A Matrix Metalloproteinase Mediates Tracheal Development in Bombyx mori
Abstract
1. Introduction
2. Results
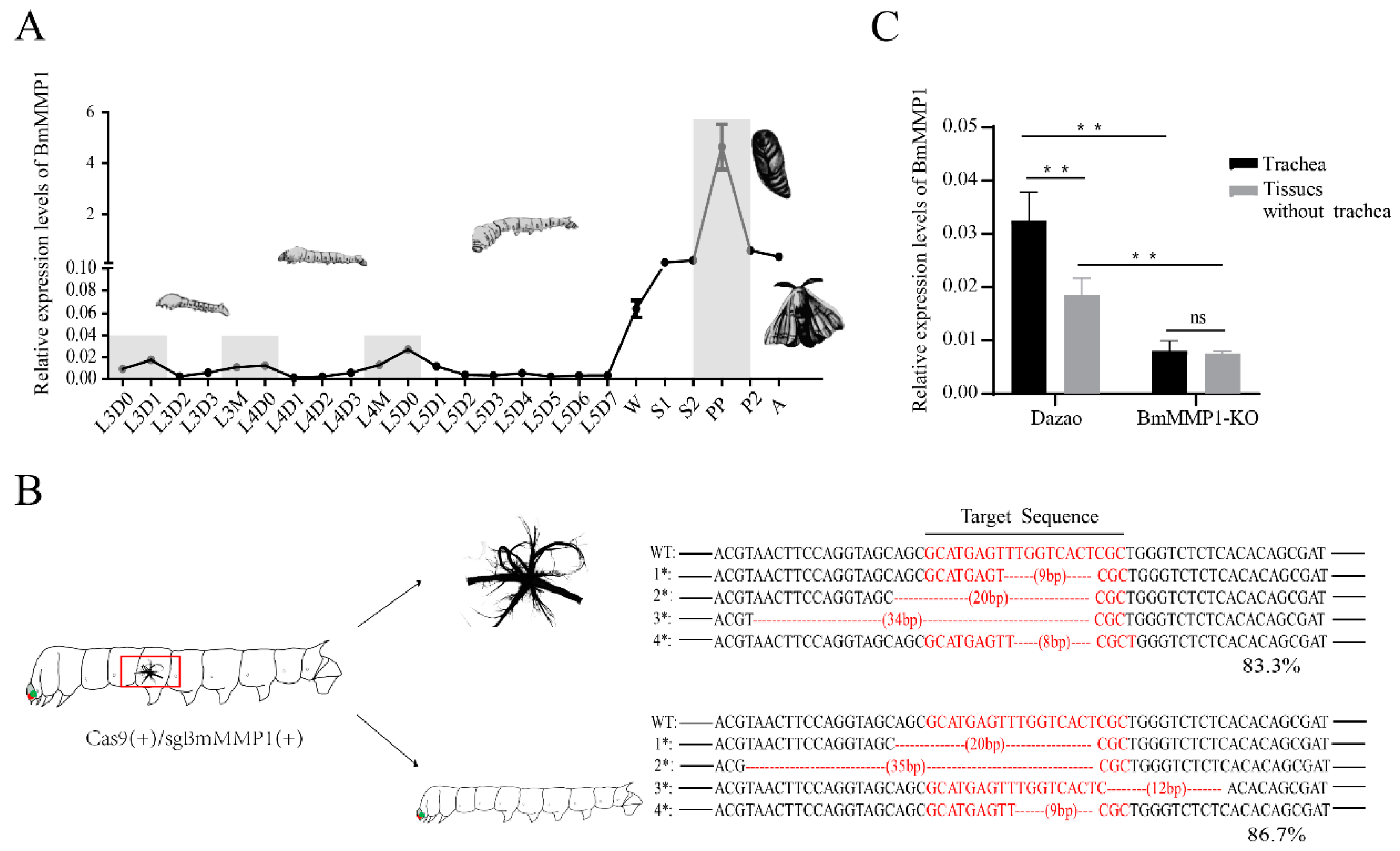
2.1. BmMMP1 Was Highly Expressed during the Critical Remodeling Periods of the Trachea
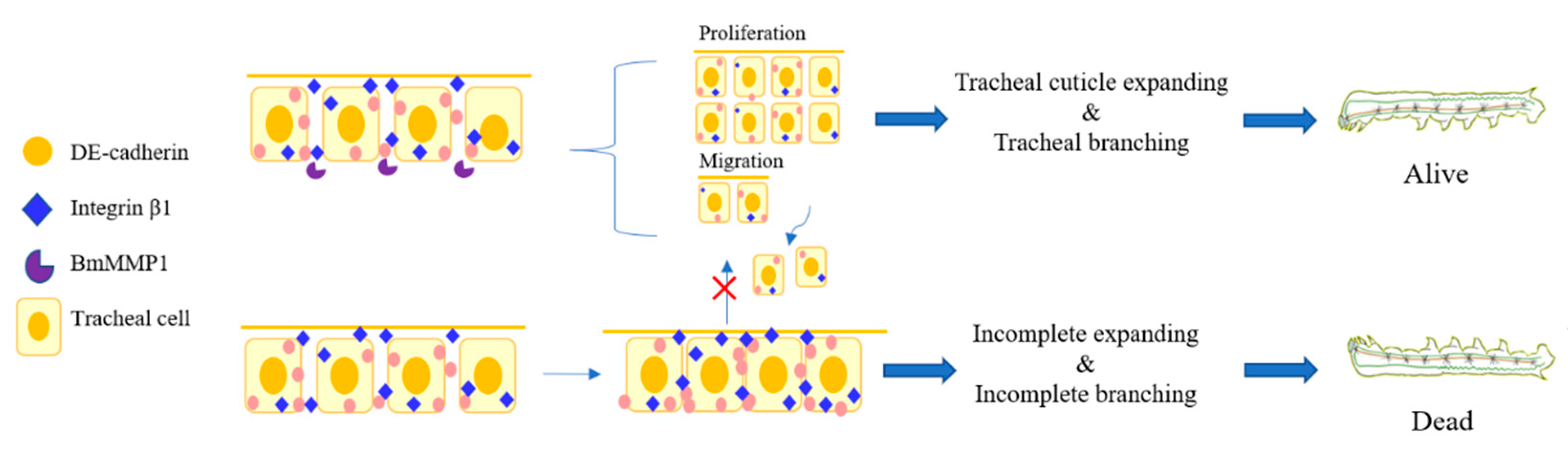
2.2. BmMMP1 Is Required for Tracheal Branch
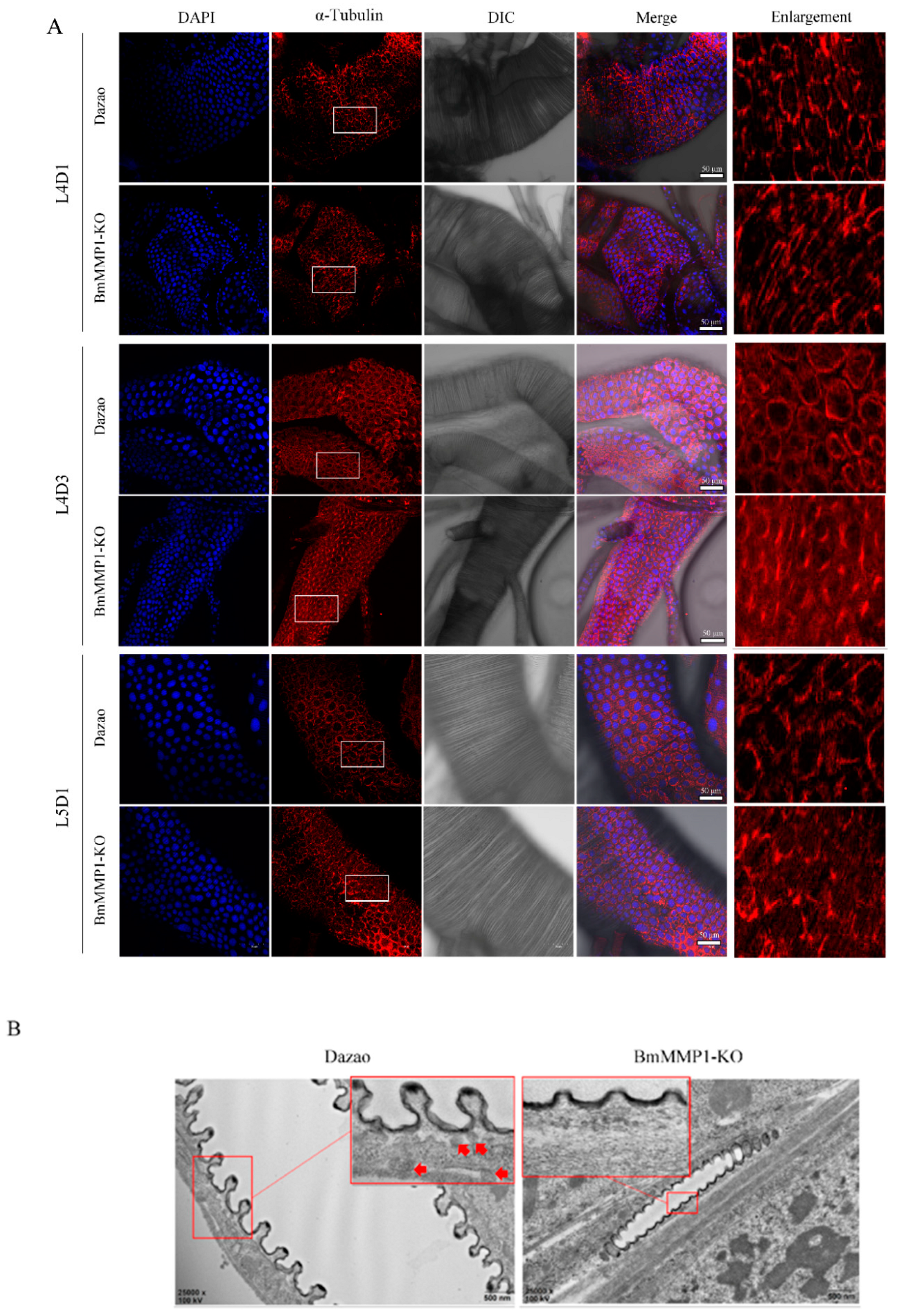
2.3. Taenidial Spacing Do Not Expand in Transgenic BmMMP1-KO Lines
2.4. Knockout of BmMMP1 Decreases Epidermal Cell Spacing and Breaks the Structure of Apical Membrane in Trachea
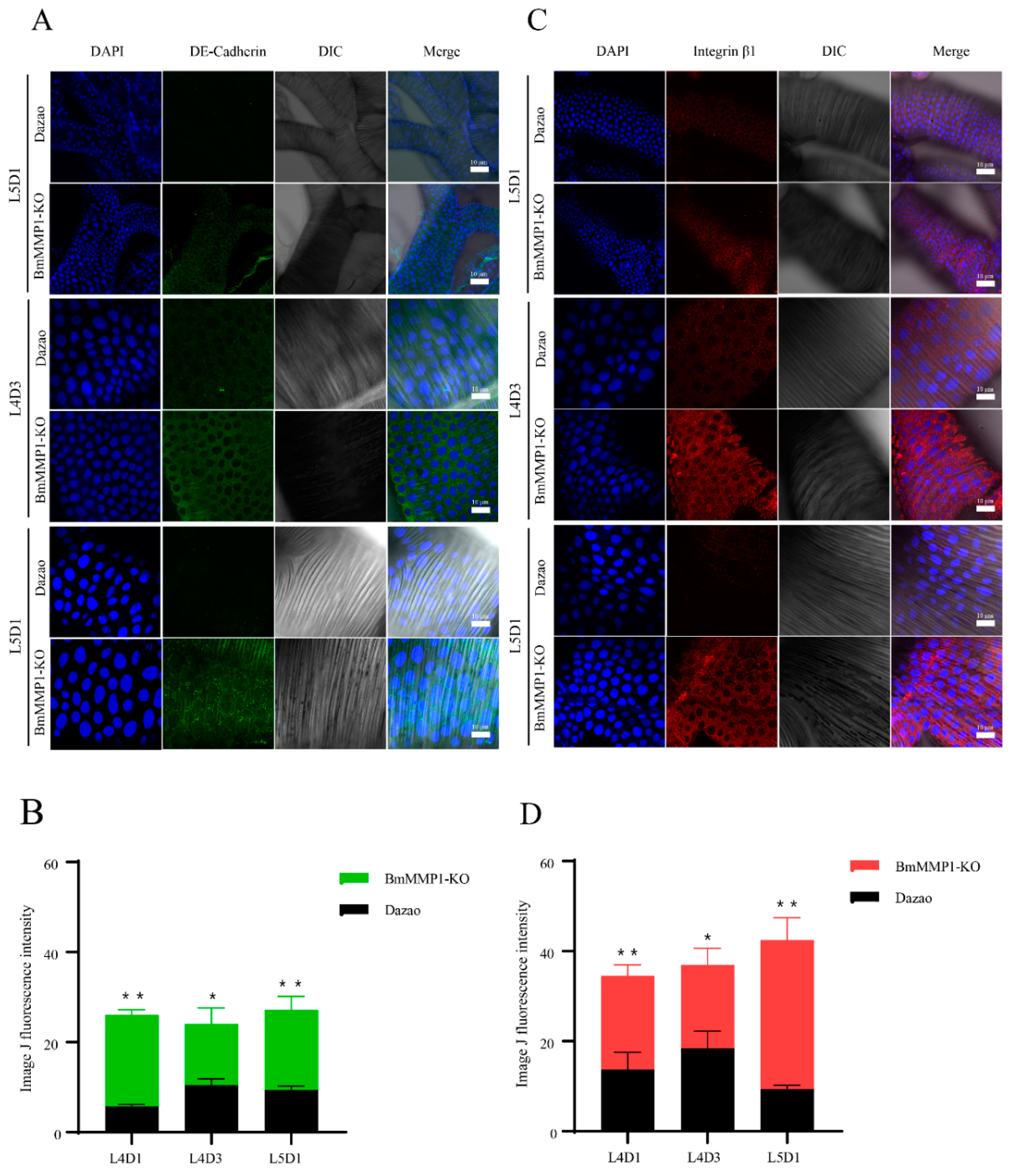
2.5. DE-Cadherin and Integrin β1 Are Accumulated in Trachea of Transgenic BmMMP1-KO Silkworm
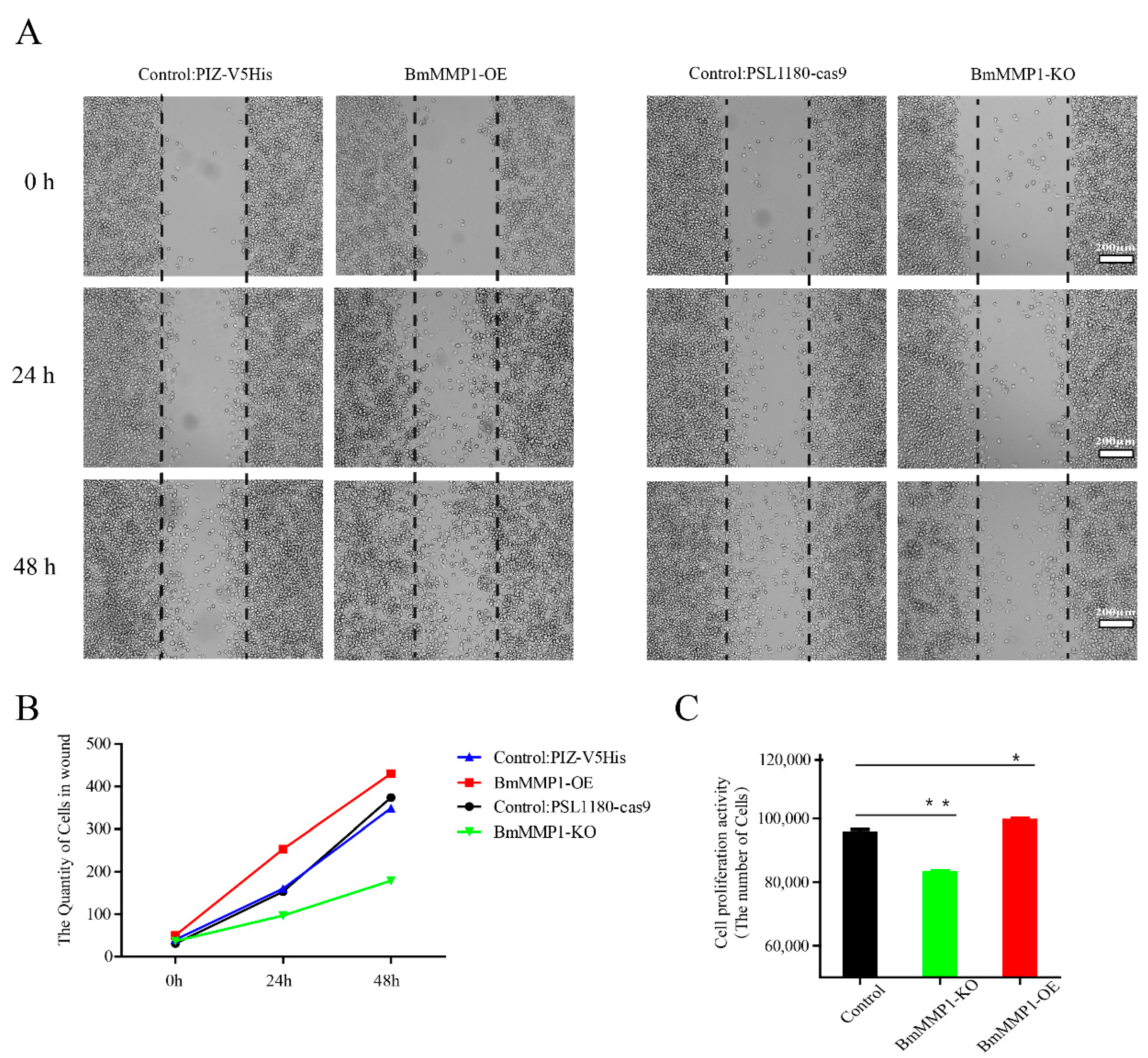
2.6. BmMMP1 Controls Proliferation and Migration of Cells in Silkworm
3. Discussion
4. Methods and Materials
4.1. Silkworm Strains and Cell Cultures
4.2. Embryonic Anatomy and Live Cell Imaging System
4.3. Total RNA Extraction and Quantitative Real-Time PCR
4.4. Knockout Efficiency Assays
4.5. BrdU Incorporation and Immunostaining
4.6. Wound Healing Assay and Cell MTS Assay
4.7. Transmission Electron Microscope (TEM)
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BmMMP1-OE | BmMMP1 overexpression |
| BmMMP1-KO | BmMMP1 knockout |
| BmNS | BmN-SWU1 |
| BrdU | 5-Bromo-2′-Deoxyuridine |
| DAPI | 2, 4-diamidino-2-phenylindole |
| ECM | Extracellular matrix |
| FGF | Fibroblast growth factor |
| MMPs | Matrix metalloproteinases |
| mRNA | Messenger RNA |
