Ribosome Biogenesis and Cancer: Overview on Ribosomal Proteins
Abstract
1. Introduction
2. Ribosome Biogenesis in Health
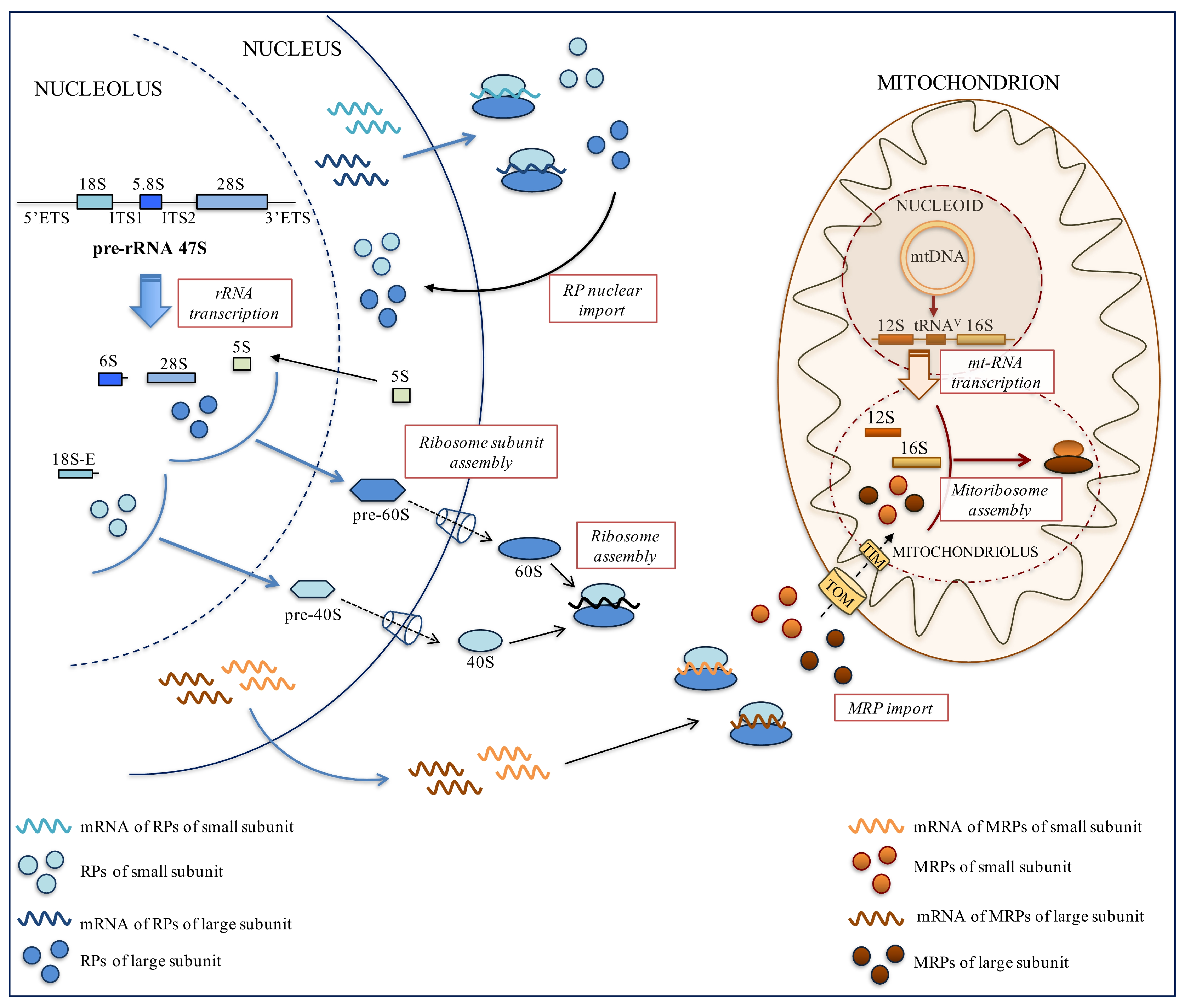
2.1. Cytosolic Ribosome Biogenesis
2.2. Pre-rRNA Synthesis and Maturation
2.3. Nucleolar Assembly of pre-60S and 40S Subunits
2.4. Mitochondrial Ribosome Biogenesis
3. Role of Ribosome Biogenesis in Neoplastic Transformation
3.1. Alteration of rRNA Synthesis
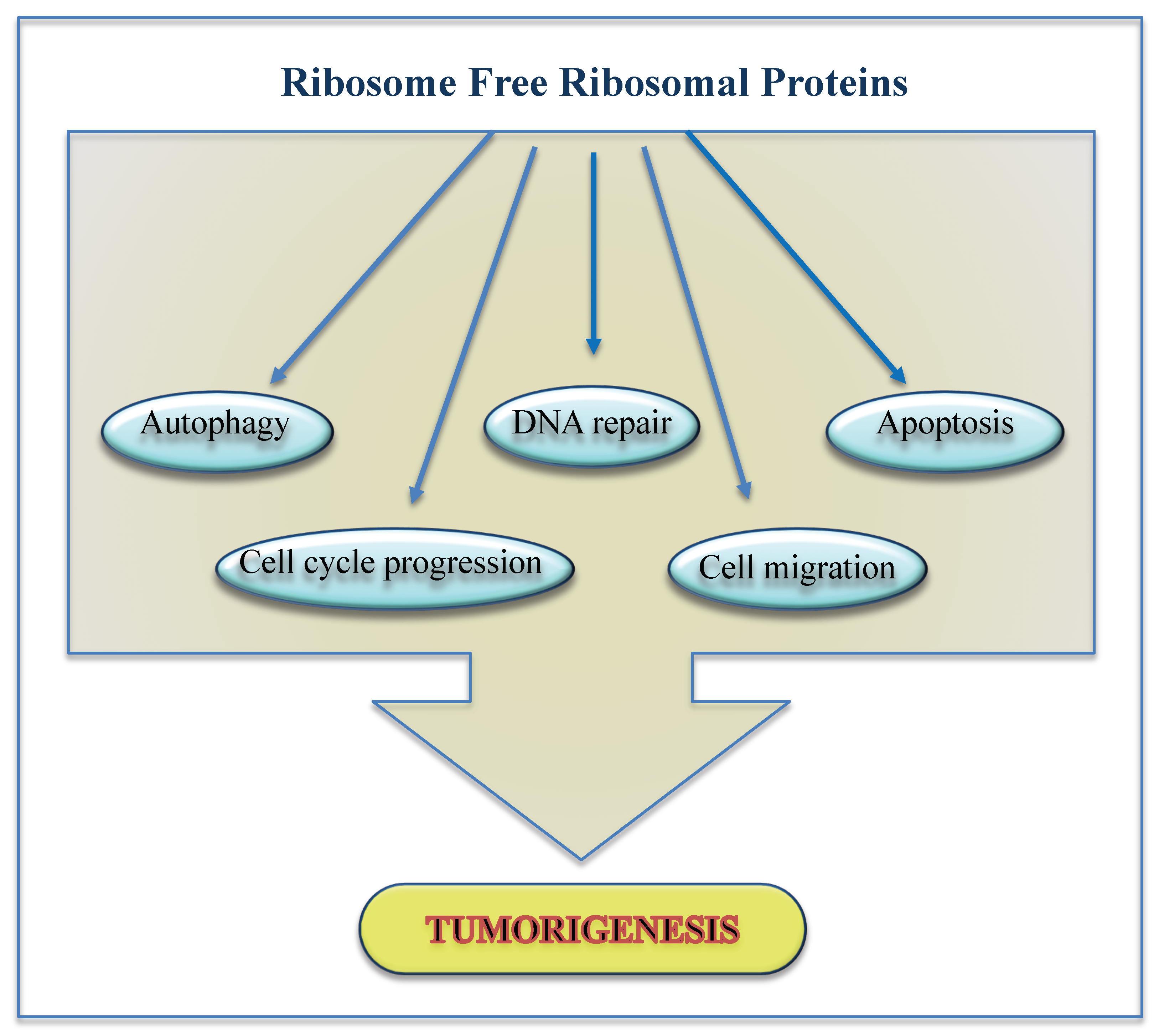
3.2. Ribosome Free RP Expression Regulate Cell Cycle
3.3. Ribosome Free RPs Take Part to DNA Repair
3.4. Ribosome Free RPs Control Apoptosis
3.5. Ribosome Free RPs Are Involved in ER Stress and Autophagy
3.6. Ribosome Free RPs Affect Cell Migration
4. RPs as Biomarkers in Cancer Diagnosis
5. RPs as Molecular Target in Cancer Treatment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomson, E.; Ferreira-Cerca, S.; Hurt, E. Eukaryotic ribosome biogenesis at a glance. J. Cell Sci. 2013, 126, 4815–4821. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz, J.; Karbstein, K.; Woolford, J.L., Jr. Functions of Ribosomal Proteins in Assembly of Eukaryotic Ribosomes In Vivo. Annu. Rev. Biochem. 2015, 84, 93–129. [Google Scholar] [CrossRef]
- Ameismeier, M.; Zemp, I.; van den Heuvel, J.; Thoms, M.; Berninghausen, O.; Kutay, U.; Beckmann, R. Structural basis for the final steps of human 40S ribosome maturation. Nat. Cell Biol. 2020, 587, 683–687. [Google Scholar] [CrossRef]
- Aubert, M.; O’Donohue, M.F.; LeBaron, S.; Gleizes, P.-E. Pre-Ribosomal RNA Processing in Human Cells: From Mechanisms to Congenital Diseases. Biomolecules 2018, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.-K.; Porras-Yakushi, T.R.; Reitsma, J.M.; Huber, F.M.; Sweredoski, M.J.; Hoelz, A.; Hess, S.; Deshaies, R.J. A conserved quality-control pathway that mediates degradation of unassembled ribosomal proteins. eLife 2016, 5, 19105. [Google Scholar] [CrossRef]
- Russo, G.; Cuccurese, M.; Monti, G.; Russo, A.; Amoresano, A.; Pucci, P.; Pietropaolo, C. Ribosomal protein L7a binds RNA through two distinct RNA-binding domains. Biochem. J. 2005, 385, 289–299. [Google Scholar] [CrossRef]
- Russo, A.; Russo, G.; Cuccurese, M.; Garbi, C.; Pietropaolo, C. The 3′-untranslated region directs ribosomal protein-encoding mRNAs to specific cytoplasmic regions. Biochim. Biophys. Acta 2006, 1763, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Cuccurese, M.; Russo, G.; Russo, A.; Pietropaolo, C. Alternative splicing and nonsense-mediated mRNA decay regulate mammalian ribosomal gene expression. Nucleic Acids Res. 2005, 33, 5965–5977. [Google Scholar] [CrossRef]
- Russo, G.; Ricciardelli, G.; Pietropaolo, C. Different Domains Cooperate to Target the Human Ribosomal L7a Protein to the Nucleus and to the Nucleoli. J. Biol. Chem. 1997, 272, 5229–5235. [Google Scholar] [CrossRef]
- Pillet, B.; Mitterer, V.; Kressler, D.; Pertschy, B. Hold on to your friends: Dedicated chaperones of ribosomal proteins: Dedicated chaperones mediate the safe transfer of ribosomal proteins to their site of pre-ribosome incorporation. BioEssays 2017, 39, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pausch, P.; Singh, U.; Ahmed, Y.L.; Pillet, B.; Murat, G.; Altegoer, F.; Stier, G.; Thoms, M.; Hurt, E.; Sinning, I.; et al. Co-translational capturing of nascent ribosomal proteins by their dedicated chaperones. Nat. Commun. 2015, 6, 7494. [Google Scholar] [CrossRef] [PubMed]
- Pillet, B.; García-Gómez, J.J.; Pausch, P.; Falquet, L.; Bange, G.; De La Cruz, J.; Kressler, D. The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly Site. PLoS Genet. 2015, 11, e1005565. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.-H.; Lu, T.-J.; Johnson, A.W.; Shie, J.-T.; Chen, B.-R.; Kumar, S.S.; Lo, K.-Y. Bcp1 Is the Nuclear Chaperone of Rpl23 in Saccharomyces cerevisiae. J. Biol. Chem. 2017, 292, 585–596. [Google Scholar] [CrossRef]
