Detection of SARS-CoV-2 RNA by a Multiplex Reverse-Transcription Loop-Mediated Isothermal Amplification Coupled with Melting Curves Analysis
Abstract
1. Introduction
2. Results
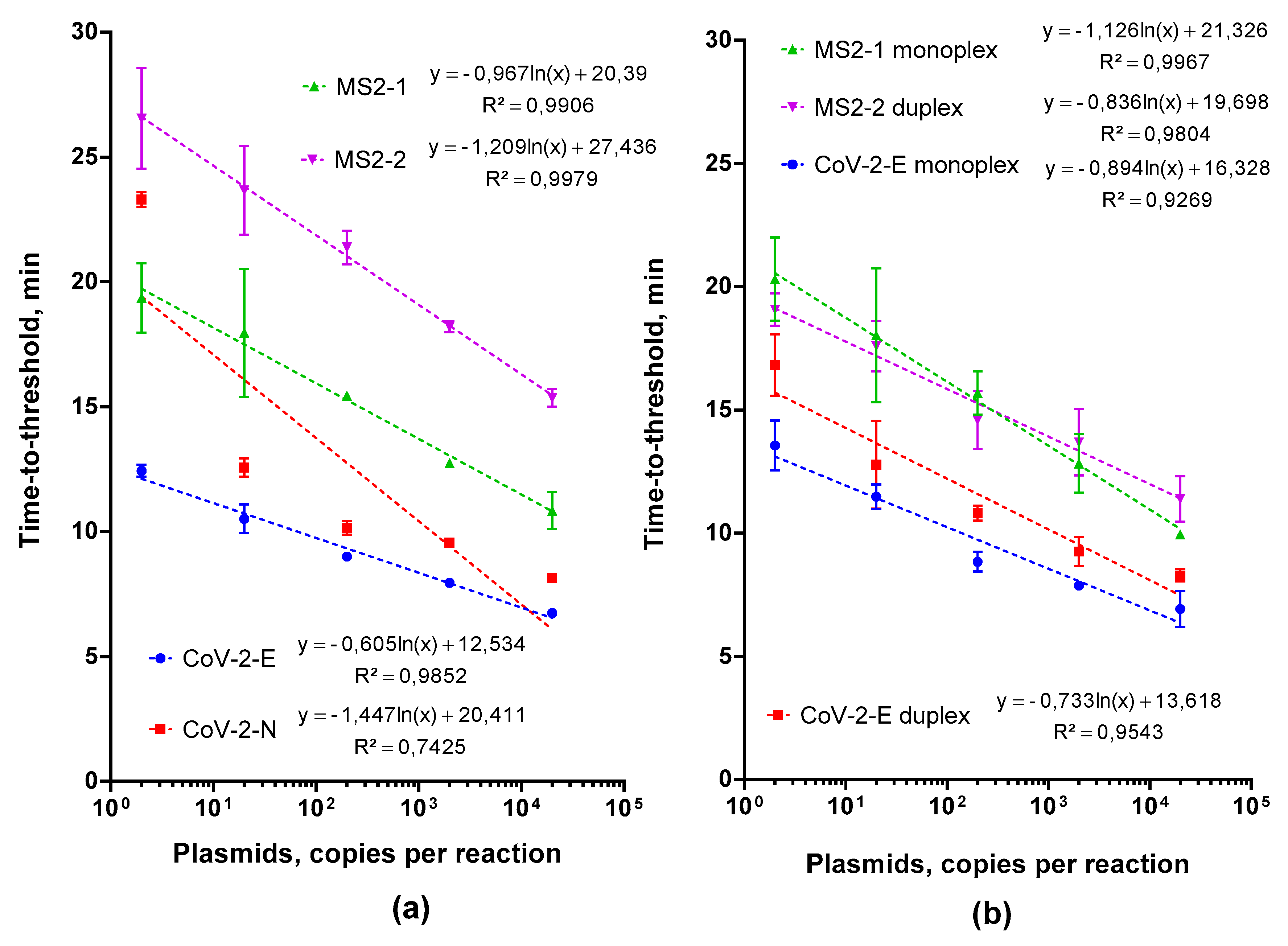
2.1. Choosing and Testing LAMP Primers
2.2. Multiplex LAMP Assay Evaluation
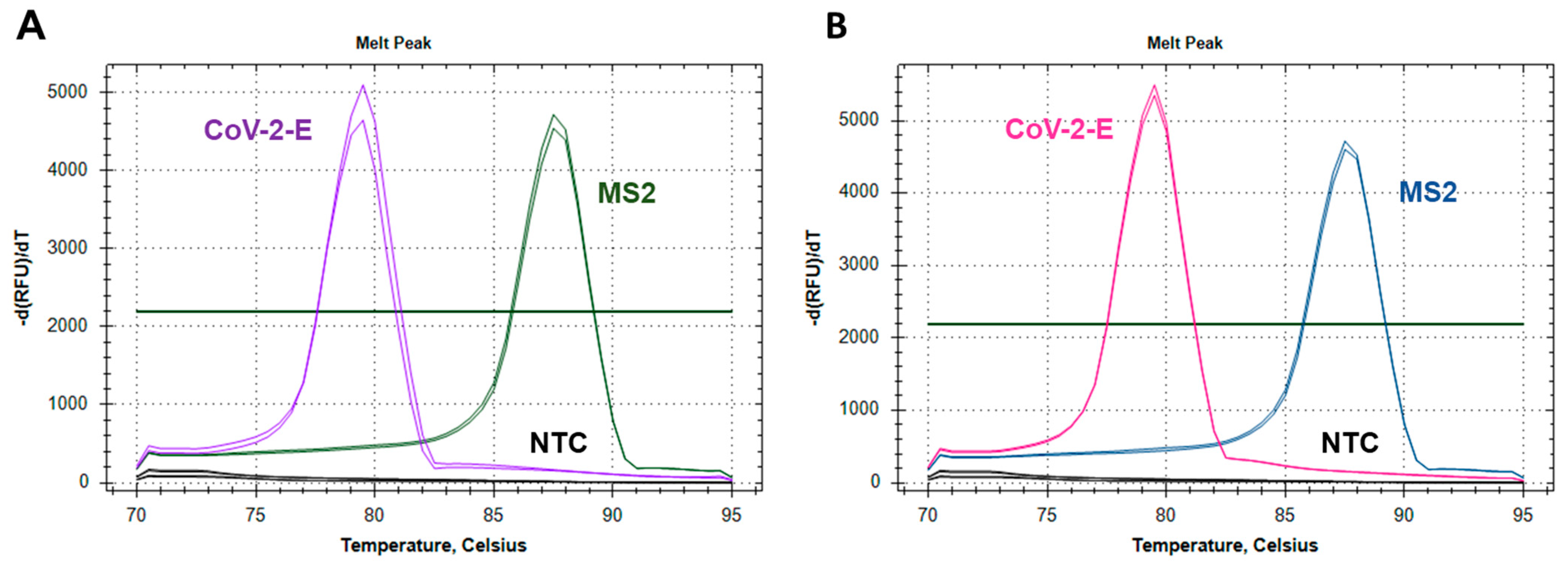
2.2.1. Testing the Feasibility of Duplex CoV-2-E-MS2 LAMP
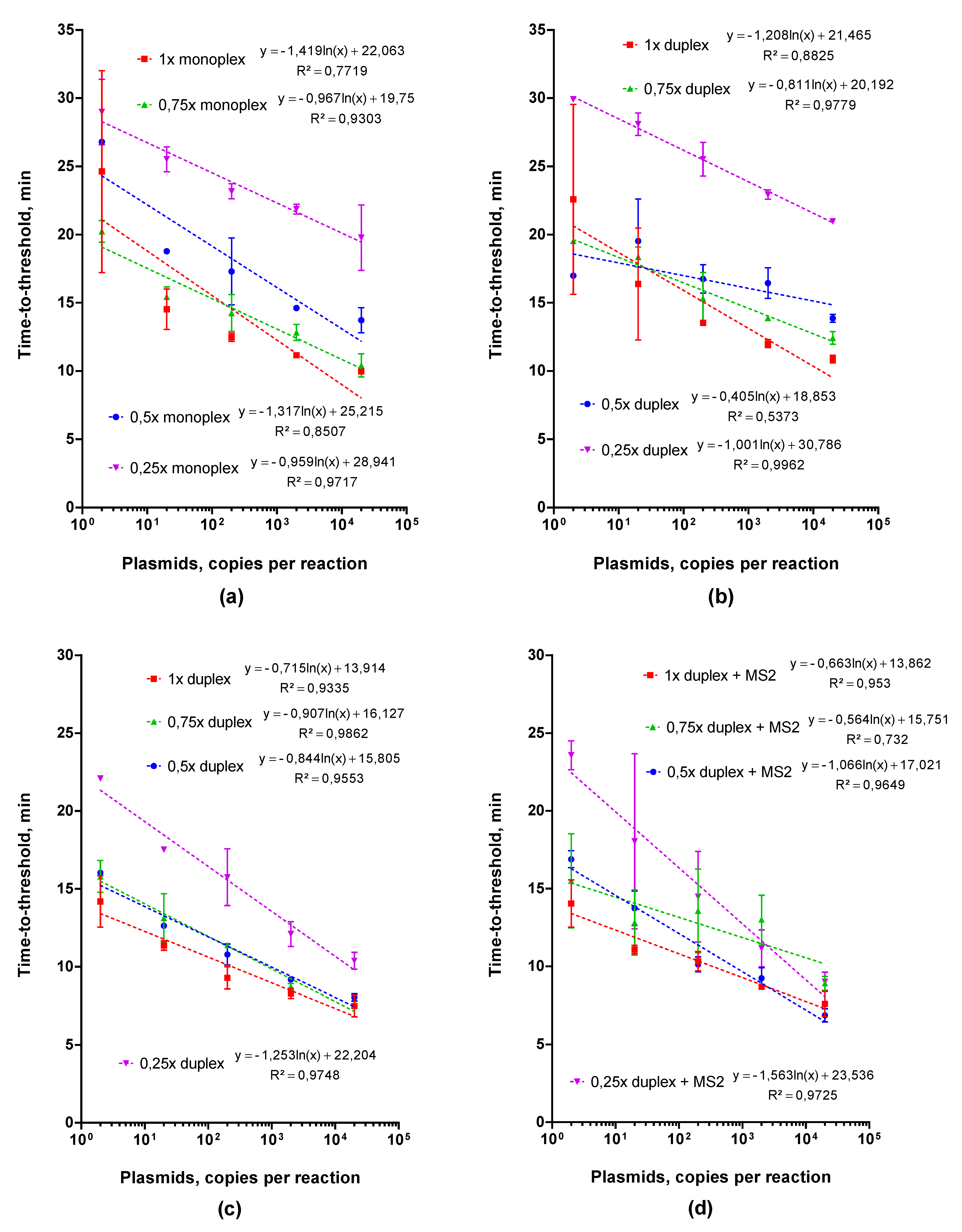
2.2.2. Adjusting Optimal Concentrations for LAMP MS2 Primers
2.2.3. Choosing an Optimal Concentration for the Control MS2 RNA
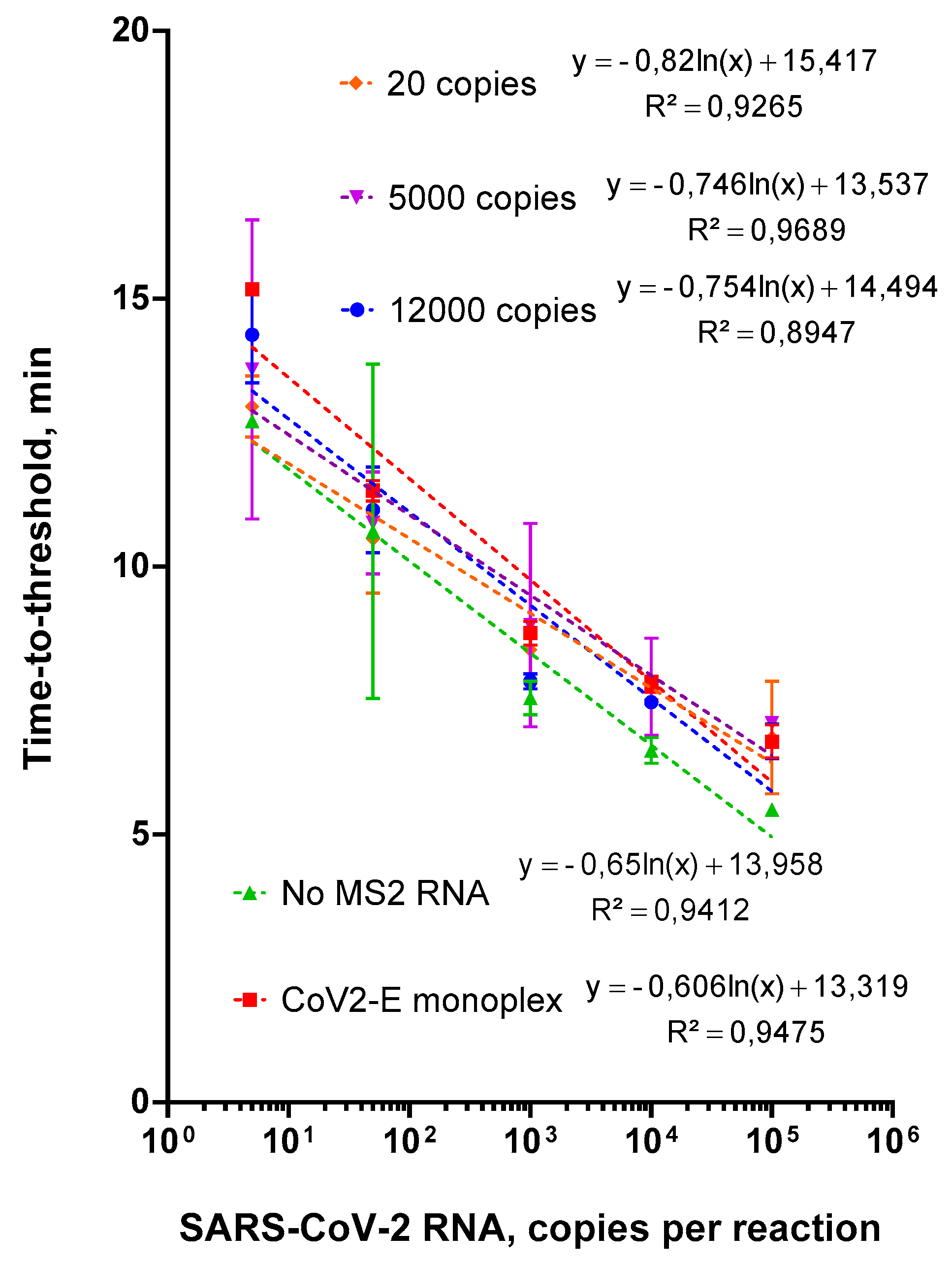
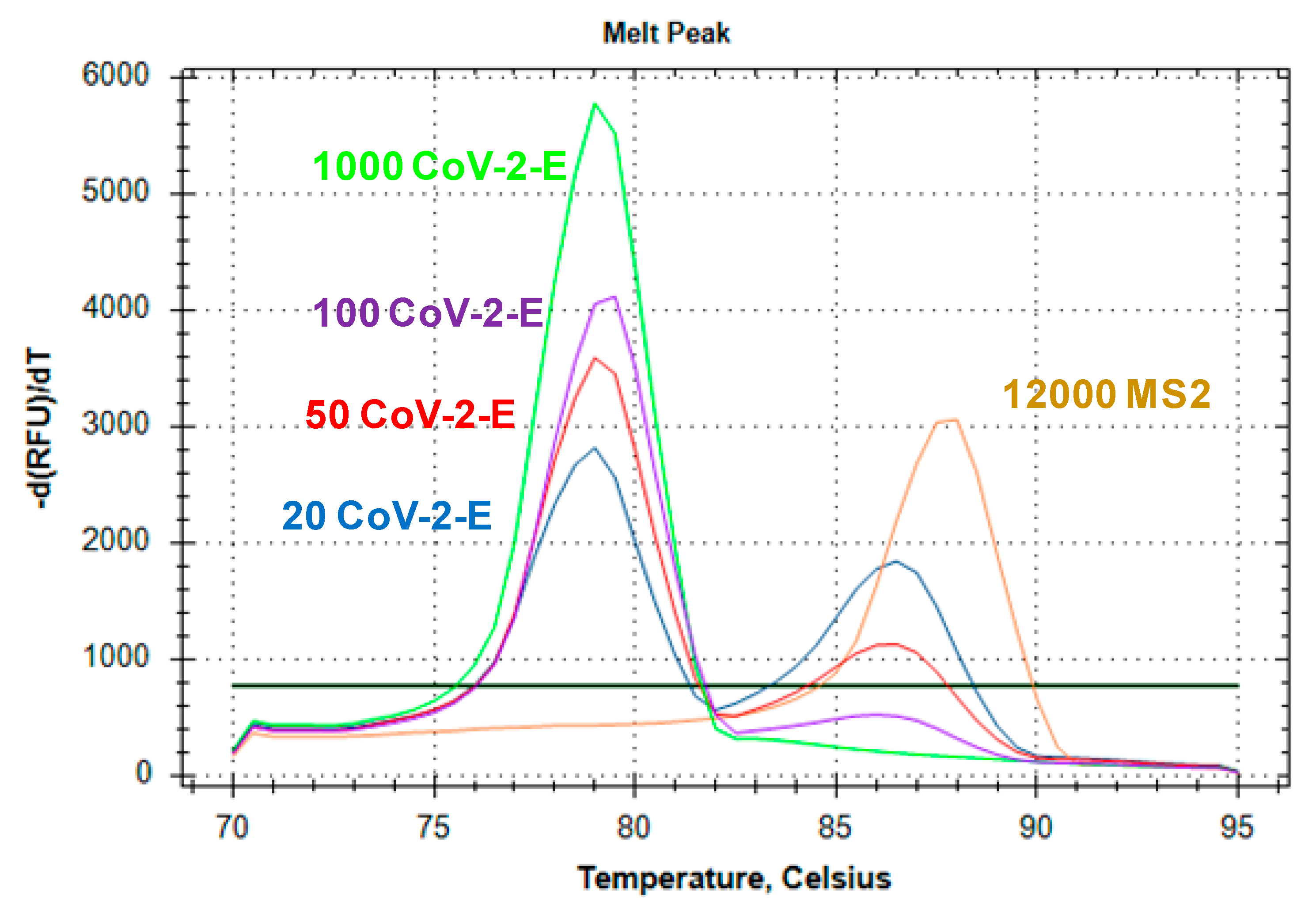
2.3. Evaluation of Duplex Assay Limit of Detection
2.4. Testing of Clinical Samples
3. Discussion
4. Materials and Methods
4.1. Clinical and Standard Samples
4.2. Droplet Digital PCR
4.3. Real-Time RT PCR
4.4. Real-Time Loop-Mediated Isothermal DNA Amplification (LAMP)
4.5. The Evaluation of the Limit of Detection and Clinical Sensitivity and Specificity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fire, A.; Xu, S.Q. Rolling replication of short DNA circles. Proc. Natl. Acad. Sci. USA 1995, 92, 4641–4645. [Google Scholar] [CrossRef]
- Compton, J. Nucleic acid sequence-based amplification. Nature 1991, 350, 91–92. [Google Scholar] [CrossRef]
- Piepenburg, O.; Williams, C.H.; Stemple, D.L.; Armes, N.A. DNA detection using recombination proteins. PLoS Biol. 2006, 4, e204. [Google Scholar] [CrossRef]
- Vincent, M.; Xu, Y.; Kong, H. Helicase-dependent isothermal DNA amplification. EMBO Rep. 2004, 5, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.T.; Fraiser, M.S.; Schram, J.L.; Little, M.C.; Nadeau, J.G.; Malinowski, D.P. Strand displacement amplification—An isothermal, in vitro DNA amplification technique. Nucleic Acids Res. 1992, 20, 1691–1696. [Google Scholar] [CrossRef]