| MTS | 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium |
| PBS | Phosphate buffer saline |
| qRT-PCR | Quantitative real-time reverse transcription polymerase chain reaction |
| TEM | transmission electron microscopy |
| WT | Wild type |
References
- Birkedal-Hansen, H.; Moore, W.G.; Bodden, M.K.; Windsor, L.J.; Birkedal-Hansen, B.; DeCarlo, A.; Engler, J.A. Matrix metalloproteinases: A review. Crit. Rev. Oral Biol. Med. 1993, 4, 197–250. [Google Scholar] [CrossRef]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef]
- Loffek, S.; Schilling, O.; Franzke, C.W. Series “matrix metalloproteinases in lung health and disease”: Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef]
- Khokha, R.; Denhardt, D.T. Matrix metalloproteinases and tissue inhibitor of metalloproteinases: A review of their role in tumorigenesis and tissue invasion. Invasion Metastasis 1989, 9, 391–405. [Google Scholar]
- Herrera, I.; Cisneros, J.; Maldonado, M.; Ramirez, R.; Ortiz-Quintero, B.; Anso, E.; Chandel, N.S.; Selman, M.; Pardo, A. Matrix metalloproteinase (MMP)-1 induces lung alveolar epithelial cell migration and proliferation, protects from apoptosis, and represses mitochondrial oxygen consumption. J. Biol. Chem. 2013, 288, 25964–25975. [Google Scholar] [CrossRef]
- Jia, Q.; Liu, Y.; Liu, H.; Li, S. Mmp1 and Mmp2 cooperatively induce Drosophila fat body cell dissociation with distinct roles. Sci. Rep. 2014, 4, 1–12. [Google Scholar] [CrossRef]
