Overexpression of MdATG8i Enhances Drought Tolerance by Alleviating Oxidative Damage and Promoting Water Uptake in Transgenic Apple
Abstract
1. Introduction
2. Results
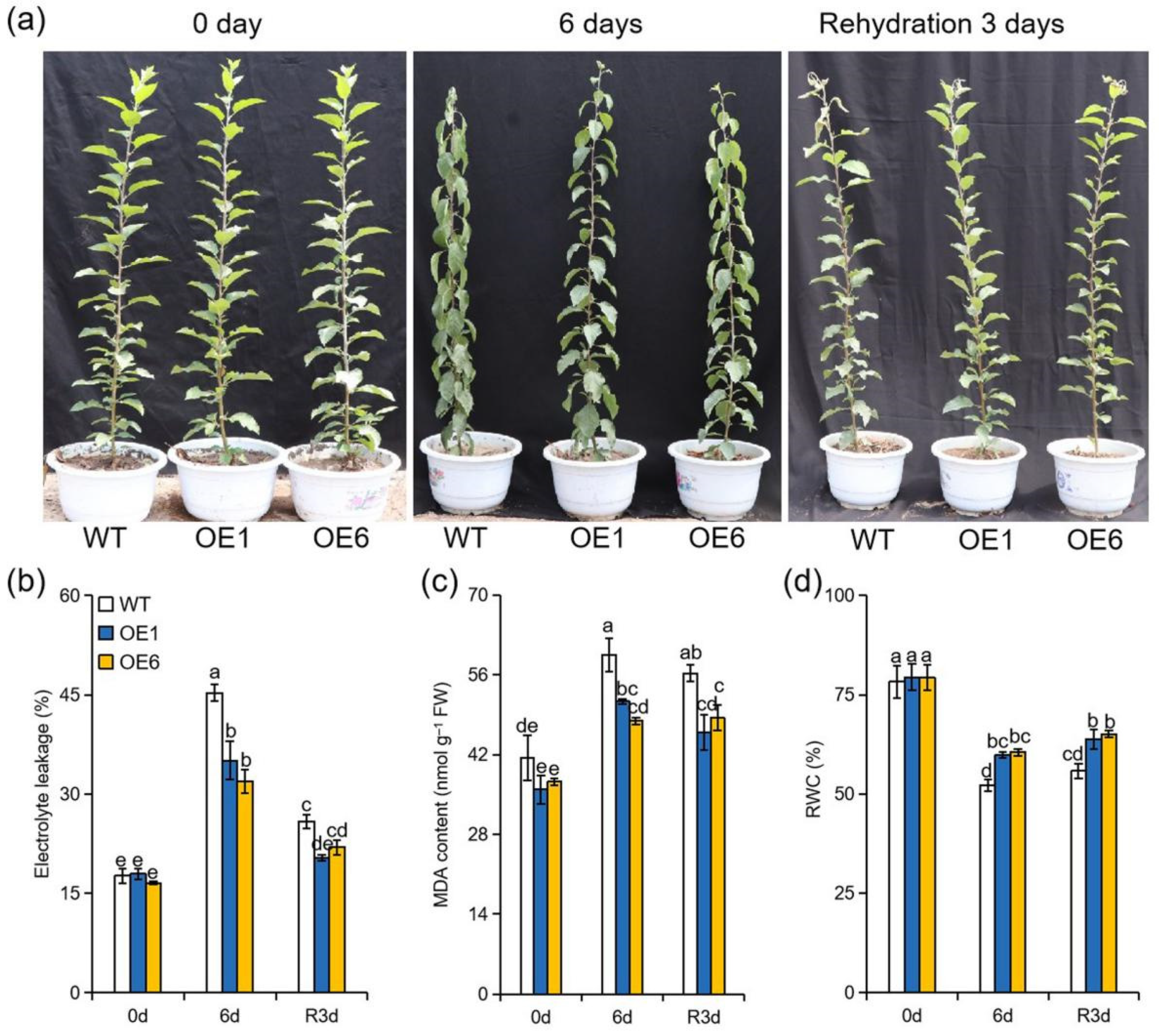
2.1. Overexpression of MdATG8i Increased Apple Plants’ Tolerance to Extreme Drought Stress
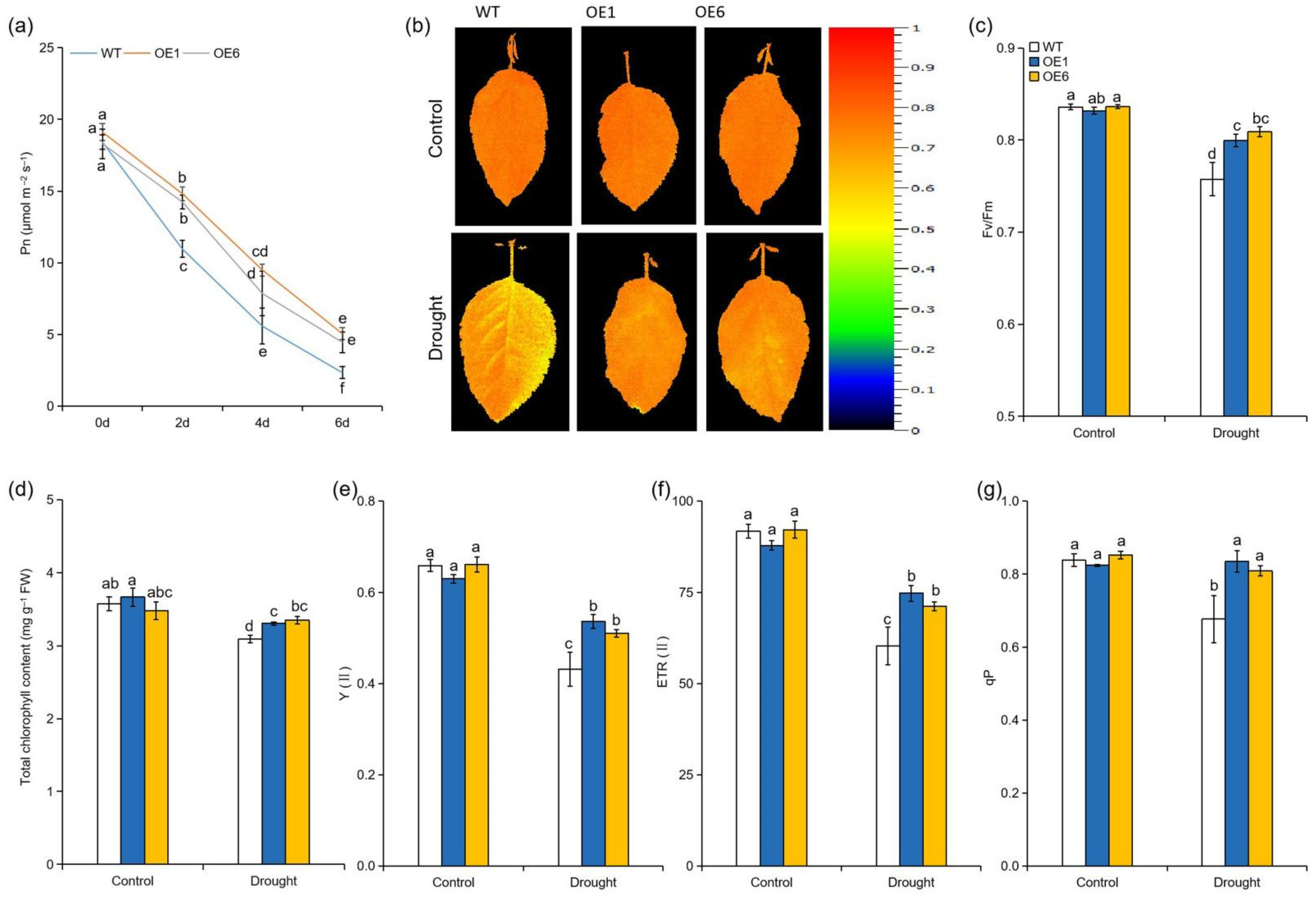
2.2. MdATG8i Overexpression Apple Plants Maintained Higher Photosynthetic Capacity under Drought Stress
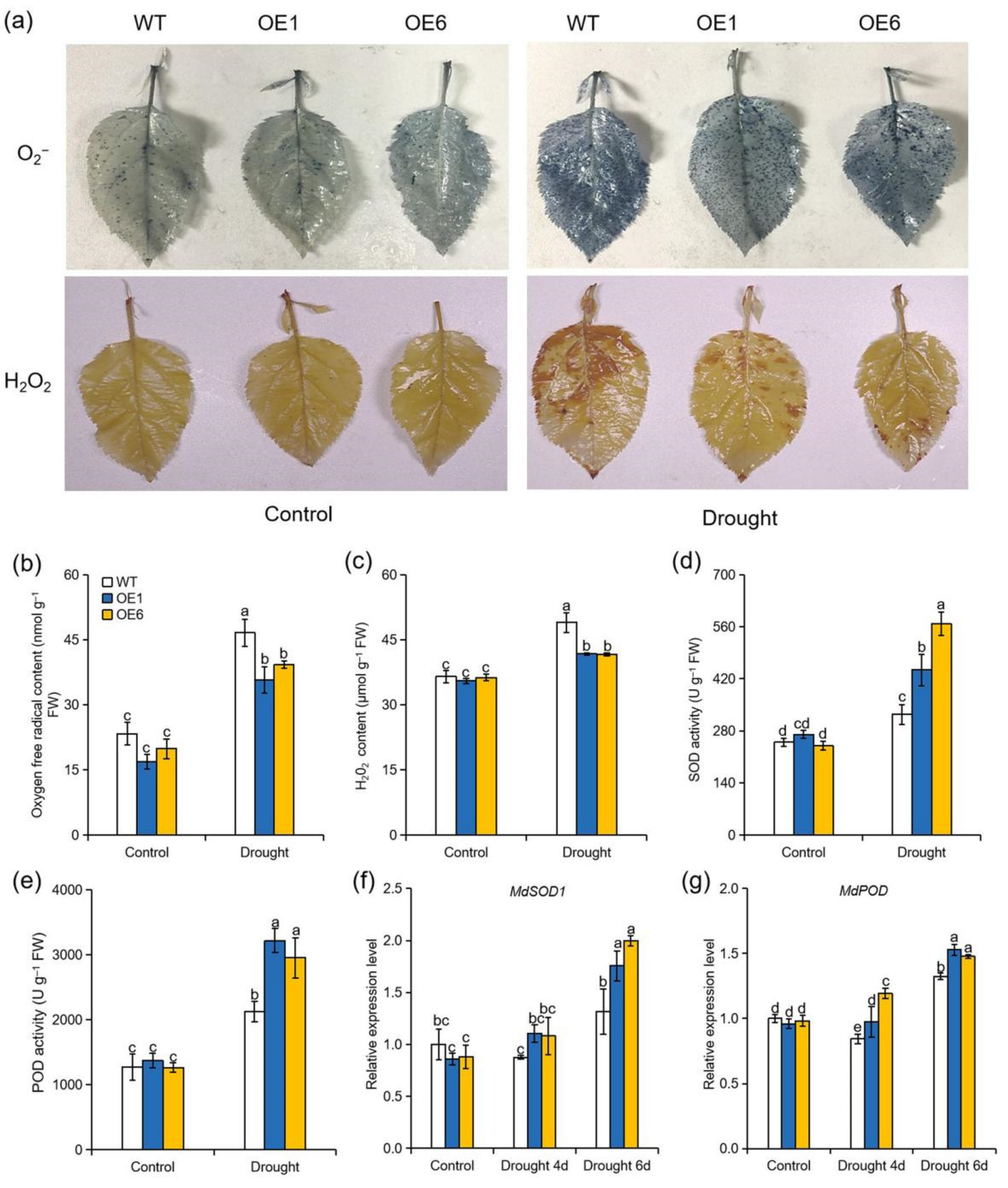
2.3. Overexpression of MdATG8i in Apple Stimulated ROS Scavenging under Drought Stress
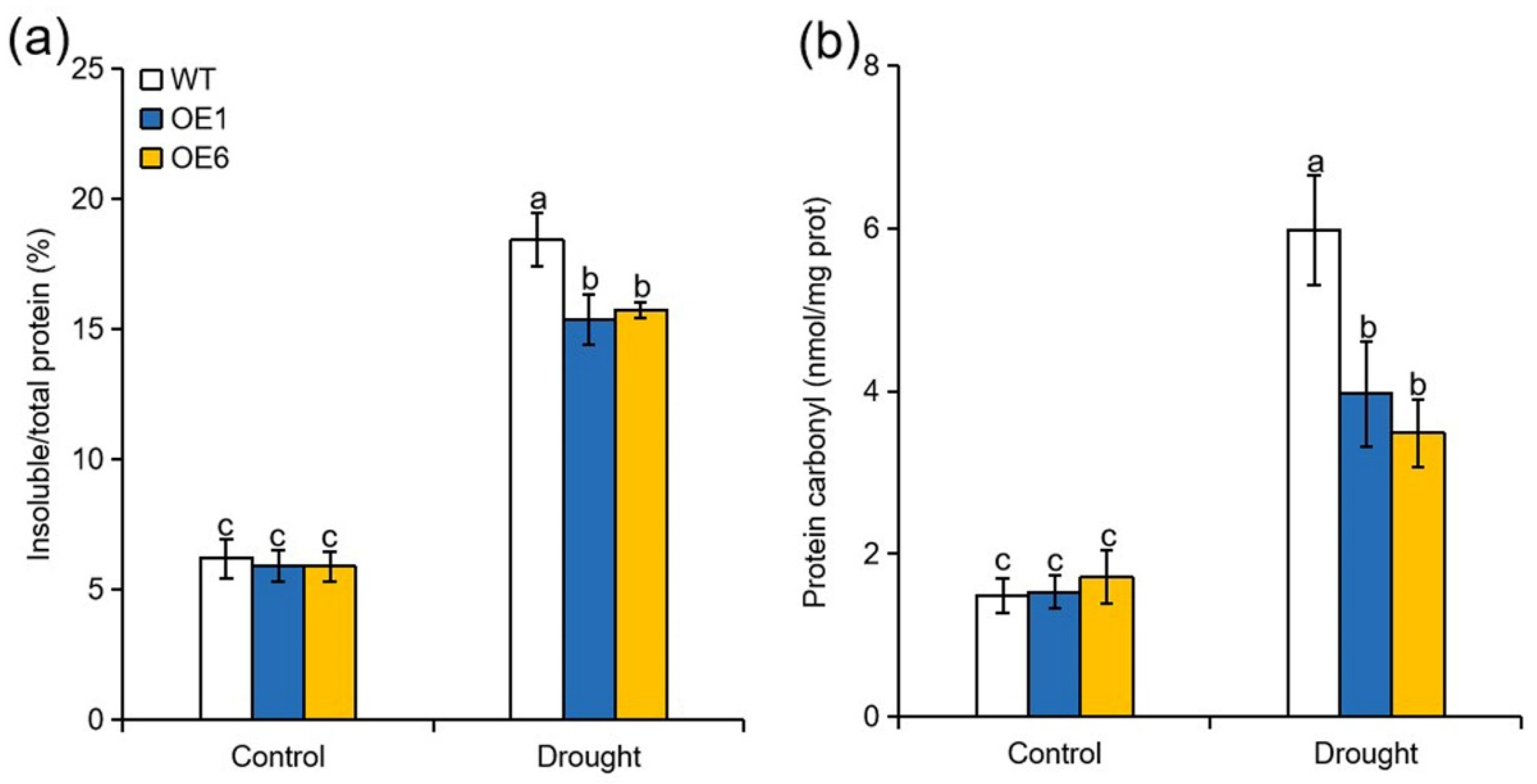
2.4. Overexpression of MdATG8i in Apple Reduced the Accumulation of Insoluble or Oxidized Proteins under Drought Stress
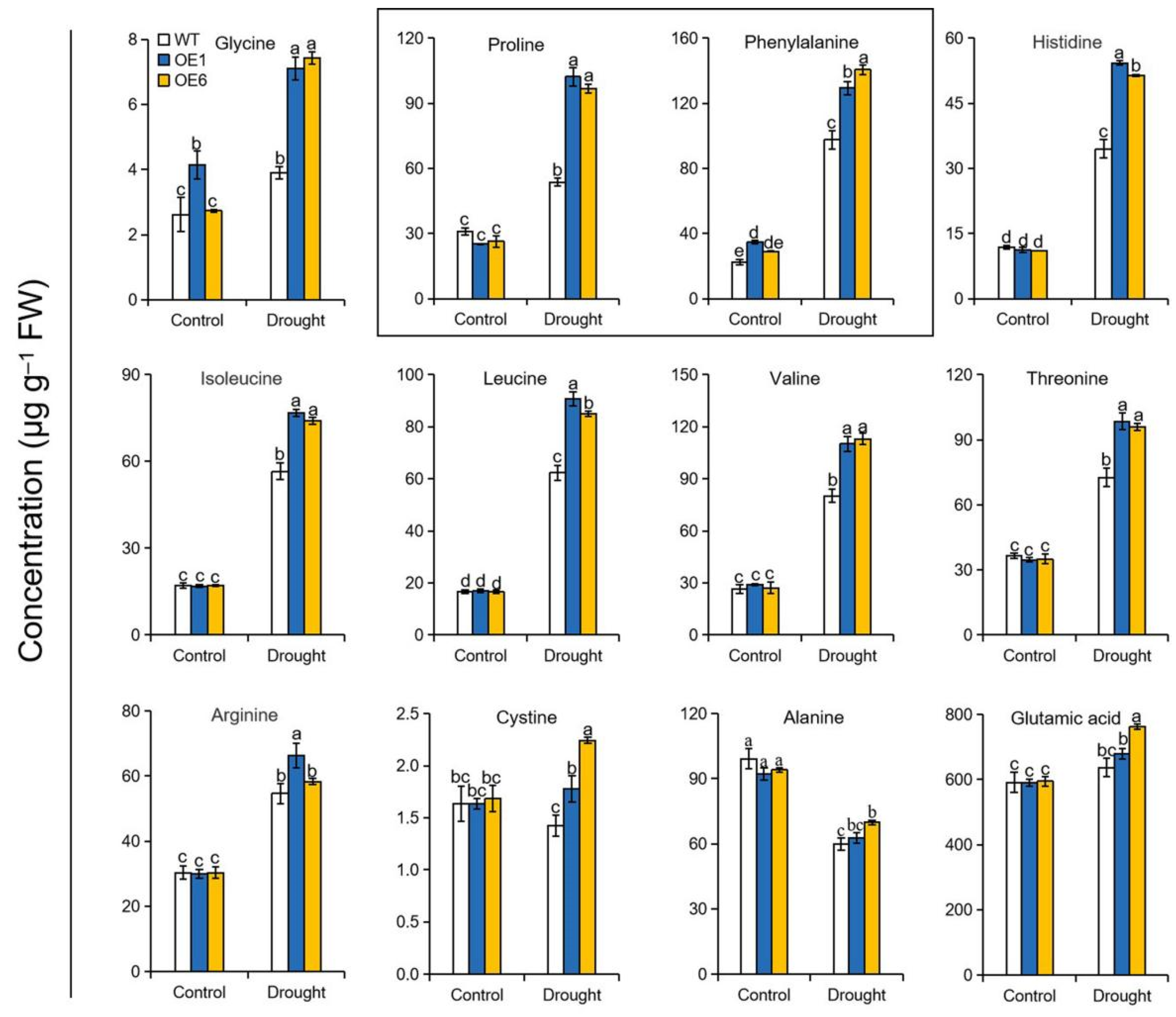
2.5. Overexpression of MdATG8i Modulated Amino Acid Metabolism under Drought Stress
2.6. Overexpression of MdATG8i Promoted the Synthesis of Flavonoid under Drought Stress
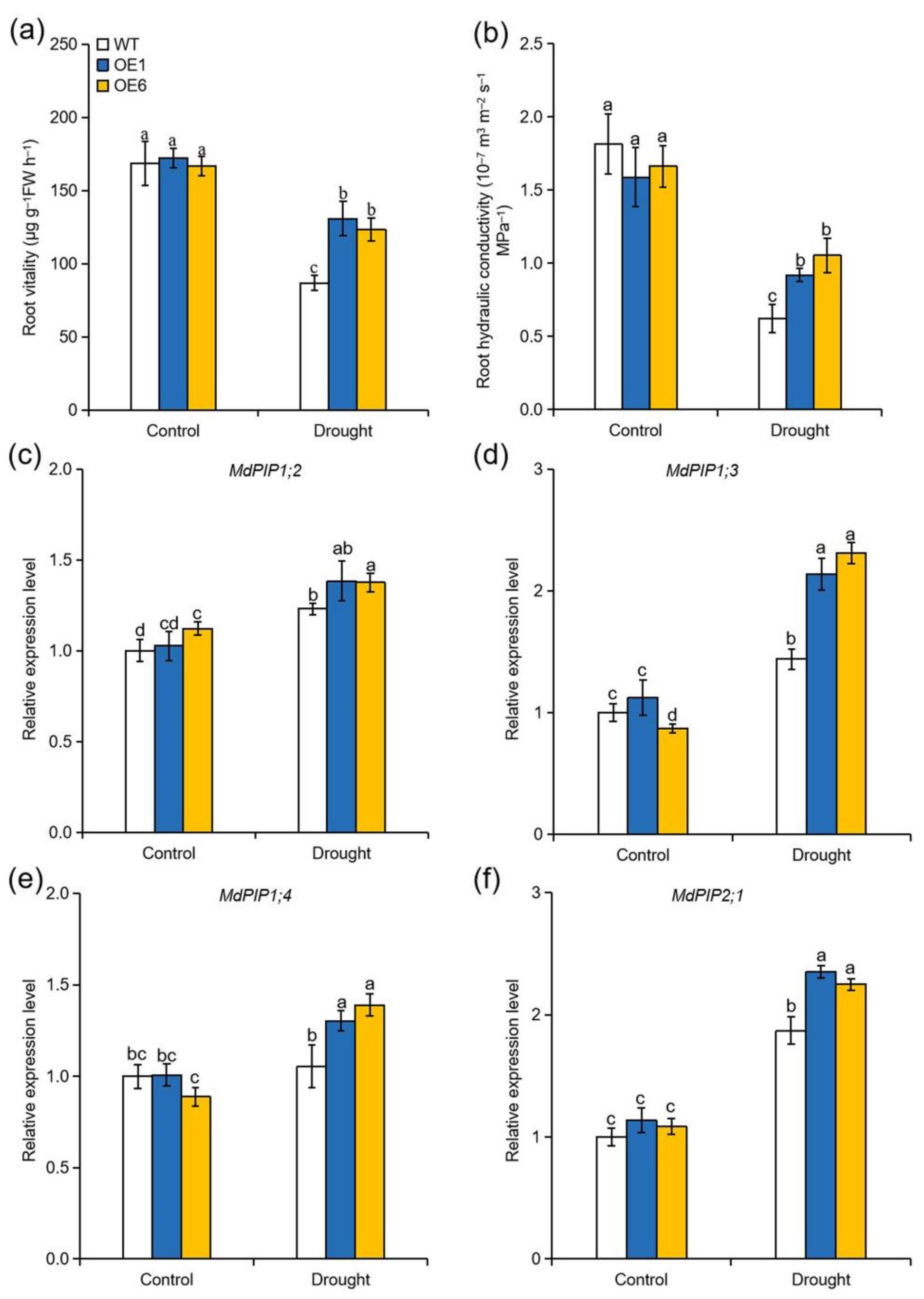
2.7. Overexpression of MdATG8i Improved Root Vitality and Hydraulic Conductivity of Apple Plants under Drought Stress
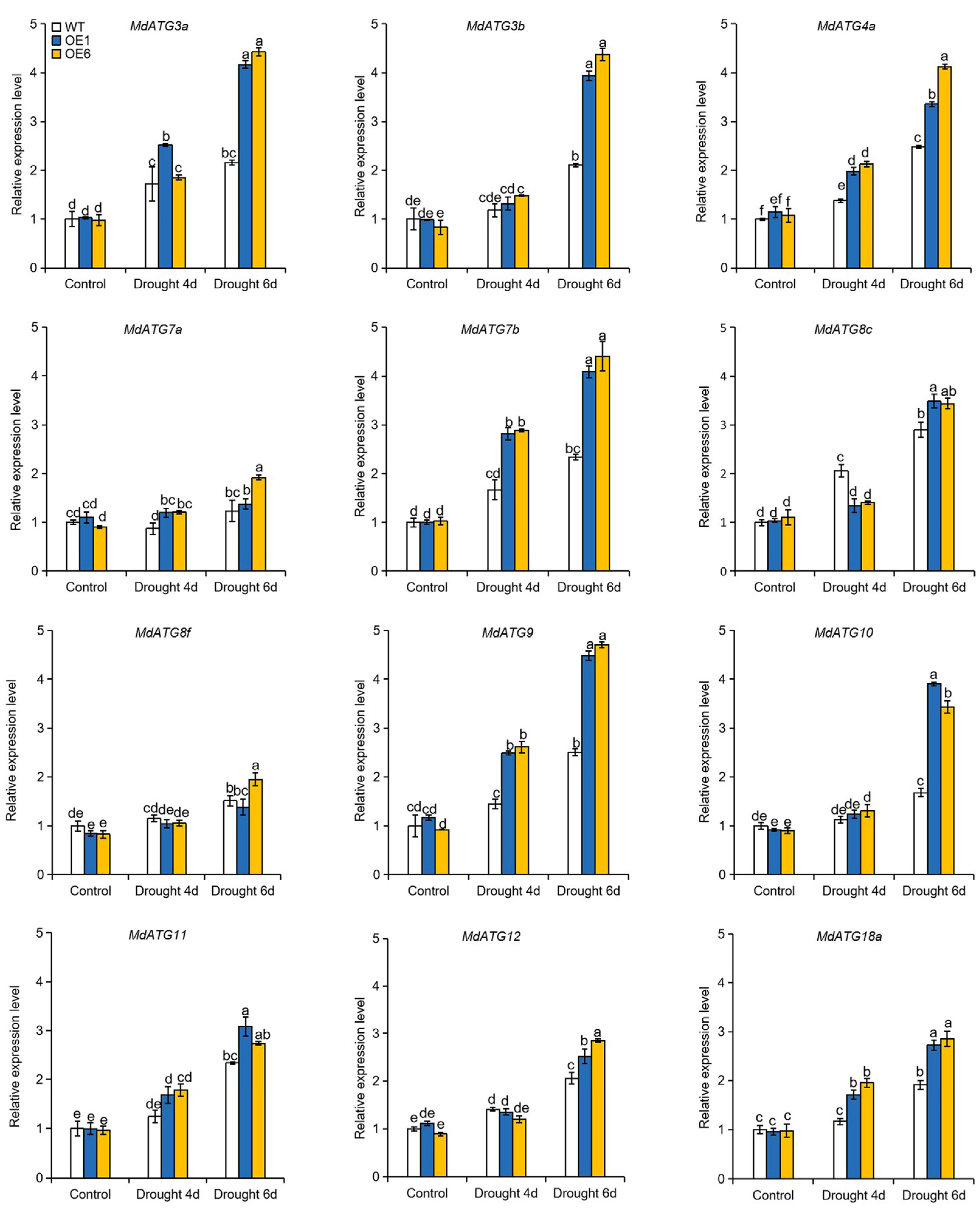
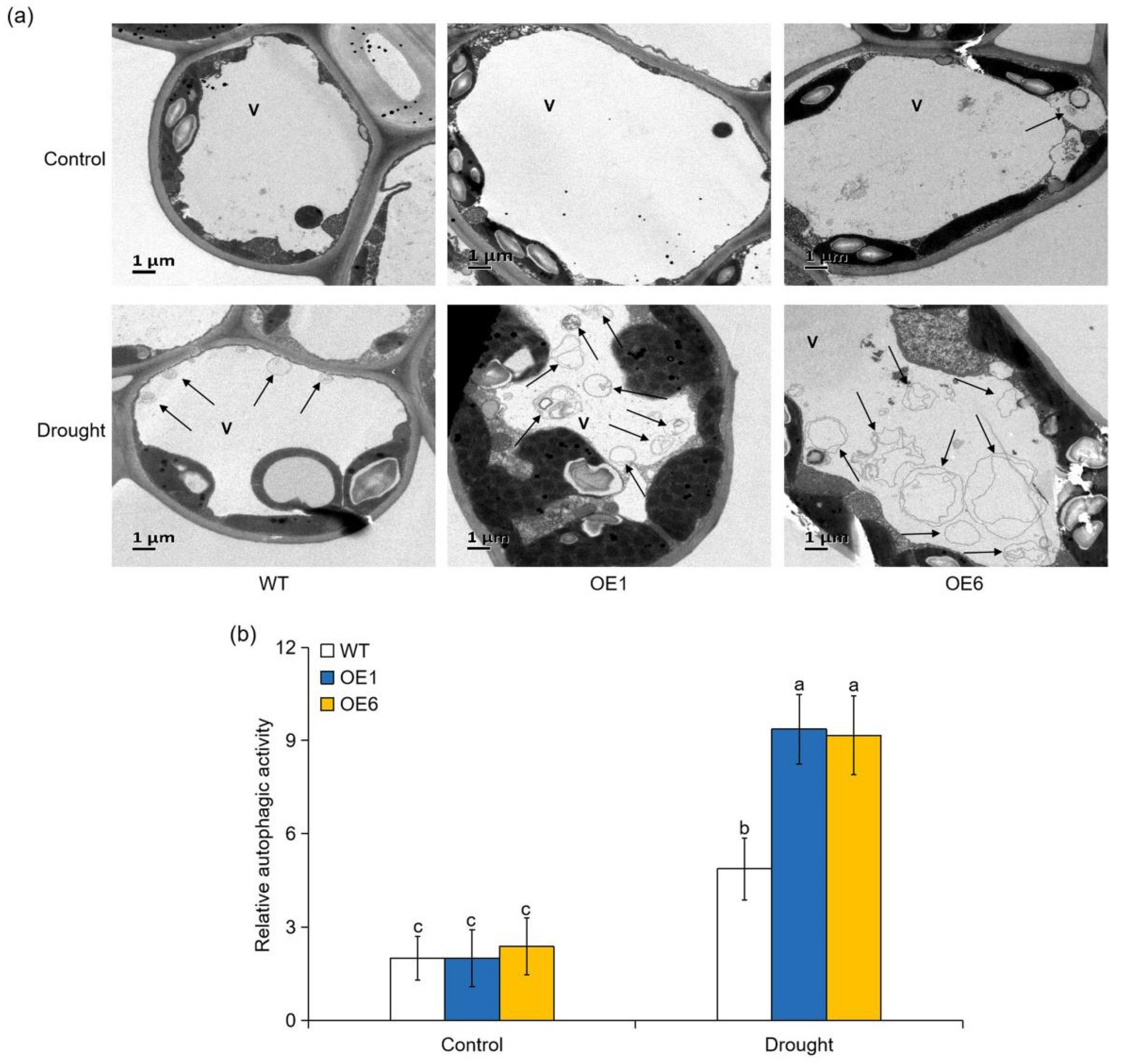
2.8. Overexpression of MdATG8i in Apple Enhanced the Transcript of other Autophagy-Related (ATG) Genes and the Accumulation of Autophagic Structures under Drought Stress
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. RNA Extraction and qRT-PCR Analysis
4.3. Analysis of Physiological Traits and Measurement of Root Hydraulic Conductivity
4.4. Analysis of ROS Accumulation and Antioxidant Enzyme Activity
4.5. Measurement of Photosynthetic Characteristics and Chlorophyll Fluorescence
4.6. Determination of Insoluble and Oxidized Proteins
4.7. Measurement of Amino Acids and Flavonoid
4.8. Detection of Autophagic Structures
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatt, R.M.; Srinivasa, N.K. Influence of pod load on response of okra to water stress. Indian J. Plant Physiol. 2005, 10, 54–59. [Google Scholar]
