The Effects of Insulin on Immortalized Rat Schwann Cells, IFRS1
Abstract
1. Introduction
2. Results
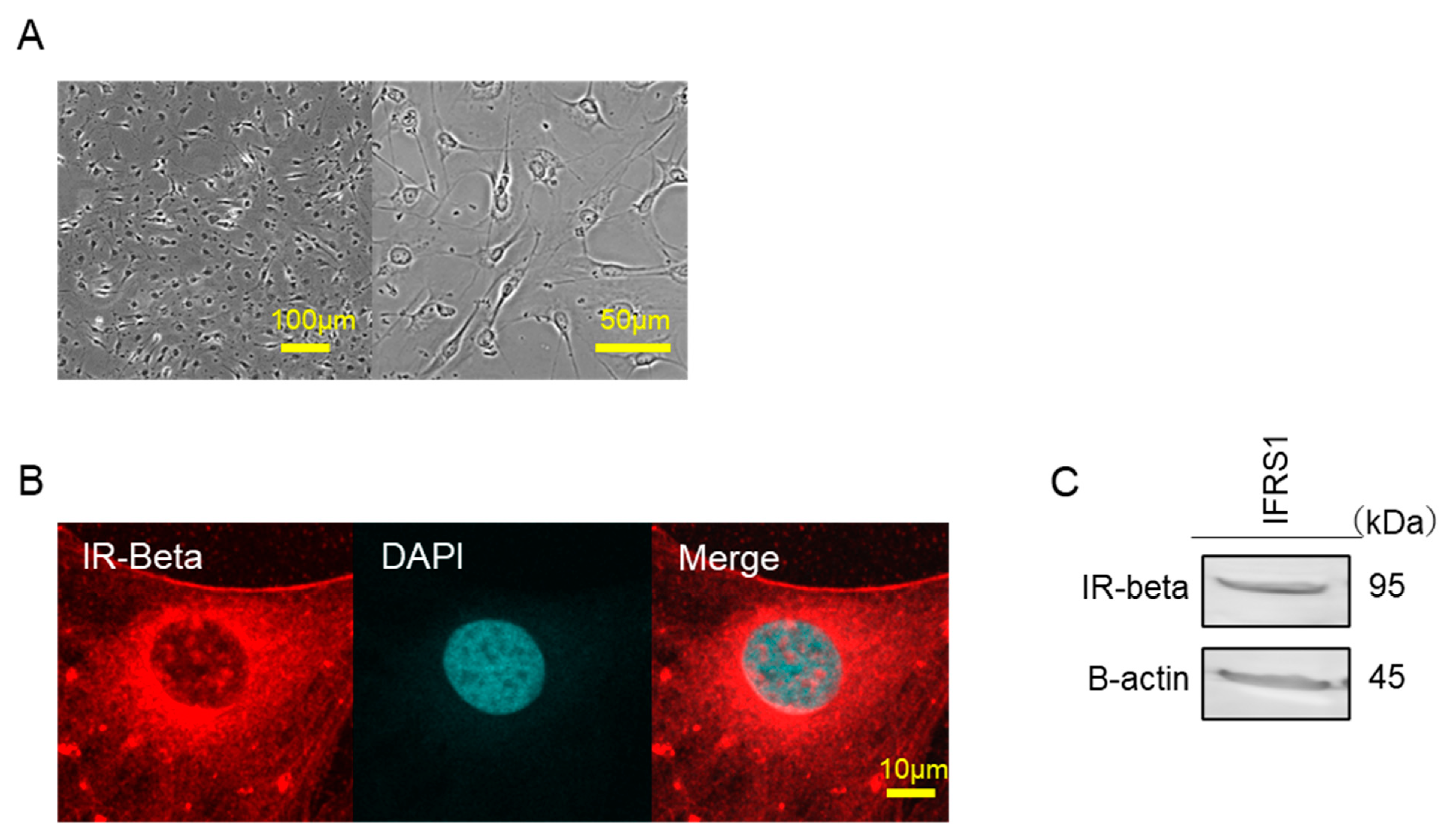
2.1. Identification of Insulin Receptors
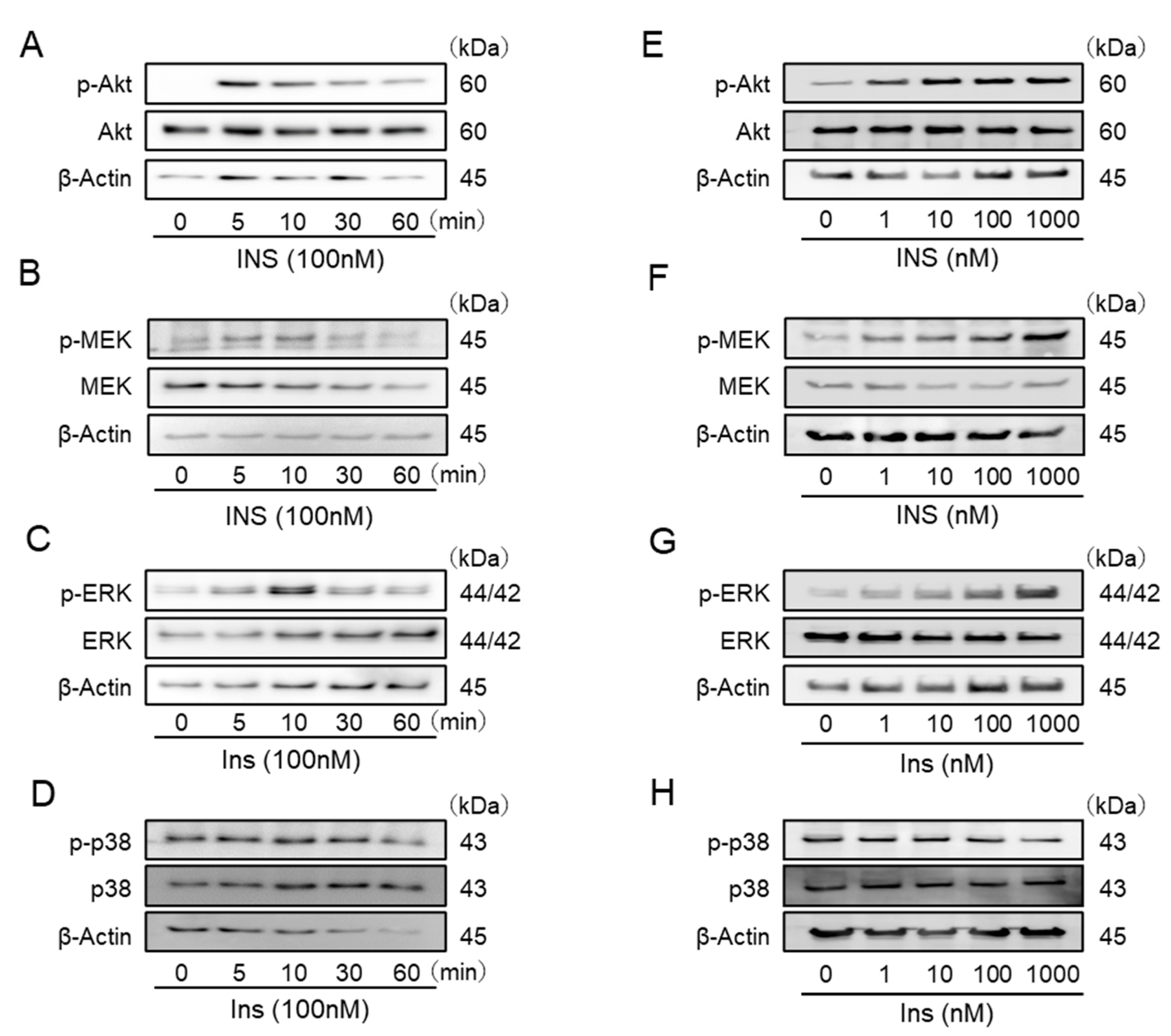
2.2. Insulin Stimulates the Phosphorylation of Akt, MEK, and Extracellular-Signal-Regulated Kinase (ERK) in IFRS1
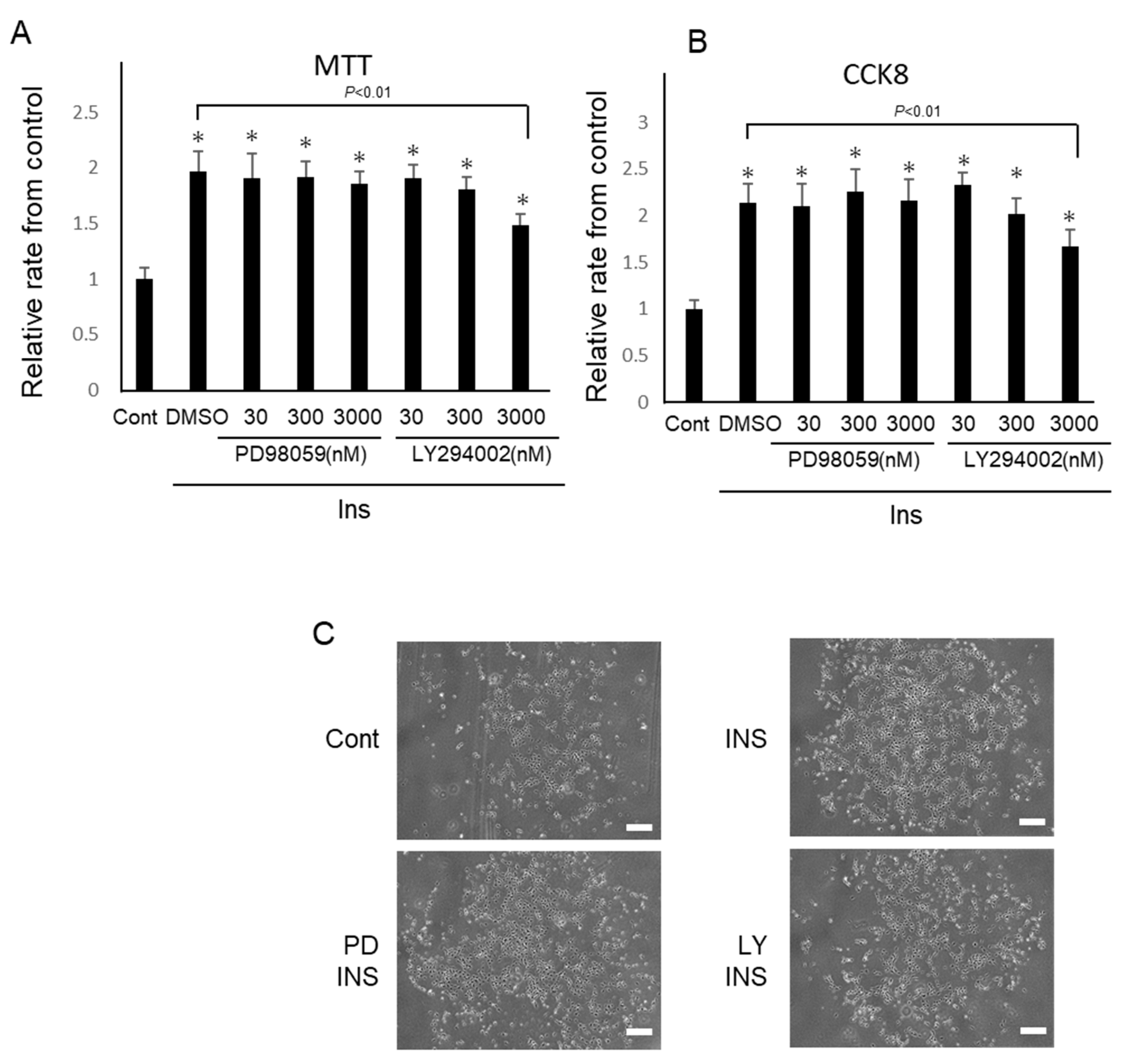
2.3. Insulin Promotes the Proliferation of IFRS1
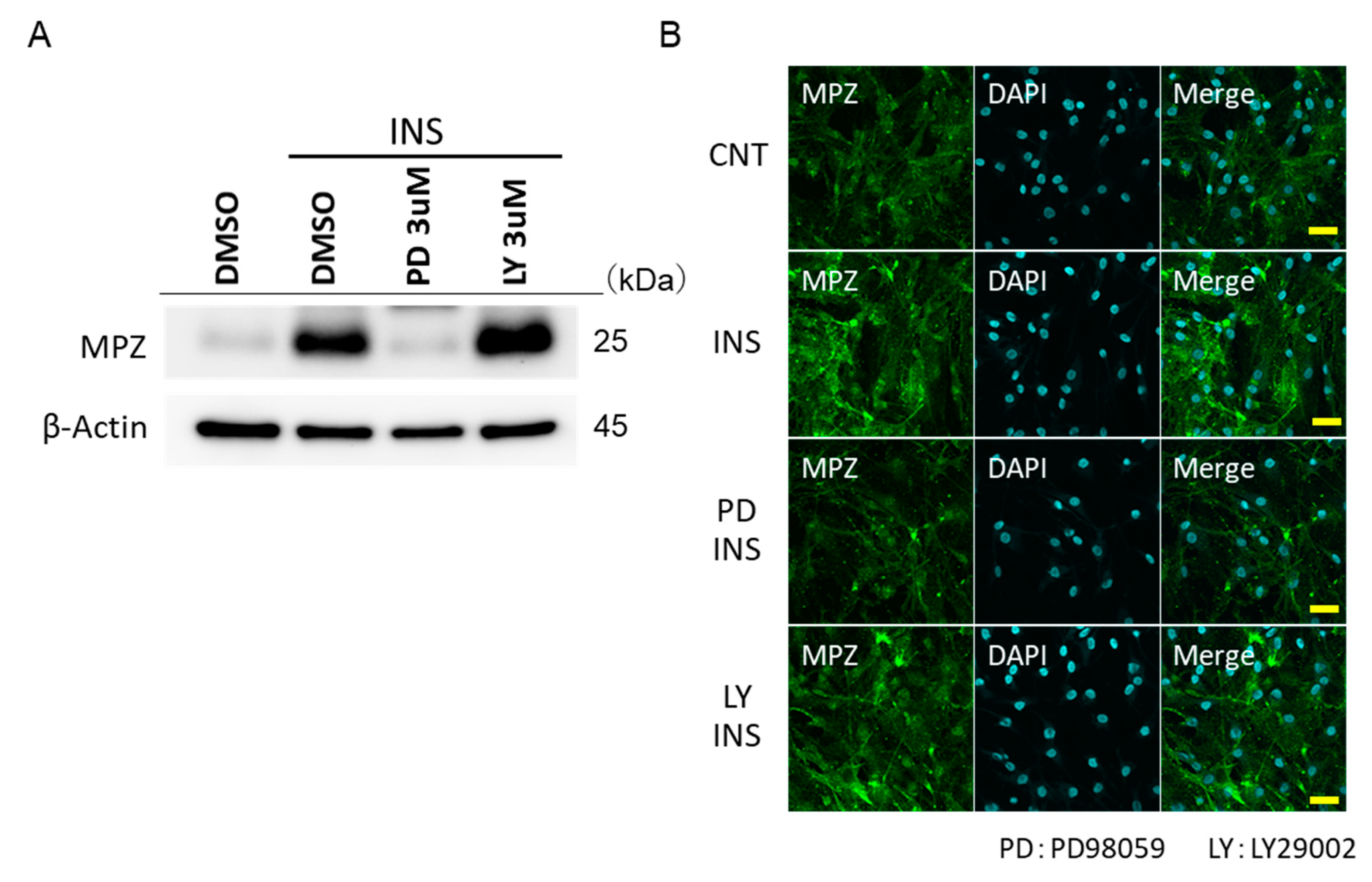
2.4. Insulin Promotes the MPZ Expressions in IFRS1 via MEK/ERK
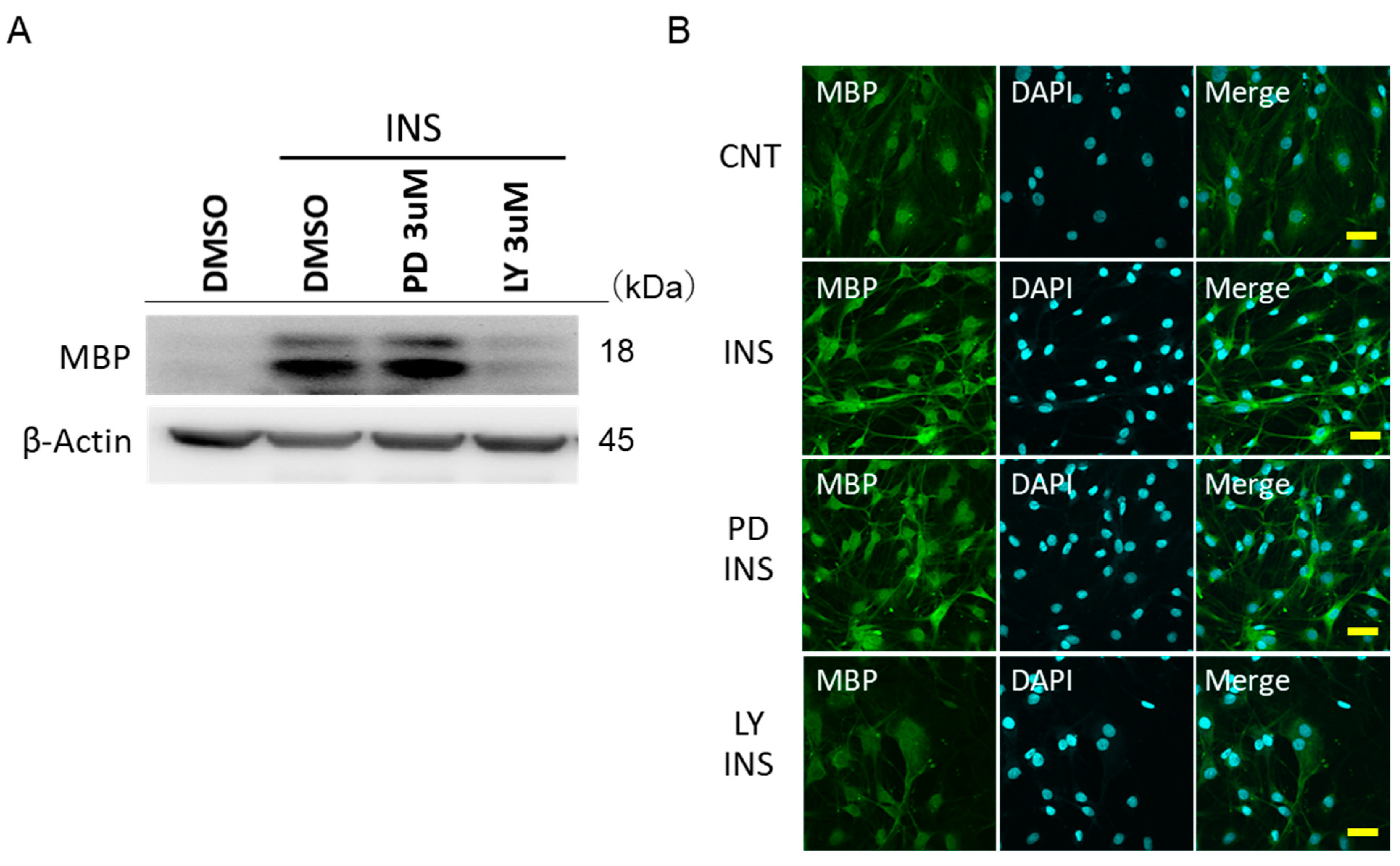
2.5. Insulin Promotes the MBP Expressions in IFRS1 via PI3-K/Akt
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Immunofluorescence Staining of IFRS1
4.3. Proliferation Analyses of IFRS1
4.4. Western Blot Analyses
4.5. Identification of Insulin Receptor in IFRS1
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IFRS1 | Immortalized Fischer rat Schwann cells 1 |
| DMEM | Dulbecco’s modified Eagle medium |
| DPN | Diabetic peripheral neuropathy |
| ERK | Extracellular signal-regulated kinase |
| MAPK | Mitogen-activated protein kinase |
| NGF | Mitogen-activated protein kinase |
| NT-3 | Neurotrophin 3 |
| GDNF | Glial cell line-derived neurotrophic factor |
| MPZ | Myelin protein zero |
| MAG | Myelin associated glycoprotein |
| MBP | Myelin basic protein |
| PNS | Peripheral nervous system |
References