- Janiszewska, M.; Primi, M.C.; Izard, T. Cell adhesion in cancer: Beyond the migration of single cells. J. Biol. Chem. 2020, 295, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Oda, H.; Kraut, R.; Hayashi, S.; Kotaoka, Y.; Takeichi, M. Zygotic Drosophila E-cadherin expression is required for processes of dynamic epithelial cell rearrangement in the Drosophila embryo. Genes Dev. 1996, 10, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Berrier, A.L.; Yamada, K.M. Cell-matrix adhesion. J. Cell. Physiol. 2007, 213, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Mustafa, A.; Yerzhan, A.; Merzhakupova, D.; Yerlan, P.; Orakov, A.N.; Wang, X.; Huang, Y.; Miao, L. Nuclear matrix metalloproteinases: Functions resemble the evolution from the intracellular to the extracellular compartment. Cell Death Discov. 2017, 3, 1–8. [Google Scholar] [CrossRef]
- Wiseman, B.S.; Sternlicht, M.D.; Lund, L.R.; Alexander, C.M.; Mott, J.; Bissell, M.J.; Soloway, P.; Itohara, S.; Werb, Z. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J. Cell. Biochem. 2003, 162, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Parks, W.C. Role of matrix metalloproteinases in epithelial migration. J. Cell. Biochem. 2009, 108, 1233–1243. [Google Scholar] [CrossRef]
- Puente, X.S.; Sanchez, L.M.; Overall, C.M.; Lopez-Otin, C. Human and mouse proteases: A comparative genomic approach. Nat. Rev. Genet. 2003, 4, 544–558. [Google Scholar] [CrossRef]
- Knorr, E.; Schmidtberg, H.; Vilcinskas, A.; Altincicek, B. MMPs regulate both development and immunity in the tribolium model insect. PLoS ONE 2009, 4, e4751. [Google Scholar] [CrossRef]
