber symptoms and its survival in storage. Nematol. Medit. 2009, 37, 25–29. [Google Scholar]
- Lima, F.D.O.; dos Santos, G.R.; Nogueira, S.R.; dos Santos, P.R.R.; Correa, V.R. Population dynamics of the root-lesion nematode, Pratylenchus brachyurus, in soybean fields in Tocantins state and its effect to soybean yield. Nematropica 2015, 45, 170–177. [Google Scholar]
- Thompson, J.P.; Owen, K.J.; Stirling, G.R.; Bell, M.J. Root-lesion nematodes (Pratylenchus Thornei and P. Neglectus): A review of recent progress in managing a significant pest of grain crops in Northern Australia. Aust. Plant Pathol. 2008, 37, 235–242. [Google Scholar] [CrossRef]
- Handoo, Z.A.; Golden, A.M. A key and diagnostic compendium to the species of the genus Pratylenchus Filipjev, 1936 (lesion nematodes). J. Nematol. 1989, 21, 202–218. [Google Scholar] [PubMed]
- Kimpinski, J. Root-lesion nematodes in potatoes. Am. Potato J. 1979, 56, 79–86. [Google Scholar] [CrossRef]
- Luc, M. A reappraisal of Tylenchina (Nemata). 7. The family Pratylenchidae Thorne, 1949. Annu. Rev. Nematol. 1987, 10, 203–218. [Google Scholar]
- Wheeler, T.A.; Madden, L.V.; Rowe, R.C.; Riedel, R.M. Modeling of yield loss in potato early dying caused by Pratylenchus penetrans and Verticillium Dahliae. J. Nematol. 1992, 24, 99–102. [Google Scholar] [PubMed]
- Pinochet, P.; Cenis, J.L.; Fernández, C.; Doucet, M.; Maruli, J. Reproductive fitness and random amplified polymorphic DNA variation among isolates of Pratylenchus vulnus. J. Nematol. 1994, 26, 271–277. [Google Scholar] [PubMed]
- Al-Banna, L.; Ploeg, A.T.; Williamson, V.M.; Kaloshian, I. Discrimination of six Pratylenchus species using PCR and species-specific primers. J. Nematol. 2004, 36, 142–146. [Google Scholar] [CrossRef]
- Baidoo, R.; Yan, G.; Nagachandrabose, S.; Skantar, A.M. Developing a real-time PCR assay for direct identification and quantification of Pratylenchus penetrans in soil. Plant Dis. 2017, 101, 1432–1441. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Yan, G.P. Specific detection of the root-lesion nematode Pratylenchus scribneri using conventional and real-time PCR. Plant Dis. 2017, 101, 359–365. [Google Scholar] [CrossRef]
- Oliveira, C.M.G.; Blok, V.; Neilson, R.; Mróz, T.; Roberts, D. Hydrolysis probe-based PCR for detection of Pratylenchus crenatus, P. neglectus and P. penetrans. Nematology 2017, 19, 81–91. [Google Scholar] [CrossRef]
- Uehara, T.; Mizukubo, T.; Kushida, A.; Momota, Y. Identification of Pratylenchus penetrans (Cobb) by PCR using ITS-based species-specific primers. Jpn. J. Nematol. 1998, 28, 1–7. [Google Scholar] [CrossRef]
- Yan, G.P.; Smiley, R.W.; Okubara, P.A. Detection and quantification of Pratylenchus thornei in DNA extracted from soil using real-time PCR. Phytopathology 2012, 102, 14–22. [Google Scholar] [CrossRef]
- Yan, G.P.; Smiley, R.W.; Okubara, P.A.; Skantar, A.M.; Reardon, C.L. Developing a real-time PCR assay for detection and quantification of Pratylenchus neglectus in soil. Plant Dis. 2013, 97, 757–764. [Google Scholar] [CrossRef]
- Yan, G.P.; Smiley, R.W.; Okubara, P.A.; Skantar, A.; Easley, S.A.; Sheedy, J.G.; Thompson, A.L. Detection and discrimination of Pratylenchus neglectus and P. thornei in DNA extracts from soil. Plant Dis. 2008, 92, 1480–1487. [Google Scholar] [CrossRef]
