Abstract
The hypothalamic-pituitary-adrenal axis is stimulated in response to stress. When activated, it is suppressed by the negative feedback effect of glucocorticoids. Glucocorticoids directly inhibit proopiomelanocortin (Pomc) gene expression in the pituitary. Glucocorticoid signaling is mediated via glucocorticoid receptors, 11β-hydroxysteroid dehydrogenases, and the FK506-binding immunophilins, Fkbp4 and Fkbp5. Fkbp4 and Fkbp5 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor, resulting in modulation of the glucocorticoid action. Here, we explored the regulation of Fkbp4 and Fkbp5 genes and their proteins with dexamethasone, a major synthetic glucocorticoid drug, in murine AtT-20 corticotroph cells. To elucidate further roles of Fkbp4 and Fkbp5, we examined their effects on Pomc mRNA levels in corticotroph cells. Dexamethasone decreased Pomc mRNA levels as well as Fkpb4 mRNA levels in mouse corticotroph cells. Dexamethasone tended to decrease Fkbp4 protein levels, while it increased Fkpb5 mRNA and its protein levels. The dexamethasone-induced decreases in Pomc mRNA levels were partially canceled by Fkbp4 knockdown. Alternatively, Pomc mRNA levels were further decreased by Fkbp5 knockdown. Thus, Fkbp4 contributes to the negative feedback of glucocorticoids, and Fkbp5 reduces the efficiency of the glucocorticoid effect on Pomc gene expression in pituitary corticotroph cells.
1. Introduction
Stress activates the hypothalamic-pituitary-adrenal (HPA) axis. The activated HPA axis is suppressed by the negative feedback effect of glucocorticoids. Corticotropin-releasing factor (CRF), produced in the hypothalamic paraventricular nucleus in response to stress, stimulates the release of the adrenocorticotropic hormone (ACTH) from the anterior pituitary [1,2,3]. ACTH, cleaved from the proopiomelanocortin (Pomc) gene, stimulates the secretion of corticosterone and cortisol, the principal glucocorticoid in rodents and human, respectively, from the adrenal glands [3]. Glucocorticoids bind to the glucocorticoid receptor (GR), and subsequently inhibit CRF production in the hypothalamus and the ACTH in the pituitary as an inhibitory feedback loop [4,5,6].
Glucocorticoid signaling is mediated via the GR, 11β-hydroxysteroid dehydrogenases, and the FK506-binding immunophilins, Fkbp52 (Fkbp4) and Fkbp51 (Fkbp5) [7,8,9]. The genes encoding Fkbp4 and Fkbp5 are Fkbp4 and Fkbp5, respectively. Fkbp4 and Fkbp5 differentially regulate dynein interaction and nuclear translocation of the GR [10]. In the absence of corticosterone, the GR is retained in the cytoplasm as a complex containing one GR molecule, heat shock protein (HSP) 90 dimer, HSP90-binding protein P23, and Fkbp5 [11,12].
Corticosterone binds to a cytoplasmic receptor, GR, in rodents. Following the binding of corticosterone to the GR, Fkbp5 is replaced by Fkbp4, resulting in translocation of the complex to the nucleus owing to the interaction between Fkbp4 and the motor protein dynein [12]. Fkbp5 changes the conformation of the receptor complex, leading to sensitivity reduction of the GR to corticosterone and negative feedback efficiency [13].
We revealed regulation and roles of Fkbp4 and Fkbp5 in corticotroph cells. In the present study, we explored the regulation of Fkbp4 and Fkbp5 genes and their proteins using dexamethasone, a major synthetic glucocorticoid drug, in murine corticotroph AtT-20 cells. To elucidate further roles of Fkbp4 and Fkbp5, we subsequently examined the effects of Fkbp4 and Fkbp5 on Pomc mRNA levels in corticotroph cells.
2. Results
2.1. Effect of Dexamethasone on Pomc mRNA Levels
This time course study showed that 100 nM dexamethasone significantly decreased Pomc mRNA levels with marked effects observed within the first 24 h of treatment (p < 0.05, Figure 1A). Pomc mRNA levels decreased in a concentration-dependent manner (p < 0.005), with significant effects initially occurring at 1 nM dexamethasone (Figure 1B).