- Altincicek, B.; Vilcinskas, A. Identification of a lepidopteran matrix metalloproteinase with dual roles in metamorphosis and innate immunity. Dev. Comp. Immunol. 2008, 32, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Page-McCaw, A.; Serano, J.; Sante, J.M.; Rubin, G.M. Drosophila matrix metalloproteinases are required for tissue remodeling, but not embryonic development. Dev. Cell 2003, 4, 95–106. [Google Scholar] [CrossRef]
- Wang, Q.; Uhlirova, M.; Bohmann, D. Spatial restriction of FGF signaling by a matrix metalloprotease controls branching morphogenesis. Dev. Cell 2010, 18, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Kondo, T. Development and Function of the Drosophila Tracheal System. Genetics 2018, 209, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Beitel, G.J.; Krasnow, M.A. Genetic control of epithelial tube size in the Drosophila tracheal system. Development 2000, 127, 3271–3282. [Google Scholar] [CrossRef]
- Vidal, M.; Salavaggione, L.; Ylagan, L.; Wilkins, M.; Watson, M.; Weilbaecher, K.; Cagan, R. A role for the epithelial microenvironment at tumor boundaries: Evidence from Drosophila and human squamous cell carcinomas. Am. J. Pathol. 2010, 176, 3007–3014. [Google Scholar] [CrossRef]
- Young, R.E.; Jones, M.K.; Hines, E.A.; Li, R.; Luo, Y.; Shi, W.; Verheyden, J.M.; Sun, X. Smooth Muscle Differentiation Is Essential for Airway Size, Tracheal Cartilage Segmentation, but Dispensable for Epithelial Branching. Dev. Cell 2020, 53, 73–85. [Google Scholar] [CrossRef]