- Chaitanya, K.V.; Sundar, D.; Jutur, P.P.; Ramachandra, A. Water stress effects on photosynthesis in different mulberry cultivars. Plant Growth Regul. 2003, 40, 75–80. [Google Scholar] [CrossRef]
- Karthikeyan, B.; Jaleel, C.A.; Gopi, R.; Deiveekasundaram, M. Alterations in seedling vigour and antioxidant enzyme activities in Catharanthus roseus under seed priming with native diazotrophs. J. Zhejiang Univ. Sci. B 2007, 8, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Hu, H.; Xiong, L. Genetic engineering and breeding of drought resistant crops. Annu. Rev. Plant Biol. 2014, 65, 715–741. [Google Scholar] [CrossRef]
- Kooyers, N.J. The evolution of drought escape and avoidance in natural herbaceous populations. Plant Sci. 2015, 234, 155–162. [Google Scholar] [CrossRef]
- Liu, P.; Yin, L.; Deng, X.; Wang, S.W.; Tanaka, K.; Zhang, S.Q. Aquaporin-mediated increase in root hydraulic conductance is involved in silicon-induced improved root water uptake under osmotic stress in Sorghum bicolor L. J. Exp. Bot. 2014, 17, 4747. [Google Scholar] [CrossRef]
- Ebskamp, M.J.M.; Vandermeer, I.M.; Spronk, B.A.; Weisbeek, P.J.; Smeekens, S.C.M. Accumulation of fructose polymers in transgenic tobacco. Biotechnology 1994, 12, 272–275. [Google Scholar] [CrossRef]
- Hildebrandt, T.M. Synthesis versus degradation: Directions of amino acid metabolism during Arabidopsis abiotic stress response. Plant Mol. Biol. 2018, 98, 121–135. [Google Scholar] [CrossRef]
- Huang, X.S.; Wang, W.; Zhang, Q.; Liu, J.H. A basic helix-loop-helix transcription factor, PtrbHLH, of Poncirus trifoliata confers cold tolerance and modulates peroxidase-mediated scavenging of hydrogen peroxide. Plant Physiol. 2013, 162, 1178–1194. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, W.J.; Yu, L.; Guo, Z.W.; Chen, Z.J.; Jiang, S.H.; Xu, H.F.; Fang, H.C.; Wang, Y.C.; Zhang, Z.Y.; et al. Heat shock factor a8a modulates flavonoid synthesis and drought tolerance. Plant Physiol. 2020, 184, 1273–1290. [Google Scholar] [CrossRef] [PubMed]