- Calviño, F.R.; Kharde, S.; Ori, A.; Hendricks, A.; Wild, K.; Kressler, D.; Bange, G.; Hurt, E.; Beck, M.; Sinning, I. Symportin 1 chaperones 5S RNP assembly during ribosome biogenesis by occupying an essential rRNA-binding site. Nat. Commun. 2015, 6, 6510. [Google Scholar] [CrossRef]
- Holzer, S.; Klinge, S.; Ban, N. Crystal Structure of the Yeast Ribosomal Protein rpS3 in Complex with Its Chaperone Yar1. J. Mol. Biol. 2013, 425, 4154–4160. [Google Scholar] [CrossRef] [PubMed]
- Hellmich, U.A.; Weis, B.L.; Lioutikov, A.; Wurm, J.P.; Kaiser, M.; Christ, N.A.; Hantke, K.; Kötter, P.; Entian, K.-D.; Schleiff, E.; et al. Essential ribosome assembly factor Fap7 regulates a hierarchy of RNA-protein interactions during small ribosomal subunit biogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 15253–15258. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, J.J.; Fernández-Pevida, A.; Lebaron, S.; Rosado, I.V.; Tollervey, D.W.; Kressler, D.; De La Cruz, J. Final Pre-40S Maturation Depends on the Functional Integrity of the 60S Subunit Ribosomal Protein L3. PLoS Genet. 2014, 10, e1004205. [Google Scholar] [CrossRef]
- Kisly, I.; Remme, J.; Tamm, T. Ribosomal protein eL24, involved in two intersubunit bridges, stimulates translation initiation and elongation. Nucleic Acids Res. 2019, 47, 406–420. [Google Scholar] [CrossRef]
- Mailliot, J.; Garreau De Loubresse, N.; Yusupova, G.; Meskauskas, A.; Dinman, J.D.; Yusupov, M. Crystal Structures of the uL3 Mutant Ribosome: Illustration of the Importance of Ribosomal Proteins for Translation Efficiency. J. Mol. Biol. 2016, 428, 2195–2202. [Google Scholar] [CrossRef]
- O’Brien, T.W. Properties of Human Mitochondrial Ribosomes. IUBMB Life 2003, 55, 505–513. [Google Scholar] [CrossRef]
- De Silva, D.; Tu, Y.-T.; Amunts, A.; Fontanesi, F.; Barrientos, A. Mitochondrial ribosome assembly in health and disease. Cell Cycle 2015, 14, 2226–2250. [Google Scholar] [CrossRef] [PubMed]
- Antonicka, H.; Shoubridge, E.A. Mitochondrial RNA Granules Are Centers for Posttranscriptional RNA Processing and Ribosome Biogenesis. Cell Rep. 2015, 10, 920–932. [Google Scholar] [CrossRef]
- Russo, A.; Cirulli, C.; Amoresano, A.; Pucci, P.; Pietropaolo, C.; Russo, G. cis-acting sequences and trans-acting factors in the localization of mRNA for mitochondrial ribosomal proteins. Biochim. Biophys. Acta 2008, 1779, 820–829. [Google Scholar] [CrossRef]
- Saurer, M.; Ramrath, D.J.F.; Niemann, M.; Calderaro, S.; Prange, C.; Mattei, S.; Scaiola, A.; Leitner, A.; Bieri, P.; Horn, E.K.; et al. Mitoribosomal small subunit biogenesis in trypanosomes involves an extensive assembly machinery. Science 2019, 365, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Jaskolowski, M.; Ramrath, D.J.; Bieri, P.; Niemann, M.; Mattei, S.; Calderaro, S.; Leibundgut, M.; Horn, E.K.; Boehringer, D.; Schneider, A.; et al. Structural Insights into the Mechanism of Mitoribosomal Large Subunit Biogenesis. Mol. Cell 2020, 79, 629–644.e4. [Google Scholar] [CrossRef]
- Karbstein, K.; Katrin, K. Mitochondria teach ribosome assembly. Sciences 2019, 365, 1077–1078. [Google Scholar] [CrossRef] [PubMed]
- Penzo, M.; Montanaro, L.; Treré, D.; Derenzini, M. The Ribosome Biogenesis—Cancer Connection. Cells 2019, 8, 55. [Google Scholar] [CrossRef]
- Russo, A.; Russo, G. Ribosomal Proteins Control or Bypass p53 during Nucleolar Stress. Int. J. Mol. Sci. 2017, 18, 140. [Google Scholar] [CrossRef]
- Pelava, A.; Schneider, C.; Watkins, N.J. The importance of ribosome production, and the 5S RNP–MDM2 pathway, in health and disease. Biochem. Soc. Trans. 2016, 44, 1086–1090. [Google Scholar] [CrossRef]
