Modulation of Endocannabinoids by Caloric Restriction Is Conserved in Mice but Is Not Required for Protection from Acute Kidney Injury
Abstract
1. Introduction
2. Results
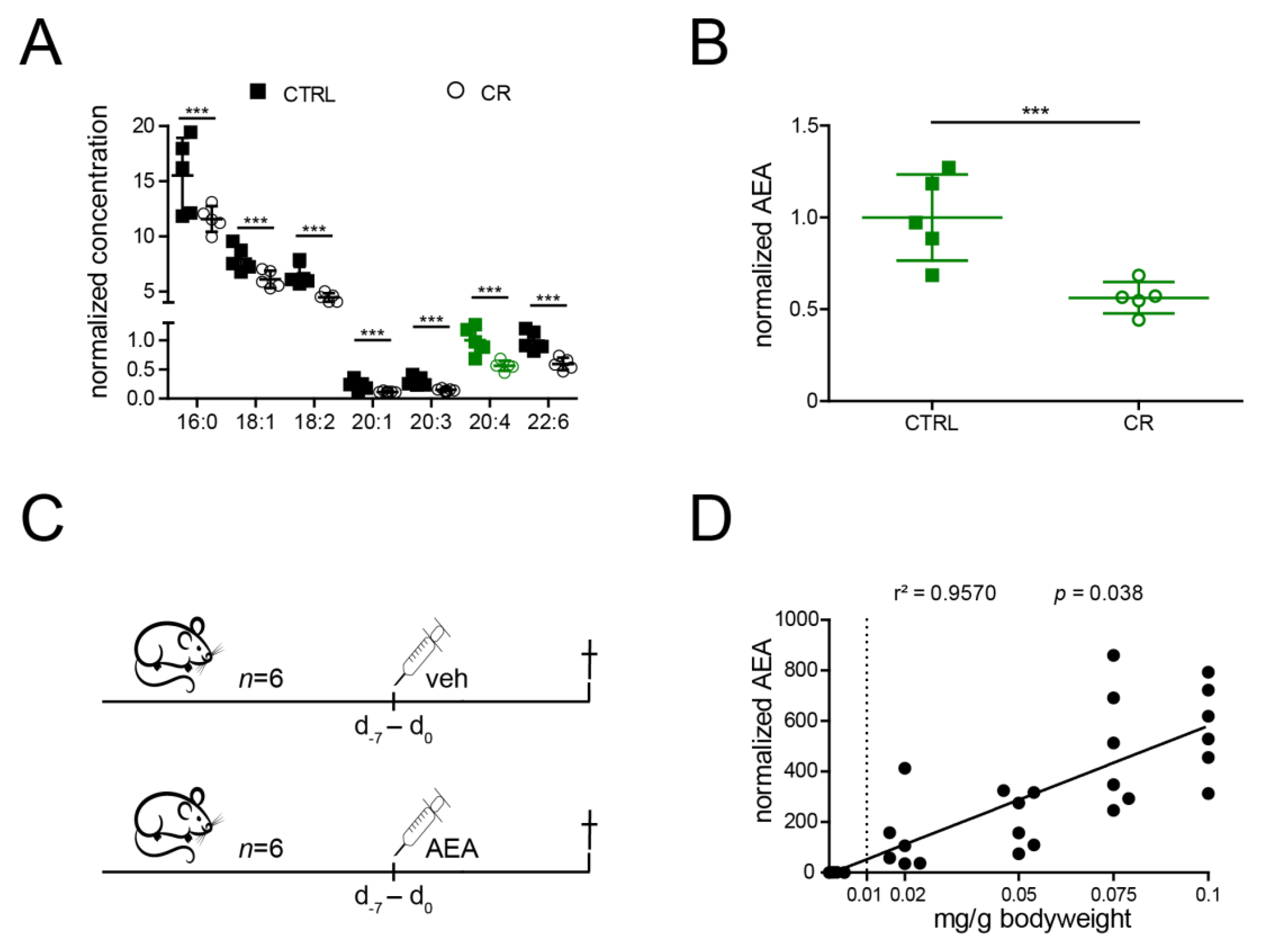
2.1. Anandamide Is Reduced by CR and Can Be Increased by Intraperitoneal Administration in Mice
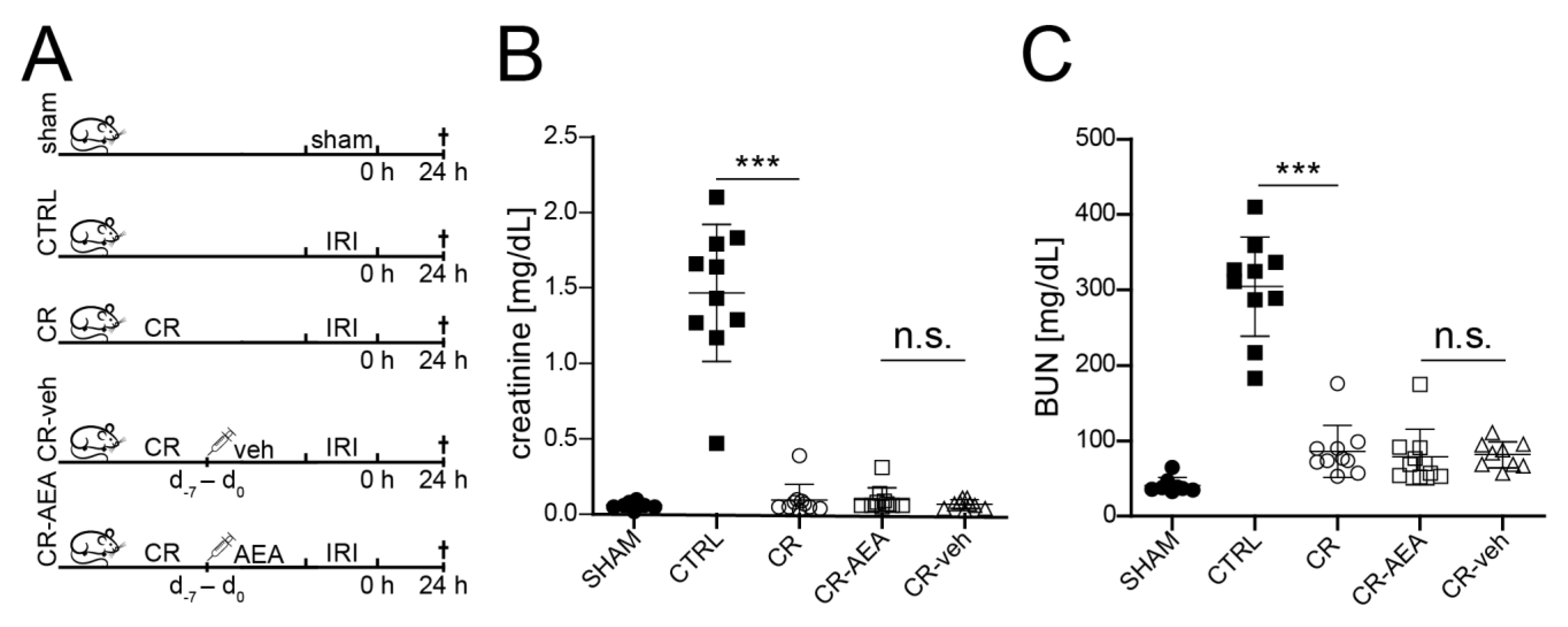
2.2. The CR-Mediated Protection from Renal IRI Does Not Depend on the Reduction of AEA
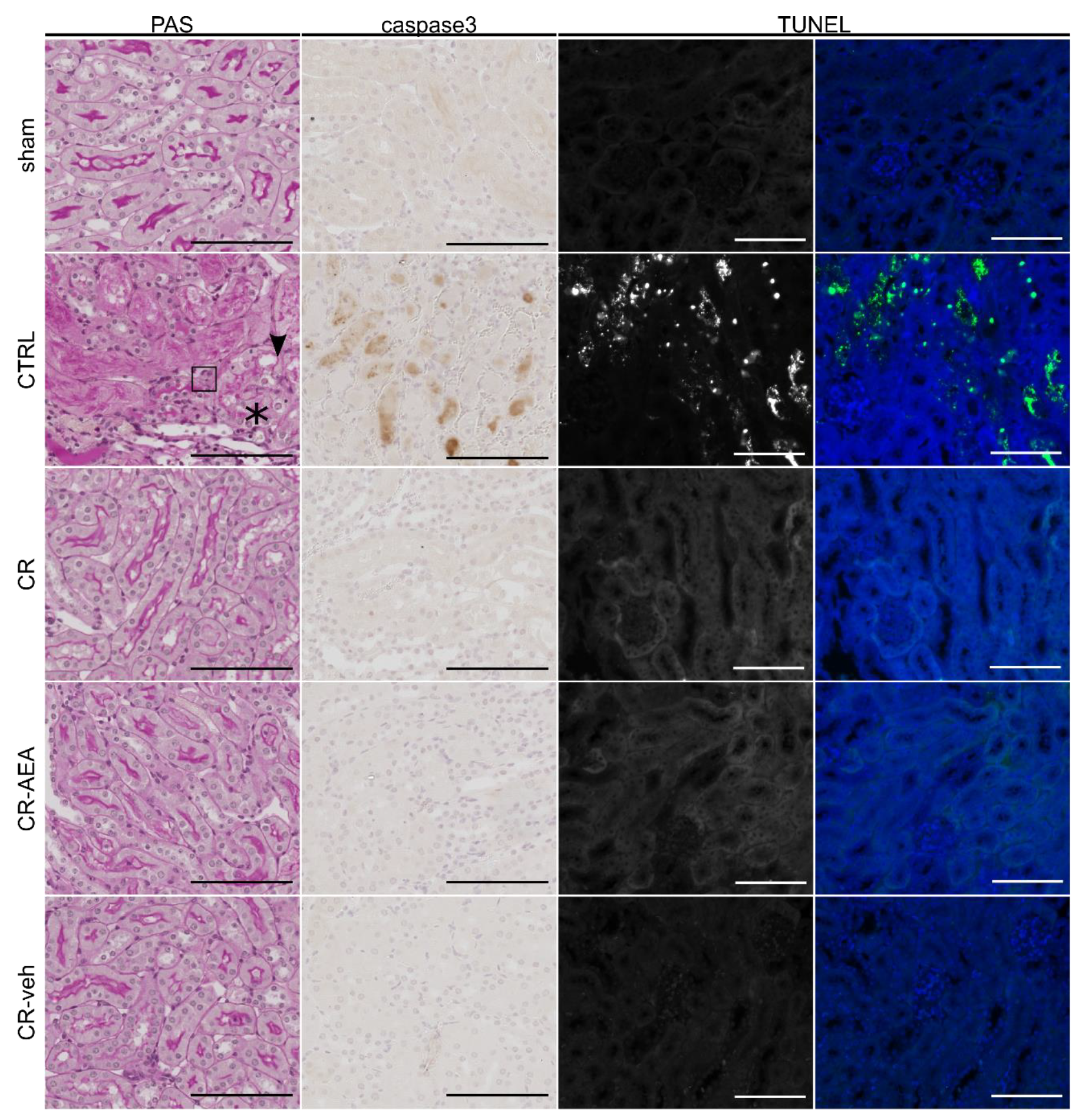
2.3. Intraperitoneal AEA Supplementation Does Not Abrogate the CR-Mediated Protection from Cell Death and Tubular Damage
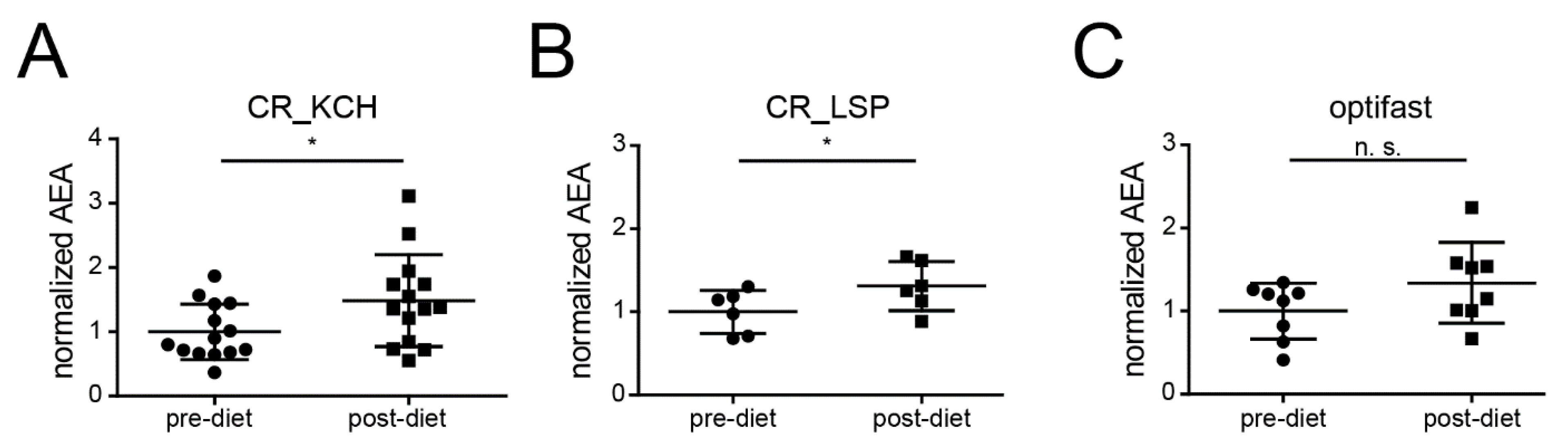
2.4. CR Is Associated with an Increase of AEA in Humans
3. Discussion
4. Methods
4.1. Animal Procedures
4.2. Ischemia-Reperfusion Injury (IRI)
4.3. Blood Analyses
4.4. Histopathology
4.5. Image Acquisition
4.6. Quantification of Ethanolamides
4.7. Human Sample Acquisition
4.8. Functional Data and Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Rewa, O.; Bagshaw, S.M. Acute kidney injury—Epidemiology, outcomes and economics. Nat. Rev. Nephrol. 2014, 10, 193. [Google Scholar] [CrossRef]
- Wald, R.; Quinn, R.R.; Luo, J.; Li, P.; Scales, D.C.; Mamdani, M.M.; Ray, J.G. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 2009, 302, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Thakar, C.V.; Christianson, A.; Himmelfarb, J.; Leonard, A.C. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin. J. Am. Soc. Nephrol. 2011, 6, 2567–2572. [Google Scholar] [CrossRef] [PubMed]
- Lafrance, J.P.; Miller, D.R. Acute kidney injury associates with increased long-term mortality. J. Am. Soc. Nephrol. 2010, 21, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Prendecki, M.; Blacker, E.; Sadeghi-Alavijeh, O.; Edwards, R.; Montgomery, H.; Gillis, S.; Harber, M. Improving outcomes in patients with Acute Kidney Injury: The impact of hospital based automated AKI alerts. Postgrad. Med. J. 2016, 92, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Burdick, E.; Honour, M.; Bonventre, J.V.; Bates, D.W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol. 2005, 16, 3365–3370. [Google Scholar] [CrossRef]
- Coca, S.G.; Yusuf, B.; Shlipak, M.G.; Garg, A.X.; Parikh, C.R. Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am. J. Kidney Dis. 2009, 53, 961–973. [Google Scholar] [CrossRef]
- Rodríguez, E.; Arias-Cabrales, C.; Bermejo, S.; Sierra, A.; Burballa, C.; Soler, M.J.; Barrios, C.; Pascual, J. Impact of recurrent acute kidney injury on patient outcomes. Kidney Blood Press. Res. 2018, 43, 34–44. [Google Scholar] [CrossRef]