- van Hinsbergh, V.W.; Koolwijk, P. Endothelial sprouting and angiogenesis: Matrix metalloproteinases in the lead. Cardiovasc. Res. 2008, 78, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Shuying, Y.; Qizhang, S. Scanning electron microscope observation of silkworm embryo development. Jiangsu Seric. J. 1995, 4, 1–4. [Google Scholar]
- Ghabrial, A.S.; Krasnow, M.A. Social interactions among epithelial cells during tracheal branching morphogenesis. Nature 2006, 441, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.R.; Lin, L.; Huang, H.; Guha, A.; Roy, S.; Kornberg, T.B. Developmental compartments in the larval trachea of Drosophila. eLife 2015, 4, e08666. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Chavez, C.; Andrew, D.J. Trachealess (Trh) regulates all tracheal genes during Drosophila embryogenesis. Dev. Biol. 2011, 360, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Glasheen, B.M.; Robbins, R.M.; Piette, C.; Beitel, G.J.; Page-McCaw, A. A matrix metalloproteinase mediates airway remodeling in Drosophila. Dev. Biol. 2010, 344, 772–783. [Google Scholar] [CrossRef]
- Jia, Q.; Chen, X.; Wu, L.; Ruan, Z.; Li, K.; Li, S. Matrix metalloproteinases promote fat body cell dissociation and ovary development in Bombyx mori. J. Insect Physiol. 2018, 111, 8–15. [Google Scholar] [CrossRef]