- Dean, F.B.; Hosono, S.; Fang, L.; Wu, X.; Faruqi, A.-F.; Bray-Ward, P.; Sun, Z.; Zong, Q.; Du, Y.; Du, J.; et al. Comprehensive human genome amplification using multiple displacement amplification. Proc. Natl. Acad. Sci. USA 2002, 99, 5261–5266. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Dong, D.; Yang, Z.; Zou, D.; Chen, Z.; Yuan, J.; Huang, L. Polymerase Spiral Reaction (PSR): A novel isothermal nucleic acid amplification method. Sci. Rep. 2015, 5, 12723. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Honda, E.; Ogura, A.; Nomoto, A.; Hanaki, K.-I. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques 2009, 46, 167–172. [Google Scholar] [CrossRef]
- Tomita, N.; Mori, Y.; Kanda, H.; Notomi, T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 2008, 3, 877–882. [Google Scholar] [CrossRef]
- Kouguchi, Y.; Fujiwara, T.; Teramoto, M.; Kuramoto, M. Homogenous, real-time duplex loop-mediated isothermal amplification using a single fluorophore-labeled primer and an intercalator dye: Its application to the simultaneous detection of Shiga toxin genes 1 and 2 in Shiga toxigenic Escherichia coli isolates. Mol. Cell. Probes 2010, 24, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Huang, S.; Liu, N.; Dong, D.; Yang, Z.; Tang, Y.; Ma, W.; He, X.; Ao, D.; Xu, Y.; et al. Establishment of an accurate and fast detection method using molecular beacons in loop-mediated isothermal amplification assay. Sci. Rep. 2017, 7, 40125. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Nagamine, K.; Tomita, N.; Notomi, T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem. Biophys. Res. Commun. 2001, 289, 150–154. [Google Scholar] [CrossRef]