- Dear, J.W.; Yuen, P.S. Setting the stage for acute-on-chronic kidney injury. Kidney Int. 2008, 74, 7–9. [Google Scholar] [CrossRef][Green Version]
- Mao, H.; Katz, N.; Ariyanon, W.; Blanca-Martos, L.; Adýbelli, Z.; Giuliani, A.; Danesi, T.H.; Kim, J.C.; Nayak, A.; Neri, M.; et al. Cardiac surgery-associated acute kidney injury. Cardiorenal Med. 2013, 3, 178–199. [Google Scholar] [CrossRef] [PubMed]
- Kinsey, G.R.; Okusa, M.D. Pathogenesis of acute kidney injury: Foundation for clinical practice. Am. J. Kidney Dis. 2011, 58, 291–301. [Google Scholar] [CrossRef]
- Krüger, B.; Krick, S.; Dhillon, N.; Lerner, S.M.; Ames, S.; Bromberg, J.S.; Lin, M.; Walsh, L.; Vella, J.; Fischereder, M.; et al. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc. Natl. Acad. Sci. USA 2009, 106, 3390–3395. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, M.; Rosso, G.; Bertoni, E. Update on ischemia-reperfusion injury in kidney transplantation: Pathogenesis and treatment. World J. Transpl. 2015, 5, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Peerapornratana, S.; Manrique-Caballero, C.L.; Gómez, H.; Kellum, J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019, 96, 1083–1099. [Google Scholar] [CrossRef]
- Peres, L.A.; da Cunha, A.D., Jr. Acute nephrotoxicity of cisplatin: Molecular mechanisms. J. Bras. Nefrol. 2013, 35, 332–340. [Google Scholar] [CrossRef]
- Späth, M.R.; Bartram, M.P.; Palacio-Escat, N.; Hoyer, K.J.R.; Debes, C.; Demir, F.; Schroeter, C.B.; Mandel, A.M.; Grundmann, F.; Ciarimboli, G.; et al. The proteome microenvironment determines the protective effect of preconditioning in cisplatin-induced acute kidney injury. Kidney Int. 2019, 95, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, J.; Williams, B.T.; Banerjee, A.; Harken, A.H.; Burke, T.J.; Cairns, C.B.; Shapiro, J.I. Ischemic preconditioning attenuates functional, metabolic, and morphologic injury from ischemic acute renal failure in the rat. Ren. Fail. 1999, 21, 135–145. [Google Scholar] [CrossRef]
- Mitchell, J.R.; Verweij, M.; Brand, K.; van de Ven, M.; Goemaere, N.; van den Engel, S.; Chu, T.; Forrer, F.; Muller, C.; de Jong, M.; et al. Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell 2010, 9, 40–53. [Google Scholar] [CrossRef]
- Bernhardt, W.M.; Campean, V.; Kany, S.; Jurgensen, J.S.; Weidemann, A.; Warnecke, C.; Arend, M.; Klaus, S.; Gunzler, V.; Amann, K.; et al. Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J. Am. Soc. Nephrol. 2006, 17, 1970–1978. [Google Scholar] [CrossRef]