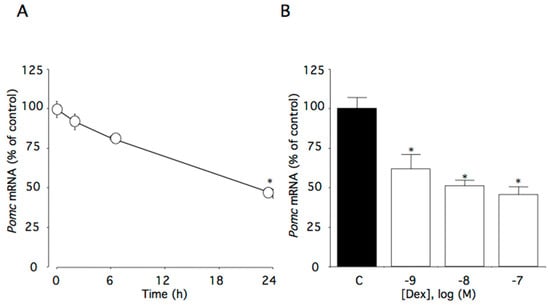
Figure 1.
Effects of dexamethasone on Pomc mRNA levels in AtT-20 cells. (A) Time-dependent effects of dexamethasone on Pomc mRNA levels. Cells were cultured in medium containing 100 nM dexamethasone. (B) Concentration-dependent effects of dexamethasone on Pomc mRNA levels. Cells were cultured for 24 h in medium containing 1–100 nM dexamethasone. Data are expressed as means ± standard errors of the means. * p < 0.05 compared with time 0 or the control (C). Cells were treated in triplicate, and the average of three independent experiments is shown (n = 3).
2.2. Effect of Dexamethasone on Fkbp4 mRNA Levels
This time course study showed that 100 nM dexamethasone significantly decreased Fkbp4 mRNA levels (p < 0.01). Within the first 24 h of incubation with dexamethasone, Fkbp4 mRNA levels decreased to 72% of the control level (Figure 2A). Additionally, Fkbp4 mRNA levels decreased as dexamethasone concentrations increased (p < 0.01), with significant effects initially occurring at 10 nM dexamethasone (Figure 2B).
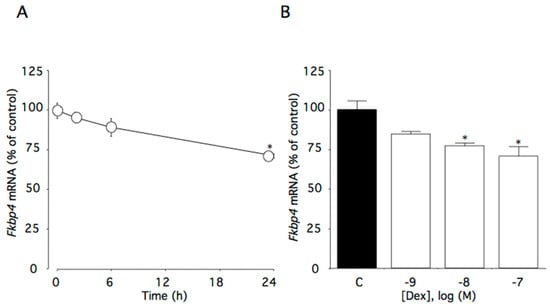
Figure 2.
Effects of dexamethasone on Fkbp4 mRNA levels in AtT-20 cells. (A) Time-dependent effects of dexamethasone on Fkbp4 mRNA levels. Cells were cultured in medium containing 100 nM dexamethasone. (B) Concentration-dependent effects of dexamethasone on Fkbp4 mRNA levels. Cells were cultured for 24 h in medium containing 1–100 nM dexamethasone. Data are expressed as means ± standard errors of the means. * p < 0.05 compared with time 0 or the control (C). Cells were treated in triplicate, and the average of three independent experiments is shown (n = 3).
2.3. Effect of Dexamethasone on Fkbp5 mRNA Lvels
This time course study showed that 100 nM dexamethasone significantly increased Fkbp5 mRNA levels (p < 0.0001). Within the first 6 h of dexamethasone incubation, Fkbp5 mRNA levels increased to 375% of the control level (Figure 3A). Additionally, Fkbp5 mRNA levels were increased as dexamethasone concentrations increased (p < 0.0001), with significant effects initially occurring at 1 nM dexamethasone (Figure 3B).
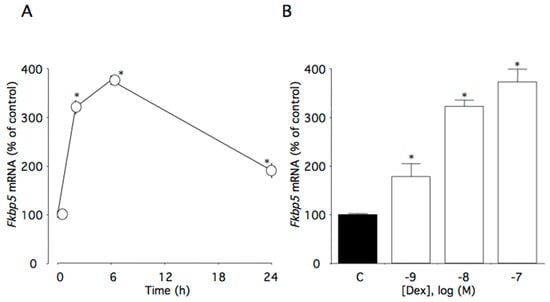
Figure 3.