- Vinik, A.I.; Park, T.S.; Stansberry, K.B.; Pittenger, G.L. Diabetic neuropathies. Diabetologia 2000, 43, 957–973. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, J.; Kato, K.; Hamada, Y.; Nakayama, M.; Chaya, S.; Nakashima, E.; Naruse, K.; Kasuya, Y.; Mizubayashi, R.; Miwa, K.; et al. A protein kinase C-beta-selective inhibitor ameliorates neural dysfunction in streptozotocin-induced diabetic rats. Diabetes 1999, 48, 2090–2095. [Google Scholar] [CrossRef]
- Naruse, K. Schwann Cells as Crucial Players in Diabetic Neuropathy. Adv. Exp. Med. Biol. 2019, 1190, 345–356. [Google Scholar] [PubMed]
- Gonçalves, N.P.; Vægter, C.B.; Andersen, H.; Østergaard, L.; Calcutt, N.A.; Jensen, T.S. Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat. Rev. Neurol. 2017, 13, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Grote, C.W.; Ryals, J.M.; Wright, D.E. In vivo peripheral nervous system insulin signaling. J. Peripher. Nerv. Syst. 2013, 18, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Grote, C.W.; Wilson, N.M.; Katz, N.K.; Guilford, B.L.; Ryals, J.M.; Novikova, L.; Stehno-Bittel, L.; Wright, D.E. Deletion of the insulin receptor in sensory neurons increases pancreatic insulin levels. Exp. Neurol. 2018, 305, 97–107. [Google Scholar] [CrossRef]
- Brussee, V.; Cunningham, F.A.; Zochodne, D.W. Direct insulin signaling of neurons reverses diabetic neuropathy. Diabetes 2004, 53, 1824–1830. [Google Scholar] [CrossRef]
- Toth, C.; Brussee, V.; Martinez, J.A.; McDonald, D.; Cunningham, F.A.; Zochodne, D.W. Rescue and regeneration of injured peripheral nerve axons by intrathecal insulin. Neuroscience 2006, 139, 429–449. [Google Scholar] [CrossRef]