- Yang, C.C.; Lin, L.C.; Wu, M.S.; Chien, C.T.; Lai, M.K. Repetitive hypoxic preconditioning attenuates renal ischemia/reperfusion induced oxidative injury via upregulating HIF-1 alpha-dependent bcl-2 signaling. Transplantation 2009, 88, 1251–1260. [Google Scholar] [CrossRef]
- Johnsen, M.; Kubacki, T.; Yeroslaviz, A.; Späth, M.R.; Mörsdorf, J.; Göbel, H.; Bohl, K.; Ignarski, M.; Meharg, C.; Habermann, B.; et al. The Integrated RNA landscape of renal preconditioning against ischemia—Reperfusion injury. J. Am. Soc. Nephrol. 2020, ASN.2019050534. [Google Scholar] [CrossRef]
- Mattison, J.A.; Roth, G.S.; Beasley, T.M.; Tilmont, E.M.; Handy, A.M.; Herbert, R.L.; Longo, D.L.; Allison, D.B.; Young, J.E.; Bryant, M.; et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 2012, 489, 318–321. [Google Scholar] [CrossRef] [PubMed]
- O’Kane, D.; Baldwin, G.S.; Bolton, D.M.; Ischia, J.J.; Patel, O. Preconditioning against renal ischaemia reperfusion injury: The failure to translate to the clinic. J. Nephrol. 2019, 32, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Späth, M.R.; Koehler, F.C.; Hoyer-Allo, K.J.R.; Grundmann, F.; Burst, V.; Müller, R.U. Preconditioning strategies to prevent acute kidney injury. F1000Research 2020, 9, F1000. [Google Scholar] [CrossRef] [PubMed]
- Deferrari, G.; Bonanni, A.; Bruschi, M.; Alicino, C.; Signori, A. Remote ischaemic preconditioning for renal and cardiac protection in adult patients undergoing cardiac surgery with cardiopulmonary bypass: Systematic review and meta-analysis of randomized controlled trials. Nephrol. Dial. Transplant. 2018, 33, 813–824. [Google Scholar] [CrossRef]
- Lucanic, M.; Held, J.M.; Vantipalli, M.C.; Klang, I.M.; Graham, J.B.; Gibson, B.W.; Lithgow, G.J.; Gill, M.S. N-acylethanolamine signalling mediates the effect of diet on lifespan in Caenorhabditis elegans. Nature 2011, 473, 226–229. [Google Scholar] [CrossRef]
- Chua, J.T.; Argueta, D.A.; DiPatrizio, N.V.; Kovesdy, C.P.; Vaziri, N.D.; Kalantar-Zadeh, K.; Moradi, H. Endocannabinoid system and the kidneys: From renal physiology to injury and disease. Cannabis Cannabinoid Res. 2019, 4, 10–20. [Google Scholar] [CrossRef]
- Silva, G.B.; Atchison, D.K.; Juncos, L.I.; Garcia, N.H. Anandamide inhibits transport-related oxygen consumption in the loop of Henle by activating CB1 receptors. Am. J. Physiol. Ren. Physiol. 2013, 304, F376–F381. [Google Scholar] [CrossRef]