Effects of dexamethasone on Fkbp5 mRNA levels in AtT-20 cells. (A) Time-dependent effects of dexamethasone on Fkbp5 mRNA levels. Cells were cultured in medium containing 100 nM dexamethasone. (B) Concentration-dependent effects of dexamethasone on Fkbp5 mRNA levels. Cells were cultured for 6 h in medium containing 1–100 nM dexamethasone. Data are expressed as means ± standard errors of the means. * p < 0.05 compared with time 0 or the control (C). Cells were treated in triplicate, and the average of three independent experiments is shown (n = 3).
2.4. Effect of Dexamethasone on Fkbp4, Fkbp5, and GR Protein Levels
This time course study showed that 100 nM dexamethasone significantly increased Fkbp5 (p < 0.005) and tended to decrease Fkbp4 protein levels (p = 0.094) (Figure 4A and 4B). Within the first 24 h of dexamethasone incubation, Fkbp5 protein levels were increased to 192% of the control level (Figure 4B), while GR protein levels were decreased to 47% (Figure 4C).
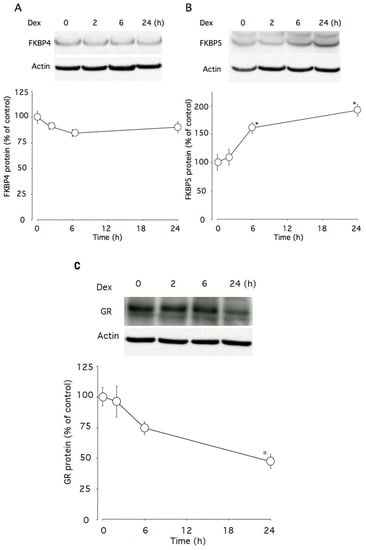
Figure 4.
Effects of dexamethasone on Fkbp4 and Fkbp5 proteins levels in AtT-20 cells. (A) Time-dependent effects of dexamethasone on Fkbp4 protein levels. (B) Time-dependent effects of dexamethasone on Fkbp5 protein levels. (C) Time-dependent effects of dexamethasone on GR protein levels. Cells were cultured in medium containing 100 nM dexamethasone. β-actin was used as a housekeeping protein. Data are expressed as means ± standard errors of the means. * p < 0.05 compared with time 0. Cells were treated in triplicate, and the average of three independent experiments (n = 3) and a representative blot are shown.
2.5. Effect of Fkbp4 and Fkbp5 Knockdown on Dexamethasone-Induced Changes of Pomc mRNA Levels
Fkbp4 mRNA levels were reduced by 18% in cells transfected with siFkbp4, while Fkbp5 mRNA levels remained unchanged. Pomc mRNA levels were not changed in cells transfected with siFkbp4, while they were decreased by dexamethasone. Dexamethasone-induced decreases in Pomc mRNA levels were partially canceled by Fkbp4 knockdown (Figure 5A).
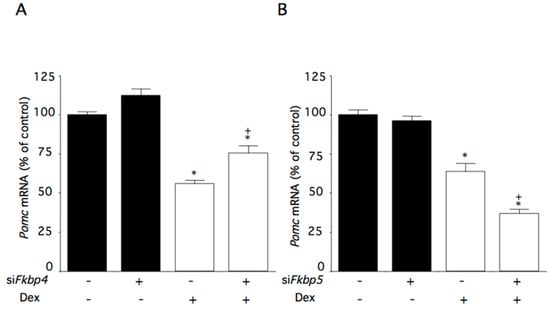
Figure 5.
Effects of Fkbp4 and Fkbp5 knockdown on dexamethasone-induced changes of Pomc mRNA levels in AtT-20 cells. Cells were incubated with medium containing control small interfering (si)RNA or Fkbp4- or Fkbp5-specific siRNA (siFkbp4 or siFkbp5), and subsequently with medium containing 100 nM dexamethasone (Dex) or control medium. (A) Effect of Fkbp4 on dexamethasone-induced changes of Pomc mRNA levels. (B) Effect of Fkbp5 on dexamethasone-induced changes of Pomc mRNA levels. Data are expressed as means ± standard errors of the means. * p < 0.05 compared with control siRNA and Dex (-). + p < 0.05 compared with control siFkbp and the Dex (-) group or control siRNA and the Dex (+) group. Cells were treated in triplicate, and the average of three independent experiments is shown (n = 3).
