Somatostatin, a Presynaptic Modulator of Glutamatergic Signal in the Central Nervous System
Abstract
:1. The Somatostatinergic System in the Central Nervous System
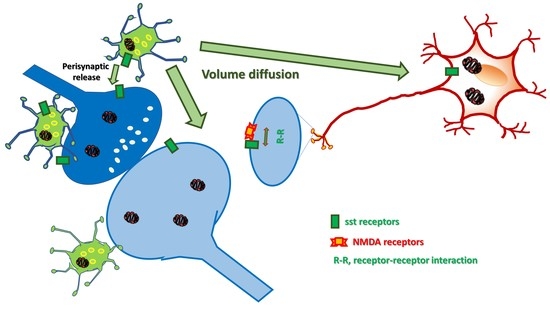
2. Somatostatin in the Central Nervous System: Synaptic Release and Volume Diffusion
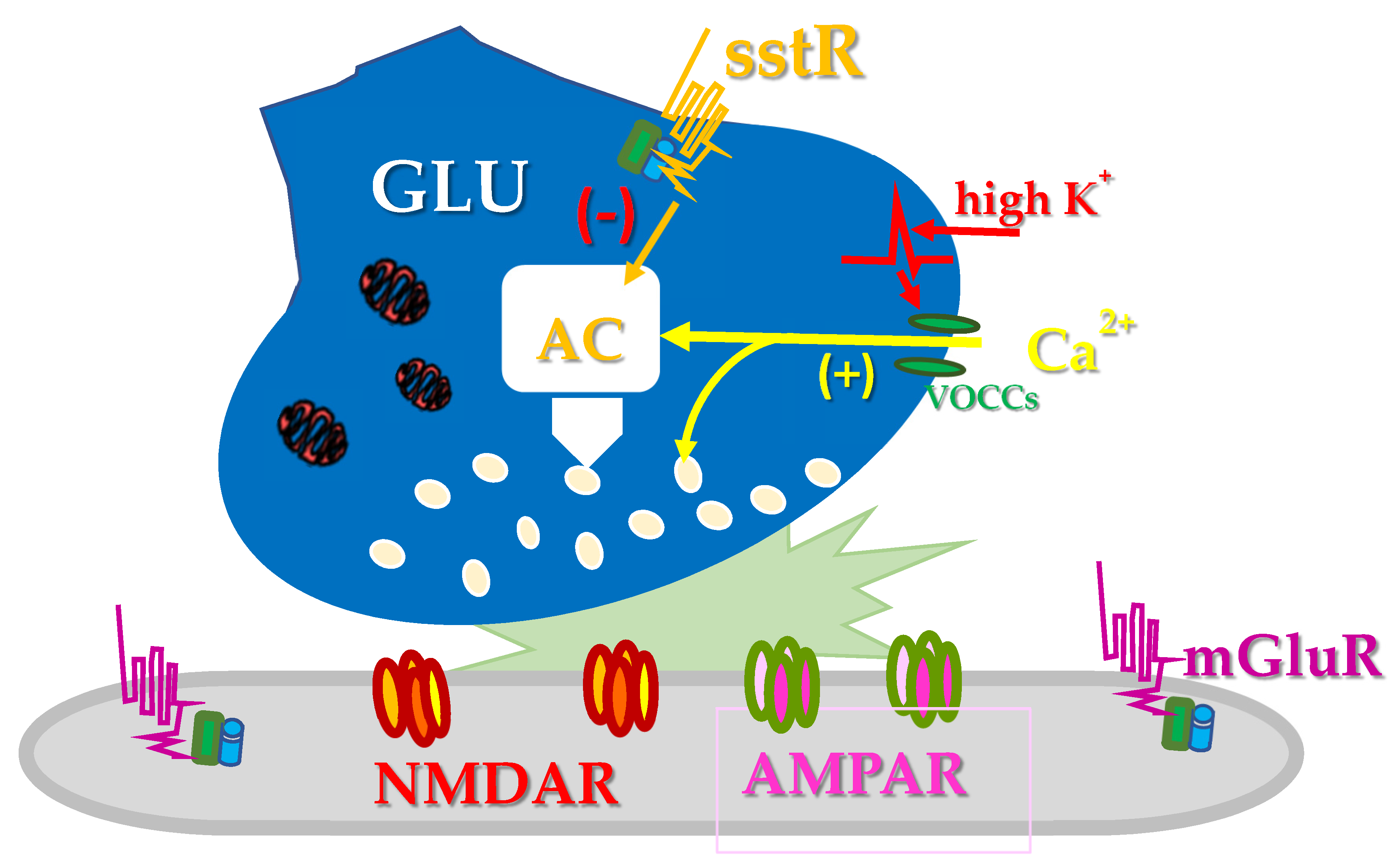
3. Somatostatin and Glutamate Presynaptically Modulate Each Other in the Central Nervous System
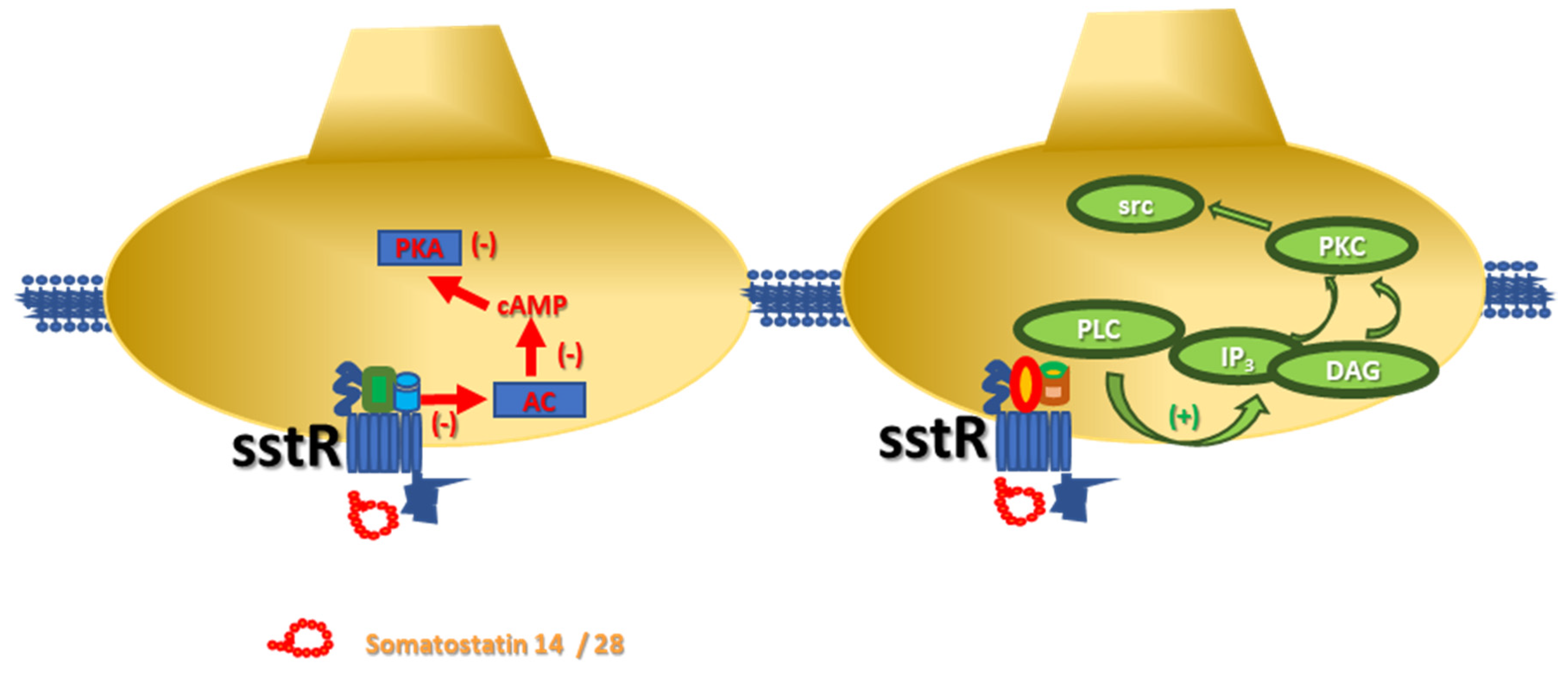
4. Somatostatin Receptors and Metamodulation in the Central Nervous System
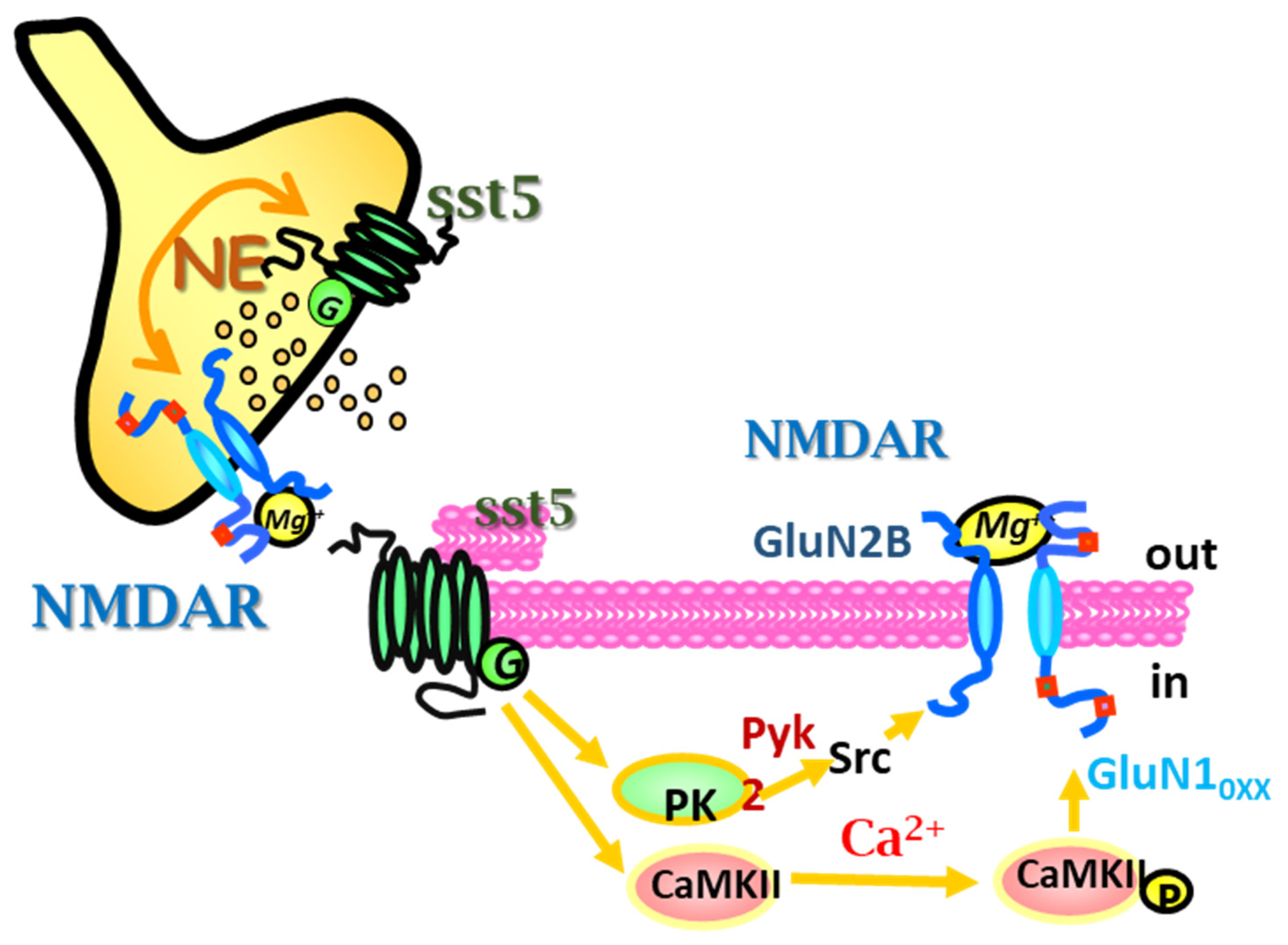
5. sst5 and NMDA Receptor-Receptor Interaction: A Model of Presynaptic Metamodulation
6. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Brazeau, P.; Vale, W.; Burgus, R.; Ling, N.; Butcher, M.; Rivier, J.; Guillemin, R. Hypothalamic Polypeptide That Inhibits the Secretion of Immunoreactive Pituitary Growth Hormone. Science 1973, 179, 77–79. [Google Scholar] [CrossRef]
- Reichlin, S. Somatostatin. N. Engl. J. Med. 1983, 309, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Zingg, H.H.; Patel, Y.C. Processing of synthetic somatostatin-28 and a related endogenous rat hypothalamic somatostatin-like molecule by hypothalamic enzymes. Life Sci. 1982, 30, 525–533. [Google Scholar] [CrossRef]
- Robbins, R.J.; Reichlin, S. Somatostatin Biosynthesis by Cerebral Cortical Cells in Monolayer Culture. Endocrinology 1983, 113, 574–581. [Google Scholar] [CrossRef]
- Vezzani, A.; Hoyer, D. Brain somatostatin: A candidate inhibitory role in seizures and epileptogenesis. Eur. J. Neurosci. 1999, 11, 3767–3776. [Google Scholar] [CrossRef] [PubMed]
- Günther, T.; Tulipano, G.; Dournaud, P.; Bousquet, C.; Csaba, Z.; Kreienkamp, H.-J.; Lupp, A.; Korbonits, M.; Castaño, J.P.; Wester, H.-J.; et al. International Union of Basic and Clinical Pharmacology. CV. Somatostatin Receptors: Structure, Function, Ligands, and New Nomenclature. Pharmacol. Rev. 2018, 70, 763–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatto, F.; Barbieri, F.; Arvigo, M.; Thellung, S.; Amarù, J.; Albertelli, M.; Ferone, D.; Florio, T. Biological and Biochemical Basis of the Differential Efficacy of First and Second Generation Somatostatin Receptor Ligands in Neuroendocrine Neoplasms. Int. J. Mol. Sci. 2019, 20, 3940. [Google Scholar] [CrossRef] [Green Version]
- Bruns, C.; Weckbecker, G.; Raulf, F.; Lübbert, H.; Hoyer, D. Characterization of Somatostatin Receptor Subtypes. Novartis Found. Symp. 2007, 190, 89–110. [Google Scholar] [CrossRef]
- Siehler, S.; Nunn, C.; Hannon, J.; Feuerbach, D.; Hoyer, D. Pharmacological profile of somatostatin and cortistatin receptors. Mol. Cell. Endocrinol. 2008, 286, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Grant, M. Somatostatin and Somatostatin Receptors. Chem. Biol. Pteridines Folates 2009, 50, 97–120. [Google Scholar]