- Onofrillo, C.; Galbiati, A.; Montanaro, L.; Derenzini, M. The pre-existing population of 5S rRNA effects p53 stabilization during ribosome biogenesis inhibition. Oncotarget 2016, 8, 4257–4267. [Google Scholar] [CrossRef]
- Nishimura, K.; Kumazawa, T.; Kuroda, T.; Katagiri, N.; Tsuchiya, M.; Goto, N.; Furumai, R.; Murayama, A.; Yanagisawa, J.; Kimura, K. Perturbation of Ribosome Biogenesis Drives Cells into Senescence through 5S RNP-Mediated p53 Activation. Cell Rep. 2015, 10, 1310–1323. [Google Scholar] [CrossRef]
- Molavi, G.; Samadi, N.; Hosseingholi, E.Z. The roles of moonlight ribosomal proteins in the development of human cancers. J. Cell. Physiol. 2019, 234, 8327–8341. [Google Scholar] [CrossRef]
- Russo, A.; Siciliano, G.; Catillo, M.; Giangrande, C.; Amoresano, A.; Pucci, P.; Pietropaolo, C.; Russo, G. hnRNP H1 and intronic G runs in the splicing control of the human rpL3 gene. Biochim. Biophys. Acta 2010, 1799, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Catillo, M.; Esposito, D.; Briata, P.; Pietropaolo, C.; Russo, G. Autoregulatory circuit of human rpL3 expression requires hnRNP H1, NPM and KHSRP. Nucleic Acids Res. 2011, 39, 7576–7585. [Google Scholar] [CrossRef] [PubMed]
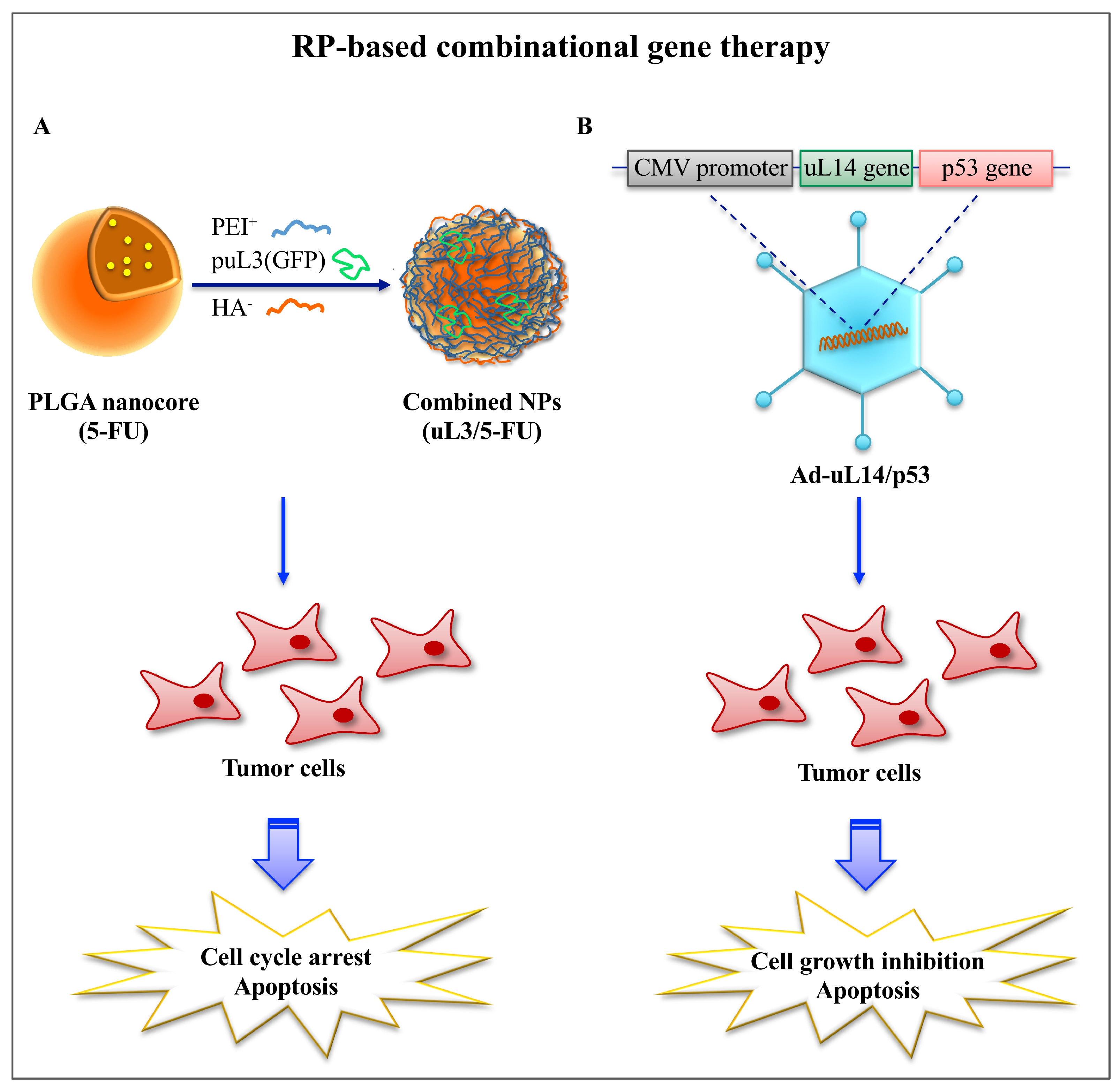
- Russo, A.; Maiolino, S.; Pagliara, V.; Ungaro, F.; Tatangelo, F.; Leone, A.; Scalia, G.; Budillon, A.; Quaglia, F.; Russo, G. Enhancement of 5-FU sensitivity by the proapoptotic rpL3 gene in p53 null colon cancer cells through combined polymer nanoparticles. Oncotarget 2016, 7, 79670–79687. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Saide, A.; Smaldone, S.; Faraonio, R.; Russo, G. Role of uL3 in Multidrug Resistance in p53-Mutated Lung Cancer Cells. Int. J. Mol. Sci. 2017, 18, 547. [Google Scholar] [CrossRef]
- Pecoraro, A.; Carotenuto, P.; Russo, G.; Russo, A. Ribosomal protein uL3 targets E2F1 and Cyclin D1 in cancer cell response to nucleolar stress. Sci. Rep. 2019, 9, 15431. [Google Scholar] [CrossRef] [PubMed]
- Ebright, R.Y.; Lee, S.; Wittner, B.S.; Niederhoffer, K.L.; Nicholson, B.T.; Bardia, A.; Truesdell, S.; Wiley, D.F.; Wesley, B.; Li, S.; et al. Deregulation of ribosomal protein expression and translation promotes breast cancer metastasis. Science 2020, 367, 1468–1473. [Google Scholar] [CrossRef]
- De Filippis, D.; Russo, A.; De Stefano, D.; Cipriano, M.; Esposito, D.; Grassia, G.; Carnuccio, R.; Russo, G.; Iuvone, T. Palmitoylethanolamide inhibits rMCP-5 expression by regulating MITF activation in rat chronic granulomatous inflammation. Eur. J. Pharmacol. 2014, 725, 64–69. [Google Scholar] [CrossRef]
- Phung, B.; Cieśla, M.; Sanna, A.; Guzzi, N.; Beneventi, G.; Cao Thi Ngoc, P.; Lauss, M.; Cabrita, R.; Cordero, E.; Bosch, A.; et al. The X-Linked DDX3X RNA Helicase Dictates Translation Reprogramming and Metastasis in Melanoma. Cell Rep. 2019, 27, 3573–3586.e7. [Google Scholar] [CrossRef]
- Sharifi, S.; Bierhoff, H. Regulation of RNA Polymerase I Transcription in Development, Disease, and Aging. Annu. Rev. Biochem. 2018, 87, 51–73. [Google Scholar] [CrossRef]
- Drygin, D.; Rice, W.G.; Grummt, I. The RNA Polymerase I Transcription Machinery: An Emerging Target for the Treatment of Cancer. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 131–156. [Google Scholar] [CrossRef]
- Zhang, C.; Comai, L.; Johnson, D.L. PTEN Represses RNA Polymerase I Transcription by Disrupting the SL1 Complex. Mol. Cell. Biol. 2005, 25, 6899–6911. [Google Scholar] [CrossRef]
- Arabi, A.; Wu, S.; Ridderstråle, K.; Bierhoff, H.; Shiue, C.; Fatyol, K.; Fahlén, S.; Hydbring, P.; Söderberg, O.; Grummt, I.; et al. c-Myc associates with ribosomal DNA and activates RNA polymerase I transcription. Nat. Cell Biol. 2005, 7, 303–310. [Google Scholar] [CrossRef]