- Mekete, T.; Reynolds, K.; Lopez-Nicora, H.D.; Gray, M.E.; Niblack, T.L. Distribution and diversity of root-lesion nematode (Pratylenchus Spp.) associated with Miscanthus × Giganteus and Panicum virgatum used for biofuels, and species identification in a multiplex polymerase chain reaction. Nematology 2011, 13, 673–686. [Google Scholar] [CrossRef]
- Wittwer, C.T.; Herrmann, M.G.; Moss, A.A.; Rasmussen, R.P. Continuous fluorescence monitoring of rapid cycle DNA amplification. BioTechniques 1997, 22, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Yan, G.P.; Baidoo, R. Developing a real-time PCR assay for direct detection and quantification of Pratylenchus scribneri in field soil. Nematology 2020, 22, 733–744. [Google Scholar] [CrossRef]
- Berry, S.D.; Fargette, M.; Spaull, V.W.; Morand, S.; Cadet, P. Detection and quantification of root-knot nematode (Meloidogyne Javanica), lesion nematode (Pratylenchus zeae) and dagger nematode (Xiphinema elongatum) parasites of sugarcane using real-time PCR. Mol. Cell. Probes 2008, 22, 168–176. [Google Scholar] [CrossRef]
- Machado, A.C.Z.; Ferraz, L.C.C.B.; de Oliveira, C.M.G. Development of a species-specific reverse primer for the molecular diagnostic of Pratylenchus brachyurus. Nematropica 2007, 37, 249–257. [Google Scholar]
- Yan, G.P.; Plaisance, A.; Huang, D.; Chowdhury, I.A.; Handoo, Z.A. First report of the new root-lesion nematode Pratylenchus sp. on soybean in North Dakota. Plant Dis. 2017, 101, 1554. [Google Scholar] [CrossRef]
- Handoo, Z.A.; Yan, G.P.; Kantor, M.R.; Huang, D.; Chowdhury, I.A.; Plaisance, A.; Bauchan, G.R.; Mowery, J.D. Morphological and molecular characterization of Pratylenchus dakotaensis n. Sp. (Nematoda: Pratylenchidae), a new root-lesion nematode species on soybean in North Dakota, USA. Plants 2021, 10, 168. [Google Scholar] [CrossRef]
- Huang, D.; Yan, G.P.; Skantar, A.M. Development of real-time and conventional PCR assays for identifying stubby root nematode Paratrichodorus Allius. Plant Dis. 2017, 101, 964–972. [Google Scholar] [CrossRef]
- Kumari, S.; Subbotin, S.A. Molecular characterization and diagnostics of stubby root and virus vector nematodes of the family Trichodoridae (Nematoda: Triplonchida) using ribosomal RNA Genes. Plant Pathol. 2012, 61, 1021–1031. [Google Scholar] [CrossRef]
- Mai, W.F.; Mullin, P.G.; Lyon, H.H.; Loeffler, K. Plant-Parasitic Nematodes: A Pictorial Key to Genera, 5th ed.; Cornell University Press: Ithaca, NY, USA, 1996; pp. 10–277. [Google Scholar]
- Schmitt, D.P.; Barker, K.R. Damage and reproductive potentials of Pratylenchus brachyurus and P. Penetrans on Soybean. J. Nematol. 1981, 13, 327–332. [Google Scholar]
- Koenning, S.R.; Overstreet, C.; Noling, J.W.; Donald, P.A.; Becker, J.O.; Fortnum, B.A. Survey of crop losses in response to phytoparasitic nematodes in the United States for 1994. J. Nematol. 1999, 31, 587–618. [Google Scholar] [PubMed]
- Schrader, C.; Schielke, A.; Ellerbroek, L.; Johne, R. PCR inhibitors-occurrence, properties and removal. J. Appl. Microbiol. 2012, 113, 1014–1026. [Google Scholar] [CrossRef] [PubMed]
- De Weerdt, M.; Kox, L.; Waeyenberge, L.; Viaene, N.; Zijlstra, C. A real-time PCR assay to identify Meloidogyne minor. J. Phytopathol. 2011, 3, 30–37. [Google Scholar] [CrossRef]
- Huang, D.; Yan, G.P.; Gudmestad, N.; Skantar, A. Quantification of Paratrichodorus allius in DNA extracted from soil using TaqMan Probe and SYBR Green real-time PCR assays. Nematology 2017, 19, 987–1001. [Google Scholar] [CrossRef]
