Graphene Oxide Loaded with Protocatechuic Acid and Chlorogenic Acid Dual Drug Nanodelivery System for Human Hepatocellular Carcinoma Therapeutic Application
Abstract
1. Introduction
2. Results and Discussion
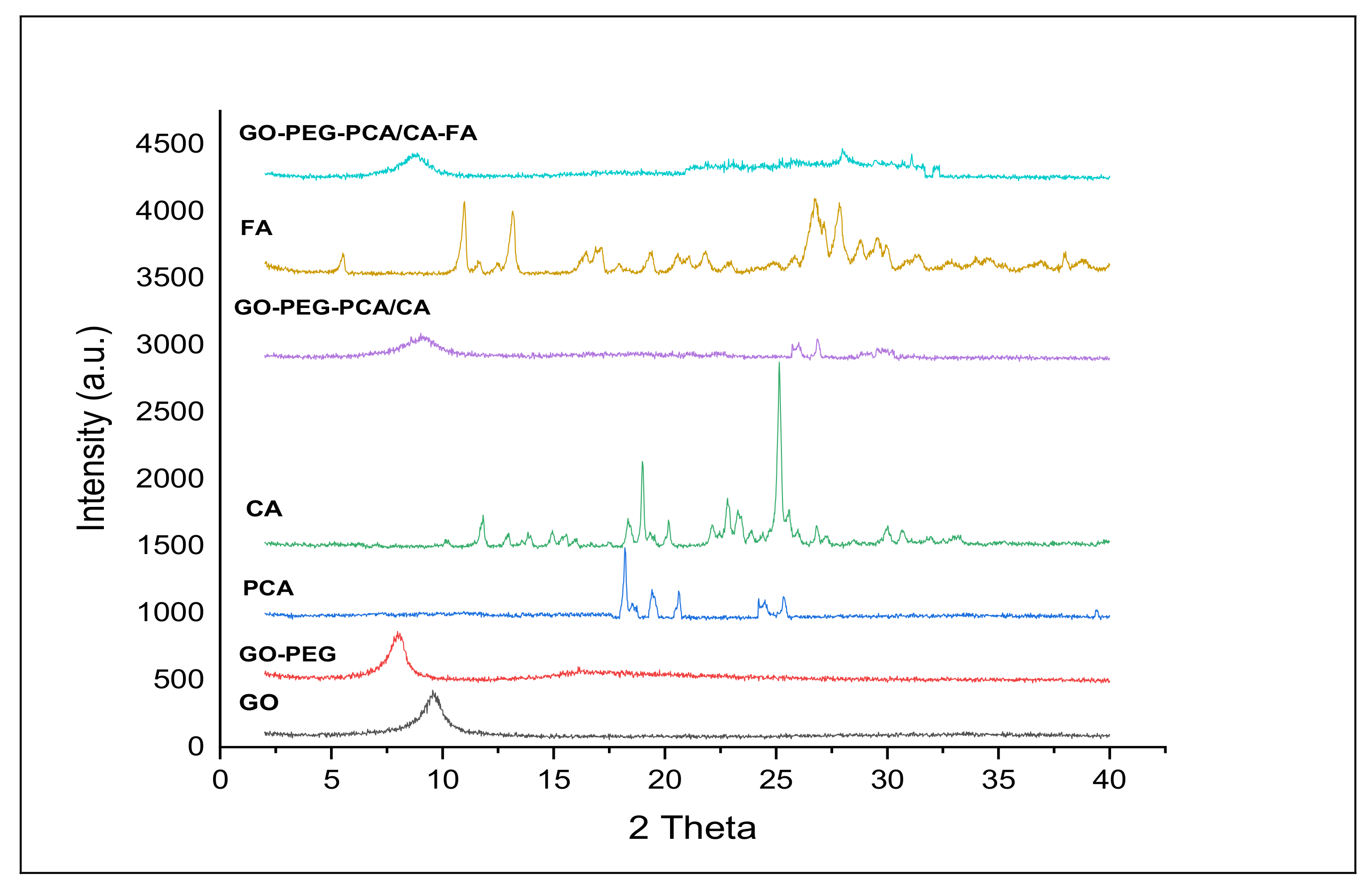
2.1. X-ray Diffraction Analysis of the Nanocomposites
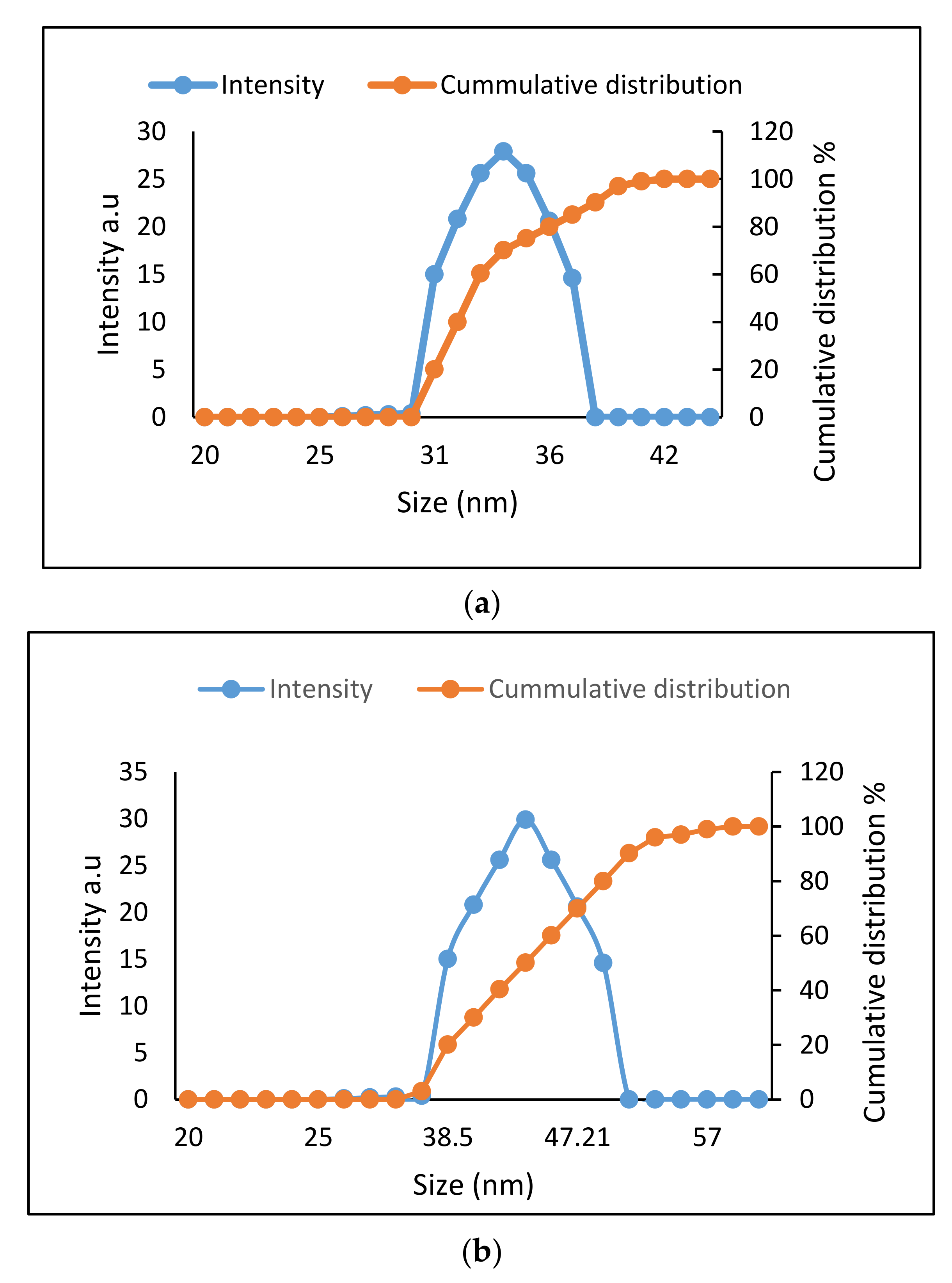
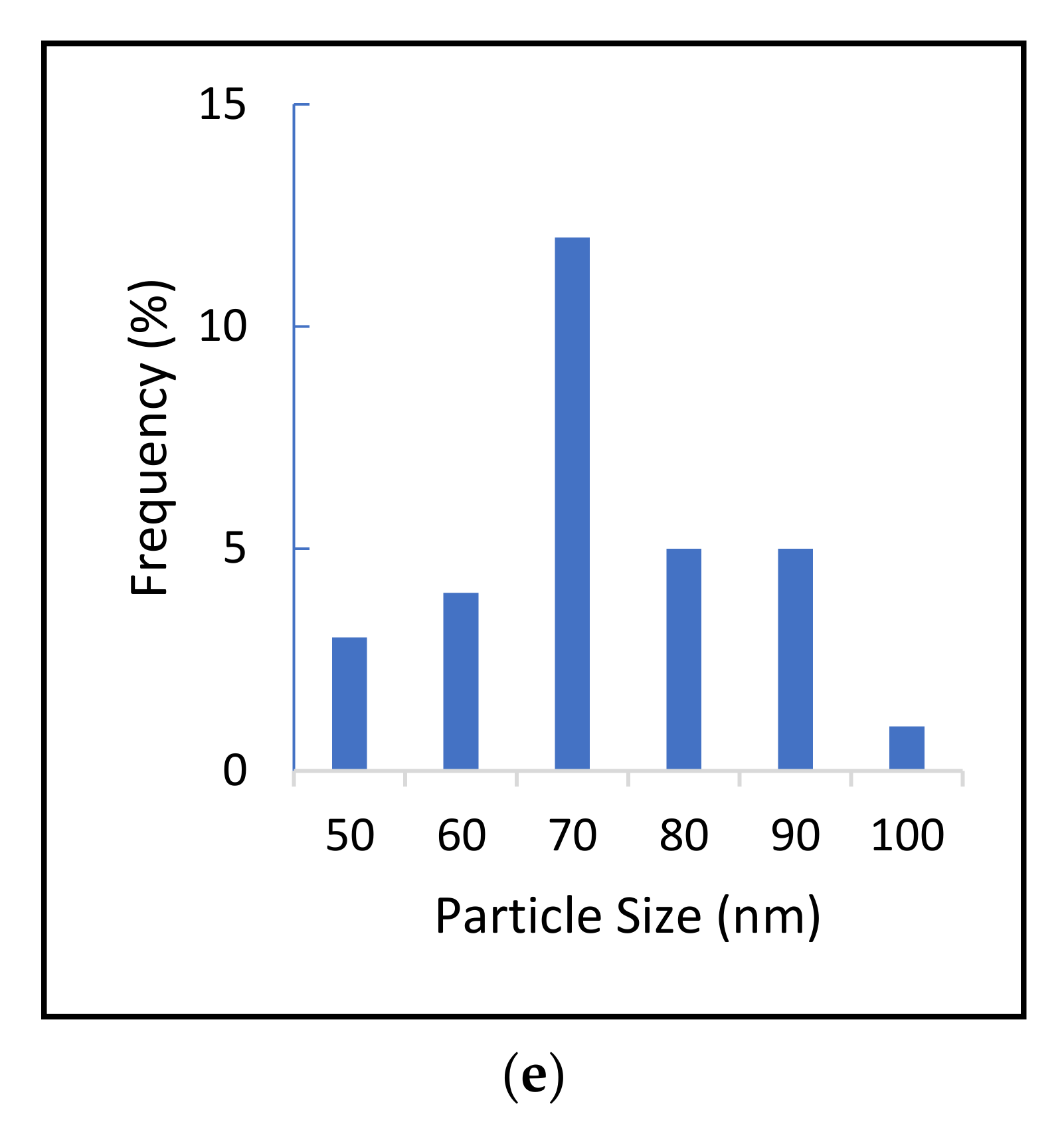
2.2. Determination of Size Distribution and Zeta Potential Measurement of Nanocomposites
2.3. Quantification of Dug Loading and Encapsulation Efficacy
2.4. Surface Properties Analysis
2.5. Protocatechuic Acid and Chlorogenic Acid Drug Release Study of Nanocomposites
2.6. Cell Viability Effect of Nanocomposites on Normal and Cancer Cells
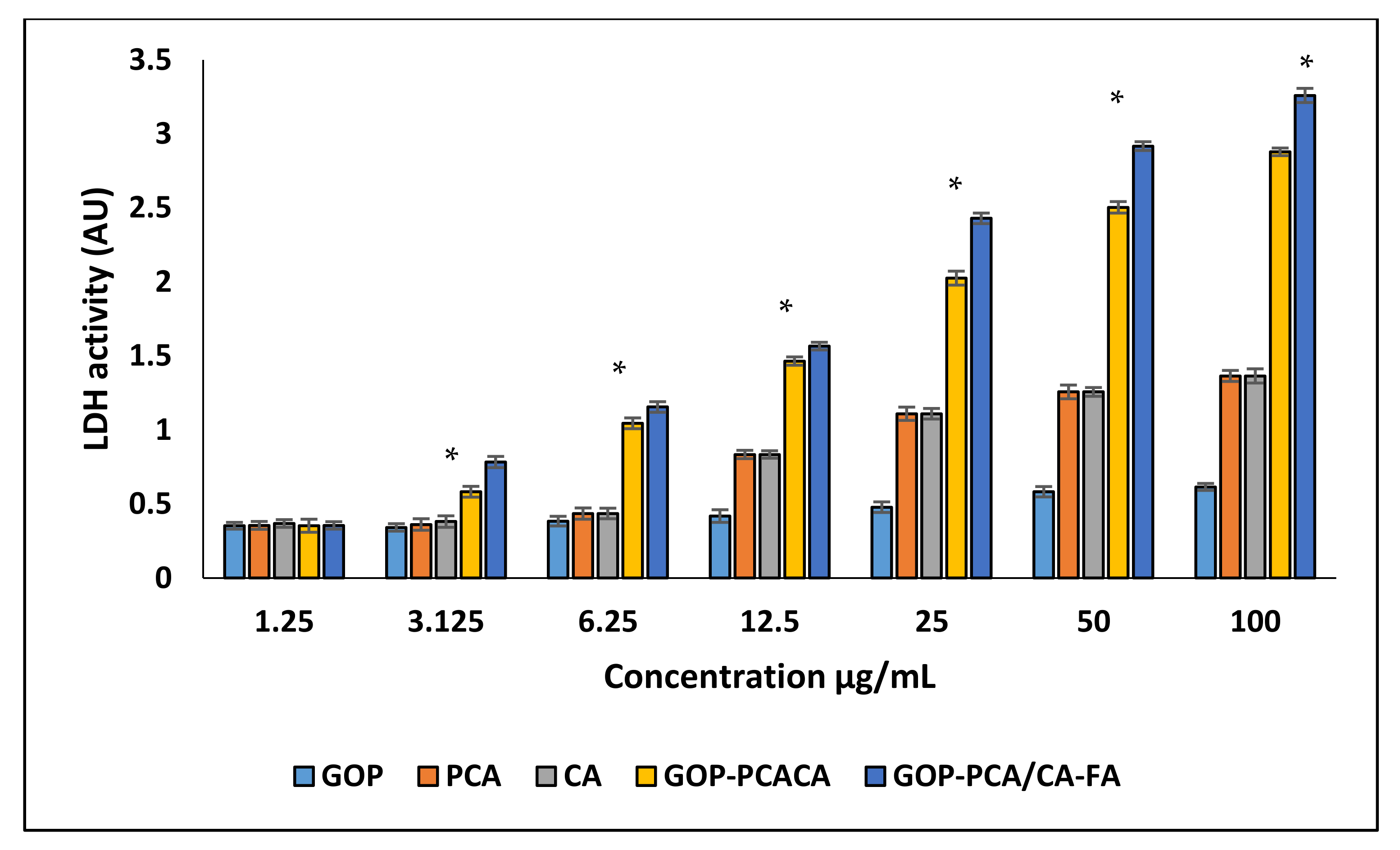
2.7. Plasma Membrane Integrity Analysis
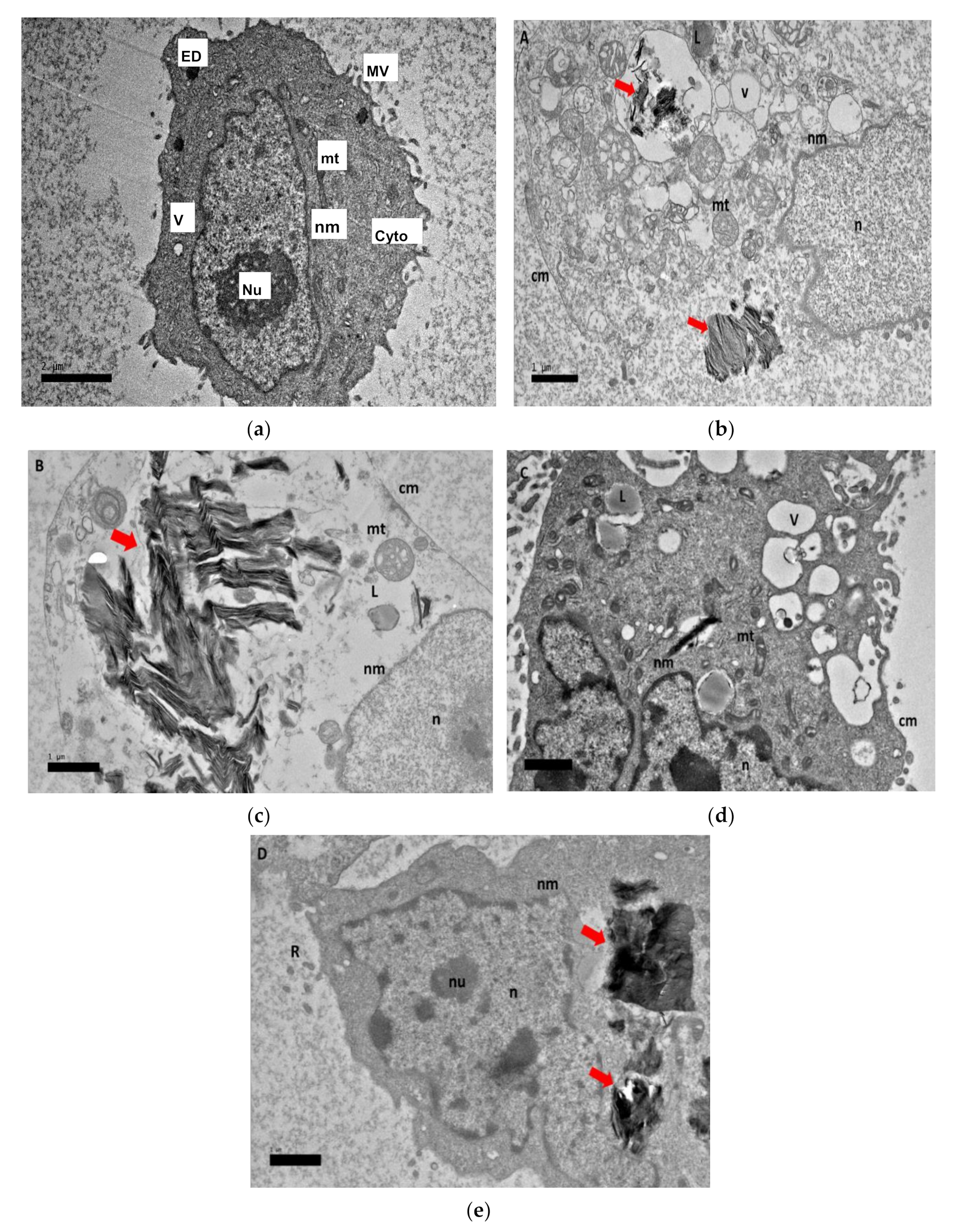
2.8. Morphological Observation of Nanocomposite Cellular Uptake
