Dopaminergic Receptor Targeting in Multiple Sclerosis: Is There Therapeutic Potential?
Abstract
1. Introduction
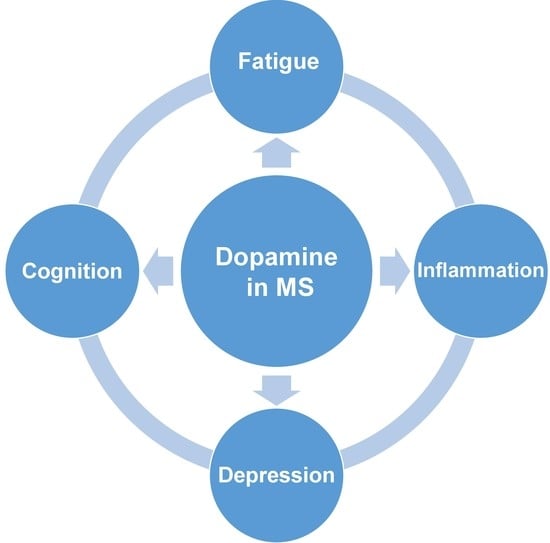
2. The Involvement of Dopamine in the Development of Clinical Symptoms in MS
3. The Role of Dopamine in Regulation of Neuroimmune Interaction in MS
4. The Prospects of Dopaminergic Therapeutics in MS Treatment
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boyko, A.; Melnikov, M. Prevalence and Incidence of Multiple Sclerosis in Russian Federation: 30 Years of Studies. Brain Sci. 2020, 10, 305. [Google Scholar] [CrossRef]
- Xu, X.; Chi, S.; Wang, Q.; Li, C.; Xu, B.; Zhang, J.; Chen, X. Efficacy and safety of monoclonal antibody therapies for relapsing remitting multiple sclerosis: A network meta-analysis. Mult. Scler. Relat. Disord. 2018, 25, 322–328. [Google Scholar] [CrossRef]
- Soleimani, B.; Murray, K.; Hunt, D. Established and Emerging Immunological Complications of Biological Therapeutics in Multiple Sclerosis. Drug Saf. 2019, 42, 941–956. [Google Scholar] [CrossRef] [PubMed]
- Sellner, J.; Rommer, P.S. Immunological consequences of “immune reconstitution therapy” in multiple sclerosis: A systematic review. Autoimmun. Rev. 2020, 19, 102492. [Google Scholar] [CrossRef]
- Yu, Y.J.; Watts, R.J. Developing therapeutic antibodies for neurodegenerative disease. Neurotherapeutics 2013, 10, 459–472. [Google Scholar] [CrossRef]
- Ziemssen, T. Modulating processes within the central nervous system is central to therapeutic control of multiple sclerosis. J. Neurol. 2005, 252 (Suppl. 5), v38–v45. [Google Scholar] [CrossRef] [PubMed]
- Hodo, T.W.; de Aquino, M.T.P.; Shimamoto, A.; Shanker, A. Critical Neurotransmitters in the Neuroimmune Network. Front. Immunol. 2020, 11, 1869. [Google Scholar] [CrossRef]
- Cosentino, M.; Marino, F. Adrenergic and dopaminergic modulation of immunity in multiple sclerosis: Teaching old drugs new tricks? J. Neuroimmune Pharm. 2013, 8, 163–179. [Google Scholar] [CrossRef]
- Marino, F.; Cosentino, M. Multiple sclerosis: Repurposing dopaminergic drugs for MS—The evidence mounts. Nat. Rev. Neurol. 2016, 12, 191–192. [Google Scholar] [CrossRef]
- Melnikov, M.; Sviridova, A.; Rogovskii, V.; Oleskin, A.; Boziki, M.; Bakirtzis, C.; Kesidou, E.; Grigoriadis, N.; Boyko, A. Serotoninergic system targeting in multiple sclerosis: The prospective for pathogenetic therapy. Mult. Scler. Relat. Disord. 2021, 51, 102888. [Google Scholar] [CrossRef] [PubMed]
- Manjaly, Z.M.; Harrison, N.A.; Critchley, H.D.; Do, C.T.; Stefanics, G.; Wenderoth, N.; Lutterotti, A.; Müller, A.; Stephan, K.E. Pathophysiological and cognitive mechanisms of fatigue in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Dobryakova, E.; Genova, H.M.; de Luca, J.; Wylie, G.R. The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurological disorders. Front. Neurol. 2015, 6, 52. [Google Scholar] [CrossRef]
- Grech, L.B.; Butler, E.; Stuckey, S.; Hester, R. Neuroprotective Benefits of Antidepressants in Multiple Sclerosis: Are We Missing the Mark? J. Neuropsychiatry Clin. Neurosci. 2019, 31, 289–297. [Google Scholar] [CrossRef]