- Xu, Q.-G.; Li, X.-Q.; Kotecha, S.A.; Cheng, C.; Sun, H.S.; Zochodne, D.W. Insulin as an in vivo growth factor. Exp. Neurol. 2004, 188, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.; Martinez, J.; Liu, W.; Nguyen, T.; Ayer, A.; Fine, J.; Zochodne, D.; Hanson, L.R.; Frey, W.H., II; Toth, C. Intranasal insulin ameliorates experimental diabetic neuropathy. Diabetes 2009, 58, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 2005, 6, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Gould, R.M.; Oakley, T.; Goldstone, J.V.; Dugas, J.C.; Brady, S.T.; Gow, A. Myelin sheaths are formed with proteins that originated in vertebrate lineages. Neuron Glia Biol. 2008, 4, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Crawford, T.O.; Griffin, J.W.; Tu, P.; Lee, V.M.; Li, C.; Roder, J.; Trapp, B.D. Myelin-associated glycoprotein is a myelin signal that modulates the caliber of myelinated axons. J. Neurosci. 1998, 18, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Lemke, G.; Axel, R. Isolation and sequence of a cDNA encoding the major structural protein of peripheral myelin. Cell 1985, 40, 501–508. [Google Scholar] [CrossRef]
- Omi, M.; Hata, M.; Nakamura, N.; Miyabe, M.; Ozawa, S.; Nukada, H.; Tsukamoto, M.; Sango, K.; Himeno, T.; Kamiya, H.; et al. Transplantation of dental pulp stem cells improves long-term diabetic polyneuropathy together with improvement of nerve morphometrical evaluation. Stem Cell Res. Ther. 2017, 8, 279. [Google Scholar] [CrossRef]
- Wang, L.; Chopp, M.; Szalad, A.; Lu, X.; Zhang, Y.; Wang, X.; Cepparulo, P.; Lu, M.; Li, C.; Zhang, Z.G. Exosomes derived from Schwann aells ameliorate neripheral neuropathy in Type 2 Diabetic Mice. Diabetes 2020, 69, 749–759. [Google Scholar] [CrossRef] [PubMed]