Fkbp5 mRNA levels were reduced by 55% in cells transfected with siFkbp5. As expected, Fkbp4 mRNA levels were not affected by Fkbp5 knockdown. Pomc mRNA levels were not changed in cells transfected with siFkbp5, while they were decreased by dexamethasone (Figure 5B). The dexamethasone-induced decreases in Pomc mRNA levels were further decreased by Fkbp5 knockdown (Figure 5B).
3. Discussion
The activated HPA axis is suppressed by the negative feedback effect of glucocorticoids [14]. Pituitary corticotroph cells express high levels of GRs, and glucocorticoids directly inhibit Pomc gene expression in pituitary corticotroph cells [15]. However, the molecular mechanisms for glucocorticoid negative regulation of Pomc gene expression are not fully understood. Glucocorticoid suppression of the Pomc gene is specific for the pituitary, while Pomc gene expression is upregulated by glucocorticoids in the hypothalamus [16]. King et al. [17] proposed that the differences of transcription factors among cells or tissues cause differential regulation, because the interactions with specific DNA-regulatory sequences and other transcription factors produce cell-type specific effects.
By binding glucocorticoid to the GR, the GR translocates from the cytoplasm to the nucleus [12]. The glucocorticoid-GR complex directly and indirectly regulates target gene transcription. The glucocorticoid-GR complex binds to glucocorticoid-response elements (GRE) in the target gene promoter, and subsequently activates target gene transcription [18], while the negative glucocorticoid-response elements (nGREs) are necessary for the negative regulation of Pomc gene expression by glucocorticoids [4,19]. The GR complex binds to nGREs of the Pomc promoter, and the nGRE complex suppresses Pomc transcription in the pituitary corticotroph [12]. Additionally, suppression of Nur77 or NeuroD1 by glucocorticoids is also involved in the glucocorticoid-mediated negative regulation of Pomc in the pituitary [16,20].
In our study, dexamethasone-induced decreases in Pomc mRNA levels were partially canceled by Fkbp4 knockdown. Following the binding of corticosterone to the GR, Fkbp5 is replaced by Fkbp4, resulting in translocation of the complex to the nucleus (Figure 6A). Newly-formed GR/HSP90/Fkbp4 complexes generally accumulate in the nucleus [21]. Thereafter, the GR acts on the expression of target genes. Thus, Fkbp4 contributes to the negative feedback effect of glucocorticoids. Fkbp4 mRNA levels decreased 24 h after incubation with dexamethasone, and Fkbp4 protein levels tended to decrease. To induce homeostasis, the decrease in Fkbp4 could lead to a decrease in the effects on Pome gene expression of glucocorticoids.
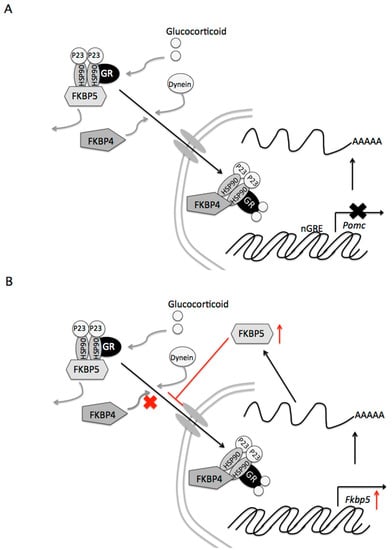
Figure 6.
Proposed signaling mechanisms of Fkbp4 and Fkbp5 by glucocorticoids in pituitary corticotroph cells. (A) Contribution of Fkbp4 to the negative feedback of glucocorticoids. Following binding of glucocorticoid to the glucocorticoid receptor (GR), Fkbp5 is replaced by Fkbp4, resulting in translocation of the complex to the nucleus. Newly-formed GR/heat shock protein 90 (HSP90)/Fkbp4 complexes generally accumulate in the nucleus. The GR subsequently suppresses the expression of the target Pomc gene. (B) Reduced efficiency of the glucocorticoid effect of Fkbp5 on Pomc gene expression. GR activity stimulates Fkbp5 gene transcription and upregulates the protein FKPB5 (indicated by a red arrow). Increases in the Fkbp5 protein inhibit GR translocation to the nucleus, resulting in diminished effects on target genes of glucocorticoids.