- Carandini, T.; Cercignani, M.; Galimberti, D.; Scarpini, E.; Bozzali, M. The distinct roles of monoamines in multiple sclerosis: A bridge between the immune and nervous systems? Brain Behav. Immun. 2021, 94, 381–391. [Google Scholar] [CrossRef]
- González-Arancibia, C.; Urrutia-Piñones, J.; Illanes-González, J.; Martinez-Pinto, J.; Sotomayor-Zárate, R.; Julio-Pieper, M.; Bravo, J.A. Do your gut microbes affect your brain dopamine? Psychopharmacology 2019, 236, 1611–1622. [Google Scholar] [CrossRef]
- Boziki, M.K.; Kesidou, E.; Theotokis, P.; Mentis, A.A.; Karafoulidou, E.; Melnikov, M.; Sviridova, A.; Rogovski, V.; Boyko, A.; Grigoriadis, N. Microbiome in Multiple Sclerosis; Where Are We, What We Know and Do Not Know. Brain Sci. 2020, 10, 234. [Google Scholar] [CrossRef]
- Kozhieva, M.K.; Melnikov, M.V.; Rogovsky, V.S.; Oleskin, A.V.; Kabilov, M.R.; Boyko, A.N. Gut human microbiota and multiple sclerosis. Zh. Nevrol. Psikhiatr. Im. S S Korsakova 2017, 117, 11–19. [Google Scholar] [CrossRef]
- Carandini, T.; Mancini, M.; Bogdan, I.; Rae, C.L.; Barritt, A.W.; Sethi, A.; Harrison, N.; Rashid, W.; Scarpini, E.; Galimberti, D.; et al. Disruption of brainstem monoaminergic fibre tracts in multiple sclerosis as a putative mechanism for cognitive fatigue: A fixel-based analysis. Neuroimage Clin. 2021, 30, 102587. [Google Scholar] [CrossRef]
- Beurel, E.; Lowell, J.A. Th17 cells in depression. Brain Behav. Immun. 2018, 69, 28–34. [Google Scholar] [CrossRef]
- Coyle, P.K. Symptom Management and Lifestyle Modifications in Multiple Sclerosis. Continuum 2016, 22, 815–836. [Google Scholar] [CrossRef]
- Mohr, D.C.; Lovera, J.; Brown, T.; Cohen, B.; Neylan, T.; Henry, R.; Siddique, J.; Jin, L.; Daikh, D.; Pelletier, D. A randomized trial of stress management for the prevention of new brain lesions in MS. Neurology 2012, 79, 412–419. [Google Scholar] [CrossRef]
- Alvarenga-Filho, H.; Salles, M.; Hygino, J.; Ferreira, T.B.; Sacramento, P.M.; Monteiro, C.; Vasconcelos, C.C.; Alvarenga, R.M.; Bento, C.A. Fatigue favors in vitro Th1 and Th17-like cell expansion and reduces corticoid sensitivity in MS patients. J. Neuroimmunol. 2017, 303, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga-Filho, H.; Sacramento, P.M.; Ferreira, T.B.; Hygino, J.; Abreu, J.E.C.; Carvalho, S.R.; Wing, A.C.; Alvarenga, R.M.P.; Bento, C.A.M. Combined exercise training reduces fatigue and modulates the cytokine profile of T-cells from multiple sclerosis patients in response to neuromediators. J. Neuroimmunol. 2016, 293, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Polak, P.E.; Kalinin, S.; Feinstein, D.L. Locus coeruleus damage and noradrenaline reductions in multiple sclerosis and experimental autoimmune encephalomyelitis. Brain 2011, 134, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, A.; Wilson, H.; Giordano, B.; Caminiti, S.P.; Chappell, Z.; Williams, S.; Hammers, A.; Silber, E.; Brex, P.; Politis, M. Impaired connectivity within neuromodulatory networks in multiple sclerosis and clinical implications. J. Neurol. 2020, 267, 2042–2053. [Google Scholar] [CrossRef]
- Melnikov, M.; Belousova, O.; Murugin, V.; Pashenkov, M.; Boyкo, A. The role of dopamine in modulation of Th-17 immune response in multiple sclerosis. J. Neuroimmunol. 2016, 292, 97–101. [Google Scholar] [CrossRef]