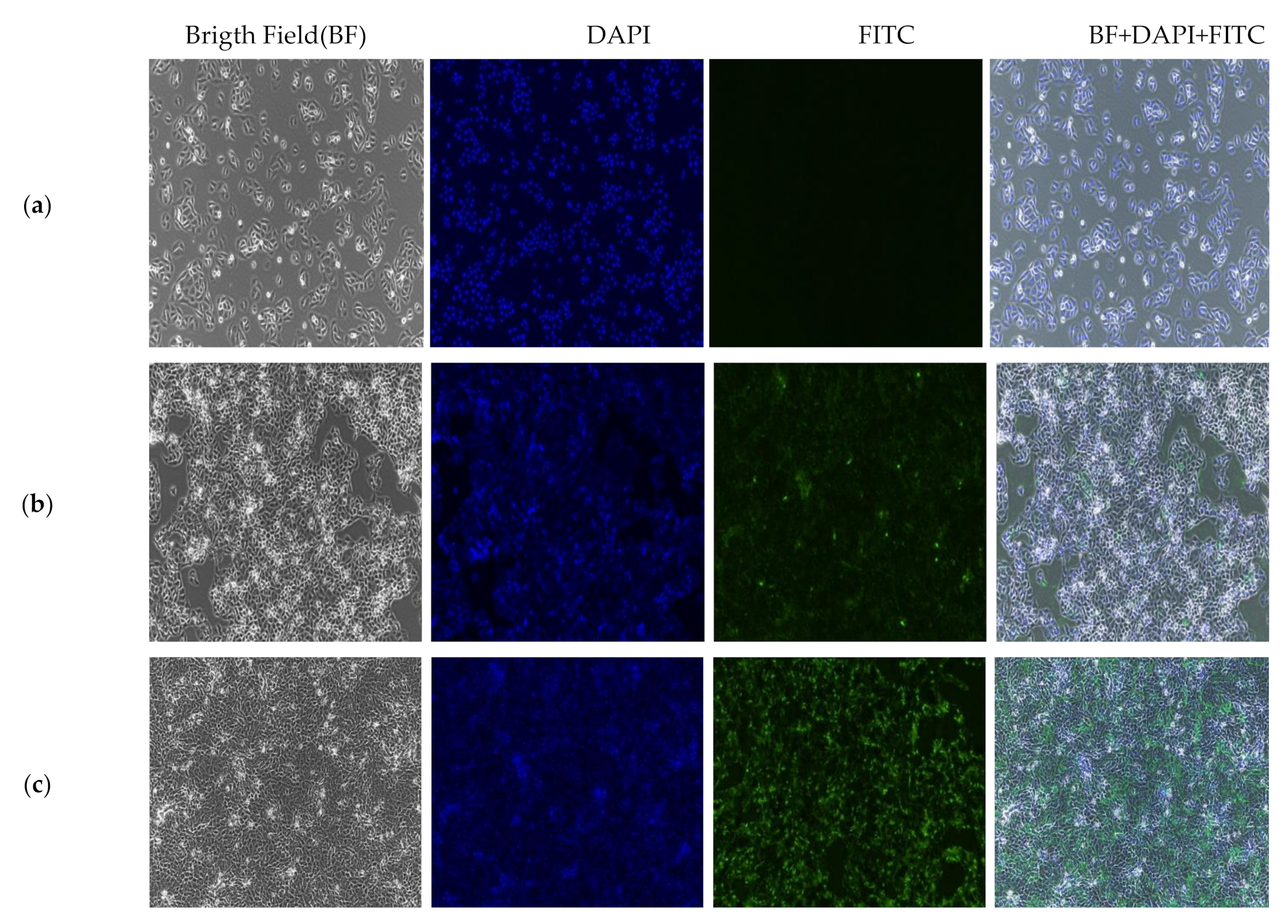
2.9. In Vitro Localization and Accumulation of Nanocomposite
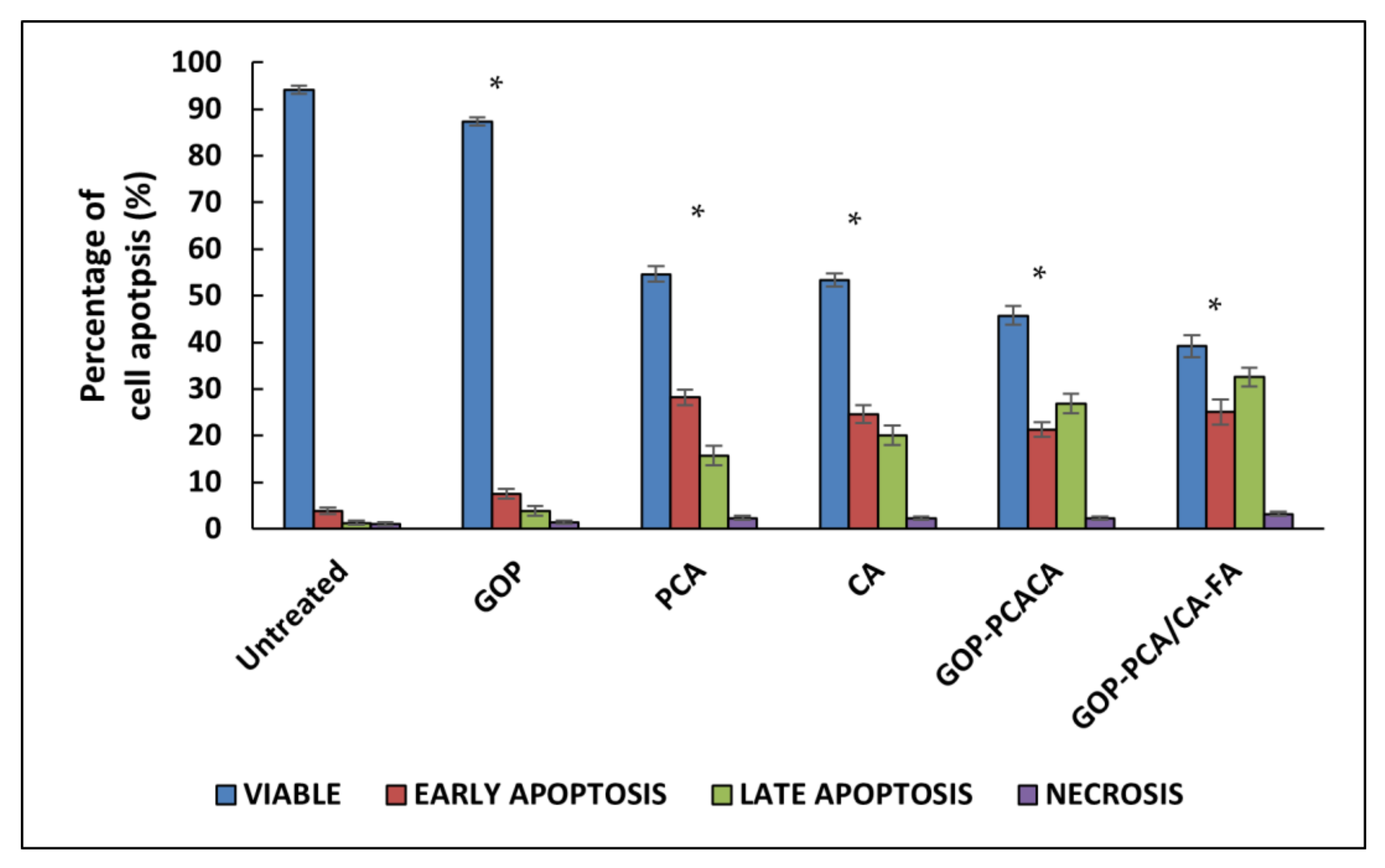
2.10. Apoptosis Induced by Nanocomoposites in HepG2 Cells via Annexin V-FITC/Propidium Iodide (PI) Staining Analysis
2.11. The Effect of Nanocomposite on Cell Cycle Distribution in HepG2 Cells
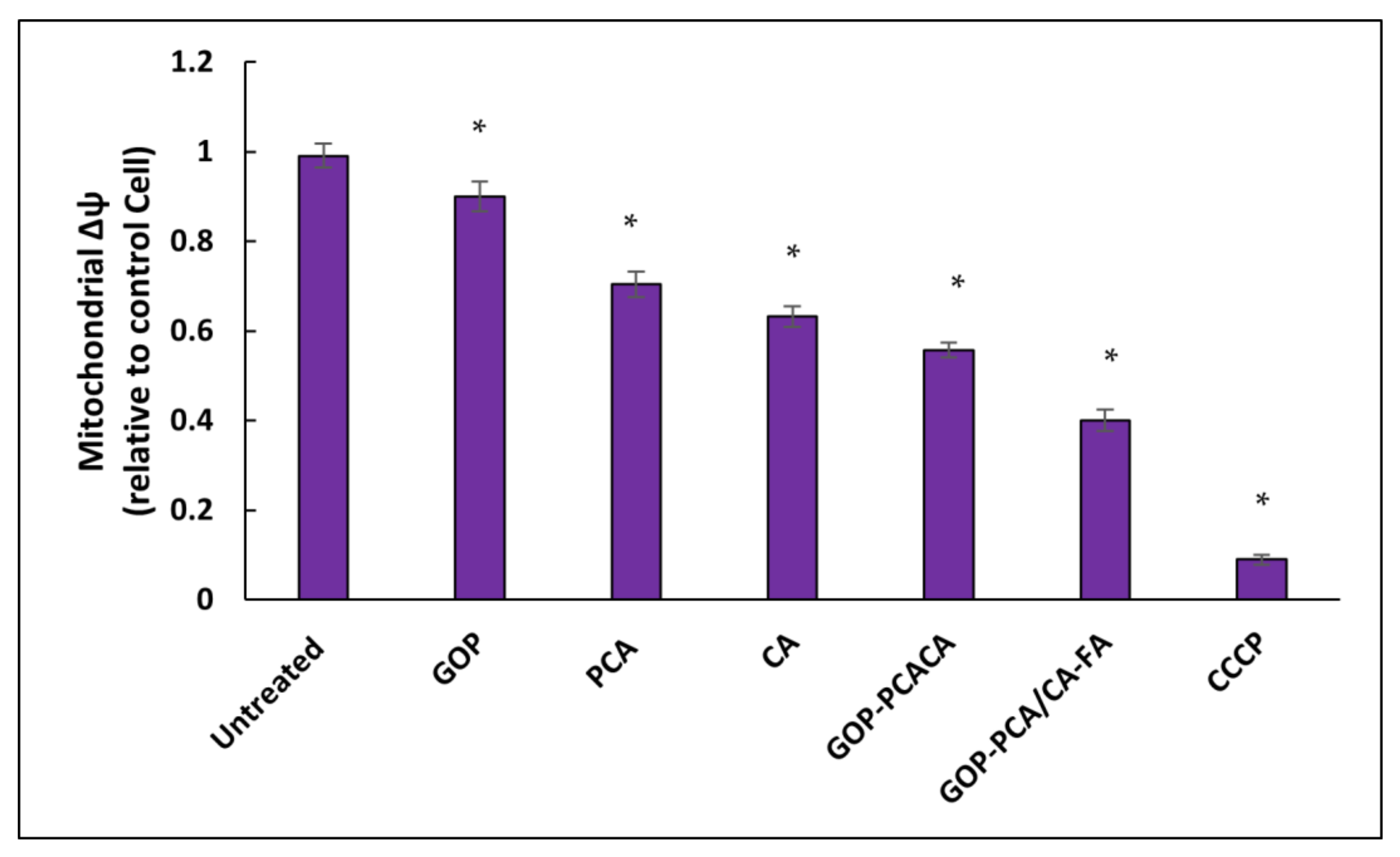
2.12. Nanocomposite Stimulates Mitochondrial Membrane Potential in HepG2 Cells
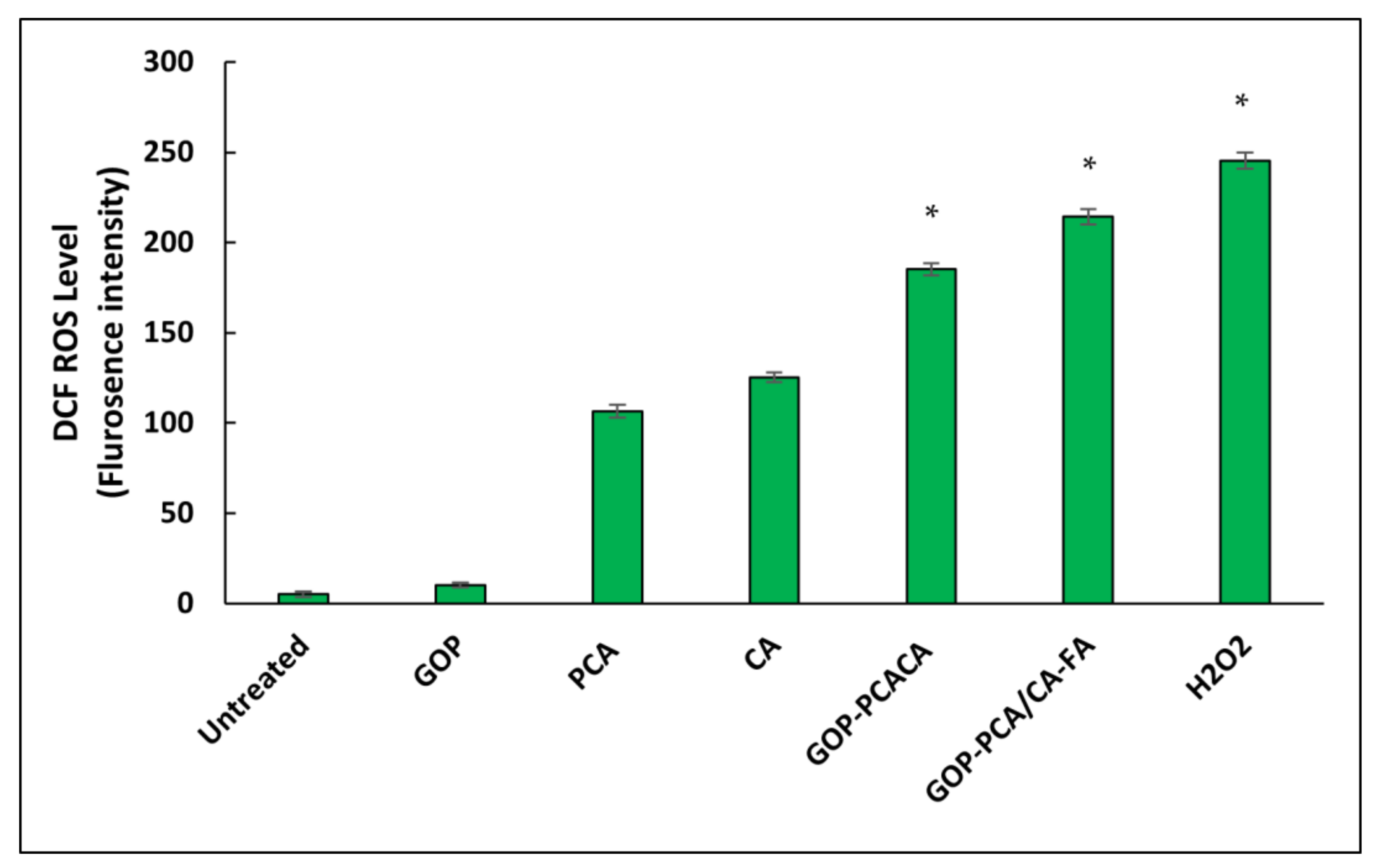
2.13. Nanocomposite Induces Intracellular Reactive Oxygen Species Generation in HepG2 Cells
3. Materials and Methods
3.1. Materials
3.2. Cell Lines
3.3. Synthesis of Graphene Oxide and Conjugation with Polyethylene Glycol
3.4. Protocatechuic Acid and Chlorogenic Acid Loading on Graphene Oxide with PEG and Coated with Folic Acid
3.5. Synthesis of FITC-Labelled GOP-PCA/CA-FA
3.6. Physicochemical Characterization of Nanocomposites
