The Effects of Insulin-Like Growth Factor I and BTP-2 on Acute Lung Injury
Abstract
1. Introduction
2. Results
2.1. Past Work on Serum IGF-I Directly Applicable to the Current Study
2.2. Current Studies on the Lung
2.2.1. Intracellular Signaling Pathways
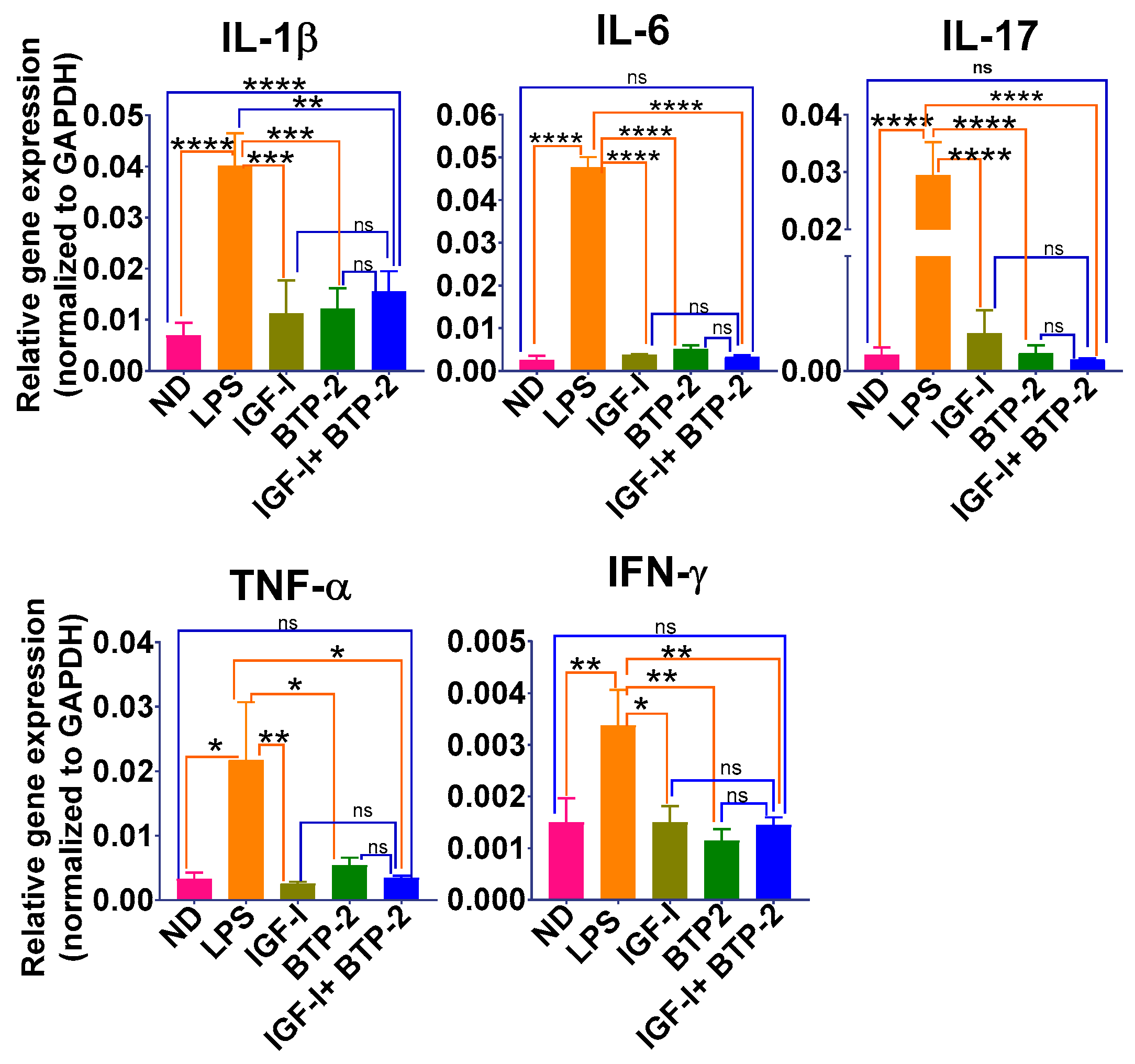