- Bassham, D.C.; Laporte, M.; Marty, F.; Moriyasu, Y.; Ohsumi, Y.; Olsen, L.J.; Yoshimoto, K. Autophagy in development and stress responses of plants. Autophagy 2006, 2, 2–11. [Google Scholar] [CrossRef]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef]
- Ryabovol, V.; Minibayeva, F. Molecular mechanisms of autophagy in plants: Role of ATG8 proteins in formation and functioning of autophagosomes. Biochemistry 2016, 81, 348–363. [Google Scholar] [CrossRef]
- Tamar, A.W. Autophagy and its role in plant abiotic stress management. Plant Cell Environ. 2019, 42, 1045–1053. [Google Scholar]
- Wang, Y.; Cai, S.; Yin, L.; Shi, K.; Zhou, J. Tomato HsfA1a plays a critical role in plant drought tolerance by activating ATG genes and inducing autophagy. Autophagy 2015, 11, 2033–2047. [Google Scholar] [CrossRef]
- Xiong, Y.; Contento, A.L.; Bassham, D.C. AtATG18a is required for the formation of autophagosomes during nutrient stress and senescence in Arabidopsis thaliana. Plant J. 2005, 42, 535–546. [Google Scholar] [CrossRef]
- Xiong, Y.; Contento, A.L.; Nguyen, P.Q.; Bassham, D.C. Degradation of oxidized proteins by autophagy during oxidative stress in Arabidopsis. Plant Physiol. 2007, 143, 291–299. [Google Scholar] [CrossRef]
- Shin, J.H.; Yoshimoto, K.; Ohsumi, Y.; Jeon, J.S.; An, G. OsATG10b, an autophagosome component, is needed for cell survival against oxidative stresses in rice. Mol. Cells 2009, 27, 67–74. [Google Scholar] [CrossRef]
- Liu, Y.; Xiong, Y.; Bassham, D.C. Autophagy is required for tolerance of drought and salt stress in plants. Autophagy 2009, 5, 954–963. [Google Scholar] [CrossRef]