- Hannon, J.P.; Nunn, C.; Stolz, B.; Bruns, C.; Weckbecker, G.; Lewis, I.; Troxler, T.; Hurth, K.; Hoyer, D. Drug Design at Peptide Receptors. J. Mol. Neurosci. 2002, 18, 15–28. [Google Scholar] [CrossRef]
- Pittaluga, A.; Feligioni, M.; Longordo, F.; Arvigo, M.; Raiteri, M. Somatostatin-Induced Activation and Up-Regulation of N-Methyl-d-aspartate Receptor Function: Mediation through Calmodulin-Dependent Protein Kinase II, Phospholipase C, Protein Kinase C, and Tyrosine Kinase in Hippocampal Noradrenergic Nerve Endings. J. Pharmacol. Exp. Ther. 2004, 313, 242–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thermos, K.; Bagnoli, P.; Epelbaum, J.; Hoyer, D. The somatostatin sst1 receptor: An autoreceptor for somatostatin in brain and retina? Pharmacol. Ther. 2006, 110, 455–464. [Google Scholar] [CrossRef]
- Yang, L.; Berk, S.C.; Rohrer, S.P.; Mosley, R.T.; Guo, L.; Underwood, D.J.; Arison, B.H.; Birzin, E.T.; Hayes, E.C.; Mitra, S.W.; et al. Synthesis and biological activities of potent peptidomimetics selective for somatostatin receptor subtype 2. Proc. Natl. Acad. Sci. USA 1998, 95, 10836–10841. [Google Scholar] [CrossRef] [Green Version]
- Rohrer, S.P.; Birzin, E.T.; Mosley, R.T.; Berk, S.C.; Hutchins, S.M.; Shen, D.-M.; Xiong, Y.; Hayes, E.C.; Parmar, R.M.; Foor, F.; et al. Rapid Identification of Subtype-Selective Agonists of the Somatostatin Receptor Through Combinatorial Chemistry. Science 1998, 282, 737–740. [Google Scholar] [CrossRef]
- Binaschi, A.; Bregola, G.; Simonato, M. On the Role of Somatostatin in Seizure Control: Clues from the Hippocampus. Rev. Neurosci. 2003, 14, 285–301. [Google Scholar] [CrossRef]
- Hoyer, D.; Bell, G.; Berelowitz, M.; Epelbaum, J.; Feniuk, W.; Humphrey, P.; O’Carroll, A.-M.; Patel, Y.; Schonbrunn, A.; Taylor, J.; et al. Classification and nomenclature of somatostatin receptors. Trends Pharmacol. Sci. 1995, 16, 86–88. [Google Scholar] [CrossRef]
- Tallent, M.K. Presynaptic Inhibition of Glutamate Release by Neuropeptides: Use-Dependent Synaptic Modification. Chem. Biol. Pteridines Folates 2007, 44, 177–200. [Google Scholar] [CrossRef]
- Martel, G.; Dutar, P.; Epelbaum, J.; Viollet, C.P. Somatostatinergic systems: An update on brain functions in normal and pathological aging. Front. Endocrinol. 2012, 3, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, S.; Henderson, Z.; Batten, T. Somatostatin immunoreactivity in axon terminals in rat nucleus tractus solitarii arising from central nucleus of amygdala: Coexistence with GABA and postsynaptic expression of sst2A receptor. J. Chem. Neuroanat. 2002, 24, 1–13. [Google Scholar] [CrossRef]
- Jinno, S.; Kosaka, T. Patterns of colocalization of neuronal nitric oxide synthase and somatostatin-like immunoreactivity in the mouse hippocampus: Quantitative analysis with optical disector. Neuroscience 2004, 124, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Chesselet, M.; Graybiel, A. Striatal neurons expressing somatostatin-like immunoreactivity: Evidence for a peptidergic interneuronal system in the cat. Neurosci. 1986, 17, 547–571. [Google Scholar] [CrossRef]