GR activity directly stimulates Fkbp5 gene transcription, and upregulates the protein FKPB5 [22]. Thereafter, Fkbp5 inhibits GR activity [10]. In our study, dexamethasone increased Fkpb5 mRNA and Fkbp5 protein levels. Fkbp5 gene expression was reported to be induced by glucocorticoids in humans and rodents [23,24,25]. In fact, the human Fkbp gene contains numerous sites for GR binding [26]. Glucocorticoids activate the Fkbp5 gene and increase the protein content, resulting in inhibition of GR translocation to the nucleus (Figure 6B). Our present study shows that dexamethasone-induced decreases in Pomc mRNA levels were further decreased by Fkbp5 knockdown. Thus, the increase in Fkbp5 also diminishes the effects on Pome gene expression of glucocorticoids. Additionally, in cooperation with the decrease in GR, Fkbp5 may contribute to the desensitization of glucocorticoid action after the long-term exposure.
Other molecules, such as HSP90, HSP90-binding protein P23, and GR itself, may be involved in these glucocorticoid effects. Post-tanslational regulation of these molecules, such as protein phosphorylation, acetylation, and SUMOylation, as well as expression regulation, may also contribute to the effects. For example, HSP90 hyperacetylation results in a loss of chaperone activity [27]. Riebold et al. [28] also showed that Cushing’s disease is caused by overexpression of HSP90 protein and can be treated with an appropriate HSP90 inhibitor. It remains undetermined whether dexamethasone also affects HSP90 expression or its activity in pituitary corticotroph cells.
In this study, mouse corticotroph tumor cells were used. However, it is unclear whether these results would be obtained in normal corticotroph cells. In fact, corticotroph tumor cells may show glucocorticoid resistance compared with normal corticotroph cells. Therefore, it is possible that the expression levels of Fkbp4 and Fkbp5 might differ between these cell types. Studies to determine this are required in future.
In conclusion, dexamethasone decreased Pomc mRNA levels as well as Fkpb4 mRNA levels in the mouse corticotroph. Dexamethasone increased Fkpb5 mRNA and its protein levels. Moreover, dexamethasone-induced decreases in Pomc mRNA levels were partially canceled by Fkbp4 knockdown, and were further decreased by Fkbp5 knockdown. Thus, Fkbp4 contributes to the negative feedback of glucocorticoids, and Fkbp5 reduces the efficiency of the glucocorticoid effect on Pomc gene expression in pituitary corticotroph cells.
4. Materials and Methods
4.1. Materials
Dexamethasone was purchased from Sigma-Aldrich (St. Lois, MO, USA).
4.2. Cell Culture
Murine pituitary AtT-20 corticotroph tumor cells were obtained from ATCC (Manassas, VA, USA). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS), 100 µg/mL streptomycin, and 100 U/mL penicillin at 37 °C in a humidified atmosphere (5% CO2 and 95% air). Cells were seeded in six-well plates at 15.0 × 104 cells/cm2 for 2 day prior to each experiment. On day 3, cells were washed with DMEM supplemented with 0.2% bovine serum albumin (BSA), and subsequently cultured overnight in DMEM without FBS prior to each experiment. Total cellular RNA was collected at the conclusion of each experiment, and stored at –80 °C until required for the relevant assay.
4.3. RNA Extraction
Cells were incubated at the indicated times with medium alone (control) or medium containing 100 nM dexamethasone. To examine the concentration-dependent effects of dexamethasone, cells were incubated with medium alone (control) or medium containing 1–100 nM dexamethasone. Total cellular RNA was extracted using the RNeasy Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s protocol. The extracted RNA (0.5 µg) was subjected to a real-time (RT) reaction using random hexamers as primers with the SuperScript First-Strand Synthesis System for the quantitative RT polymerase chain reaction (RT-qPCR) (Thermo Fisher Scientific, Waltham, MA, USA) as described previously [29].