- Veigas, B.; Branquinho, R.; Pinto, J.; Wojcik, P.J.; Martins, R.; Fortunato, E.; Baptista, P.V. Ion sensing (EIS) real-time quantitative monitorization of isothermal DNA amplification. Biosens. Bioelectron. 2014, 52, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Yongkiettrakul, S.; Kampeera, J.; Chareanchim, W.; Rattanajak, R.; Pornthanakasem, W.; Kiatpathomchai, W.; Kongkasuriyachai, D. Simple detection of single nucleotide polymorphism in Plasmodium falciparum by SNP-LAMP assay combined with lateral flow dipstick. Parasitol. Int. 2017, 66, 964–971. [Google Scholar] [CrossRef]
- World Health Organization. The Use of Loop-Mediated Isothermal Amplification (TB-LAMP) for the Diagnosis of Pulmonary Tuberculosis: Policy Guidance; World Health Organization: Geneva, Switzerland, 2016; ISBN 9789241511186. [Google Scholar]
- Guo, X.G.; Zhou, Y.Z.; Li, Q.; Wang, W.; Wen, J.Z.; Zheng, L.; Wang, Q. Rapid and reliable diagnostic method to detect Zika virus by real-time fluorescence reverse transcription loop-mediated isothermal amplification. AMB Express 2018, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.L.M.; Leung, C.S.W.; Chan, K.H.; Lee, J.H.C.; Yuen, K.Y.; Guan, Y.; Peiris, J.S.M. Detection of human influenza A viruses by loop-mediated isothermal amplification. J. Clin. Microbiol. 2005, 43, 427–430. [Google Scholar] [CrossRef]
- Broughton, J.; Deng, X.; Yu, G.; Fasching, C.; Singh, J.; Streithorst, J.; Granados, A.; Sotomayor-Gonzalez, A.; Zorn, K.; Gopez, A.; et al. Rapid Detection of 2019 Novel Coronavirus SARS-CoV-2 Using a CRISPR-based DETECTR Lateral Flow Assay. MedRxiv 2020. [Google Scholar] [CrossRef]