- Oran, A.R.; Adams, C.M.; Zhang, X.-Y.; Gennaro, V.J.; Pfeiffer, H.K.; Mellert, H.S.; Seidel, H.E.; Mascioli, K.; Kaplan, J.; Gaballa, M.R.; et al. Multi-focal control of mitochondrial gene expression by oncogenic MYC provides potential therapeutic targets in cancer. Oncotarget 2016, 7, 72395–72414. [Google Scholar] [CrossRef]
- Challagundla, K.B.; Sun, X.-X.; Zhang, X.; Devine, T.; Zhang, Q.; Sears, R.C.; Dai, M.-S. Ribosomal Protein L11 Recruits miR-24/miRISC To Repress c-Myc Expression in Response to Ribosomal Stress. Mol. Cell. Biol. 2011, 31, 4007–4021. [Google Scholar] [CrossRef]
- Li, H.-B.; Wang, R.-X.; Jiang, H.-B.; Zhang, E.-D.; Tan, J.-Q.; Xu, H.-Z.; Zhou, R.-R.; Xia, X.-B. Mitochondrial Ribosomal Protein L10 Associates with Cyclin B1/Cdk1 Activity and Mitochondrial Function. DNA Cell Biol. 2016, 35, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Esposito, D.; Catillo, M.; Pietropaolo, C.; Crescenzi, E.; Russo, G. Human rpL3 induces G₁/S arrest or apoptosis by modulating p21waf1/cip1 levels in a p53-independent manner. Cell Cycle 2012, 12, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Pagliara, V.; Albano, F.; Esposito, D.; Sagar, V.; Loreni, F.; Irace, C.; Santamaria, R.; Russo, G. Regulatory role of rpL3 in cell response to nucleolar stress induced by Act D in tumor cells lacking functional p53. Cell Cycle 2016, 15, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Shi, Y.; Gou, Y.; Li, J.; Han, S.; Zhang, Y.; Huo, J.; Ning, X.; Sun, L.; Chen, Y.; et al. Human ribosomal protein S13 promotes gastric cancer growth through down-regulating p27Kip1. J. Cell. Mol. Med. 2011, 15, 296–306. [Google Scholar] [CrossRef]
- Chen, J.; Wei, Y.; Feng, Q.; Ren, L.; He, G.; Chang, W.; Zhu, D.; Yi, T.; Lin, Q.; Tang, W.; et al. Ribosomal protein S15A promotes malignant transformation and predicts poor outcome in colorectal cancer through misregulation of p53 signaling pathway. Int. J. Oncol. 2016, 48, 1628–1638. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, Y.; Lv, X.; Dong, B.; Wang, F.; Li, J.; Zhang, Q.; Xu, R.; Xu, Y. Down-regulation of ribosomal protein S15A inhibits proliferation of human glioblastoma cells in vivo and in vitro via AKT pathway. Tumor Biol. 2016, 37, 4979–4990. [Google Scholar] [CrossRef]
- Li, C.; Ge, M.; Chen, D.; Sun, T.; Jiang, H.; Xie, Y.; Lu, H.; Zhang, B.; Han, L.; Chen, J.; et al. RPL21 siRNA Blocks Proliferation in Pancreatic Cancer Cells by Inhibiting DNA Replication and Inducing G1 Arrest and Apoptosis. Front. Oncol. 2020, 10, 1730. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chang, M.-Y.; Shiau, A.-L.; Yo, Y.-T.; Wu, C.-L. Mitochondrial ribosomal protein S36 delays cell cycle progression in association with p53 modification and p21WAF1/CIP1 expression. J. Cell. Biochem. 2007, 100, 981–990. [Google Scholar] [CrossRef]
- Yoo, Y.A.; Kim, M.J.; Park, J.K.; Chung, Y.M.; Lee, J.H.; Chi, S.-G.; Kim, J.S.; Yoo, Y.D. Mitochondrial Ribosomal Protein L41 Suppresses Cell Growth in Association with p53 and p27Kip1. Mol. Cell. Biol. 2005, 25, 6603–6616. [Google Scholar] [CrossRef] [PubMed]
- Yadavilli, S.; Hegde, V.; Deutsch, W.A. Translocation of human ribosomal protein S3 to sites of DNA damage is dependant on ERK-mediated phosphorylation following genotoxic stress. DNA Repair 2007, 6, 1453–1462. [Google Scholar] [CrossRef]
- Ogawa, L.M.; Baserga, S.J. Crosstalk between the nucleolus and the DNA damage response. Mol. BioSyst. 2017, 13, 443–455. [Google Scholar] [CrossRef]
- Esposito, D.; Crescenzi, E.; Sagar, V.; Loreni, F.; Russo, A.; Russo, G. Human rpL3 plays a crucial role in cell response to nucleolar stress induced by 5-FU and L-OHP. Oncotarget 2014, 5, 11737–11751. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zang, W.; Ji, Y.; Li, T.; Yang, Y.; Zheng, X. Ribosomal protein L6 (RPL6) is recruited to DNA damage sites in a poly(ADP-ribose) polymerase–dependent manner and regulates the DNA damage response. J. Biol. Chem. 2019, 294, 2827–2838. [Google Scholar] [CrossRef]