- Kawasaki, H.; Manickam, A.; Shahin, R.; Ote, M.; Iwanaga, M. Expression of matrix metalloproteinase genes during basement membrane degradation in the metamorphosis of Bombyx mori. Gene 2018, 638, 26–35. [Google Scholar] [CrossRef]
- Vu, T.H.; Werb, Z. Matrix metalloproteinases: Effectors of development and normal physiology. Genes Dev. 2000, 14, 2123–2133. [Google Scholar] [CrossRef]
- Inoue, Y.; Hayashi, S. Tissue-specific laminin expression facilitates integrin-dependent association of the embryonic wing disc with the trachea in Drosophila. Dev. Biol. 2007, 304, 90–101. [Google Scholar] [CrossRef]
- Urbano, J.M.; Dominguez-Gimenez, P.; Estrada, B.; Martin-Bermudo, M.D. PS integrins and laminins: Key regulators of cell migration during Drosophila embryogenesis. PLoS ONE 2011, 6, e23893. [Google Scholar] [CrossRef] [PubMed]
- Urbano, J.M.; Torgler, C.N.; Molnar, C.; Tepass, U.; Lopez-Varea, A.; Brown, N.H.; de Celis, J.F.; Martin-Bermudo, M.D. Drosophila laminins act as key regulators of basement membrane assembly and morphogenesis. Development 2009, 136, 4165–4176. [Google Scholar] [CrossRef] [PubMed]
- Mummidi, S.; Das, N.A.; Carpenter, A.J.; Yoshida, T.; Yariswamy, M.; Mostany, R.; Izadpanah, R.; Higashi, Y.; Sukhanov, S.; Noda, M.; et al. RECK suppresses interleukin-17/TRAF3IP2-mediated MMP-13 activation and human aortic smooth muscle cell migration and proliferation. J. Cell Physiol. 2019, 234, 22242–22259. [Google Scholar] [CrossRef]
- Qin, J.; Zha, G.B.; Yu, J.; Zhang, H.H.; Yi, S. Differential temporal expression of matrix metalloproteinases following sciatic nerve crush. Neural Regen. Res. 2016, 11, 1165–1171. [Google Scholar]