- Park, G.-S.; Ku, K.; Baek, S.-H.; Kim, S.-J.; Kim, S.I.; Kim, B.-T.; Maeng, J.-S. Development of Reverse Transcription Loop-mediated Isothermal Amplification (RT-LAMP) Assays Targeting SARS-CoV-2. J. Mol. Diagn. 2020, 22, 729–735. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Orihara, Y.; Kawamura, R.; Imai, K.; Sakai, J.; Tarumoto, N.; Matsuoka, M.; Takeuchi, S.; Maesaki, S.; Maeda, T. Evaluation of rapid diagnosis of novel coronavirus disease (COVID-19) using loop-mediated isothermal amplification. J. Clin. Virol. 2020, 129, 104446. [Google Scholar] [CrossRef]
- Schermer, B.; Fabretti, F.; Damagnez, M.; Di Cristanziano, V.; Heger, E.; Arjune, S.; Tanner, N.A.; Imhof, T.; Koch, M.; Ladha, A.; et al. Rapid SARS-CoV-2 testing in primary material based on a novel multiplex RT-LAMP assay. PLoS ONE 2020, 15, e0238612. [Google Scholar] [CrossRef] [PubMed]
- Lalli, M.A.; Langmade, J.S.; Chen, X.; Fronick, C.C.; Sawyer, C.S.; Burcea, L.C.; Wilkinson, M.N.; Fulton, R.S.; Heinz, M.; Buchser, W.J.; et al. Rapid and extraction-free detection of SARS-CoV-2 from saliva by colorimetric reverse-transcription loop-mediated isothermal amplification. Clin. Chem. 2021, 67, 415–424. [Google Scholar] [CrossRef]
- Bektaş, A.; Covington, M.F.; Aidelberg, G.; Arce, A.; Matute, T.; Núñez, I.; Walsh, J.; Boutboul, D.; Delaugerre, C.; Lindner, A.B.; et al. Accessible LAMP-enabled rapid test (ALERT) for detecting SARS-CoV-2. Viruses 2021, 13, 742. [Google Scholar] [CrossRef]
- Aliotta, J.M.; Pelletier, J.J.; Ware, J.L.; Moran, L.S.; Benner, J.S.; Kong, H. Thermostable Bst DNA polymerase I lacks a 3′-->5′ proofreading exonuclease activity. Genet. Anal. 1996, 12, 185–195. [Google Scholar] [CrossRef]
- Shao, Y.; Zhu, S.; Jin, C.; Chen, F. Development of multiplex loop-mediated isothermal amplification-RFLP (mLAMP-RFLP) to detect Salmonella spp. and Shigella spp. in milk. Int. J. Food Microbiol. 