- King, M.R.; Anderson, N.J.; Liu, C.; Law, E.; Cundiff, M.; Mixcoatl-Zecuatl, T.M.; Jolivalt, C.G. Activation of the insulin-signaling pathway in sciatic nerve and hippocampus of type 1 diabetic rats. Neuroscience 2015, 303, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Grote, C.W.; Wright, D.E. A Role for Insulin in Diabetic Neuropathy. Front. Neurosci. 2016, 10, 581. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Primers 2019, 5, 41. [Google Scholar] [CrossRef]
- Takaku, S.; Yanagisawa, H.; Watabe, K.; Horie, H.; Kadoya, T.; Sakumi, K.; Nakabeppu, Y.; Poirier, F.; Sango, K. GDNF promotes neurite outgrowth and upregulates galectin-1 through the RET/PI3K signaling in cultured adult rat dorsal root ganglion neurons. Neurochem. Int. 2013, 62, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Sango, K.; Kawakami, E.; Yanagisawa, H.; Takaku, S.; Tsukamoto, M.; Utsunomiya, K.; Watabe, K. Myelination in coculture of established neuronal and Schwann cell lines. Histochem. Cell Biol. 2012, 137, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Sango, K.; Yanagisawa, H.; Takaku, S.; Kawakami, E.; Watabe, K. Immortalized adult rodent Schwann cells as in vitro models to study diabetic neuropathy. Exp. Diabetes Res. 2011, 2011, 374943. [Google Scholar] [CrossRef] [PubMed]