- Kim, T.-H.; Leslie, P.; Zhang, Y. Ribosomal proteins as unrevealed caretakers for cellular stress and genomic instability. Oncotarget 2014, 5, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, J.; Lim, C.U. The role of NBS1 in DNA double strand break repair, telomere stability, and cell cycle checkpoint control. Cell Res. 2006, 16, 45–54. [Google Scholar] [CrossRef]
- Cui, D.; Li, L.; Lou, H.; Sun, H.; Ngai, S.-M.; Shao, G.; Tang, J. The ribosomal protein S26 regulates p53 activity in response to DNA damage. Oncogene 2014, 33, 2225–2235. [Google Scholar] [CrossRef]
- Huang, C.-J.; Yang, S.-H.; Lee, C.-L.; Cheng, Y.-C.; Tai, S.-Y.; Chien, C.-C. Ribosomal Protein S27-Like in Colorectal Cancer: A Candidate for Predicting Prognoses. PLoS ONE 2013, 8, e67043. [Google Scholar] [CrossRef]
- Sun, S.; He, H.; Ma, Y.; Xu, J.; Chen, G.; Sun, Y.; Xiong, X. Inactivation of ribosomal protein S27-like impairs DNA interstrand cross-link repair by destabilization of FANCD2 and FANCI. Cell Death Dis. 2020, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.-Y.; Kim, H.D.; Kim, J. Ribosomal protein S3 interacts with TRADD to induce apoptosis through caspase dependent JNK activation. Biochem. Biophys. Res. Commun. 2012, 421, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Qin, L.; Qiu, H.; Shi, D.; Sun, R.; Li, W.; Liu, T.; Wang, J.; Xu, T.; Guo, W.; et al. RPS3 regulates melanoma cell growth and apoptosis by targeting Cyto C/Ca2+/MICU1 dependent mitochondrial signaling. Oncotarget 2015, 6, 29614–29625. [Google Scholar] [CrossRef]
- Saini, N.; Balhara, J.; Adlakha, Y.K.; Singh, N. S29 ribosomal protein induces mitochondria mediated apoptosis of Hep2 cells via the activation of p38 MAPK and JNK signaling. Int. J. Integr. Biol. 2009, 5, 49–57. [Google Scholar]
- Pagliara, V.; Saide, A.; Mitidieri, E.; d’Emmanuele di Villa Bianca, R.; Sorrentino, R.; Russo, G.; Russo, A. 5-FU targets rpL3 to induce mitochondrial apoptosis via cystathionine-β-synthase in colon cancer cells lacking p53. Oncotarget 2016, 7, 50333–50348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fu, J.; Xue, F.; Ryu, B.; Zhang, T.; Zhang, S.; Sun, J.; Xu, X.; Shen, Z.; Zheng, L.; et al. Knockdown of ribosomal protein S15A induces human glioblastoma cell apoptosis. World J. Surg. Oncol. 2016, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hou, J.; Lu, L.; Qi, Z.; Sun, J.; Gao, W.; Meng, J.; Wang, Y.; Sun, H.; Gu, H.; et al. Small Ribosomal Protein Subunit S7 Suppresses Ovarian Tumorigenesis through Regulation of the PI3K/AKT and MAPK Pathways. PLoS ONE 2013, 8, e79117. [Google Scholar] [CrossRef]
- Huang, G.; Li, H.; Zhang, H. Abnormal Expression of Mitochondrial Ribosomal Proteins and Their Encoding Genes with Cell Apoptosis and Diseases. Int. J. Mol. Sci. 2020, 21, 8879. [Google Scholar] [CrossRef]
- Conde, J.A.; Claunch, C.J.; Romo, H.E.; Benito-Martín, A.; Ballestero, R.P.; González-García, M. Identification of a motif in BMRP required for interaction with Bcl-2 by site-directed mutagenesis studies. J. Cell. Biochem. 2012, 113, 3498–3508. [Google Scholar] [CrossRef]
- Hao, C.; Duan, H.; Li, H.; Wang, H.; Liu, Y.; Fan, Y.; Zhang, C. Knockdown of MRPL42 suppresses glioma cell proliferation by inducing cell cycle arrest and apoptosis. Biosci. Rep. 2018, 38, BSR20171456. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Maiti, P.; Barrientos, A. Mitochondrial ribosomes in cancer. Semin. Cancer Biol. 2017, 47, 67–81. [Google Scholar] [CrossRef]