- Deutsch, D.G.; Goligorsky, M.S.; Schmid, P.C.; Krebsbach, R.J.; Schmid, H.H.; Das, S.K.; Dey, S.K.; Arreaza, G.; Thorup, C.; Stefano, G.; et al. Production and physiological actions of anandamide in the vasculature of the rat kidney. J. Clin. Investig. 1997, 100, 1538–1546. [Google Scholar] [CrossRef]
- Hansen, H.S.; Moesgaard, B.; Petersen, G.; Hansen, H.H. Putative neuroprotective actions of N-acyl-ethanolamines. Pharmacol. Ther. 2002, 95, 119–126. [Google Scholar] [CrossRef]
- Ritter, J.K.; Li, G.; Xia, M.; Boini, K. Anandamide and its metabolites: What are their roles in the kidney? Front. Biosci. 2016, 8, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Matias, I. Endocannabinoid control of food intake and energy balance. Nat. Neurosci. 2005, 8, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Silva, F.J.; Viveros, M.P.; McPartland, J.M.; Rodriguez de Fonseca, F. The endocannabinoid system, eating behavior and energy homeostasis: The end or a new beginning? Pharmacol. Biochem. Behav. 2010, 95, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, C.; Chouinard, F.; Lefebvre, J.S.; Flamand, N. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J. Leukoc. Biol. 2015, 97, 1049–1070. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, F.; Muller, R.U.; Reppenhorst, A.; Hulswitt, L.; Spath, M.R.; Kubacki, T.; Scherner, M.; Faust, M.; Becker, I.; Wahlers, T.; et al. Preoperative short-term calorie restriction for prevention of acute kidney injury after cardiac surgery: A randomized, controlled, open-label, pilot trial. J. Am. Heart Assoc. 2018, 7, e008181. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, M.; Späth, M.R.; Denzel, M.S.; Göbel, H.; Kubacki, T.; Hoyer, K.J.R.; Hinze, Y.; Benzing, T.; Schermer, B.; Antebi, A.; et al. Oral supplementation of glucosamine fails to alleviate acute kidney injury in renal ischemia-reperfusion damage. PLoS ONE 2016, 11, e0161315. [Google Scholar] [CrossRef]
- King-Himmelreich, T.S.; Möser, C.V.; Wolters, M.C.; Schmetzer, J.; Möller, M.; Schreiber, Y.; Ferreirós, N.; Geisslinger, G.; Niederberger, E. AMP-activated kinase and the endogenous endocannabinoid system might contribute to antinociceptive effects of prolonged moderate caloric restriction in mice. Mol. Pain 2017, 13, 1744806917703111. [Google Scholar] [CrossRef]
- McDougle, D.R.; Watson, J.E.; Abdeen, A.A.; Adili, R.; Caputo, M.P.; Krapf, J.E.; Johnson, R.W.; Kilian, K.A.; Holinstat, M.; Das, A. Anti-inflammatory ω-3 endocannabinoid epoxides. Proc. Natl. Acad. Sci. USA 2017, 114, E6034–E6043. [Google Scholar] [CrossRef]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Schmid, H.H.; Schmid, P.C.; Natarajan, V. N-acylated glycerophospholipids and their derivatives. Prog. Lipid Res. 1990, 29, 1–43. [Google Scholar] [CrossRef]