- Sango, K.; Yanagisawa, H.; Kawakami, E.; Takaku, S.; Ajiki, K.; Watabe, K. Spontaneously immortalized Schwann cells from adult Fischer rat as a valuable tool for exploring neuron-Schwann cell interactions. J. Neurosci. Res. 2011, 89, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Flórez, F.; Vidal, D.; Simón, E. MAP-kinase activity in etiolated Cucumis sativus cotyledons: The effect of red and far-red light irradiation. Plant Physiol. Biochem. 2013, 63, 1–7. [Google Scholar] [CrossRef]
- Recio-Pinto, E.; Rechler, M.M.; Ishii, D.N. Effects of insulin, insulin-like growth factor-II, and nerve growth factor on neurite formation and survival in cultured sympathetic and sensory neurons. J. Neurosci. 1986, 6, 1211–1219. [Google Scholar] [CrossRef]
- Shapiro, L.; Doyle, J.P.; Hensley, P.; Colman, D.R.; Hendrickson, W.A. Crystal structure of the extracellular domain from P0, the major structural protein of peripheral nerve myelin. Neuron 1996, 17, 435–449. [Google Scholar] [CrossRef]
- Martini, R. P0-deficient knockout mice as tools to understand pathomechanisms in Charcot-Marie-Tooth 1B and P0-related Dejerine-Sottas syndrome. Ann. N. Y. Acad. Sci. 1999, 883, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Senderek, J.; Hermanns, B.; Lehmann, U.; Bergmann, C.; Marx, G.; Kabus, C.; Timmerman, V.; Stoltenburg-Didinger, G.; Schroder, J.M. Charcot-Marie-Tooth neuropathy type 2 and P0 point mutations: Two novel amino acid substitutions (Asp61Gly; Tyr119Cys) and a possible “hotspot” on Thr124Met. Brain Pathol. 2000, 10, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, K.; Himoro, M.; Sato, W.; Takada, G.; Uyemura, K.; Shimizu, N.; Bird, T.D.; Conneally, P.M.; Chance, P.F. Charcot-Marie-Tooth neuropathy type 1B is associated with mutations of the myelin P0 gene. Nat. Genet. 