- Escribano, B.M.; Aguilar-Luque, M.; Bahamonde, C.; Conde, C.; Lillo, R.; Sanchez-Lopez, F.; Giraldo, A.I.; Cruz, A.H.; Luque, E.; Gascon, F.; et al. Natalizumab Modifies Catecholamines Levels Present in Patients with Relapsing- Remitting Multiple Sclerosis. Curr. Pharm. Des. 2016, 22, 4876–4880. [Google Scholar] [CrossRef]
- Milovanovic, J.; Arsenijevic, A.; Stojanovic, B.; Kanjevac, T.; Arsenijevic, D.; Radosavljevic, G.; Milovanovic, M.; Arsenijevic, N. Interleukin-17 in Chronic Inflammatory Neurological Diseases. Front. Immunol. 2020, 11, 947. [Google Scholar] [CrossRef]
- Kebir, H.; Kreymborg, K.; Ifergan, I.; Dodelet-Devillers, A.; Cayrol, R.; Bernard, M.; Giuliani, F.; Arbour, N.; Becher, B.; Prat, A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007, 13, 1173–1175. [Google Scholar] [CrossRef]
- Sallusto, F.; Impellizzieri, D.; Basso, C.; Laroni, A.; Uccelli, A.; Lanzavecchia, A.; Engelhardt, B. T-cell trafficking in the central nervous system. Immunol. Rev. 2012, 248, 216–227. [Google Scholar] [CrossRef]
- McGinley, A.M.; Sutton, C.E.; Edwards, S.C.; Leane, C.M.; DeCourcey, J.; Teijeiro, A.; Hamilton, J.A.; Boon, L.; Djouder, N.; Mills, K.H.G. Interleukin-17A Serves a Priming Role in Autoimmunity by Recruiting IL-1β-Producing Myeloid Cells that Promote Pathogenic T Cells. Immunity 2020, 52, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Cua, D.J.; Sherlock, J.; Chen, Y.; Murphy, C.A.; Joyce, B.; Seymour, B.; Lucian, L.; To, W.; Kwan, S.; Churakova, T.; et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003, 421, 744–748. [Google Scholar] [CrossRef]
- Guo, W.; Luo, C.; Wang, C.; Wang, Y.H.; Wang, X.; Gao, X.D.; Yao, W.B. Suppression of human and mouse Th17 differentiation and autoimmunity by an endogenous Interleukin 23 receptor cytokine-binding homology region. Int. J. Biochem. Cell. Biol. 2014, 55, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Moser, T.; Akgün, K.; Proschmann, U.; Sellner, J.; Ziemssen, T. The role of TH17 cells in multiple sclerosis: Therapeutic implications. Autoimmun. Rev. 2020, 19, 102647. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, M.; Rogovskii, V.; Boyko, A.; Pashenkov, M. Dopaminergic Therapeutics in Multiple Sclerosis: Focus on Th17-Cell Functions. J. Neuroimmune Pharm. 2020, 15, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Arreola, R.; Alvarez-Herrera, S.; Pérez-Sánchez, G.; Becerril-Villanueva, E.; Cruz-Fuentes, C.; Flores-Gutierrez, E.O.; Garcés-Alvarez, M.E.; de la Cruz-Aguilera, D.L.; Medina-Rivero, E.; Hurtado-Alvarado, G.; et al. Immunomodulatory Effects Mediated by Dopamine. J. Immunol. Res. 2016, 2016, 3160486. [Google Scholar] [CrossRef]
- Melnikov, M.; Sviridova, A.; Pashenkov, M.; Boyko, A. Dopamine Suppresses Th17-Cells Function in Multiple Sclerosis. Poster Presentation. European Charcot Foundation. 28th Annual Meeting, Digital, 15–19 November 2020: The Role of Environmental Factors in Multiple Sclerosis. Available online: https://www.charcot-ms.org/28th-annual-meeting-digital/poster-presentations/basic-studies/mikhail-melnikov-57 (accessed on 24 April 2021).