- Di Marzo, V.; Fontana, A.; Cadas, H.; Schinelli, S.; Cimino, G.; Schwartz, J.C.; Piomelli, D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 1994, 372, 686–691. [Google Scholar] [CrossRef]
- Piomelli, D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 2003, 4, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Elphick, M.R.; Egertová, M. The phylogenetic distribution and evolutionary origins of endocannabinoid signalling. Handb. Exp. Pharmacol. 2005, 168, 283–297. [Google Scholar]
- Long, J.Z.; LaCava, M.; Jin, X.; Cravatt, B.F. An anatomical and temporal portrait of physiological substrates for fatty acid amide hydrolase. J. Lipid Res. 2011, 52, 337–344. [Google Scholar] [CrossRef]
- Ritter, J.K.; Li, C.; Xia, M.; Poklis, J.L.; Lichtman, A.H.; Abdullah, R.A.; Dewey, W.L.; Li, P.L. Production and actions of the anandamide metabolite prostamide E2 in the renal medulla. J. Pharmacol. Exp. Ther. 2012, 342, 770–779. [Google Scholar] [CrossRef]
- Zygmunt, P.M.; Petersson, J.; Andersson, D.A.; Chuang, H.; Sørgård, M.; Di Marzo, V.; Julius, D.; Högestätt, E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 1999, 400, 452–457. [Google Scholar] [CrossRef]
- Larrinaga, G.; Varona, A.; Perez, I.; Sanz, B.; Ugalde, A.; Candenas, M.L.; Pinto, F.M.; Gil, J.; Lopez, J.I. Expression of cannabinoid receptors in human kidney. Histol. Histopathol. 2010, 25, 1133–1138. [Google Scholar]
- Barutta, F.; Piscitelli, F.; Pinach, S.; Bruno, G.; Gambino, R.; Rastaldi, M.P.; Salvidio, G.; Di Marzo, V.; Cavallo Perin, P.; Gruden, G. Protective role of cannabinoid receptor type 2 in a mouse model of diabetic nephropathy. Diabetes 2011, 60, 2386–2396. [Google Scholar] [CrossRef]
- Jenkin, K.A.; McAinch, A.J.; Grinfeld, E.; Hryciw, D.H. Role for cannabinoid receptors in human proximal tubular hypertrophy. Cell. Physiol. Biochem. 2010, 26, 879–886. [Google Scholar] [CrossRef]
- Howlett, A.C. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002, 68–69, 619–631. [Google Scholar] [CrossRef]
- Afsar, B.; Afsar, R.E.; Copur, S.; Sag, A.A.; Ortiz, A.; Kanbay, M. The effect of energy restriction on development and progression of chronic kidney disease: Review of the current evidence. Br. J. Nutr. 2020, 125, 1–14. [Google Scholar] [CrossRef]