- Forloni, G.; Hohmann, C.; Coyle, J.T. Developmental expression of somatostatin in mouse brain. I. Immunocytochemical studies. Dev. Brain Res. 1990, 53, 6–25. [Google Scholar] [CrossRef]
- Eriksson, L.G. An immunohistochemical study of somatostatin and neurotensin positive neurons in the septal nuclei of the rat brain. Brain Struct. Funct. 1984, 170, 1–10. [Google Scholar] [CrossRef]
- Vincent, S.R.; McIntosh, C.H.S.; Buchan, A.M.J.; Brown, J.C. Central somatostatin systems revealed with monoclonal antibodies. J. Comp. Neurol. 1985, 238, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Johansson, O.; Hökfelt, T.; Elde, R. Immunohistochemical distribution of somatostatin-like immunoreactivity in the central nervous system of the adult rat. Neuroscience 1984, 13, 265-IN2. [Google Scholar] [CrossRef]
- Riedemann, T. Diversity and Function of Somatostatin-Expressing Interneurons in the Cerebral Cortex. Int. J. Mol. Sci. 2019, 20, 2952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iversen, L.L.; Iversen, S.D.; Bloom, F.E.; Douglas, C.L.; Brown, M.R.; Vale, W. Calcium-dependent release of somatostatin and neurotensin from rat brain in vitro. Nat. Cell Biol. 1978, 273, 161–163. [Google Scholar] [CrossRef]
- Bonanno, G.; Parodi, B.; Cafaggi, S.; Raiteri, M. Somatostatin Release from Rat Cerebral Cortex Synaptosomes. J. Neurochem. 1991, 57, 1258–1264. [Google Scholar] [CrossRef]
- Raiteri, M.; Angelini, F.; Levi, G. A simple apparatus for studying the release of neurotransmitters from synaptosomes. Eur. J. Pharmacol. 1974, 25, 411–414. [Google Scholar] [CrossRef]
- Raiteri, L.; Raiteri, M. Synaptosomes Still Viable after 25 Years of Superfusion. Neurochem. Res. 2000, 25, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Freund, T.F.; Buzsáki, G. Interneurons of the Hippocampus. Hippocampus 1996, 6, 347–470. [Google Scholar] [CrossRef]
- Mathé, A.A.; Nomikos, G.G.; Svensson, T.H. In vivo release of somatostatin from rat hippocampus and striatum. Neurosci. Lett. 1993, 149, 201–204. [Google Scholar] [CrossRef]
- Bonanno, G.; Gemignani, A.; Schmid, G.; Severi, P.; Cavazzani, P.; Raiteri, M. Human brain somatostatin release from isolated cortical nerve endings and its modulation through GABAB receptors. Br. J. Pharmacol. 1996, 118, 1441–1446. [Google Scholar] [CrossRef]
- Bonanno, G.; Raiteri, M. Multiple GABAB receptors. Trends Pharmacol. Sci. 1993, 14, 259–261. [Google Scholar] [CrossRef]
- Dournaud, P.; Boudin, H.; Schonbrunn, A.; Tannenbaum, G.S.; Beaudet, A. Interrelationships between Somatostatin sst2A Receptors and Somatostatin-Containing Axons in Rat Brain: Evidence for Regulation of Cell Surface Receptors by Endogenous Somatostatin. J. Neurosci. 1998, 18, 1056–1071. [Google Scholar] [CrossRef] [Green Version]