- Huang, Y.; Chen, C.C.; Wang, T.T.; Qiu, Y.H.; Peng, Y.P. Dopamine receptors modulate T lymphocytes via inhibition of cAMP-CREB signaling pathway. Neuro. Endocrinol. Lett. 2016, 37, 491–500. [Google Scholar]
- Cosentino, M.; Fietta, A.M.; Ferrari, M.; Rasini, E.; Bombelli, R.; Carcano, E.; Saporiti, F.; Meloni, F.; Marino, F.; Lecchini, S. Human CD4+CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood 2007, 109, 632–642. [Google Scholar] [CrossRef]
- Nasi, G.; Ahmed, T.; Rasini, E.; Fenoglio, D.; Marino, F.; Filaci, G.; Cosentino, M. Dopamine inhibits human CD8+ Treg function through D1-like dopaminergic receptors. J. Neuroimmunol. 2019, 332, 233–241. [Google Scholar] [CrossRef]
- Lieberknecht, V.; Junqueira, S.C.; Cunha, M.P.; Barbosa, T.A.; de Souza, L.F.; Coelho, I.S.; Santos, A.R.; Rodrigues, A.L.; Dafré, A.L.; Dutra, R.C. Pramipexole, a Dopamine D2/D3 Receptor-Preferring Agonist, Prevents Experimental Autoimmune Encephalomyelitis Development in Mice. Mol. Neurobiol. 2017, 54, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Flórez-Grau, G.; Zubizarreta, I.; Cabezón, R.; Villoslada, P.; Benitez-Ribas, D. Tolerogenic Dendritic Cells as a Promising Antigen-Specific Therapy in the Treatment of Multiple Sclerosis and Neuromyelitis Optica From Preclinical to Clinical Trials. Front. Immunol. 2018, 9, 1169. [Google Scholar] [CrossRef]
- Nally, F.K.; De Santi, C.; McCoy, C.E. Nanomodulation of Macrophages in Multiple Sclerosis. Cells 2019, 8, 543. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, M.V.; Paschenkov, M.V.; Boyko, A.N. Dendritic cells in multiple sclerosis. Zh. Nevrol. Psikhiatr. Im. S S Korsakova 2017, 117, 22–30. [Google Scholar] [CrossRef]
- Absinta, M.; Lassmann, H.; Trapp, B.D. Mechanisms underlying progression in multiple sclerosis. Curr. Opin. Neurol. 2020, 33, 277–285. [Google Scholar] [CrossRef]
- Polman, C.H.; Dijkstra, C.D.; Sminia, T.; Koetsier, J.C. Immunohistological analysis of macrophages in the central nervous system of Lewis rats with acute experimental allergic encephalomyelitis. J. Neuroimmunol. 1986, 11, 215–222. [Google Scholar] [CrossRef]
- Brück, W.; Sommermeier, N.; Bergmann, M.; Zettl, U.; Goebel, H.H.; Kretzschmar, H.A.; Lassmann, H. Macrophages in multiple sclerosis. Immunobiology 1996, 195, 588–600. [Google Scholar] [CrossRef]
- Henderson, A.P.; Barnett, M.H.; Parratt, J.D.; Prineas, J.W. Multiple sclerosis: Distribution of inflammatory cells in newly forming lesions. Ann. Neurol. 2009, 66, 739–753. [Google Scholar] [CrossRef]