- Huang, D.; Yan, G.P.; Gudmestad, N.; Whitworth, J.; Frost, K.; Brown, C.; Ye, W.; Agudelo, P.; Crow, W. Molecular characterization and identification of stubby root nematode species from multiple states in the United States. Plant Dis. 2018, 102, 2101–2111. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Yan, G.P.; Gudmestad, N.; Ye, W.; Whitworth, J.; Frost, K.; Crow, W.; Hajihassani, A. Developing a one-step multiplex PCR assay for rapid detection of four stubby-root nematode species, Paratrichodorus allius, P. minor, P. porosus, and Trichodorus obtusus. Plant Dis. 2019, 103, 404–410. [Google Scholar] [CrossRef]
- Li, W.; Yan, Z.; Nakhla, M.K.; Skantar, A.M. Real-time PCR for detection and identification of Anguina funesta, A. agrostis, A. tritici, and A. pacificae. Plant Dis. 2015, 99, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Subbotin, S.A.; Madani, M.; Krall, E.; Sturhan, D.; Moens, M. Molecular diagnostics, taxonomy, and phylogeny of the stem nematode Ditylenchus dipsaci species complex based on the sequences of the internal transcribed spacer-rDNA. Phytopathology 2005, 95, 1308–1315. [Google Scholar] [CrossRef]
- de Luca, F.; Reyes, A.; Troccoli, A.; Castillo, P. Molecular variability and phylogenetic relationships among different species and populations of Pratylenchus (Nematoda: Pratylenchidae) as inferred from the analysis of the ITS rDNA. Eur. J. Plant Pathol. 2011, 130, 415–426. [Google Scholar] [CrossRef]
- Subbotin, S.A.; Moens, M. Molecular taxonomy and phylogeny. In Plant Nematology, 2nd ed.; Perry, R.N., Moens, M., Eds.; CABI: Wallingford, UK, 2006; pp. 33–58. [Google Scholar]
- Blouin, M.S. Molecular prospecting for cryptic species of nematodes: Mitochondrial DNA versus internal transcribed spacer. Int. J. Parasitol. 2002, 32, 527–531. [Google Scholar] [CrossRef]
- Gorny, A.M.; Wang, X.; Hay, F.S.; Pethybridge, S.J. Development of a species-specific PCR for detection and quantification of Meloidogyne hapla in soil using the 16D10 root-knot nematode effector gene. Plant Dis. 2019, 103, 1902–1909. [Google Scholar] [CrossRef]
- Seesao, Y.; Gay, M.; Merlin, S.; Viscogliosi, E.; Aliouat-Denis, C.M.; Audebert, C. A review of methods for nematode identification. J. Microbiol. Meth. 2017, 138, 37–49. [Google Scholar] [CrossRef]
- Valentini, A.; Mattiucci, S.; Bondanelli, P.; Webb, S.C.; Mignucci-Giannone, A.A.; Colom-Llavina, M.M.; Nascetti, G. Genetic relationships among Anisakis species (Nematoda: Anisakidae) inferred from mitochondrial cox2 sequences, and comparison with allozyme data. J. Parasitol. 2006, 92, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chronis, D.; Lu, S.; Wang, X. Chorismate Mutase: An alternatively spliced parasitism gene and a diagnostic marker for three important Globodera nematode species. Eur. J. Plant Pathol. 2011, 129, 89–102. [Google Scholar] [CrossRef]
- Dieffenbach, C.W.; Lowe, T.M.J.; Dveksler, G.S. General concepts for PCR primer design. PCR Methods Appl. 1993, 3, 30–37. [Google Scholar] [CrossRef]
- Singh, V.K.; Kumar, A. Primer design. Mol. Biol. Today 2001, 2, 27–32. [Google Scholar]
- Rychlik, W.; Spencer, W.J.; Rhoads, R.E. Optimization of the annealing temperature for DNA amplification in vitro. Nucleic Acids Res. 1990, 18, 6409–6412. [Google Scholar] [CrossRef]