- Rojas-Morales, P.; Tapia, E.; Leon-Contreras, J.C.; Gonzalez-Reyes, S.; Jimenez-Osorio, A.S.; Trujillo, J.; Pavon, N.; Granados-Pineda, J.; Hernandez-Pando, R.; Sanchez-Lozada, L.G.; et al. Mechanisms of fasting-mediated protection against renal injury and fibrosis development after ischemic acute kidney injury. Biomolecules 2019, 9, 404. [Google Scholar] [CrossRef]
- Di Marzo, V.; Stella, N.; Zimmer, A. Endocannabinoid signalling and the deteriorating brain. Nat. Rev. Neurosci. 2015, 16, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Garza-Lombó, C.; Gonsebatt, M.E. Mammalian target of rapamycin: Its role in early neural development and in adult and aged brain function. Front. Cell. Neurosci. 2016, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Udi, S.; Hinden, L.; Earley, B.; Drori, A.; Reuveni, N.; Hadar, R.; Cinar, R.; Nemirovski, A.; Tam, J. Proximal tubular cannabinoid-1 receptor regulates obesity-induced CKD. J. Am. Soc. Nephrol. 2017, 28, 3518–3532. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Godlewski, G.; Jourdan, T.; Liu, Z.; Cinar, R.; Xiong, K.; Kunos, G. Cannabinoid-1 receptor antagonism improves glycemic control and increases energy expenditure through sirtuin-1/mechanistic target of rapamycin complex 2 and 5’adenosine monophosphate-activated protein kinase signaling. Hepatology 2019, 69, 1535–1548. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Pan, H.; Rajesh, M.; Batkai, S.; Patel, V.; Harvey-White, J.; Mukhopadhyay, B.; Hasko, G.; Gao, B.; Mackie, K.; et al. CB1 cannabinoid receptors promote oxidative/nitrosative stress, inflammation and cell death in a murine nephropathy model. Br. J. Pharmacol. 2010, 160, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Cakir, M.; Tekin, S.; Doganyigit, Z.; Cakan, P.; Kaymak, E. The protective effect of cannabinoid type 2 receptor activation on renal ischemia-reperfusion injury. Mol. Cell. Biochem. 2019, 462, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Feizi, A.; Jafari, M.R.; Hamedivafa, F.; Tabrizian, P.; Djahanguiri, B. The preventive effect of cannabinoids on reperfusion-induced ischemia of mouse kidney. Exp. Toxicol. Pathol. 2008, 60, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.A.; Al-Mulhim, A.S.; Jresat, I. Cannabidiol treatment ameliorates ischemia/reperfusion renal injury in rats. Life Sci. 2012, 91, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Moradi, H.; Oveisi, F.; Khanifar, E.; Moreno-Sanz, G.; Vaziri, N.D.; Piomelli, D. Increased renal 2-arachidonoylglycerol level is associated with improved renal function in a mouse model of acute kidney injury. Cannabis Cannabinoid Res. 2016, 1, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Engeli, S.; Böhnke, J.; Feldpausch, M.; Gorzelniak, K.; Janke, J.; Bátkai, S.; Pacher, P.; Harvey-White, J.; Luft, F.C.; Sharma, A.M.; et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes 2005, 54, 2838–2843. [Google Scholar] [CrossRef] [PubMed]