- Ponomarev, E.D.; Shriver, L.P.; Maresz, K.; Dittel, B.N. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J. Neurosci. Res. 2005, 81, 374–389. [Google Scholar] [CrossRef]
- Ajami, B.; Bennett, J.L.; Krieger, C.; McNagny, K.M.; Rossi, F.M. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 2011, 14, 1142–1149. [Google Scholar] [CrossRef]
- Nakano, K.; Higashi, T.; Hashimoto, K.; Takagi, R.; Tanaka, Y.; Matsushita, S. Antagonizing dopamine D1-like receptor inhibits Th17 cell differentiation: Preventive and therapeutic effects on experimental autoimmune encephalomyelitis. Biochem. Biophys. Res. Commun. 2008, 373, 286–291. [Google Scholar] [CrossRef]
- Prado, C.; Contreras, F.; González, H.; Díaz, P.; Elgueta, D.; Barrientos, M.; Herrada, A.A.; Lladser, Á.; Bernales, S.; Pacheco, R. Stimulation of dopamine receptor D5 expressed on dendritic cells potentiates Th17-mediated immunity. J. Immunol. 2012, 188, 3062–3070. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.; Gaiazzi, M.; González, H.; Ugalde, V.; Figueroa, A.; Osorio-Barrios, F.J.; López, E.; Lladser, A.; Rasini, E.; Marino, F.; et al. Dopaminergic Stimulation of Myeloid Antigen-Presenting Cells Attenuates Signal Transducer and Activator of Transcription 3-Activation Favouring the Development of Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2018, 9, 571. [Google Scholar] [CrossRef]
- Osorio-Barrios, F.; Prado, C.; Contreras, F.; Pacheco, R. Dopamine Receptor D5 Signaling Plays a Dual Role in Experimental Autoimmune Encephalomyelitis Potentiating Th17-Mediated Immunity and Favoring Suppressive Activity of Regulatory T-Cells. Front. Cell. Neurosci. 2018, 12, 192. [Google Scholar] [CrossRef]
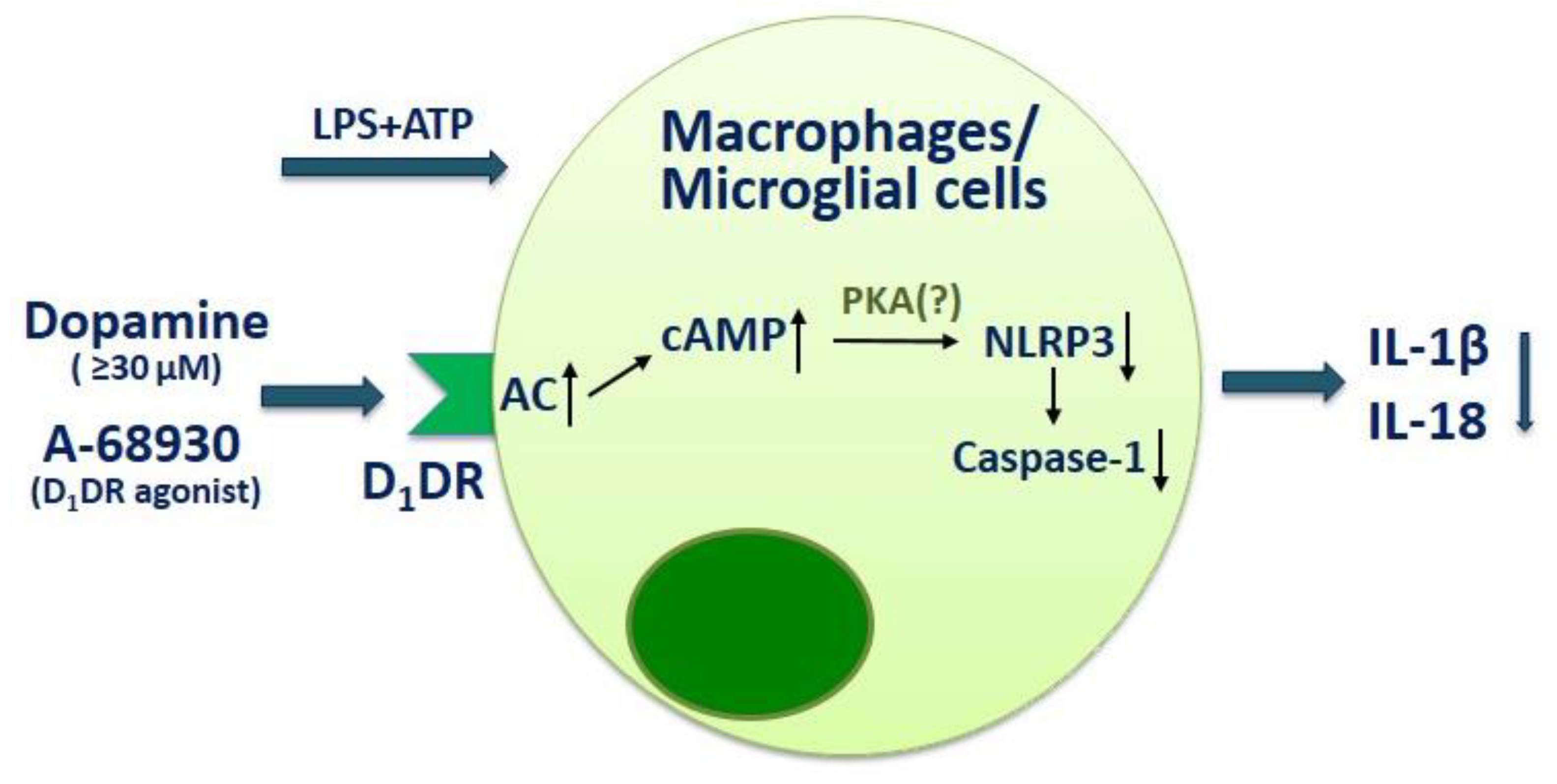
- Xia, Q.P.; Cheng, Z.Y.; He, L. The modulatory role of dopamine receptors in brain neuroinflammation. Int. Immunopharmacol. 2019, 76, 105908. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Sugino, Y.; Shibagaki, F.; Yamamuro, A.; Ishimaru, Y.; Maeda, S. Dopamine attenuates lipopolysaccharide-induced expression of proinflammatory cytokines by inhibiting the nuclear translocation of NF-κB p65 through the formation of dopamine quinone in microglia. Eur. J. Pharm. 2020, 866, 172826. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Liu, L.; Wang, X.; Ding, C.; Tian, Z.; Zhou, R. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 2015, 160, 62–73. [Google Scholar] [CrossRef]