- Erlich, H.A.; Gelfand, D.; Sninsky, J.J. Recent advances in the polymerase chain reaction. Science 1991, 252, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Delcourt, S.; Vernière, C.; Boyer, C.; Pruvost, O.; Hostachy, B.; Robène-Soustrade, I. Revisiting the specificity of PCR primers for diagnostics of Xanthomonas citri Pv. citri by experimental and in silico analyses. Plant Dis. 2013, 97, 373–378. [Google Scholar] [CrossRef]
- Okubara, P.A.; Schroeder, K.L.; Paulitz, T.C. Identification and quantification of Rhizoctonia solani and R. oryzae using real-time polymerase chain reaction. Phytopathology 2008, 98, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, K.L.; Okubara, P.A.; Tambong, J.T.; Lévesque, C.A.; Paulitz, T.C. Identification and quantification of pathogenic Pythium Spp. from soils in eastern washington using real-time polymerase chain reaction. Phytopathology 2006, 96, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Jeszke, A.; Dobosz, R.; Obrepalska-Steplowska, A. A fast and sensitive method for the simultaneous identification of three important nematode species of the genus Ditylenchus. Pest Manag. Sci. 2015, 71, 243–249. [Google Scholar] [CrossRef]
- Mokrini, F.; Waeyenberge, L.; Viaene, N.; Abbad Andaloussi, F.; Moens, M. Quantitative detection of the root-lesion nematode, Pratylenchus penetrans, using qPCR. Eur. J. Plant Pathol. 2013, 137, 403–413. [Google Scholar] [CrossRef]
- Jenkins, W.R. A Rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis. Rep. 1964, 48, 692. [Google Scholar]
- Subbotin, S.A.; Ragsdale, E.J.; Mullens, T.; Roberts, P.A.; Mundo-Ocampo, M.; Baldwin, J.G. A phylogenetic framework for root-lesion nematodes of the genus Pratylenchus (Nematoda): Evidence from 18S and D2-D3 expansion segments of 28S ribosomal RNA genes and morphological characters. Mol. Phylogenet. Evol. 2008, 48, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Vrain, T.C.; Wakarchuk, D.A.; Lévesque, A.C.; Hamilton, R.I. Intraspecific rDNA restriction fragment length polymorphism in the Xiphinema americanum group. Fundam. Appl. Nemalol. 1992, 15, 563–573. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis Program for Windows 95/98/NT. Nucliec Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Cherry, T.; Szalanski, A.L.; Todd, T.C.; Powers, T.O. The internal transcribed spacer region of belonolaimus (Nemata: Belonolaimidae). J. Nematol. 1997, 29, 23–29. [Google Scholar]
- Chowdhury, I.A.; Yan, G.P.; Friskop, A. Occurrence of vermiform plant-parasitic nematodes in North Dakota corn fields and impact of environmental and soil factors. Can. J. Plant Pathol. 2020, 42, 429–444. [Google Scholar] [CrossRef]

| Species | GenBank Accession | Sample ID or Clone ID a | Origin | Sequence Length (bp) | ΔG (kcal/mole) b | |
|---|---|---|---|---|---|---|
| IC-ITS1F | IC-ITS1R | |||||
| P. dakotaensisc | KX889990.1 | Hg50 | ND, USA | 1126 | −33.5 | −38.4 |
| Pratylenchus sp. d | KY200666.1 | Hg51 | ND, USA | 981 | ins | ins |
| P. agilis | KC952982.1 | SX/Clone7 | P.R. China | 882 | ins | ins |
| P. agilis | FJ712891.1 | PagKL5/Clone5 | Belgium | 880 | ins | ins |
| P. alleni | JX081545.2 | N/A | Canada | 1080 | ins | ins |
| P. bolivianus | HM469446.1 | TW2 | P.R. China | 1163 | ins | ins |
| P. brachyurus | MH020807.1 | AD70 | Spain | 627 | ins | ins |