1993, 5, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Cermenati, G.; Abbiati, F.; Cermenati, S.; Brioschi, E.; Volonterio, A.; Cavaletti, G.; Saez, E.; De Fabiani, E.; Crestani, M.; Garcia-Segura, L.M.; et al. Diabetes-induced myelin abnormalities are associated with an altered lipid pattern: Protective effects of LXR activation. J. Lipid Res. 2012, 53, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, M.A.; Urbanek, K.; Torres, L.; Wendell, S.G. ERK1/2 Activation in preexisting oligodendrocytes of adult mice drives new myelin synthesis and enhanced CNS function. J. Neurosci. 2016, 36, 9186–9200. [Google Scholar] [CrossRef] [PubMed]
- Furusho, M.; Ishii, A.; Bansal, R. Signaling by FGF receptor 2, not FGF receptor 1, regulates myelin thickness through activation of ERK1/2-MAPK, which promotes mTORC1 activity in an Akt-independent manner. J. Neurosci. 2017, 37, 2931–2946. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 2010, 1802, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.P.; Kim, J.; Syed, N.; Tung, Y.-J.; Bhaskaran, A.; Mindos, T.; Mirsky, R.; Jessen, K.R.; Maurel, P.; Parkinson, D.B.; et al. p38 MAPK activation promotes denervated Schwann cell phenotype and functions as a negative regulator of Schwann cell differentiation and myelination. J. Neurosci. 2012, 32, 7158–7168. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Bauer, N.M.; Schäfer, I.; White, R. Making myelin basic protein—From mRNA transport to localized translation. Front. Cell. Neurosci. 