- Han, M.-J.; Chiu, D.T.; Koc, E.C. Regulation of mitochondrial ribosomal protein S29 (MRPS29) expression by a 5′-upstream open reading frame. Mitochondrion 2010, 10, 274–283. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, P.; Yan, L.; Yang, L.; Wang, Y.; Chen, J.; Dai, J.; Li, Y.; Kang, Z.; Bai, T.; et al. MRPL35 Is Up-Regulated in Colorectal Cancer and Regulates Colorectal Cancer Cell Growth and Apoptosis. Am. J. Pathol. 2019, 189, 1105–1120. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell. Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef]
- Solanki, N.R.; Stadanlick, J.E.; Zhang, Y.; Duc, A.-C.; Lee, S.-Y.; Lauritsen, J.P.H.; Zhang, Z.; Wiest, D.L. Rpl22 Loss Selectively Impairs αβ T Cell Development by Dysregulating Endoplasmic Reticulum Stress Signaling. J. Immunol. 2016, 197, 2280–2289. [Google Scholar] [CrossRef]
- Hong, M.; Kim, H.; Kim, I. Ribosomal protein L19 overexpression activates the unfolded protein response and sensitizes MCF7 breast cancer cells to endoplasmic reticulum stress-induced cell death. Biochem. Biophys. Res. Commun. 2014, 450, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, A.; Carotenuto, P.; Franco, B.; De Cegli, R.; Russo, G.; Russo, A. Role of uL3 in the Crosstalk between Nucleolar Stress and Autophagy in Colon Cancer Cells. Int. J. Mol. Sci. 2020, 21, 2143. [Google Scholar] [CrossRef]
- Pecoraro, A.; Pagano, M.; Russo, G.; Russo, A. Role of Autophagy in Cancer Cell Response to Nucleolar and Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 2020, 21, 7334. [Google Scholar] [CrossRef]
- Artero-Castro, A.; Perez-Alea, M.; Feliciano, A.; Leal, J.A.; Genestar, M.; Castellvi, J.; Peg, V.; Cajal, S.R.Y.; Lleonart, M.E.L. Disruption of the ribosomal P complex leads to stress-induced autophagy. Autophagy 2015, 11, 1499–1519. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Liu, X.; Li, H.; He, H.; Sun, Y.; Zhao, Y. Ribosomal protein S27-like regulates autophagy via the β-TrCP-DEPTOR-mTORC1 axis. Cell Death Dis. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Ding, L.; Wei, Z.; Zhang, Y.; Li, Y.; Qinghua, L.; Ma, Y.; Guo, L.; Lv, G.; Liu, Y. Ribosomal protein L34 promotes the proliferation, invasion and metastasis of pancreatic cancer cells. Oncotarget 2016, 7, 85259–85272. [Google Scholar] [CrossRef]
- Ji, P.; Wang, L.; Liu, J.; Mao, P.; Li, R.; Jiang, H.; Lou, M.; Xu, M.; Yu, X. Knockdown of RPL34 inhibits the proliferation and migration of glioma cells through the inactivation of JAK/STAT3 signaling pathway. J. Cell. Biochem. 2019, 120, 3259–3267. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Tan, Z.; Gao, J.; Wu, W.; Liu, L.; Jin, W.; Cao, Y.; Zhao, S.; Zhang, W.; Qiu, Z.; et al. Hyperphosphorylation of ribosomal protein S6 predicts unfavorable clinical survival in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2015, 34, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Jang, Y.H.; Chau, G.C.; Pyo, S.; Um, S.H. Prognostic significance and function of phosphorylated ribosomal protein S6 in esophageal squamous cell carcinoma. Mod. Pathol. 2013, 26, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, L.; Yang, Y.-E.; Xiong, C.; Yu, J.; Wang, Y.; Lin, Y. Knockdown of ribosomal protein S6 suppresses proliferation, migration, and invasion in epithelial ovarian cancer. J. Ovarian Res. 2020, 13, 1–11. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J. Reduction of invasion in human fibrosarcoma cells by ribosomal protein S3 in conjunction with Nm23-H1 and ERK. Biochim. Biophys. Acta 2006, 1763, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, M.; Jensen, L.; Davidsson, S.; Grygoruk, O.V.; Andrén, O.; Kashuba, V.; Kashuba, E. The MRPS18-2 protein levels correlate with prostate tumor progression and it induces CXCR4-dependent migration of cancer cells. Sci. Rep. 2018, 8, 2268. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Long, X.; Jiao, L.; Zhou, M.; Wu, K. MRPS16 facilitates tumor progression via the PI3K/AKT/Snail signaling axis. J. Cancer 2020, 11, 2032–2043. [Google Scholar] [CrossRef] [PubMed]