2011, 148, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Iseki, H.; Alhassan, A.; Ohta, N.; Thekisoe, O.M.M.; Yokoyama, N.; Inoue, N.; Nambota, A.; Yasuda, J.; Igarashi, I. Development of a multiplex loop-mediated isothermal amplification (mLAMP) method for the simultaneous detection of bovine Babesia parasites. J. Microbiol. Methods 2007, 71, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Aonuma, H.; Yoshimura, A.; Kobayashi, T.; Okado, K.; Badolo, A.; Nelson, B.; Kanuka, H.; Fukumoto, S. A single fluorescence-based LAMP reaction for identifying multiple parasites in mosquitoes. Exp. Parasitol. 2010, 125, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Higgins, O.; Clancy, E.; Cormican, M.; Boo, T.W.; Cunney, R.; Smith, T.J. Evaluation of an internally controlled multiplex Tth endonuclease cleavage loop-mediated isothermal amplification (TEC-LAMP) assay for the detection of bacterial meningitis pathogens. Int. J. Mol. Sci. 2018, 19, 524. [Google Scholar] [CrossRef]
- Yaren, O.; Alto, B.W.; Bradley, K.M.; Moussatche, P.; Glushakova, L.; Benner, S.A. Multiplexed isothermal amplification based diagnostic platform to detect zika, chikungunya, and dengue 1. J. Vis. Exp. 2018, 2018, e57051. [Google Scholar] [CrossRef]
- Dong, J.; Xu, Q.; Li, C.C.; Zhang, C.Y. Single-color multiplexing by the integration of high-resolution melting pattern recognition with loop-mediated isothermal amplification. Chem. Commun. 2019, 55, 2457–2460. [Google Scholar] [CrossRef]
- Tanner, N.A.; Zhang, Y.; Evans, T.C. Simultaneous multiple target detection in real-time loop-mediated isothermal amplification. Biotechniques 2012, 53, 81–89. [Google Scholar] [CrossRef]