4.4. RT-qPCR
The resulting cDNA was subjected to RT-qPCR as follows. mRNA expression levels of mouse Pomc and Fkbp4/5 were evaluated using RT-qPCR with transcript-specific primer and probe sets (Assays-on-Demand Gene Expression Products; Applied Biosystems, Foster City, CA, USA). To standardize gene expression levels, β2-microglobulin (B2mg) was used as a reference gene. Across all treated samples, B2mg mRNA levels did not significantly differ from those of controls.
The 25-µL RT-qPCR reactions consisted of 1 × TaqMan Universal PCR Master Mix (Applied Biosystems) and 1 × Assays-on-Demand Gene Expression Products for each of the transcripts (Mm00435874_m1 for mouse Pomc, Mm00487391_m1 for mouse Fkbp4, Mm00487401_m1 for mouse Fkbp5, and Mm00487401_m1 for mouse B2mg), and 500 ng cDNA (25 µL total volume). An ABI PRISM 7000 Sequence Detection System (Applied Biosystems) was used for amplification with the following thermal cycling conditions: 95 °C for 10 min followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. All data are expressed as a function of the threshold cycle (CT) for quantitative analyses using ABI PRISM 7000 SDS software (Applied Biosystems). Analyses that used diluted samples of the gene of interest and the reference gene (B2mg) revealed identical amplification efficiencies. Relative quantitative gene expression was calculated using the 2−ΔΔCT method.
4.5. RNA Interference Experiments
Fkbp4/5 and control siRNA fragments were designed and purchased from QIAGEN. The HiPerFect transfection reagent (QIAGEN) was used to transfect AtT-20 cells with siRNA fragments according to the manufacturer’s protocol.
Target mRNA levels in samples were determined from cells that were seeded in 6-well plates at a density of 15 × 104 cells/well. Cultures were incubated for 24 h in 1 mL of culture medium control or experimental siRNAs: Fkbp4-specific siRNA (siFkbp4, Mm_Fkbp4_1) or Fkbp5-specific siRNA (siFkbp5, Mm_Fkbp5_8), and subsequently incubated in BSA medium containing dexamethasone or control for 24 h. Thereafter, Pomc, Fkbp4, Fkbp5, and B2mg transcript levels were assayed via qRT-PCR.
4.6. Western Blot Analysis
Western blot analysis was performed to examine the effect of dexamethasone (100 nM) on changes in the protein expression of Fkbp4, Fkbp5, and GR. β-actin was used as a housekeeping protein. Cells were washed twice with phosphate-buffered saline (PBS; Life Technologies, Grand Island, NY) and lysed with Laemmli sample buffer. Cell debris was pelleted via centrifugation and the supernatant was recovered. Samples were boiled and subjected to electrophoresis on a 4–20% gradient polyacrylamide gel, and proteins were transferred to a polyvinylidene fluoride membrane (Daiichi Kagaku, Tokyo, Japan). After blocking with Detector Block® buffer (Kirkegaard & Perry Laboratories, Gaithersburg, MD), the membrane was incubated at room temperature for 1 h with each antibody (anti-Fkbp4 antibody [1:5000 dilution]; anti-Fkbp5 [1:2500 dilution] antibody; anti-GR antibody [1:5000 dilution] (Proteintech Group, Rosemont, IL); and anti-β-actin [1:20,000 dilution] antibody, ab8227, Abcam, Cambridge, MA), washed with PBS containing 0.05% Tween 20, and incubated with horseradish peroxidase-labeled anti-rabbit immunoglobulin G (1:20,000 dilution; Daiichi Kagaku). The chemiluminescent substrate SuperSignal West Pico (Pierce Chemical Co., Rockford, IL) was used for detection, and the membrane was exposed to BioMax film (Eastman Kodak Co., Rochester, NY).
4.7. Statistical Analyses
Each in vitro experiment was performed three times. Samples were analyzed in triplicate for each group of experiments. Data are expressed as means ± standard errors of the means. Analysis of variance was performed, followed by Shceffe’s multiple comparison tests. Results with p values < 0.05 were considered significant.