- Mei, F.; Guo, S.; He, Y.; Wang, L.; Wang, H.; Niu, J.; Kong, J.; Li, X.; Wu, Y.; Xiao, L. Quetiapine, an atypical antipsychotic, is protective against autoimmune-mediated demyelination by inhibiting effector T cell proliferation. PLoS ONE 2012, 7, e42746. [Google Scholar] [CrossRef]
- Green, L.K.; Zareie, P.; Templeton, N.; Keyzers, R.A.; Connor, B.; La Flamme, A.C. Enhanced disease reduction using clozapine, an atypical antipsychotic agent, and glatiramer acetate combination therapy in experimental autoimmune encephalomyelitis. Mult. Scler. J. Exp. Transl. Clin. 2017, 3, 2055217317698724. [Google Scholar] [CrossRef]
- Robichon, K.; Patel, V.; Connor, B.; La Flamme, A.C. Clozapine reduces infiltration into the CNS by targeting migration in experimental autoimmune encephalomyelitis. J. Neuroinflamm. 2020, 17, 53. [Google Scholar] [CrossRef]
- Riskind, P.N.; Massacesi, L.; Doolittle, T.H.; Hauser, S.L. The role of prolactin in autoimmune demyelination: Suppression of experimental allergic encephalomyelitis by bromocriptine. Ann. Neurol. 1991, 29, 542–547. [Google Scholar] [CrossRef]
- Dijkstra, C.D.; van der Voort, E.R.; de Groot, C.J.; Uitdehaag, B.M.; Polman, C.H.; Berkenbosch, F. The therapeutic effect of bromocriptine on acute and chronic experimental allergic encephalomyelitis. Ann. Neurol. 1992, 31, 450–451. [Google Scholar] [CrossRef] [PubMed]
- Bissay, V.; De Klippel, N.; Herroelen, L.; Schmedding, E.; Buisseret, T.; Ebinger, G.; De Keyser, J. Bromocriptine therapy in multiple sclerosis: An open label pilot study. Clin. Neuropharmacol. 1994, 17, 473–476. [Google Scholar] [CrossRef]
- Ferreira, T.B.; Barros, P.O.; Teixeira, B.; Cassano, T.; Centurião, N.; Kasahara, T.M.; Hygino, J.; Vasconcelos, C.C.; Filho, H.A.; Alvarenga, R.; et al. Dopamine favors expansion of glucocorticoid-resistant IL-17-producing T cells in multiple sclerosis. Brain Behav. Immun. 2014, 41, 182–190. [Google Scholar] [CrossRef]
- Faissner, S.; Plemel, J.R.; Gold, R.; Yong, V.W. Progressive multiple sclerosis: From pathophysiology to therapeutic strategies. Nat. Rev. Drug Discov. 2019, 18, 905–922. [Google Scholar] [CrossRef]
- Teter, B.; Agashivala, N.; Kavak, K.; Chouhfeh, L.; Hashmonay, R.; Weinstock-Guttman, B. Characteristics influencing therapy switch behavior after suboptimal response to first-line treatment in patients with multiple sclerosis. Mult. Scler. 2014, 20, 830–836. [Google Scholar] [CrossRef] [PubMed]

| Disease | Cell Type | The Effect of Dopaminergic Receptor Targeting | Authors |