- Turi, Z.; Lacey, M.; Mistrik, M.; Moudry, P. Impaired ribosome biogenesis: Mechanisms and relevance to cancer and aging. Aging 2019, 11, 2512–2540. [Google Scholar] [CrossRef]
- Sulima, S.O.; De Keersmaecker, K. Ribosomal proteins: A novel class of oncogenic drivers. Oncotarget 2017, 8, 89427–89428. [Google Scholar] [CrossRef] [PubMed]
- Engidaye, G.; Melku, M.; Enawgaw, B. Diamond Blackfan Anemia: Genetics, Pathogenesis, Diagnosis and Treatment. EJIFCC 2019, 30, 67–81. [Google Scholar] [PubMed]
- Carotenuto, P.; Pecoraro, A.; Palma, G.; Russo, G.; Russo, A. Therapeutic Approaches Targeting Nucleolus in Cancer. Cells 2019, 8, 1090. [Google Scholar] [CrossRef]
- Arthurs, C.; Murtaza, B.N.; Thomson, C.; Dickens, K.; Henrique, R.; Patel, H.R.H.; Beltran, M.; Millar, M.; Thrasivoulou, C.; Ahmed, A. Expression of ribosomal proteins in normal and cancerous human prostate tissue. PLoS ONE 2017, 12, e0186047. [Google Scholar] [CrossRef]
- Zhou, C.; Sun, J.; Zheng, Z.; Weng, J.; Atyah, M.; Zhou, Q.; Chen, W.; Zhang, Y.; Huang, J.; Yin, Y.; et al. High RPS11 level in hepatocellular carcinoma associates with poor prognosis after curative resection. Ann. Transl. Med. 2020, 8, 466. [Google Scholar] [CrossRef]
- Awah, C.U.; Chen, L.; Bansal, M.; Mahajan, A.; Winter, J.; Lad, M.; Warnke, L.; Gonzalez-Buendia, E.; Park, C.; Zhang, D.; et al. Ribosomal protein S11 influences glioma response to TOP2 poisons. Oncogene 2020, 39, 5068–5081. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-K.; Lim, J.J.; Jeoun, U.-W.; Min, S.; Lee, E.-B.; Kwon, S.M.; Lee, C.; Yoon, G. Lactate-mediated mitoribosomal defects impair mitochondrial oxidative phosphorylation and promote hepatoma cell invasiveness. J. Biol. Chem. 2017, 292, 20208–20217. [Google Scholar] [CrossRef]
- Pu, M.; Wang, J.; Huang, Q.; Zhao, G.; Xia, C.; Shang, R.; Zhang, Z.; Bian, Z.; Yang, X.; Tao, K. High MRPS23 expression contributes to hepatocellular carcinoma proliferation and indicates poor survival outcomes. Tumor Biol. 2017, 39, 1010428317709127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Chen, Z.; Peng, C.; Chen, C.; Li, H. Long Noncoding RNA TRIM52-AS1 Sponges miR-514a-5p to Facilitate Hepatocellular Carcinoma Progression Through Increasing MRPS18A. Cancer Biother. Radiopharm. 2021, 36, 211–219. [Google Scholar] [CrossRef]
- Tang, N.-Y.; Chueh, F.-S.; Yu, C.-C.; Liao, C.-L.; Lin, J.-J.; Hsia, T.-C.; Wu, K.-C.; Liu, H.-C.; Lu, K.-W.; Chung, J.-G. Benzyl isothiocyanate alters the gene expression with cell cycle regulation and cell death in human brain glioblastoma GBM 8401 cells. Oncol. Rep. 2016, 35, 2089–2096. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, Y.; Amatangelo, M.D.; Stearns, M.E. Role of Ribosomal Protein RPS2 in Controlling let-7a Expression in Human Prostate Cancer. Mol. Cancer Res. 2011, 9, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Dave, B.; Granados-Principal, S.; Zhu, R.; Benz, S.; Rabizadeh, S.; Soon-Shiong, P.; Yu, K.-D.; Shao, Z.; Li, X.; Gilcrease, M.; et al. Targeting RPL39 and MLF2 reduces tumor initiation and metastasis in breast cancer by inhibiting nitric oxide synthase signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 8838–8843. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, L.; Jiang, C.; Zhu, Q.; Chen, R.; Wang, J.; Wang, S. Biological effect of ribosomal protein L32 on human breast cancer cell behavior. Mol. Med. Rep. 2020, 22, 2478–2486. [Google Scholar] [CrossRef]