3.7. Encapsulation Efficacy and Drug Loading Content Analysis
3.8. In Vitro Drug Release of Protocatechuic Acid and Chlorogenic Acid from Nanocomposites
3.9. In Vitro Cell Cytotoxicity Assay
3.10. Lactate Dehydrogenase Assay for Plasma Membrane Integrity Analysis
3.11. Morphological Observation of Nanocomposite Cellular Uptake
3.12. In Vitro Localization of Nanocomposite by Fluorescent Microscopy Analysis
3.13. Apoptosis Cell Death Analysis
3.14. Cell Cycle Arrest Analysis Using Propidium Iodide
3.15. Mitochondrial Membrane Potential Analysis
3.16. Measurement of Cellular Reactive Oxygen Species
3.17. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghouri, Y.A.; Mian, I.; Rowe, J.H. Review of Hepatocellular Carcinoma: Epidemiology, Etiology, and Carcinogenesis. J. Carcinog. 2017, 16. [Google Scholar] [CrossRef]
- Balogh, J.; Victor, D.; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.M.; Monsour, H.P. Hepatocellular Carcinoma: A Review. J. Hepatocell. Carcinoma 2016, 3, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Ashtari, S.; Pourhoseingholi, M.A.; Sharifian, A.; Zali, M.R. Hepatocellular Carcinoma in Asia: Prevention Strategy and Planning. World J. Hepatol. 2015, 7, 1708–1717. [Google Scholar] [CrossRef]
- Raza, A.; Sood, G.K. Hepatocellular Carcinoma Review: Current Treatment, and Evidence-Based Medicine. World J. Gastroenterol. WJG 2014, 20, 4115–4127. [Google Scholar] [CrossRef]
- Sharma, H.; Mondal, S. Functionalized Graphene Oxide for Chemotherapeutic Drug Delivery and Cancer Treatment: A Promising Material in Nanomedicine. Int. J. Mol. Sci. 2020, 21, 6280. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.-L.; Razlan, H.; Hartono, J.L.; Qua, C.-S.; Yoong, B.-K.; Koh, P.-S.; Abdullah, B.J.J. Liver Cancer in Malaysia: Epidemiology and Clinical Presentation in a Multiracial Asian Population. J. Dig. Dis. 2015, 16, 152–158. [Google Scholar] [CrossRef]