- Jang, W.S.; Lim, D.H.; Yoon, J.; Kim, A.; Lim, M.; Nam, J.; Yanagihara, R.; Ryu, S.-W.; Jung, B.K.; Ryoo, N.-H.; et al. Development of a multiplex Loop-Mediated Isothermal Amplification (LAMP) assay for on-site diagnosis of SARS CoV-2. PLoS ONE 2021, 16, e0248042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tanner, N.A. Development of multiplexed reverse-transcription loop-mediated isothermal amplification for detection of SARS-CoV-2 and influenza viral RNA. Biotechniques 2021, 70, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed]
- Hellou, M.M.; Górska, A.; Mazzaferri, F.; Cremonini, E.; Gentilotti, E.; De Nardo, P.; Poran, I.; Leeflang, M.; Tacconelli, E.; Paul, M. Nucleic-acid-amplification tests from respiratory samples for the diagnosis of coronavirus infections: Systematic review and meta-analysis. Clin. Microbiol. Infect. 2020, 27, 341–351. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Das, S.; Ahmed, R.; Mori, Y.; Notomi, T.; Kevadiya, B.; Thakor, A. Loop-mediated isothermal amplification (LAMP): A rapid, sensitive, specific, and cost-effective point-of-care test for coronaviruses in the context of COVID-19 pandemic. Biology 2020, 9, 182. [Google Scholar] [CrossRef]
- Ahmad, S.; Ali, N.; Kausar, M.; Misbah, H.; Wahid, A. Road toward rapid-molecular point of care test to detect novel SARS-coronavirus 2019 (COVID-19): Review from updated literature. Allergol. Immunopathol. 2020, 48, 518–520. [Google Scholar] [CrossRef]
- Xu, G.; Gunson, R.N.; Cooper, J.M.; Reboud, J. Rapid ultrasonic isothermal amplification of DNA with multiplexed melting analysis-applications in the clinical diagnosis of sexually transmitted diseases. Chem. Commun. 2015, 51, 2589–2592. [Google Scholar] [CrossRef]
- Markoulatos, P.; Siafakas, N.; Moncany, M. Multiplex polymerase chain reaction: A practical approach. J. Clin. Lab. Anal. 2002, 16, 47–51. [Google Scholar] [CrossRef]
- Elnifro, E.M.; Ashshi, A.M.; Cooper, R.J.; Klapper, P.E. Multiplex PCR: Optimization and application in diagnostic virology. Clin. Microbiol. Rev. 2000, 13, 559–570. [Google Scholar] [CrossRef] [PubMed]