- Engeli, S.; Heusser, K.; Janke, J.; Gorzelniak, K.; Bátkai, S.; Pacher, P.; Harvey-White, J.; Luft, F.C.; Jordan, J. Peripheral endocannabinoid system activity in patients treated with sibutramine. Obesity 2008, 16, 1135–1137. [Google Scholar] [CrossRef] [PubMed]
- Van Eyk, H.J.; van Schinkel, L.D.; Kantae, V.; Dronkers, C.E.A.; Westenberg, J.J.M.; de Roos, A.; Lamb, H.J.; Jukema, J.W.; Harms, A.C.; Hankemeier, T.; et al. Caloric restriction lowers endocannabinoid tonus and improves cardiac function in type 2 diabetes. Nutr. Diabetes 2018, 8, 6. [Google Scholar] [CrossRef]
- Di Marzo, V.; Côté, M.; Matias, I.; Lemieux, I.; Arsenault, B.J.; Cartier, A.; Piscitelli, F.; Petrosino, S.; Alméras, N.; Després, J.P. Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: Associations with changes in metabolic risk factors. Diabetologia 2009, 52, 213–217. [Google Scholar] [CrossRef]
- Quercioli, A.; Montecucco, F.; Pataky, Z.; Thomas, A.; Ambrosio, G.; Staub, C.; Di Marzo, V.; Ratib, O.; Mach, F.; Golay, A.; et al. Improvement in coronary circulatory function in morbidly obese individuals after gastric bypass-induced weight loss: Relation to alterations in endocannabinoids and adipocytokines. Eur. Heart J. 2013, 34, 2063–2073. [Google Scholar] [CrossRef]
- Hanus, L.; Avraham, Y.; Ben-Shushan, D.; Zolotarev, O.; Berry, E.M.; Mechoulam, R. Short-term fasting and prolonged semistarvation have opposite effects on 2-AG levels in mouse brain. Brain Res. 2003, 983, 144–151. [Google Scholar] [CrossRef]
- Kirkham, T.C.; Williams, C.M.; Fezza, F.; Di Marzo, V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: Stimulation of eating by 2-arachidonoyl glycerol. Br. J. Pharmacol. 2002, 136, 550–557. [Google Scholar] [CrossRef]
- Patschan, D.; Hildebrandt, A.; Rinneburger, J.; Wessels, J.T.; Patschan, S.; Becker, J.U.; Henze, E.; Krüger, A.; Müller, G.A. The hormone melatonin stimulates renoprotective effects of “early outgrowth” endothelial progenitor cells in acute ischemic kidney injury. Am. J. Physiol. Ren. Physiol. 2012, 302, F1305–F1312. [Google Scholar] [CrossRef]
- Williams, J.; Wood, J.; Pandarinathan, L.; Karanian, D.A.; Bahr, B.A.; Vouros, P.; Makriyannis, A. Quantitative method for the profiling of the endocannabinoid metabolome by LC-atmospheric pressure chemical ionization-MS. Anal. Chem. 2007, 79, 5582–5593. [Google Scholar] [CrossRef] [PubMed]