- Saifullah, B.; Buskaran, K.; Shaikh, R.B.; Barahuie, F.; Fakurazi, S.; Mohd-Moklas, M.A.; Hussein, M.Z. Graphene Oxide–PEG–Protocatechuic Acid Nanocomposite Formulation with Improved Anticancer Properties. Nanomaterials 2018, 8, 820. [Google Scholar] [CrossRef]
- Bullo, S.; Buskaran, K.; Baby, R.; Dorniani, D.; Fakurazi, S.; Hussein, M.Z. Dual Drugs Anticancer Nanoformulation Using Graphene Oxide-PEG as Nanocarrier for Protocatechuic Acid and Chlorogenic Acid. Pharm. Res. 2019, 36, 91. [Google Scholar] [CrossRef]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current Trends and Challenges in Cancer Management and Therapy Using Designer Nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef]
- Narvekar, M.; Xue, H.Y.; Eoh, J.Y.; Wong, H.L. Nanocarrier for Poorly Water-Soluble Anticancer Drugs—Barriers of Translation and Solutions. AAPS PharmSciTech 2014, 15, 822–833. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Qasim, M.; Kim, J.-H. Nanoparticle-Mediated Combination Therapy: Two-in-One Approach for Cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef] [PubMed]
- Buskaran, K.; Hussein, M.Z.; Mohd-Moklas, M.A.; Fakurazi, S. Morphological Changes and Cellular Uptake of Functionalized Graphene Oxide Loaded with Protocatechuic Acid and Folic Acid in Hepatocellular Carcinoma Cancer Cell. Int. J. Mol. Sci. 2020, 21, 5874. [Google Scholar] [CrossRef] [PubMed]
- Perveen, S.; Al-Taweel, A.M. Phenolic Compounds from the Natural Sources and Their Cytotoxicity. Phenolic Compd. Nat. Sources Importance Appl. 2017. [Google Scholar] [CrossRef]
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological Properties of Protocatechuic Acid and Its Potential Roles as Complementary Medicine. Available online: https://www.hindawi.com/journals/ecam/2015/593902/ (accessed on 12 December 2020).