|---|---|---|---|
| EAE | Splenic lymphocytes | D2-like dopaminergic receptor agonist bromocriptine has a preventive and curative effect on EAE in mice. The treatment with bromocriptine reduces prolactin serum level and splenic lymphocyte proliferation upon Con A stimulation. | Riskind et al., 1991 [61] |
| EAE | Not investigated | The treatment with D2-like dopaminergic receptor agonist bromocriptine reduces prolactin plasma level and clinical symptoms of acute and chronic EAE. | Dijkstra et al., 1993 [62] |
| EAE | Dendritic cells T cells | D1-like dopaminergic receptor antagonist (SCH23390) has a preventive and curative effect on EAE in mice. D2-like dopaminergic receptor antagonist (L750667) enhances EAE severity. The spleen cells from SCH23390-treated mice produce less IL-17 than the PBS-treated mice. Dendritic cells treated with SCH23390 and transferred to mice have the same effect compared with the direct influence of SCH23390 on EAE. | Nakano et al., 2008 [51] |
| EAE | Dendritic cells | D5-dopaminergic receptor deficiency on dendritic cells impair LPS-induced IL-12 production and IL-23 mRNA expression and attenuate CD4+ T-cell activation. D5-dopaminergic receptor deficient mice show a delayed onset of the EAE and reduced disease severity compared with WT mice. Transfer of D5-dopaminergic receptor deficiency dendritic cells to EAE mice lessens the infiltration of Th17 cells in the CNS. | Prado et al., 2012 [52] |
| EAE | Peripheral lymphoid tissue | The treatment with a D2- and D3-dopaminergic receptors agonist pramipexole reduces IL-17, IL-1β and TNF-α production in lymph nodes and prevents clinical signs of the EAE in mice. | Lieberknecht et al., 2017 [41] |
| EAE | Dendritic cells | Transfer of D5-dopaminergic-receptor-deficient dendritic cells to EAE mice reduces EAE manifestation and decreases the infiltration of IL-17+, IFN-γ+IL-17+ and GM-CSF+IFN-γ+IL-17+CD4+ T cells at the peak of the disease. | Prado et al., 2018 [53] |
| EAE | CD4+ T cells | D5-dopaminergic receptor signaling in naive CD4+ T cells potentiates T cell activation with the acquisition of Th17-phenotype favoring EAE development. D5-dopaminergic receptor signaling in Treg cells contributes to their suppressive activity. | Osorio-Barrios et al., 2018 [54] |
| RRMS Progressive MS | Not investigated | No evidence of clinical efficacy of bromocriptine therapy in MS. After one year of treatment, 14 of the 15 patients showed disease progression. | Bissay et al., 1994 [63] |