Author Contributions
K.K. and Y.I. conceived and designed the experiments; K.K., Y.W., and K.N. performed the experiments; K.K. analyzed the data; K.K., K.N. and Y.W. contributed reagents/materials/analysis tools; K.K. wrote the paper; Y.I. and M.D. reviewed the paper; and M.D. is a supervisor of the department. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Suda, T.; Kageyama, K.; Sakihara, S.; Nigawara, T. Physiological roles of urocortins, human homologues of fish urotensin I, and their receptors. Peptides 2004, 25, 1689–1701. [Google Scholar] [CrossRef] [PubMed]
- Vale, W.; Spiess, J.; Rivier, C.; Rivier, J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 1981, 213, 1394–1397. [Google Scholar] [CrossRef]
- Whitnall, M.H. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog. Neurobiol. 1993, 40, 573–629. [Google Scholar] [CrossRef]
- Drouin, J.; Trifiro, M.A.; Plante, R.K.; Nemer, M.; Eriksson, P.; Wrange, O. Glucocorticoid receptor binding to a specific DNA sequence is required for hormone-dependent repression of pro-opiomelanocortin gene transcription. Mol. Cell. Biol. 1989, 9, 5305–5314. [Google Scholar] [CrossRef] [PubMed]
- Eberwine, J.H.; Roberts, J.L. Glucocorticoid regulation of pro-opiomelanocortin gene transcription in the rat pituitary. J. Biol. Chem. 1984, 259, 2166–2170. [Google Scholar] [CrossRef]
- Itoi, K.; Mouri, T.; Takahashi, K.; Murakami, O.; Imai, Y.; Sasaki, S.; Yoshinaga, K.; Sasano, N. Suppression by glucocorticoid of the immunoreactivity of corticotropin-releasing factor and vasopressin in the paraventricular nucleus of rat hypothalamus. Neurosci. Lett. 1987, 73, 231–236. [Google Scholar] [CrossRef]
- Ruiz-Conca, M.; Gardela, J.; Martínez, C.A.; Wright, D.; López-Bejar, M.; Rodríguez-Martínez, H.; Álvarez-Rodríguez, M. Natural mating differentially triggers expression of glucocorticoid receptor (NR3C1)-related genes in the preovulatory porcine female reproductive tract. Int. J. Mol. Sci. 2020, 21, 4437. [Google Scholar] [CrossRef]
- Tyrka, A.R.; Ridout, K.K.; Parade, S.H. Childhood adversity and epigenetic regulation of glucocorticoidsignaling genes: Associations in children and adults. Dev. Psychopathol. 2016, 28, 1319–1331. [Google Scholar] [CrossRef]
- Vitellius, G.; Lombes, M. Genetics in endocrinology: Glucocorticoid resistance syndrome. Eur. J. Endocrinol. 2020, 182, R15–R27. [Google Scholar] [CrossRef] [PubMed]
- Wochnik, G.M.; Rüegg, J.; Abel, G.A.; Schmidt, U.; Holsboer, F.; Rein, T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J. Biol. Chem. 2005, 280, 4609–4616. [Google Scholar] [CrossRef]
- Baker, J.D.; Ozsan, I.; Rodriguez Ospina, S.; Gulick, D.; Blair, L.J. Hsp90 heterocomplexes regulate steroid hormone receptors: From stress response to psychiatric disease. Int. J. Mol. Sci. 2018, 20, 79. [Google Scholar] [CrossRef]
- Merkulov, V.M.; Merkulova, T.I.; Bondar, N.P. Mechanisms of brain glucocorticoid resistance in stress-induced psychopathologies. Biochemistry 2017, 82, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.B. The role of Fkbp5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 2009, 34 (Suppl. S1), S186–S195. [Google Scholar] [CrossRef]
- Keller-Wood, M. Hypothalamic-pituitary—Adrenal axis-feedback control. Compr. Physiol. 2015, 5, 1161–1182. [Google Scholar] [CrossRef] [PubMed]