| Name | 5′-Sequence-3′ |
|---|---|
| PMTL-1 | CTTCGCTATTACGCCAGCT |
| PMTL-2 | GCGGATAACAATTTCACACAG |
| CoR1-F2 | GCCTTTGTAAGCACAAGCTG |
| CoR1-B2 | AAGAAGGTTTTACAAGACTCACGT |
| CoR1-LF | CATCCTTACTGCGCTTCGAT |
| CoR1-LB | TAACGTACCTGTCTCTTCCGAAA |
| CoR1-FIP | GCAAGAAAAAGAAGTACGCTATTAACTAGAGTACGAACTTATGTACTCATTC |
| CoR1-BIP | CGTGGTATTCTTGCTAGTTACACTAGATATTGCAGCAGTACGCACAC |
| CoR2-F2 | TGCAACTGAGGGAGCCTTG |
| CoR2-B2 | TGGAGTTGAATTTCTTGAACTG |
| CoR2-LF | CGGCAGTCAAGCCTCTTCTC |
| CoR2-LB | ATTGTTAGCAGGATTGCGGGT |
| CoR2-FIP | GGAAGTTGTAGCACGATTGCAGATACACCAAAAGATCACATTGG |
| CoR2-BIP | GCTTCTACGCAGAAGGGAGCATGCGACTACGTGATGAGGAA |
| MS2-1-FIP | CTCCTGAGGGAATGTGGGAACCCCGGCGTGCGCGTTAT |
| MS2-1-BIP | GCCAGCGAGCTCTCCTCGGGCACCCGTGCTCTTTCGA |
| MS2-1-F3 | CCGACAGCATGAAGTCCG |
| MS2-1-B3 | AGCCCGCCCACCTTTC |
| MS2-1-LB | GTTAGCCACTCCGAAGTGCG |
| MS2-1-LF | GCTGACCGAGGGACCCC |
| MS2-2-LB | GTCTATACCAACGGATTTGAGCC |
| MS2-2-LF | GCATCCGATTCCATCTCCGAT |
| MS2-2-F3 | TGCCTGTAAGGAGCCTGAT |
| MS2-2-B3 | TGAGCGGATACGATCGAGAT |
| MS2-2-FIP | GCCAGACGCTGGTTGATCGATTAAGGGGTCGGTGCTTTCA |
| MS2-2-BIP | GGTTCGCTTGCGACGATAGACTTCTGGTGGGAGAAAACTCCA |
| N_Sarb_F | CACATTGGCACCCGCAATC |
| N_Sarb_R | GAGGAACGAGAAGAGGCTTG |
| N_Sarb_P | FAM-ACTTCCTCAAGGAACAACATTGCCA-BHQ1 |
| E_Sarb_F | ACAGGTACGTTAATAGTTAATAGCGT |
| E_Sarb_R | ATATTGCAGCAGTACGCACACA |
| E_Sarb_P | HEX-ACACTAGCCATCCTTACTGCGCTTCG-BHQ2 |
| MS2-5-F | GTACGAGGAGAAAGCCGGTTTC |
| MS2-5-R | GTTCTGCGGCACTTCGATG |
| MS2-5-P | FAM-TCCCTCGACGCACGCTCCTGCT-BHQ1 |
| Concentration of Standard, Copies/Reaction | CoV-2-E | Concentration of Standard, Copies/Reaction | MS2-2 |
|---|---|---|---|
| 1 × 104 | 7.88 ± 0.06 | 1250 | 13.18 ± 0.61 |
| 1 × 103 | 8.68 ± 0.68 | 250 | 15.83 ± 0.12 |