2013, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Domenech-Estevez, E.; Baloui, H.; Meng, X.; Zhang, Y.; Deinhardt, K.; Dupree, J.L.; Einheber, S.; Chrast, R.; Salzer, J.L. Akt regulates axon wrapping and myelin sheath thickness in the PNS. J. Neurosci. 2016, 36, 4506–4521. [Google Scholar] [CrossRef]
- Quarles, R.H. Myelin-associated glycoprotein (MAG): Past, present and beyond. J. Neurochem. 2007, 100, 1431–1448. [Google Scholar] [CrossRef] [PubMed]
- Kamil, K.; Yazid, M.D.; Idrus, R.B.H.; Das, S.; Kumar, J. Peripheral demyelinating diseases: From biology to translational medicine. Front. Neurol. 2019, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Rachana, K.S.; Manu, M.S.; Advirao, G.M. Insulin influenced expression of myelin proteins in diabetic peripheral neuropathy. Neurosci. Lett. 2016, 629, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Sotsios, Y.; Ward, S.G. Phosphoinositide 3-kinase: A key biochemical signal for cell migration in response to chemokines. Immunol. Rev. 2000, 177, 217–235. [Google Scholar] [CrossRef]
- Figlia, G.; Gerber, D.; Suter, U. Myelination and mTOR. Glia 2018, 66, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Distelhorst, C.W. Bcl-2 protein family members: Versatile regulators of calcium signaling in cell survival and apoptosis. Annu. Rev. Physiol. 2008, 70, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Arya, R.; Mallik, M.; Lakhotia, S.C. Heat shock genes—integrating cell survival and death. J. Biosci. 2007, 32, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Maurel, P.; Salzer, J.L. Axonal regulation of Schwann cell proliferation and survival and the initial events of myelination requires PI 3-kinase activity. J. Neurosci. 