- Parvin, R.; Saito-Hakoda, A.; Shimada, H.; Shimizu, K.; Noro, E.; Iwasaki, Y.; Fujiwara, K.; Yokoyama, A.; Sugawara, A. Role of NeuroD1 on the negative regulation of Pomc expression by glucocorticoid. PLoS ONE 2017, 12, e0175435. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, S.L.; McCarthy, K.C.; Conwell, I.M. Glucocorticoid regulation of hypothalamic proopiomelanocortin. Neuroendocrinology 1998, 67, 51–57. [Google Scholar] [CrossRef] [PubMed]
- King, B.R.; Smith, R.; Nicholson, R.C. Novel glucocorticoid and cAMP interactions on the CRH gene promoter. Mol. Cell. Endocrinol. 2002, 194, 19–28. [Google Scholar] [CrossRef]
- Kumar, R.; Thompson, E.B. Gene regulation by the glucocorticoid receptor: Structure: Function relationship. J. Steroid Biochem. Mol. Biol. 2005, 94, 383–394. [Google Scholar] [CrossRef]
- Dostert, A.; Heinzel, T. Negative glucocorticoid receptor response elements and their role in glucocorticoid action. Curr. Pharm. Des. 2004, 10, 2807–2816. [Google Scholar] [CrossRef] [PubMed]
- Okabe, T.; Takayanagi, R.; Adachi, M.; Imasaki, K.; Nawata, H. Nur77, a member of the steroid receptor superfamily, antagonizes negative feedback of ACTH synthesis and secretion by glucocorticoid in pituitary corticotrope cells. J. Endocrinol. 1998, 156, 169–175. [Google Scholar] [CrossRef][Green Version]
- Wolf, I.M.; Periyasamy, S.; Hinds, T., Jr.; Yong, W.; Shou, W.; Sanchez, E.R. Targeted ablation reveals a novel role of Fkbp52 in gene-specific regulation of glucocorticoid receptor transcriptional activity. J. Steroid Biochem. Mol. Biol. 2009, 113, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Tatro, E.T.; Everall, I.P.; Kaul, M.; Achim, C.L. Modulation of glucocorticoid receptor nuclear translocation in neurons by immunophilins Fkbp51 and Fkbp52: Implications for major depressive disorder. Brain Res. 2009, 1286, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.B.; Salyakina, D.; Lichtner, P.; Wochnik, G.M.; Ising, M.; Pütz, B.; Papiol, S.; Seaman, S.; Lucae, S.; Kohli, M.A.; et al. Polymorphisms in Fkbp5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat. Genet. 2004, 36, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Santen, R.J.; Jewell, C.M.; Yue, W.; Heitjan, D.F.; Raff, H.; Katen, K.S.; Cidlowski, J.A. Glucocorticoid receptor mutations and hypersensitivity to endogenous and exogenous glucocorticoids. J. Clin. Endocrinol. Metab. 2018, 103, 3630–3639. [Google Scholar] [CrossRef]
- Scharf, S.H.; Liebl, C.; Binder, E.B.; Schmidt, M.V.; Müller, M.B. Expression and regulation of the Fkbp5 gene in the adult mouse brain. PLoS ONE 2011, 6, e16883. [Google Scholar] [CrossRef]
- Hubler, T.R.; Scammell, J.G. Intronic hormone response elements mediate regulation of Fkbp5 by progestins and glucocorticoids. Cell Stress Chaperones 2004, 9, 243–252. [Google Scholar] [CrossRef]
- Kovacs, J.J.; Murphy, P.J.; Gaillard, S.; Zhao, X.; Wu, J.T.; Nicchitta, C.V.; Yoshida, M.; Toft, D.O.; Pratt, W.B.; Yao, T.P. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol. Cell 2005, 18, 601–607. [Google Scholar] [CrossRef]
- Riebold, M.; Kozany, C.; Freiburger, L.; Sattler, M.; Buchfelder, M.; Hausch, F.; Stalla, G.K.; Paez-Pereda, M. A C-terminal HSP90 inhibitor restores glucocorticoid sensitivity and relieves a mouse allograft model of Cushing disease. Nat. Med. 2015, 21, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Ishigame, N.; Kageyama, K.; Takayasu, S.; Furumai, K.; Nakada, Y.; Daimon, M. Regulation of the expression of corticotropin-releasing factor gene by pyroglutamylated RFamide peptide in rat hypothalamic 4B cells. Endocr. J. 2016, 63, 919–927. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).