- Daley, W.P.; Peters, S.B.; Larsen, M. Extracellular matrix dynamics in development and regenerative medicine. J. Cell Sci. 2008, 121, 255–264. [Google Scholar] [CrossRef]
- Cheng, T.; Zhang, X.; Peng, Z.; Fan, Y.; Zhang, L.; Liu, C. Effects of Osiris9a on Silk Properties in Bombyx mori Determined by Transgenic Overexpression. Int. J. Mol. Sci. 2020, 21, 1888. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Zhou, X.-L.; Liu, T.-H.; Chen, P.; Jiang, X.; Dong, Z.-Q.; Pan, M.-H.; Lu, C. A Matrix Metalloproteinase Mediates Tracheal Development in Bombyx mori. Int. J. Mol. Sci. 2021, 22, 5618. https://doi.org/10.3390/ijms22115618
Wei Y, Zhou X-L, Liu T-H, Chen P, Jiang X, Dong Z-Q, Pan M-H, Lu C. A Matrix Metalloproteinase Mediates Tracheal Development in Bombyx mori. International Journal of Molecular Sciences. 2021; 22(11):5618. https://doi.org/10.3390/ijms22115618
Chicago/Turabian StyleWei, Yi, Xiao-Lin Zhou, Tai-Hang Liu, Peng Chen, Xia Jiang, Zhan-Qi Dong, Min-Hui Pan, and Cheng Lu. 2021. "A Matrix Metalloproteinase Mediates Tracheal Development in Bombyx mori" International Journal of Molecular Sciences 22, no. 11: 5618. https://doi.org/10.3390/ijms22115618
APA StyleWei, Y., Zhou, X.-L., Liu, T.-H., Chen, P., Jiang, X., Dong, Z.-Q., Pan, M.-H., & Lu, C. (2021). A Matrix Metalloproteinase Mediates Tracheal Development in Bombyx mori. International Journal of Molecular Sciences, 22(11), 5618. https://doi.org/10.3390/ijms22115618