- D’Angelo, I.; Costabile, G.; Durantie, E.; Brocca, P.; Rondelli, V.; Russo, A.; Russo, G.; Miro, A.; Quaglia, F.; Petri-Fink, A.; et al. Hybrid Lipid/Polymer Nanoparticles for Pulmonary Delivery of siRNA: Development and Fate Upon In Vitro Deposition on the Human Epithelial Airway Barrier. J. Aerosol Med. Pulm. Drug Deliv. 2018, 31, 170–181. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, W.; Liang, X.; Shuai, C.; Zhou, Y.; Pan, H.; Yang, Y.; Han, W. RPL32 Promotes Lung Cancer Progression by Facilitating p53 Degradation. Mol. Ther. Nucleic Acids 2020, 21, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Saide, A.; Cagliani, R.; Cantile, M.; Botti, G.; Russo, G. rpL3 promotes the apoptosis of p53 mutated lung cancer cells by down-regulating CBS and NFκB upon 5-FU treatment. Sci. Rep. 2016, 6, 38369. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Pellosi, D.S.; Pagliara, V.; Milone, M.R.; Pucci, B.; Caetano, W.; Hioka, N.; Budillon, A.; Ungaro, F.; Russo, G.; et al. Biotin-targeted Pluronic® P123/F127 mixed micelles delivering niclosamide: A repositioning strategy to treat drug-resistant lung cancer cells. Int. J. Pharm. 2016, 511, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, A.; Virgilio, A.; Esposito, V.; Galeone, A.; Russo, G.; Russo, A. uL3 Mediated Nucleolar Stress Pathway as a New Mechanism of Action of Antiproliferative G-quadruplex TBA Derivatives in Colon Cancer Cells. Biomolecules 2020, 10, 583. [Google Scholar] [CrossRef]
- Mosca, L.; Pagano, M.; Pecoraro, A.; Borzacchiello, L.; Mele, L.; Cacciapuoti, G.; Porcelli, M.; Russo, G.; Russo, A. S-Adenosyl-l-Methionine Overcomes uL3-Mediated Drug Resistance in p53 Deleted Colon Cancer Cells. Int. J. Mol. Sci. 2020, 22, 103. [Google Scholar] [CrossRef] [PubMed]
- Barbato, A.; Iuliano, A.; Volpe, M.; D’Alterio, R.; Brillante, S.; Massa, F.; De Cegli, R.; Carrella, S.; Salati, M.; Russo, A.; et al. Integrated Genomics Identifies miR-181/TFAM Pathway as a Critical Driver of Drug Resistance in Melanoma. Int. J. Mol. Sci. 2021, 22, 1801. [Google Scholar] [CrossRef] [PubMed]
- Maiolino, S.; Russo, A.; Pagliara, V.; Conte, C.; Ungaro, F.; Russo, G.; Quaglia, F. Biodegradable nanoparticles sequentially decorated with Polyethyleneimine and Hyaluronan for the targeted delivery of docetaxel to airway cancer cells. J. Nanobiotechnol. 2015, 13, 29. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Zhang, B.-C.; Zhang, A.-R.; Wu, T.-T.; Liu, J.; Yu, L.-F.; Wang, W.-X.; Gao, J.-F.; Fang, D.-C.; Rao, Z.-G. Co-transduction of ribosomal protein L23 enhances the therapeutic efficacy of adenoviral-mediated p53 gene transfer in human gastric cancer. Oncol. Rep. 2013, 30, 1989–1995. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Xu, S.; Zhang, X.; He, J.; Yan, D.; Yang, Z.; Xiao, S. Ribosomal protein RPL41 induces rapid degradation of ATF4, a transcription factor critical for tumour cell survival in stress. J. Pathol. 2011, 225, 285–292. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecoraro, A.; Pagano, M.; Russo, G.; Russo, A. Ribosome Biogenesis and Cancer: Overview on Ribosomal Proteins. Int. J. Mol. Sci. 2021, 22, 5496. https://doi.org/10.3390/ijms22115496
Pecoraro A, Pagano M, Russo G, Russo A. Ribosome Biogenesis and Cancer: Overview on Ribosomal Proteins. International Journal of Molecular Sciences. 2021; 22(11):5496. https://doi.org/10.3390/ijms22115496
Chicago/Turabian StylePecoraro, Annalisa, Martina Pagano, Giulia Russo, and Annapina Russo. 2021. "Ribosome Biogenesis and Cancer: Overview on Ribosomal Proteins" International Journal of Molecular Sciences 22, no. 11: 5496. https://doi.org/10.3390/ijms22115496
APA StylePecoraro, A., Pagano, M., Russo, G., & Russo, A. (2021). Ribosome Biogenesis and Cancer: Overview on Ribosomal Proteins. International Journal of Molecular Sciences, 22(11), 5496. https://doi.org/10.3390/ijms22115496