2000, 20, 4635–4645. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Naruse, K.; Hamada, Y.; Nakashima, E.; Kato, K.; Akiyama, N.; Kamiya, H.; Watarai, A.; Nakae, M.; Oiso, Y.; et al. Human proinsulin C-peptide prevents proliferation of rat aortic smooth muscle cells cultured in high-glucose conditions. Diabetologia 2005, 48, 2396–2401. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saiki, T.; Nakamura, N.; Miyabe, M.; Ito, M.; Minato, T.; Sango, K.; Matsubara, T.; Naruse, K. The Effects of Insulin on Immortalized Rat Schwann Cells, IFRS1. Int. J. Mol. Sci. 2021, 22, 5505. https://doi.org/10.3390/ijms22115505
Saiki T, Nakamura N, Miyabe M, Ito M, Minato T, Sango K, Matsubara T, Naruse K. The Effects of Insulin on Immortalized Rat Schwann Cells, IFRS1. International Journal of Molecular Sciences. 2021; 22(11):5505. https://doi.org/10.3390/ijms22115505
Chicago/Turabian StyleSaiki, Tomokazu, Nobuhisa Nakamura, Megumi Miyabe, Mizuho Ito, Tomomi Minato, Kazunori Sango, Tatsuaki Matsubara, and Keiko Naruse. 2021. "The Effects of Insulin on Immortalized Rat Schwann Cells, IFRS1" International Journal of Molecular Sciences 22, no. 11: 5505. https://doi.org/10.3390/ijms22115505
APA StyleSaiki, T., Nakamura, N., Miyabe, M., Ito, M., Minato, T., Sango, K., Matsubara, T., & Naruse, K. (2021). The Effects of Insulin on Immortalized Rat Schwann Cells, IFRS1. International Journal of Molecular Sciences, 22(11), 5505. https://doi.org/10.3390/ijms22115505