- Dutar, P.; Vaillend, C.; Viollet, C.; Billard, J.-M.; Potier, B.; Carlo, A.-S.; Ungerer, A.; Epelbaum, J. Spatial learning and synaptic hippocampal plasticity in type 2 somatostatin receptor knock-out mice. Neuroscience 2002, 112, 455–466. [Google Scholar] [CrossRef]
- Mastrodimou, N.; Thermos, K. The somatostatin receptor (sst1) modulates the release of somatostatin in rat retina. Neurosci. Lett. 2004, 356, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Agnati, L.F.; Cortelli, P.; Biagini, G.; Bjelke, B.; Fuxe, K. Different classes of volume transmission signals exist in the central nervous system, and are affected by metabolic signals, temperature gradients, and pressure waves. NeuroReport 1994, 6, 9–12. [Google Scholar] [CrossRef]
- Vizi, E.; Kiss, J.P.; Lendvai, B. Nonsynaptic communication in the central nervous system. Neurochem. Int. 2004, 45, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Herkenham, M. Mismatches between neurotransmitter and receptor localizations in brain: Observations and implications. Neuroscience 1987, 23, 1–38. [Google Scholar] [CrossRef]
- Sun, Q.-Q.; Huguenard, J.R.; Prince, D.A. Somatostatin Inhibits Thalamic Network Oscillations In Vitro: Actions on the GABAergic Neurons of the Reticular Nucleus. J. Neurosci. 2002, 22, 5374–5386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Ke, J.-B.; Wu, H.-J.; Miao, Y.; Li, F.; Yang, X.-L.; Wang, Z. Somatostatin receptor-mediated suppression of gabaergic synaptic transmission in cultured rat retinal amacrine cells. Neuroscience 2014, 273, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Momiyama, T.; Zaborszky, L. Somatostatin Presynaptically Inhibits Both GABA and Glutamate Release Onto Rat Basal Forebrain Cholinergic Neurons. J. Neurophysiol. 2006, 96, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Pittman, Q.; Siggins, G. Somatostatin hyperpolarizes hippocampal pyramidal cells in vitro. Brain Res. 1981, 221, 402–408. [Google Scholar] [CrossRef]
- Moore, S.; Madamba, S.; Joëls, M.; Siggins, G. Somatostatin augments the M-current in hippocampal neurons. Science 1988, 239, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Dodd, J.; Kelly, J.S. Is somatostatin an excitatory transmitter in the hippocampus? Nat. Cell Biol. 1978, 273, 674–675. [Google Scholar] [CrossRef]
- Delfs, J.; Dichter, M. Effects of somatostatin on mammalian cortical neurons in culture: Physiological actions and unusual dose response characteristics. J. Neurosci. 1983, 3, 1176–1188. [Google Scholar] [CrossRef] [Green Version]
- Boehm, S.; Betz, H. Somatostatin Inhibits Excitatory Transmission at Rat Hippocampal Synapses via Presynaptic Receptors. J. Neurosci. 1997, 17, 4066–4075. [Google Scholar] [CrossRef]
- Tallent, M.K.; Siggins, G.R. Somatostatin depresses excitatory but not inhibitory neurotransmission in rat CA1 hippocampus. J. Neurophysiol. 1997, 78, 3008–3018. [Google Scholar] [CrossRef]