- Lin, W.-L.; Hsieh, Y.-J.; Chou, F.-P.; Wang, C.-J.; Cheng, M.-T.; Tseng, T.-H. Hibiscus Protocatechuic Acid Inhibits Lipopolysaccharide-Induced Rat Hepatic Damage. Arch. Toxicol. 2003, 77, 42–47. [Google Scholar] [CrossRef]
- Pimpão, R.C.; Dew, T.; Figueira, M.E.; McDougall, G.J.; Stewart, D.; Ferreira, R.B.; Santos, C.N.; Williamson, G. Urinary Metabolite Profiling Identifies Novel Colonic Metabolites and Conjugates of Phenolics in Healthy Volunteers. Mol. Nutr. Food Res. 2014, 58, 1414–1425. [Google Scholar] [CrossRef]
- González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S.; Tuñón, M.J. Fruit Polyphenols, Immunity and Inflammation. Br. J. Nutr. 2010, 104, S15–S27. [Google Scholar] [CrossRef]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic Acid: A Comprehensive Review of the Dietary Sources, Processing Effects, Bioavailability, Beneficial Properties, Mechanisms of Action, and Future Directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Fernández, M.; Javaid, F.; Chudasama, V. Advances in Targeting the Folate Receptor in the Treatment/Imaging of Cancers. Chem. Sci. 2018, 9, 790–810. [Google Scholar] [CrossRef]
- Xing, L.; Xu, Y.; Sun, K.; Wang, H.; Zhang, F.; Zhou, Z.; Zhang, J.; Zhang, F.; Caliskan, B.; Qiu, Z.; et al. Identification of a Peptide for Folate Receptor Alpha by Phage Display and Its Tumor Targeting Activity in Ovary Cancer Xenograft. Sci. Rep. 2018, 8, 8426. [Google Scholar] [CrossRef]
- Sakurai, Y.; Kajimoto, K.; Harashima, H. Anti-Angiogenic Nanotherapy via Active Targeting Systems to Tumors and Adipose Tissue Vasculature. Biomater. Sci. 2015, 3, 1253–1265. [Google Scholar] [CrossRef]
- Morales-Cruz, M.; Delgado, Y.; Castillo, B.; Figueroa, C.M.; Molina, A.M.; Torres, A.; Milián, M.; Griebenow, K. Smart Targeting To Improve Cancer Therapeutics. Drug Des. Devel. Ther. 2019, 13, 3753–3772. [Google Scholar] [CrossRef] [PubMed]
- Buskaran, K.; Bullo, S.; Hussein, M.Z.; Masarudin, M.J.; Mohd-Moklas, M.A.; Fakurazi, S. Anticancer Molecular Mechanism of Protocatechuic Acid Loaded on Folate Coated Functionalized Graphene Oxide Nanocomposite Delivery System in Human Hepatocellular Carcinoma. Materials 2021, 14, 817. [Google Scholar] [CrossRef] [PubMed]
- Colone, M.; Calcabrini, A.; Stringaro, A. Drug Delivery Systems of Natural Products in Oncology. Molecules 2020, 25, 4560. [Google Scholar] [CrossRef]
- Naso, L.G.; Valcarcel, M.; Roura-Ferrer, M.; Kortazar, D.; Salado, C.; Lezama, L.; Rojo, T.; González-Baró, A.C.; Williams, P.A.M.; Ferrer, E.G. Promising Antioxidant and Anticancer (Human Breast Cancer) Oxidovanadium(IV) Complex of Chlorogenic Acid. Synthesis, Characterization and Spectroscopic Examination on the Transport Mechanism with Bovine Serum Albumin. J. Inorg. Biochem. 2014, 135, 86–99. [Google Scholar] [CrossRef]
- Li, Z.-J.; Li, C.; Zheng, M.-G.; Pan, J.-D.; Zhang, L.-M.; Deng, Y.-F. Functionalized Nano-Graphene Oxide Particles for Targeted Fluorescence Imaging and Photothermy of Glioma U251 Cells. Int. J. Clin. Exp. Med. 2015, 8, 1844–1852. [Google Scholar]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Barahuie, F.; Hussein, M.Z.; Hussein-Al-Ali, S.H.; Arulselvan, P.; Fakurazi, S.; Zainal, Z. Preparation and Controlled-Release Studies of a Protocatechuic Acid-Magnesium/Aluminum-Layered Double Hydroxide Nanocomposite. Int. J. Nanomed. 2013, 8, 1975–1987. [Google Scholar] [CrossRef] [PubMed]
- Sathyamoorthy, N.; Dhanaraju, M.D.; Devendiran, S.; Battu, H. Influence of Surface Charge on the in Vitro Protein Adsorption and Cell Cytotoxicity of Paclitaxel Loaded Poly(ε-Caprolactone) Nanoparticles. Bull. Fac. Pharm. Cairo Univ. 2017, 55. [Google Scholar] [CrossRef]
- Gunasekaran, T.; Haile, T.; Nigusse, T.; Dhanaraju, M.D. Nanotechnology: An Effective Tool for Enhancing Bioavailability and Bioactivity of Phytomedicine. Asian Pac. J. Trop. Biomed. 2014, 4, S1–S7. [Google Scholar] [CrossRef]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle Uptake: The Phagocyte Problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef]
- Wang, N.; Cheng, X.; Li, N.; Wang, H.; Chen, H. Nanocarriers and Their Loading Strategies. Adv. Healthc. Mater. 2019, 8, 1801002. [Google Scholar] [CrossRef] [PubMed]
- Yee-Kuen, C.; Galen, T.; Fakurazi, S.; Othman, S.S.; Masarudin, M.J. Increased Cytotoxic Efficacy of Protocatechuic Acid in A549 Human Lung Cancer Delivered via Hydrophobically Modified-Chitosan Nanoparticles As an Anticancer Modality. Polymers 2020, 12, 1951. [Google Scholar] [CrossRef]
- Hunt, P.A.; Ashworth, C.R.; Matthews, R.P. Hydrogen Bonding in Ionic Liquids. Chem. Soc. Rev. 2015, 44, 1257–1288. [Google Scholar] [CrossRef] [PubMed]
- White, K.A.; Grillo-Hill, B.K.; Barber, D.L. Cancer Cell Behaviors Mediated by Dysregulated PH Dynamics at a Glance. J. Cell Sci. 2017, 130, 663–669. [Google Scholar] [CrossRef]
- Damaghi, M.; Wojtkowiak, J.W.; Gillies, R.J. PH Sensing and Regulation in Cancer. Front. Physiol. 2013, 4, 370. [Google Scholar] [CrossRef] [PubMed]
- Kabir, F.; Katayama, S.; Tanji, N.; Nakamura, S. Antimicrobial Effects of Chlorogenic Acid and Related Compounds. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 359–365. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of Chlorogenic Acid on Regulating Glucose and Lipids Metabolism: A Review. Available online: https://www.hindawi.com/journals/ecam/2013/801457/ (accessed on 16 February 2021).