| P. coffeae | JX046940.1 | YT/Clone4 | P.R. China | 1102 | ins | ins |
| P. convallariae | HM469448.1 | N/A | P.R. China | 722 | ins | ins |
| P. crenatus | LC030310.1 | He1/Clone1 | Japan | 928 | ins | ins |
| P. crenatus | LC030308.1 | Pcr-H01/Clone1 | Japan | 958 | ins | ins |
| P. fallax | KY828273.1 | V4C/Clone180 | Belgium | 755 | ins | ins |
| P. goodeyi | KF700243.1 | CICR-Cot.Warud | India | 782 | ins | ins |
| P. gutierrezi | KT971367.1 | O22_1 | Spain | 906 | ins | ins |
| P. hippeastri | KR029085.1 | QIXIA | China | 1185 | ins | ins |
| P. jaehni | FJ712937.1 | PjaKL1/Clone | Belgium | 997 | ins | ins |
| P. lentis | AM933154.1 | Individual Nematode 10 | Italy | 703 | ins | ins |
| P. loosi | KY424222.1 | EX11 | P.R. China | 1212 | ins | ins |
| P. mediterraneus | FR692306.1 | N/A | Italy | 946 | ins | ins |
| P. neglectus | LC030328.1 | NM1/Clone8 | Japan | 852 | −14.4 | ins |
| P. neglectus | LC030323.1 | HT2KU1/Clone1 | Japan | 871 | −14.4 | ins |
| P. neglectus | LC030325.1 | HKf1/Clone6 | Japan | 855 | −14.4 | ins |
| P. penetrans | LC030336.1 | HM1/Clone3 | Japan | 874 | ins | ins |
| P. pinguicaudatus | KY828261.1 | T572/Clone168 | Belgium | 762 | ins | ins |
| P. pratensis | KY828311.1 | T616/Clone 232 | Belgium | 807 | ins | ins |
| P. pseudocoffeae | LC030337.1 | Pps-KM1/Clone1 | Japan | 1090 | ins | ins |
| P. pseudocoffeae | LC030339.1 | Pps-KM1/Clone7 | Japan | 1090 | ins | ins |
| P. scribneri | KT873860.1 | ND | ND, USA | 1103 | ins | ins |
| P. scribneri | KX842626.1 | F21 | ND, USA | 1103 | ins | ins |
| P. scribneri | JX046934.1 | XC/Clone2 | P.R. China | 957 | ins | ins |
| P. thornei | FR692305.1 | Pt_Je_SP_cl13 | Italy | 1001 | ins | ins |
| P. thornei | FR692304.1 | PT_SI_IT_cl19 | Italy | 974 | ins | ins |
| P. vulnus | KY424232.1 | JSR | China | 925 | ins | ins |
| P. zeae | FJ643590.1 | N/A | Republic of China | 967 | ins | ins |
| Sample ID a | Species | Origin | Crop b | # of Nema c | PCR Assay | |
|---|---|---|---|---|---|---|
| Conventional d | RT (Cq) e | |||||
| Ps1 | Pratylenchus scribneri | Sargent, ND, USA | Corn | 2 | − | N/A |
| Ps2 | P. scribneri | Sargent, ND, USA | Potato | 2 | − | N/A |
| Pn1 | P. neglectus | Richland, ND, USA | Corn | 2 | − | N/A |
| Pn2 | P. neglectus | Bottineau, ND, USA | Field Pea | 2 | − | N/A |
| Pt | P. thornei | OR, USA | Wheat | 2 | − | N/A |
| Pp | P. penetrans | Sherburne, MN, USA | Potato | 2 | − | N/A |
| Tyl | Tylenchorhychus sp. | Richland, ND, USA | Corn | 2 | − | N/A |
| Spi | Helicotylenchus sp. | Richland, ND, USA | Soybean | 2 | − | N/A |
| Xph | Xiphinema sp. | Sargent, ND, USA | Potato | 2 | − | N/A |
| Prt | Paratylenchus sp. | McIntosh, ND, USA | Corn | 2 | − | N/A |
| Ptr | Paratrichodorus sp. | Sargent, ND, USA | Potato | 2 | − | N/A |
| Hop | Hoplolaimus sp. | Sargent, ND, USA | Soybean | 2 | − | N/A |
| SCN | Heterodera glycines | Richland, ND, USA | Soybean | 2 | − | N/A |
| SBCN | H. schachtii | Richland, MT, USA | Sugarbeet | 2 | − | N/A |
| NPN1 | Non-plant parasitic nematode 1 | Richland, ND, USA | Corn | 2 | − | N/A |
| NPN2 | Non-plant parasitic nematode 2 | Richland, ND, USA | Soybean | 2 | − | N/A |
| Hg51 | Pratylenchus sp. | Richland, ND, USA | Soybean | 2 | − | N/A |
| Hg50-1 | P. dakotaensis | Richland, ND, USA | Soybean | 2 | + | 28.91 ± 0.40 |
| Hg50-2 | P. dakotaensis | Richland, ND, USA | Soybean | 2 | + | 27.70 ± 0.10 |