- Zoerner, A.A.; Batkai, S.; Suchy, M.T.; Gutzki, F.M.; Engeli, S.; Jordan, J.; Tsikas, D. Simultaneous UPLC-MS/MS quantification of the endocannabinoids 2-arachidonoyl glycerol (2AG), 1-arachidonoyl glycerol (1AG), and anandamide in human plasma: Minimization of matrix-effects, 2AG/1AG isomerization and degradation by toluene solvent extraction. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 883–884, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, W.; Hammels, I.; Jenke, B.; Binczek, E.; Schmidt-Soltau, I.; Brodesser, S.; Odenthal, M.; Thevis, M. Obesity resistance and deregulation of lipogenesis in Δ6-fatty acid desaturase (FADS2) deficiency. EMBO Rep. 2014, 15, 110–120. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoyer-Allo, K.J.R.; Späth, M.R.; Hanssen, R.; Johnsen, M.; Brodesser, S.; Kaufmann, K.; Kiefer, K.; Koehler, F.C.; Göbel, H.; Kubacki, T.; et al. Modulation of Endocannabinoids by Caloric Restriction Is Conserved in Mice but Is Not Required for Protection from Acute Kidney Injury. Int. J. Mol. Sci. 2021, 22, 5485. https://doi.org/10.3390/ijms22115485
Hoyer-Allo KJR, Späth MR, Hanssen R, Johnsen M, Brodesser S, Kaufmann K, Kiefer K, Koehler FC, Göbel H, Kubacki T, et al. Modulation of Endocannabinoids by Caloric Restriction Is Conserved in Mice but Is Not Required for Protection from Acute Kidney Injury. International Journal of Molecular Sciences. 2021; 22(11):5485. https://doi.org/10.3390/ijms22115485
Chicago/Turabian StyleHoyer-Allo, Karla Johanna Ruth, Martin Richard Späth, Ruth Hanssen, Marc Johnsen, Susanne Brodesser, Kathrin Kaufmann, Katharina Kiefer, Felix Carlo Koehler, Heike Göbel, Torsten Kubacki, and et al. 2021. "Modulation of Endocannabinoids by Caloric Restriction Is Conserved in Mice but Is Not Required for Protection from Acute Kidney Injury" International Journal of Molecular Sciences 22, no. 11: 5485. https://doi.org/10.3390/ijms22115485
APA StyleHoyer-Allo, K. J. R., Späth, M. R., Hanssen, R., Johnsen, M., Brodesser, S., Kaufmann, K., Kiefer, K., Koehler, F. C., Göbel, H., Kubacki, T., Grundmann, F., Schermer, B., Brüning, J., Benzing, T., Burst, V., & Müller, R.-U. (2021). Modulation of Endocannabinoids by Caloric Restriction Is Conserved in Mice but Is Not Required for Protection from Acute Kidney Injury. International Journal of Molecular Sciences, 22(11), 5485. https://doi.org/10.3390/ijms22115485