- Schulz, S.; Händel, M.; Schreff, M.; Schmidt, H.; Höllt, V. Localization of five somatostatin receptors in the rat central nervous system using subtype-specific antibodies. J. Physiol. 2000, 94, 259–264. [Google Scholar] [CrossRef]
- Bencivinnil, I.; Ferrini, F.; Saliol, C.; Beltramol, M.; Merighil, A. The somatostatin analogue octreotide inhibits capsaicin-mediated activation of nociceptive primary afferent fibres in spinal cord lamina II (substantia gelatinosa). Eur. J. Pain 2011, 15, 591–599. [Google Scholar] [CrossRef]
- Grilli, M.; Raiteri, L.; Pittaluga, A. Somatostatin inhibits glutamate release from mouse cerebrocortical nerve endings through presynaptic sst2 receptors linked to the adenylyl cyclase–protein kinase A pathway. Neuropharmacology 2004, 46, 388–396. [Google Scholar] [CrossRef]
- Ristori, C.; Cammalleri, M.; Martini, D.; Pavan, B.; Liu, Y.; Casini, G.; Monte, M.D.; Bagnoli, P. Involvement of the cAMP-dependent pathway in the reduction of epileptiform bursting caused by somatostatin in the mouse hippocampus. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2008, 378, 563–577. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Storm, D.R. Calmodulin-Regulated Adenylyl Cyclases: Cross-Talk and Plasticity in the Central Nervous System. Mol. Pharmacol. 2003, 63, 463–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cammalleri, M.; Cervia, D.; Monte, M.D.; Martini, D.; Langenegger, D.; Fehlmann, D.; Feuerbach, D.; Pavan, B.; Hoyer, D.; Bagnoli, P. Compensatory changes in the hippocampus of somatostatin knockout mice: Upregulation of somatostatin receptor 2 and its function in the control of bursting activity and synaptic transmission. Eur. J. Neurosci. 2006, 23, 2404–2422. [Google Scholar] [CrossRef] [Green Version]
- Grigoriev, V.V.; Petrova, L.N.; Gabrelian, A.V.; Zamoyski, V.L.; Serkova, T.P.; Bachurin, S.O. Effect of Somatostatin on Presynaptic and Postsynaptic Glutamate Receptors and Postsynaptic GABA Receptors in the Neurons of Rat Brain. Bull. Exp. Biol. Med. 2012, 154, 10–12. [Google Scholar] [CrossRef]
- Pittaluga, A. Presynaptic release-regulating NMDA receptors in isolated nerve terminals: A narrative review. Br. J. Pharmacol. 2021, 178, 1001–1017. [Google Scholar] [CrossRef] [PubMed]
- Tallent, M.K.; Siggins, G.R. Somatostatin Acts in CA1 and CA3 to Reduce Hippocampal Epileptiform Activity. J. Neurophysiol. 1999, 81, 1626–1635. [Google Scholar] [CrossRef]
- Tallent, M.K. Somatostatin in the dentate gyrus. Prog. Brain Res. 2007, 163, 265–284. [Google Scholar] [CrossRef] [PubMed]
- Urban-Ciecko, J.; Fanselow, E.E.; Barth, A.L. Neocortical Somatostatin Neurons Reversibly Silence Excitatory Transmission via GABAb Receptors. Curr. Biol. 2015, 25, 722–731. [Google Scholar] [CrossRef] [Green Version]
- Farah, L.B.; Bowman, B.R.; Bokiniec, P.; Karim, S.; Le, S.; Goodchild, A.K.; McMullan, S. Somatostatin in the rat rostral ventrolateral medulla: Origins and mechanism of action. J. Comp. Neurol. 2016, 524, 323–342. [Google Scholar] [CrossRef] [PubMed]