| RRMS | PBMCs CD4+ T cells CD8+ T cells | Dopamine (at 10−6 M) enhances IL-17 and IL-21 but suppresses IL-10 and TGF-β production by PHA-activated PBMCs in RRMS patients and enhances IL-17 production by anti-CD3/anti-CD28-antibody-activated CD4+ and CD8+ T cells in RRMS patients. | Ferreira et al., 2014 [64] |
| RRMS | CD3+ T cells | Dopamine (at 10−6 M) enhances IL-6, IL-17, IL-21, and IL-22 but suppresses IL-10 production by anti-CD3/anti-CD28-antibody-activated CD3+ T cells in RRMS patients. | Alvarenga-Filho et al., 2016 [23] |
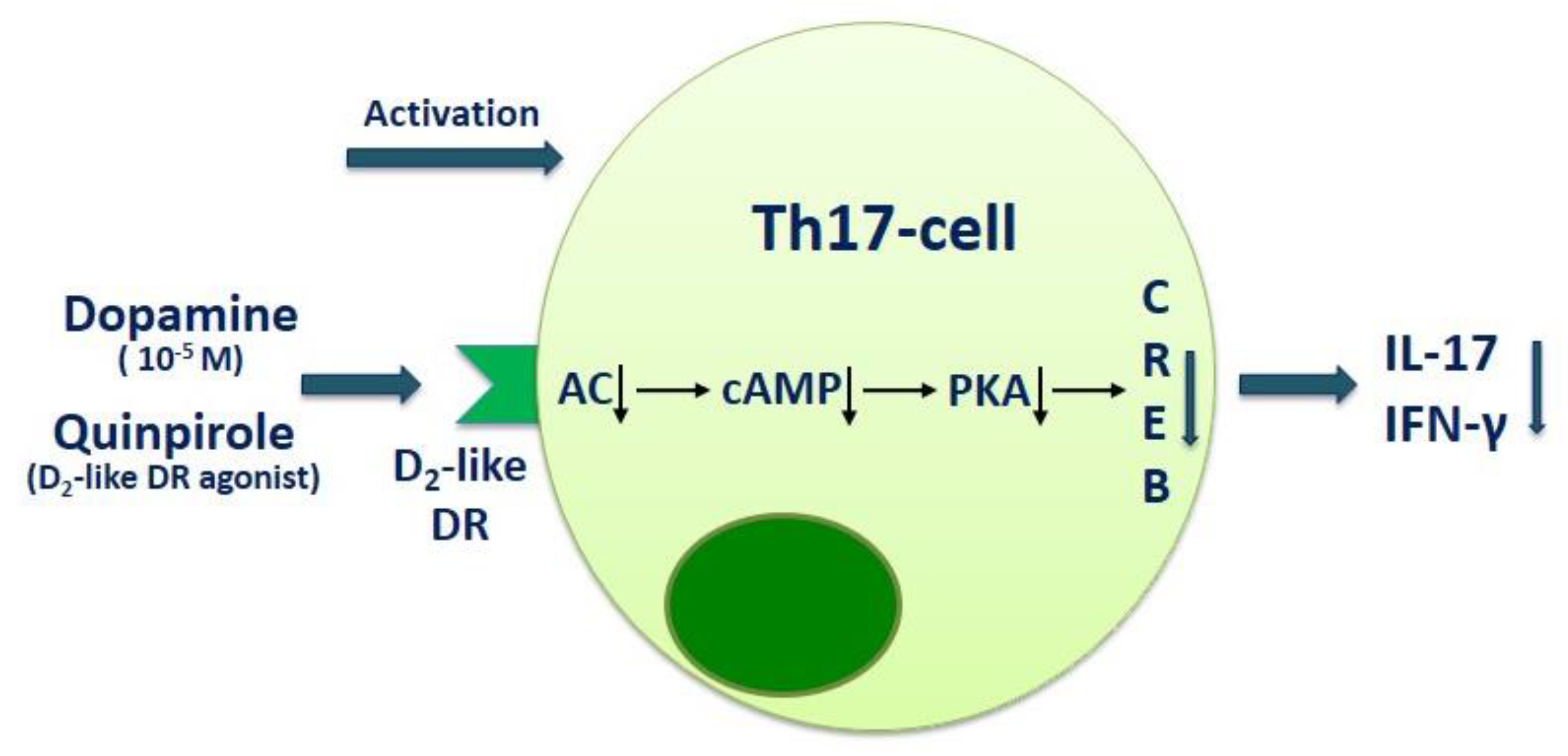
| RRMS | PBMCs | Dopamine (at 10−5 M) suppresses IL-17 and IFN-γ production by anti-CD3/anti-CD28 microbead-activated PBMCs in RRMS patients and healthy subjects. Blockade of D1-like dopaminergic receptor with SCH23390 enhances the inhibitory effect of dopamine on IL-17 production, while blockade of D2-like dopaminergic receptor with sulpiride conversely reduces it. | Melnikov et al., 2016 [26] |
| RRMS | CD4+ T cells | Dopamine (at 10−5 M) suppresses IL-17 and IFN-γ production by anti-CD3/anti-CD28 microbead-activated CD4+ T cells in RRMS patients and healthy subjects. Blockade of D2-like dopaminergic receptor with sulpiride reduces dopamine-mediated IL-17 suppression in MS patients. Blockade of D1-like dopaminergic receptor with SCH23390 reduces IL-17 and GM-CSF production by activated CD4+ T cells in MS patients and in healthy subjects. | Melnikov et al., 2020 [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnikov, M.; Pashenkov, M.; Boyko, A. Dopaminergic Receptor Targeting in Multiple Sclerosis: Is There Therapeutic Potential? Int. J. Mol. Sci. 2021, 22, 5313. https://doi.org/10.3390/ijms22105313
Melnikov M, Pashenkov M, Boyko A. Dopaminergic Receptor Targeting in Multiple Sclerosis: Is There Therapeutic Potential? International Journal of Molecular Sciences. 2021; 22(10):5313. https://doi.org/10.3390/ijms22105313
Chicago/Turabian StyleMelnikov, Mikhail, Mikhail Pashenkov, and Alexey Boyko. 2021. "Dopaminergic Receptor Targeting in Multiple Sclerosis: Is There Therapeutic Potential?" International Journal of Molecular Sciences 22, no. 10: 5313. https://doi.org/10.3390/ijms22105313
APA StyleMelnikov, M., Pashenkov, M., & Boyko, A. (2021). Dopaminergic Receptor Targeting in Multiple Sclerosis: Is There Therapeutic Potential? International Journal of Molecular Sciences, 22(10), 5313. https://doi.org/10.3390/ijms22105313