| 50 | 10.37 ± 1.03 | 50 | 17.89 ± 0.34 |
| 5 | 12.56 ± 1.22 | 5 | 20.69 ± 2.28 |
| RT-PCR | Multiplex LAMP | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 40 | 2 | 42 |
| Negative | 1 | 82 | 83 |
| Total | 41 | 84 | 125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oscorbin, I.P.; Shevelev, G.Y.; Pronyaeva, K.A.; Stepanov, A.A.; Shamovskaya, D.V.; Mishukova, O.V.; Pyshnyi, D.V.; Filipenko, M.L. Detection of SARS-CoV-2 RNA by a Multiplex Reverse-Transcription Loop-Mediated Isothermal Amplification Coupled with Melting Curves Analysis. Int. J. Mol. Sci. 2021, 22, 5743. https://doi.org/10.3390/ijms22115743
Oscorbin IP, Shevelev GY, Pronyaeva KA, Stepanov AA, Shamovskaya DV, Mishukova OV, Pyshnyi DV, Filipenko ML. Detection of SARS-CoV-2 RNA by a Multiplex Reverse-Transcription Loop-Mediated Isothermal Amplification Coupled with Melting Curves Analysis. International Journal of Molecular Sciences. 2021; 22(11):5743. https://doi.org/10.3390/ijms22115743
Chicago/Turabian StyleOscorbin, Igor P., Georgiy Yu. Shevelev, Ksenia A. Pronyaeva, Andrey A. Stepanov, Darya V. Shamovskaya, Olga V. Mishukova, Dmitrii V. Pyshnyi, and Maksim L. Filipenko. 2021. "Detection of SARS-CoV-2 RNA by a Multiplex Reverse-Transcription Loop-Mediated Isothermal Amplification Coupled with Melting Curves Analysis" International Journal of Molecular Sciences 22, no. 11: 5743. https://doi.org/10.3390/ijms22115743
APA StyleOscorbin, I. P., Shevelev, G. Y., Pronyaeva, K. A., Stepanov, A. A., Shamovskaya, D. V., Mishukova, O. V., Pyshnyi, D. V., & Filipenko, M. L. (2021). Detection of SARS-CoV-2 RNA by a Multiplex Reverse-Transcription Loop-Mediated Isothermal Amplification Coupled with Melting Curves Analysis. International Journal of Molecular Sciences, 22(11), 5743. https://doi.org/10.3390/ijms22115743