- Luo, L.M.; Zhang, P.P.; Zhu, R.H.; Fu, J.; Su, J.; Zheng, J.; Gong, Q.Q. Autophagy is rapidly induced by salt stress and is required for salt tolerance in Arabidopsis. Front. Plant Sci. 2017, 8, 1459. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Song, W.M.; Wang, P.; Yu, X.; Li, B.; Jiang, C.; Shiu, S.H.; Zhang, H.; Bassham, D.C. COST1 regulates autophagy to control plant drought tolerance. Proc. Natl. Acad. Sci. USA 2020, 117, 7482–7493. [Google Scholar] [CrossRef]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 conjugation system in mammalian autophagy. Int. J. Biochem. Cell Biol. 2004, 36, 2503–2518. [Google Scholar] [CrossRef]
- Yoshimoto, K.; Hanaoka, H.; Sato, S.; Kato, T.; Tabata, S.; Noda, T.; Ohsumi, Y. Processing of ATG8s, ubiquitin-like proteins, and their deconjugation by ATG4s are essential for plant autophagy. Plant Cell 2004, 16, 2967–2983. [Google Scholar] [CrossRef] [PubMed]
- Honig, A.; Avin-Wittenberg, T.; Ufaz, S.; Galili, G. A new type of compartment, defined by plant-specific Atg8-interacting proteins, is induced upon exposure of Arabidopsis plants to carbon starvation. Plant Cell 2012, 24, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Doelling, J.H.; Walker, J.M.; Friedman, E.M.; Thompson, A.R.; Vierstra, R.D. The APG8/12-activating enzyme APG7 is required for proper nutrient recycling and senescence in Arabidopsis thaliana. J. Biol. Chem. 2002, 277, 33105–33114. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, X.; Jia, X.; Wang, N.; Gong, X.Q.; Ma, F.W. Characterization of an Autophagy-Related gene MdATG8i from Apple. Front. Plant Sci. 2016, 7, 16. [Google Scholar] [CrossRef]
- Huo, L.Q.; Guo, Z.J.; Wang, P.; Zhang, Z.J.; Jia, X.; Sun, Y.M.; Sun, X.; Gong, X.Q.; Ma, F.W. MdATG8i functions positively in apple salt tolerance by maintaining photosynthetic ability and increasing the accumulation of arginine and polyamines. Environ. Exp. Bot. 2020, 172, 103989. [Google Scholar] [CrossRef]
- Farooq, M.; Kobayashi, N.; Ito, O.; Wahid, A.; Serraj, R. Broader leaves result in better performance of indica rice under drought stress. J. Plant Physiolo. 