- Kozhemyakin, M.; Rajasekaran, K.; Todorovic, M.S.; Kowalski, S.L.; Balint, C.; Kapur, J. Somatostatin type-2 receptor activation inhibits glutamate release and prevents status epilepticus. Neurobiol. Dis. 2013, 54, 94–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cammalleri, M.; Martini, D.; Timperio, A.M.; Bagnoli, P. Functional effects of somatostatin receptor 1 activation on synaptic transmission in the mouse hippocampus. J. Neurochem. 2009, 111, 1466–1477. [Google Scholar] [CrossRef]
- Qiu, C.; Zeyda, T.; Johnson, B.; Hochgeschwender, U.; De Lecea, L.; Tallent, M.K. Somatostatin Receptor Subtype 4 Couples to the M-Current to Regulate Seizures. J. Neurosci. 2008, 28, 3567–3576. [Google Scholar] [CrossRef] [PubMed]
- Tallent, M.K.; Qiu, C. Somatostatin: An endogenous antiepileptic. Mol. Cell. Endocrinol. 2008, 286, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Moneta, D.; Richichi, C.; Aliprandi, M.; Dournaud, P.; Dutar, P.; Billard, J.M.; Carlo, A.S.; Viollet, C.; Hannon, J.P.; Fehlmann, D.; et al. Somatostatin receptor subtypes 2 and 4 affect seizure susceptibility and hippocampal excitatory neurotransmission in mice. Eur. J. Neurosci. 2002, 16, 843–849. [Google Scholar] [CrossRef]
- Cammalleri, M.; Liu, Y.; Monte, M.D.; Cervia, D.; Langenegger, D.; Hoyer, D.; Bagnoli, P. Somatostatin receptors differentially affect spontaneous epileptiform activity in mouse hippocampal slices. Eur. J. Neurosci. 2004, 20, 2711–2721. [Google Scholar] [CrossRef] [Green Version]
- Paudice, P.; Gemignani, A.; Raiteri, M. Evidence for functional native NMDA receptors activated by glycine ord-serine alone in the absence of glutamatergic coagonist. Eur. J. Neurosci. 1998, 10, 2934–2944. [Google Scholar] [CrossRef]
- Sun, H.Y.; Bartley, A.F.; Dobrunz, L.E. Calcium-Permeable Presynaptic Kainate Receptors Involved in Excitatory Short-Term Facilitation Onto Somatostatin Interneurons During Natural Stimulus Patterns. J. Neurophysiol. 2009, 101, 1043–1055. [Google Scholar] [CrossRef] [Green Version]
- Fuxe, K.; Agnati, L.F. Receptor-receptor interactions in the central nervous system. A new integrative mechanism in synapses. Med. Res. Rev. 1985, 5, 441–482. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Tarakanov, A.O.; Brito, I.; Fuxe, K. Glutamate heteroreceptor complexes in the brain. Pharmacol. Rep. 2018, 70, 936–950. [Google Scholar] [CrossRef]
- Fuxe, K.; Borroto-Escuela, D.O. Volume transmission and receptor-receptor interactions in heteroreceptor complexes: Understanding the role of new concepts for brain communication. Neural Regen. Res. 2016, 11, 1220–1223. [Google Scholar] [CrossRef]
- Fuxe, K.; Borroto-Escuela, D.O.; Romero-Fernandez, W.; Palkovits, M.; Tarakanov, A.O.; Ciruela, F.; Agnati, L.F. Moonlighting Proteins and Protein–Protein Interactions as Neurotherapeutic Targets in the G Protein-Coupled Receptor Field. Neuropsychopharmacology 2013, 39, 131–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, J.A.; Sebastião, A.M. Modulation and metamodulation of synapses by adenosine. Acta Physiol. 2010, 199, 161–169. [Google Scholar] [CrossRef]