| Hg50-3 | P. dakotaensis | Richland, ND, USA | Corn | 2 | + | 28.02 ± 0.59 |
| Hg50-4 | P. dakotaensis | Richland, ND, USA | Soybean | 4 | + | 26.43 ± 0.05 |
| Hg50-4 | P. dakotaensis | Richland, ND, USA | Soybean | 2 | + | 27.60 ± 0.20 |
| Hg50-4 | P. dakotaensis | Richland, ND, USA | Soybean | 1 | + | 29.87 ± 0.50 |
| Hg50-4 | P. dakotaensis | Richland, ND, USA | Soybean | 0.5 | + | 30.64 ± 0.50 |
| Field ID | Crop | County Origin | Density in 100 cm3 of Soil b | Conventional c | RT d | Species Identity | ||
|---|---|---|---|---|---|---|---|---|
| PNEG-F1/D3B5 | PsF7/PsR7 | IC-ITS1 F/IC-ITS1R | IC-ITS1F/IC-ITS1R (Cq) e | |||||
| 50 RL 1 | Soybean | Richland | 21 | − | + | − | N/A | P. scribneri |
| 50 RL 2 | Soybean | Richland | 28 | − | + | − | N/A | P. scribneri |
| 50 RL 3 f | Soybean | Richland | 342 | − | − | + | 27.4 ± 0.1 | P. dakotaensis |
| 50 RL 4 | Soybean | Richland | 360 | − | + | − | N/A | P. scribneri |
| C14L | Soybean | Dickey | 140 | − | + | − | N/A | P. scribneri |
| C3L | Soybean | Sargent | 75 | − | + | − | N/A | P. scribneri |
| HG50-0 g | Soybean | Richland | 360 | − | − | + | 27.7 ± 0.1 | P. dakotaensis |
| Russ Field | Soybean | Wells | 203 | + | − | − | N/A | P. neglectus |
| SCN 188 E | Corn | Cass | 45 | + | − | − | N/A | P. neglectus |
| SCN 188 W | Corn | Cass | 45 | + | − | − | N/A | P. neglectus |
| SCN 207 | Soybean | Cass | 242 | + | − | − | N/A | P. neglectus |
| SCN 215 | Soybean | Grand Forks | 120 | + | − | − | N/A | P. neglectus |
| SCN 222 | Soybean | Cass | 100 | + | − | − | N/A | P. neglectus |
| SCN 310 | Soybean | Nelson | 31 | + | − | − | N/A | P. neglectus |
| SCN 311 | Soybean | Nelson | 30 | + | − | − | N/A | P. neglectus |
| SCN 366 | Dry bean | Grand Forks | 60 | + | − | − | N/A | P. neglectus |
| SCN 372 | Soybean | Grand Forks | 100 | + | − | − | N/A | P. neglectus |
| SCN 388 | Soybean | Grand Forks | 25 | + | − | − | N/A | P. neglectus |
| SCN 48 | Soybean | Richland | 15 | − | + | − | N/A | P. scribneri |
| SCN 55 | Corn | Richland | 75 | − | + | − | N/A | P. scribneri |
| SCN 7 | Soybean | Cass | 60 | + | − | − | N/A | P. neglectus |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, I.A.; Yan, G. Development of Real-Time and Conventional PCR Assays for Identifying a Newly Named Species of Root-Lesion Nematode (Pratylenchus dakotaensis) on Soybean. Int. J. Mol. Sci. 2021, 22, 5872. https://doi.org/10.3390/ijms22115872
Chowdhury IA, Yan G. Development of Real-Time and Conventional PCR Assays for Identifying a Newly Named Species of Root-Lesion Nematode (Pratylenchus dakotaensis) on Soybean. International Journal of Molecular Sciences. 2021; 22(11):5872. https://doi.org/10.3390/ijms22115872
Chicago/Turabian StyleChowdhury, Intiaz Amin, and Guiping Yan. 2021. "Development of Real-Time and Conventional PCR Assays for Identifying a Newly Named Species of Root-Lesion Nematode (Pratylenchus dakotaensis) on Soybean" International Journal of Molecular Sciences 22, no. 11: 5872. https://doi.org/10.3390/ijms22115872
APA StyleChowdhury, I. A., & Yan, G. (2021). Development of Real-Time and Conventional PCR Assays for Identifying a Newly Named Species of Root-Lesion Nematode (Pratylenchus dakotaensis) on Soybean. International Journal of Molecular Sciences, 22(11), 5872. https://doi.org/10.3390/ijms22115872