2010, 167, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Bassham, D.C. Plant autophagy-more than a starvation response. Cur. Opin. Plant Biol. 2007, 10, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Huo, L.Q.; Jia, X.; Che, R.M.; Gong, X.Q.; Wang, P.; Ma, F.W. Overexpression of MdATG18a in apple improves resistance to Diplocarpon mali infection by enhancing antioxidant activity and salicylic acid levels. Hortic. Res. 2018, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Galili, G. New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef]
- Zhou, K.; Hu, L.Y.; Li, Y.T.S.; Chen, X.F.; Zhang, Z.J.; Liu, B.B.; Li, P.M.; Gong, X.Q.; Ma, F.W. Mdugt88f1-mediated phloridzin biosynthesis regulates apple development and valsa canker resistance. Plant Physiol. 2019, 180, 2290–2305. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, X.; Hu, Y.; Han, W.; Yin, J.; Li, H.; Gong, H. Silicon improves salt tolerance by increasing root water uptake in Cucumis sativus L. Plant Cell Rep. 2015, 34, 1629–1646. [Google Scholar] [CrossRef]
- Hove, R.M.; Bhave, M. Plant aquaporins with non-aqua functions: Deciphering the signature sequences. Plant Mol. Biol. 2011, 75, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Jia, X.; Huo, L.Q.; Che, R.M.; Ma, F.W. MdATG18a overexpression improves tolerance to nitrogen deficiency and regulates anthocyanin accumulation through increased autophagy in transgenic apple. Plant Cell Environ. 2017, 41, 469. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.R.; Olson, A.J.; Schrader, S.M.; Sharkey, T.D. Electron transport is the functional limitation of photosynthesis in field-grown Pima cotton plants at high temperature. Plant Cell Environ. 2004, 27, 717–724. [Google Scholar] [CrossRef]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant. 2015, 8, 1304–1320. [Google Scholar] [CrossRef]
- Ismail, A.; Takeda, S.; Nick, P. Life and death under salt stress: Same players, different timing? J. Exp. Bot. 2014, 65, 2963–2979. [Google Scholar] [CrossRef]