- Liechty, W.B.; Peppas, N.A. Expert Opinion: Responsive Polymer Nanoparticles in Cancer Therapy. Eur. J. Pharm. Biopharm. 2012, 80, 241–246. [Google Scholar] [CrossRef]
- Koirala, N.; Das, D.; Fayazzadeh, E.; Sen, S.; McClain, A.; Puskas, J.E.; Drazba, J.A.; McLennan, G. Folic Acid Conjugated Polymeric Drug Delivery Vehicle for Targeted Cancer Detection in Hepatocellular Carcinoma. J. Biomed. Mater. Res. A 2019, 107, 2522–2535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xia, J.; Zhao, Q.; Liu, L.; Zhang, Z. Functional Graphene Oxide as a Nanocarrier for Controlled Loading and Targeted Delivery of Mixed Anticancer Drugs. Small Weinh. Bergstr. Ger. 2010, 6, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Lammel, T.; Boisseaux, P.; Fernández-Cruz, M.-L.; Navas, J.M. Internalization and Cytotoxicity of Graphene Oxide and Carboxyl Graphene Nanoplatelets in the Human Hepatocellular Carcinoma Cell Line Hep G2. Part. Fibre Toxicol. 2013, 10, 27. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Vandenabeele, P.; Abrams, J.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; El-Deiry, W.S.; Golstein, P.; Green, D.R.; et al. Classification of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009, 16, 3–11. [Google Scholar] [CrossRef]
- Johnson, M. FITC/Fluorescein. Mater. Methods 2021. [Google Scholar] [CrossRef]
- Hassan, U.A.; Hussein, M.Z.; Alitheen, N.B.; Yahya-Ariff, S.A.; Masarudin, M.J. In Vitro Cellular Localization and Efficient Accumulation of Fluorescently Tagged Biomaterials from Monodispersed Chitosan Nanoparticles for Elucidation of Controlled Release Pathways for Drug Delivery Systems. Int. J. Nanomed. 2018, 13, 5075–5095. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Reutelingsperger, C. Flow Cytometry of Apoptotic Cell Death. J. Immunol. Methods 2000, 243, 167–190. [Google Scholar] [CrossRef]
- Wlodkowic, D.; Telford, W.; Skommer, J.; Darzynkiewicz, Z. Apoptosis and beyond: Cytometry in Studies of Programmed Cell Death. Methods Cell Biol. 2011, 103, 55–98. [Google Scholar] [CrossRef]
- Demchenko, A.P. Beyond Annexin V: Fluorescence Response of Cellular Membranes to Apoptosis. Cytotechnology 2013, 65, 157–172. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Chen, B.; Xia, G.; Wang, S.; Cheng, J.; Shao, Z.; Gao, C.; Bao, W.; Tian, L.; et al. Effect of Magnetic Nanoparticles on Apoptosis and Cell Cycle Induced by Wogonin in Raji Cells. Int. J. Nanomed. 2012, 7, 789–798. [Google Scholar] [CrossRef]
- Evan, G.I.; Vousden, K.H. Proliferation, Cell Cycle and Apoptosis in Cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the Mitochondrial Membrane Potential Using the Cationic JC-1 Dye as a Sensitive Fluorescent Probe. Bio-Protocol 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial Membrane Potential Probes and the Proton Gradient: A Practical Usage Guide. BioTechniques 2011, 50, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Gao, H.; Ching, C.B. Comparative Protein Profile of Human Hepatoma HepG2 Cells Treated with Graphene and Single-Walled Carbon Nanotubes: An ITRAQ-Coupled 2D LC-MS/MS Proteome Analysis. Toxicol. Lett. 2011, 207, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Bermejo, R.; Hernández-Hernández, A. The Importance of NADPH Oxidases and Redox Signaling in Angiogenesis. Antioxid. Basel Switz. 2017, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Hockenbery, D.M. Mitochondria and Cell Death; Humana Press: Totowa, NJ, USA, 2016. [Google Scholar]







| Nanocomposites | ζ-Potential (mV) | ||
|---|---|---|---|
| Deionized Water | Eagle’s Minimum Essential Medium | Eagle’s Minimum Essential Medium + 10% FBS | |
| GOP | −8.99 ± 2.021 | −14.15 ± 1.224 | −6.42 ± 1.302 |
| GOP-PCA/CA | −17.10 ± 1.072 | −22.19 ± 1.151 | −15.44 ± 1.932 |
| GOP-PCA/CA-FA | −22.72 ± 2.311 | −29.28 ± 1.618 | −19.76 ± 2.041 |
| Nanocomposites | Loading Content (%) | Encapsulation Efficiency (%) | ||
|---|---|---|---|---|
| PCA | CA | PCA | CA | |
| GOP-PCA/CA | 23.82 | 19.55 | 75.23 | 72.11 |
| GOP-PCA/CA-FA | 24.47 | 23.33 | 79.16 | 75.15 |
| Nanocomposites IC50 (μg/mL) | HDFa Dermal Fibroblast Cell | HepG2 Cell |
|---|---|---|
| Graphene oxide-PEG (GOP) | N.C | N.C |
| Protocatechuic acid (PCA) | N.C | 40.78 ± 1.92 |
| Chlorogenic acid (CA) | N.C | 43.61 ± 1.74 |
| GOP-PCA/CA | N.C | 34.73 ± 1.04 |
| GOP-PCA/CA-FA | N.C | 26.79 ± 1.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buskaran, K.; Hussein, M.Z.; Moklas, M.A.M.; Masarudin, M.J.; Fakurazi, S. Graphene Oxide Loaded with Protocatechuic Acid and Chlorogenic Acid Dual Drug Nanodelivery System for Human Hepatocellular Carcinoma Therapeutic Application. Int. J. Mol. Sci. 2021, 22, 5786. https://doi.org/10.3390/ijms22115786
Buskaran K, Hussein MZ, Moklas MAM, Masarudin MJ, Fakurazi S. Graphene Oxide Loaded with Protocatechuic Acid and Chlorogenic Acid Dual Drug Nanodelivery System for Human Hepatocellular Carcinoma Therapeutic Application. International Journal of Molecular Sciences. 2021; 22(11):5786. https://doi.org/10.3390/ijms22115786
Chicago/Turabian StyleBuskaran, Kalaivani, Mohd Zobir Hussein, Mohamad Aris Mohd Moklas, Mas Jaffri Masarudin, and Sharida Fakurazi. 2021. "Graphene Oxide Loaded with Protocatechuic Acid and Chlorogenic Acid Dual Drug Nanodelivery System for Human Hepatocellular Carcinoma Therapeutic Application" International Journal of Molecular Sciences 22, no. 11: 5786. https://doi.org/10.3390/ijms22115786
APA StyleBuskaran, K., Hussein, M. Z., Moklas, M. A. M., Masarudin, M. J., & Fakurazi, S. (2021). Graphene Oxide Loaded with Protocatechuic Acid and Chlorogenic Acid Dual Drug Nanodelivery System for Human Hepatocellular Carcinoma Therapeutic Application. International Journal of Molecular Sciences, 22(11), 5786. https://doi.org/10.3390/ijms22115786