- Olivero, G.; Vergassola, M.; Cisani, F.; Roggeri, A.; Pittaluga, A. Presynaptic Release-regulating Metabotropic Glutamate Receptors: An Update. Curr. Neuropharmacol. 2020, 18, 655–672. [Google Scholar] [CrossRef]
- Di Prisco, S.; Olivero, G.; Merega, E.; Bonfiglio, T.; Marchi, M.; Pittaluga, A. CXCR4 and NMDA Receptors Are Functionally Coupled in Rat Hippocampal Noradrenergic and Glutamatergic Nerve Endings. J. Neuroimmune Pharmacol. 2016, 11, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Olivero, G.; Vergassola, M.; Cisani, F.; Usai, C.; Pittaluga, A. Immuno-Pharmacological Characterization of Presynaptic GluN3A-Containing NMDA Autoreceptors: Relevance to Anti-NMDA Receptor Autoimmune Diseases. Mol. Neurobiol. 2019, 56, 6142–6155. [Google Scholar] [CrossRef] [PubMed]
- Olivero, G.; Grilli, M.; Vergassola, M.; Bonfiglio, T.; Padolecchia, C.; Garrone, B.; Di Giorgio, F.P.; Tongiani, S.; Usai, C.; Marchi, M.; et al. 5-HT2A-mGlu2/3 receptor complex in rat spinal cord glutamatergic nerve endings: A 5-HT2A to mGlu2/3 signalling to amplify presynaptic mechanism of auto-control of glutamate exocytosis. Neuropharmacology 2018, 133, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Durán-Prado, M.; Malagón, M.M.; Gracía-Navarro, F.; Castaño, J.P. Dimerization of G protein-coupled receptors: New avenues for somatostatin receptor signalling, control and functioning. Mol. Cell. Endocrinol. 2008, 286, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Blake, A.C.B.A.M.Z.S.A.D.; Badway, A.; Strowsk, M. Delineating Somatostatins Neuronal Actions. Curr. Drug Target -CNS Neurol. Disord. 2004, 3, 153–160. [Google Scholar] [CrossRef]
- Gastambide, F.; Lepousez, G.; Viollet, C.; Loudes, C.; Epelbaum, J.; Guillou, J.-L. Cooperation between hippocampal somatostatin receptor subtypes 4 and 2: Functional relevance in interactive memory systems. Hippocampus 2009, 20, 745–757. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Koch, T.; Schröder, H.; Laugsch, M.; Höllt, V.; Schulz, S. Heterodimerization of Somatostatin and Opioid Receptors Cross-modulates Phosphorylation, Internalization, and Desensitization. J. Biol. Chem. 2002, 277, 19762–19772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baragli, A.; Alturaihi, H.; Watt, H.L.; Abdallah, A.; Kumar, U. Heterooligomerization of human dopamine receptor 2 and somatostatin receptor 2: Co-immunoprecipitation and fluorescence resonance energy transfer analysis. Cell. Signal. 2007, 19, 2304–2316. [Google Scholar] [CrossRef] [PubMed]