- Jia, D.F.; Jiang, Q.; Steven, V.N.; Gong, X.Q.; Ma, F.W. An apple (Malus domestica) NAC transcription factor enhances drought tolerance in transgenic apple plants. Plant Physiol. Biochem. 2019, 139, 504–512. [Google Scholar] [CrossRef]
- Shaid, S.; Brandts, C.H.; Serve, H.; Dikic, I. Ubiquitination and selective autophagy. Cell Death Differ. 2013, 20, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, J.; Yu, J.Q.; Chen, Z. Role and regulation of autophagy in heat stress responses of tomato plants. Front. Plant Sci. 2014, 5, 174. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, T.; Whittaker, A.; Bochicchio, A.; Vazzana, C.; Suzuki, A.; Masclaux-Daubresse, C. Amino acid pattern and glutamate metabolism during dehydration stress in the ‘resurrection’ plant Sporobolus stapfianus: A comparison between desiccation-sensitive and desiccation-tolerant leaves. J. Exp. Bot. 2007, 58, 3037–3046. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, A.; Bernardi, R.; Lanese, P.; Soldatini, G.F. Changes in free amino acid content and protein pattern of maize seedlings under water stress. Environ. Exp. Bot. 1989, 29, 351–357. [Google Scholar] [CrossRef]
- Willian, B.S.; Björn, H.; Nils, R.; Adriano, N.N.; Wagner, L.A.; Braun, H.P.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar]
- Barros, J.A.S.; Cavalcanti, J.H.F.; Medeiros, D.B.; Nunes-Nesi, A.; Avin-Wittenberg, T.; Fernie, A.R.; Araújo, W.L. Autophagy deficiency compromises alternative pathways of respiration following energy deprivation in Arabidopsis thaliana. Plant Physiol. 2017, 175, 62–76. [Google Scholar] [CrossRef]
- Hirota, T.; Izumi, M.; Wada, S.; Makino, A.; Ishida, H. Vacuolar protein degradation via autophagy provides substrates to amino acid catabolic pathways as an adaptive response to sugar starvation in Arabidopsis thaliana. Plant Cell Physiol. 2018, 59, 1363–1376. [Google Scholar] [CrossRef]
- Kavi, K.P.B.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef]
- Tan, Z.L.; Wen, X.J.; Wang, Y.C. Betula platyphylla BpHOX2 transcription factor binds to different cis-acting elements and confers osmotic tolerance. J. Integr. Plant Biol. 2020, 62, 1762–1779. [Google Scholar] [CrossRef]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotecion. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effect of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Tattini, M.; Galardi, C.; Pinelli, P.; Massai, R.; Remorini, D.; Agati, G. Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol. 2004, 163, 547–561. [Google Scholar] [CrossRef]
- Geng, D.; Chen, P.; Shen, X.; Zhang, Y.; Li, X.; Jiang, L.; Xie, Y.; Niu, C.; Zhang, J.; Huang, X.; et al. MdMYB88 and MdMYB124 enhance drought tolerance by modulating root vessels and cell walls in apple. Plant Physiol. 2018, 178, 1296–1309. [Google Scholar] [CrossRef] [PubMed]
- Vadez, V.; Kholova, J.; Medina, S.; Kakkera, A.; Anderberg, H. Transpiration efficiency: New insights into an old story. J. Exp. Bot. 2014, 6, 6141–6153. [Google Scholar] [CrossRef]
- Liu, S.Y.; Fukumoto, T.; Gena, P.; Feng, P.; Sun, Q.; Li, Q.; Matsumoto, T.; Kaneko, T.; Zhang, H.; Zhang, Y.; et al. Ectopic expression of a rice plasma membrane intrinsic protein (OsPIP1;3) promotes plant growth and water uptake. Plant J. 2020, 102, 779–796. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.; Li, S.; Zhang, L.; Qi, C.D.; Weeda, S.; Zhao, B.; Ren, S.X.; Guo, Y.D. Plasma Membrane Intrinsic Proteins SlPIP2;1, SlPIP2;7 and SlPIP2;5 conferring enhanced drought stress tolerance in tomato. Sci. Rep. 2016, 6, 31814. [Google Scholar] [CrossRef]
- Wang, C.; Liu, S.; Dong, Y.; Zhao, Y.; Geng, A.; Xia, X.; Yin, W. PdEPF1 regulates water-use efficiency and drought tolerance by modulating stomatal density in poplar. Plant Biotechnol. J. 2016, 14, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Gaxiola, R.A.; Li, J.; Undurraga, S.; Dang, L.M.; Allen, G.J.; Alper, S.L.; Fink, G.R. Drought- and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc. Natl. Acad. Sci. USA 2001, 98, 11444–11449. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. T. 1983, 603, 591–592. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Huo, L.; Guo, Z.; Jia, X.; Sun, X.; Wang, P.; Gong, X.; Ma, F. Increased autophagic activity in roots caused by overexpression of the autophagy-related gene MdATG10 in apple enhances salt tolerance. Plant Sci. 2020, 294, 110444. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhang, D.; Pan, X.; Chang, F.; Wang, S. Toxic effects of mercury on PSI and PSII activities, membrane potential and transthylakoid proton gradient in Microsorium pteropus. J. Photochem. Photobiol. B 2013, 127, 1–7. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, X.; Gong, X.; Jia, X.; Li, X.; Wang, Y.; Wang, P.; Huo, L.; Sun, X.; Che, R.; Li, T.; et al. Overexpression of MdATG8i Enhances Drought Tolerance by Alleviating Oxidative Damage and Promoting Water Uptake in Transgenic Apple. Int. J. Mol. Sci. 2021, 22, 5517. https://doi.org/10.3390/ijms22115517
Jia X, Gong X, Jia X, Li X, Wang Y, Wang P, Huo L, Sun X, Che R, Li T, et al. Overexpression of MdATG8i Enhances Drought Tolerance by Alleviating Oxidative Damage and Promoting Water Uptake in Transgenic Apple. International Journal of Molecular Sciences. 2021; 22(11):5517. https://doi.org/10.3390/ijms22115517
Chicago/Turabian StyleJia, Xin, Xiaoqing Gong, Xumei Jia, Xianpeng Li, Yu Wang, Ping Wang, Liuqing Huo, Xun Sun, Runmin Che, Tiantian Li, and et al. 2021. "Overexpression of MdATG8i Enhances Drought Tolerance by Alleviating Oxidative Damage and Promoting Water Uptake in Transgenic Apple" International Journal of Molecular Sciences 22, no. 11: 5517. https://doi.org/10.3390/ijms22115517
APA StyleJia, X., Gong, X., Jia, X., Li, X., Wang, Y., Wang, P., Huo, L., Sun, X., Che, R., Li, T., Zou, Y., & Ma, F. (2021). Overexpression of MdATG8i Enhances Drought Tolerance by Alleviating Oxidative Damage and Promoting Water Uptake in Transgenic Apple. International Journal of Molecular Sciences, 22(11), 5517. https://doi.org/10.3390/ijms22115517