- Rocheville, M.; Lange, D.C.; Kumar, U.; Patel, S.C.; Patel, R.C.; Patel, Y.C. Receptors for Dopamine and Somatostatin: Formation of Hetero-Oligomers with Enhanced Functional Activity. Science 2000, 288, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Doucet, G.; Oleskevich, S.; Descarries, L. Quantified regional and laminar distribution of the noradrenaline innervation in the anterior half of the adult rat cerebral cortex. J. Comp. Neurol. 1988, 274, 307–318. [Google Scholar] [CrossRef]
- Epelbaum, J.; Bluet-Pajot, M.; Llorens-Cortes, C.; Kordon, C.; Mounier, F.; Senut, M.; Videau, C. 125I-[Tyr0,D-Trp8]somatostatin-14 binding sites in the locus coeruleus of the rat are located on both ascending and descending projecting noradrenergic cells. Peptides 1990, 11, 21–27. [Google Scholar] [CrossRef]
- Gagne, C.; Moyse, E.; Kocher, L.; Bour, H.; Pujol, J. Light-microscopic localization of somatostatin binding sites in the locus coeruleus of the rat. Brain Res. 1990, 530, 196–204. [Google Scholar] [CrossRef]
- Pittaluga, A.; Bonfanti, A.; Raiteri, M. Somatostatin potentiates NMDA receptor function via activation of InsP3 receptors and PKC leading to removal of the Mg2+ block without depolarization. Br. J. Pharmacol. 2000, 130, 557–566. [Google Scholar] [CrossRef] [Green Version]
- Benquet, P.; Gee, C.E.; Gerber, U. Two Distinct Signaling Pathways Upregulate NMDA Receptor Responses via Two Distinct Metabotropic Glutamate Receptor Subtypes. J. Neurosci. 2002, 22, 9679–9686. [Google Scholar] [CrossRef] [Green Version]
- Bleakman, D.; Rusin, K.I.; Chard, P.S.; Glaum, S.R.; Miller, R.J. Metabotropic glutamate receptors potentiate ionotropic glutamate responses in the rat dorsal horn. Mol. Pharmacol. 1992, 42, 192–196. [Google Scholar] [PubMed]
- Pittaluga, A.; Feligioni, M.; Ghersi, C.; Gemignani, A.; Raiteri, M. Potentiation of NMDA receptor function through somatostatin release: A possible mechanism for the cognition-enhancing activity of GABAB receptor antagonists. Neuropharmacology 2001, 41, 301–310. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pittaluga, A.; Roggeri, A.; Vallarino, G.; Olivero, G. Somatostatin, a Presynaptic Modulator of Glutamatergic Signal in the Central Nervous System. Int. J. Mol. Sci. 2021, 22, 5864. https://doi.org/10.3390/ijms22115864
Pittaluga A, Roggeri A, Vallarino G, Olivero G. Somatostatin, a Presynaptic Modulator of Glutamatergic Signal in the Central Nervous System. International Journal of Molecular Sciences. 2021; 22(11):5864. https://doi.org/10.3390/ijms22115864
Chicago/Turabian StylePittaluga, Anna, Alessandra Roggeri, Giulia Vallarino, and Guendalina Olivero. 2021. "Somatostatin, a Presynaptic Modulator of Glutamatergic Signal in the Central Nervous System" International Journal of Molecular Sciences 22, no. 11: 5864. https://doi.org/10.3390/ijms22115864
APA StylePittaluga, A., Roggeri, A., Vallarino, G., & Olivero, G. (2021). Somatostatin, a Presynaptic Modulator of Glutamatergic Signal in the Central Nervous System. International Journal of Molecular Sciences, 22(11), 5864. https://doi.org/10.3390/ijms22115864