2.2.2. Inflammatory Cytokine Gene Expression in the Lungs
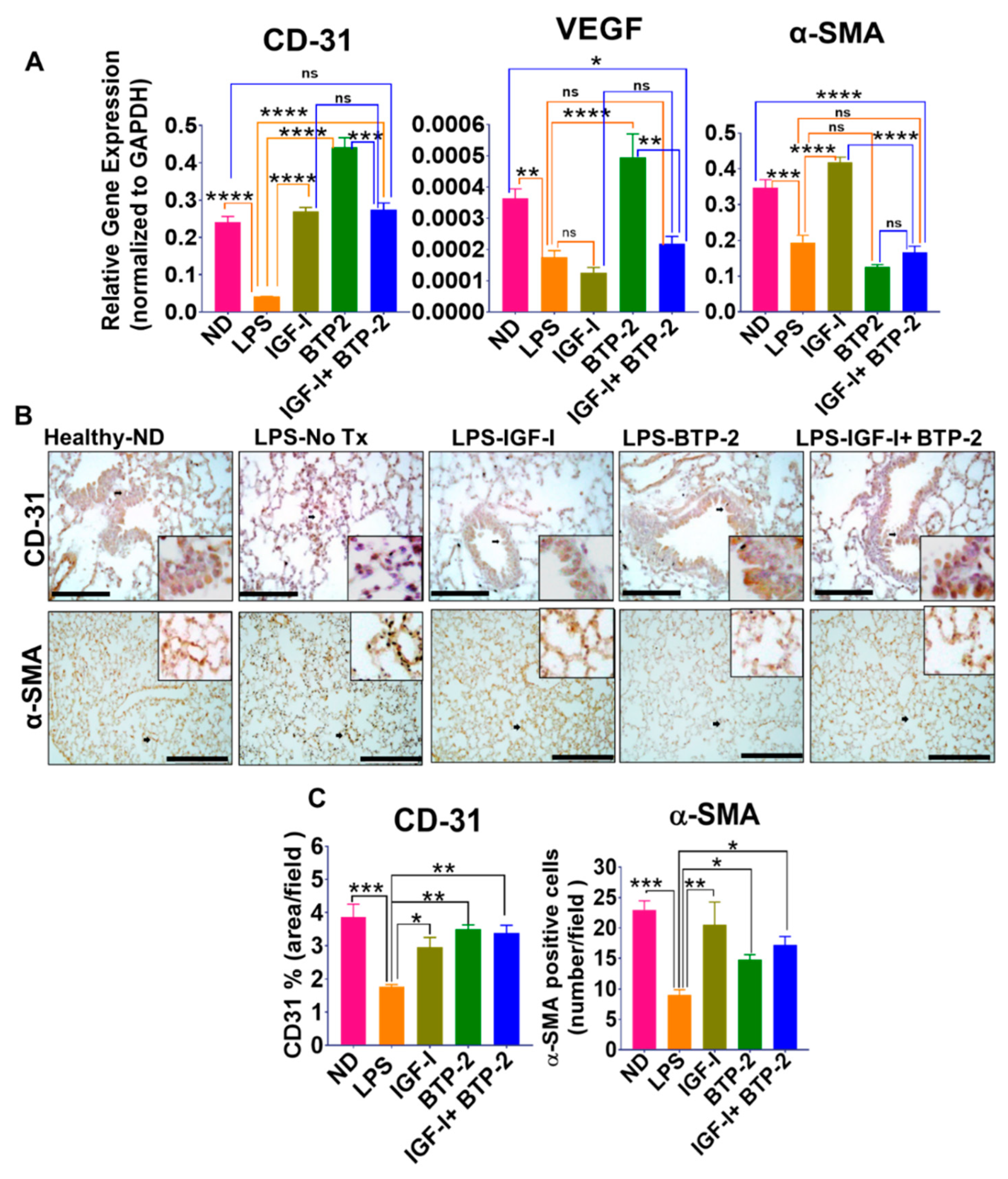
2.2.3. Vascular Integrity
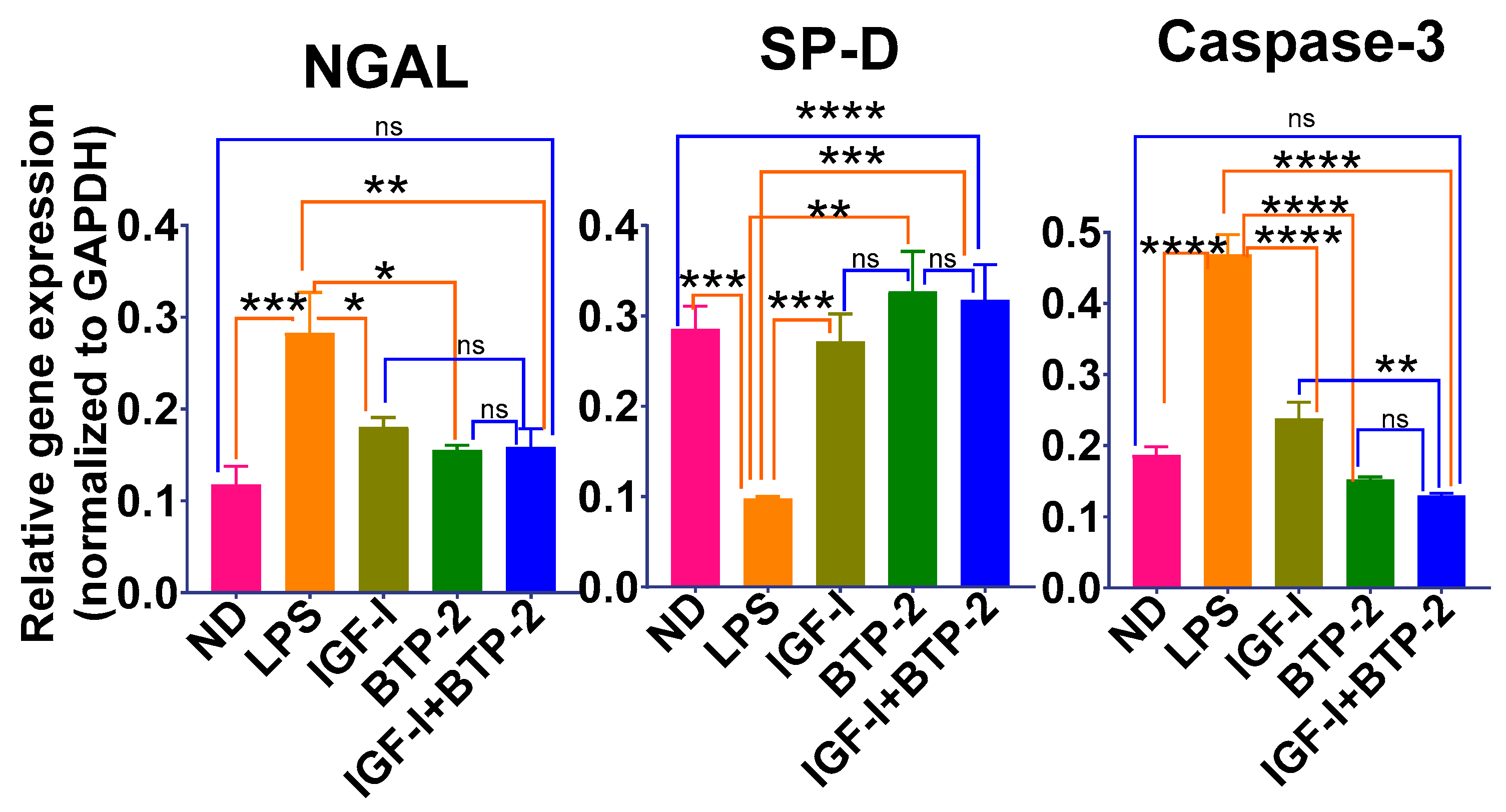
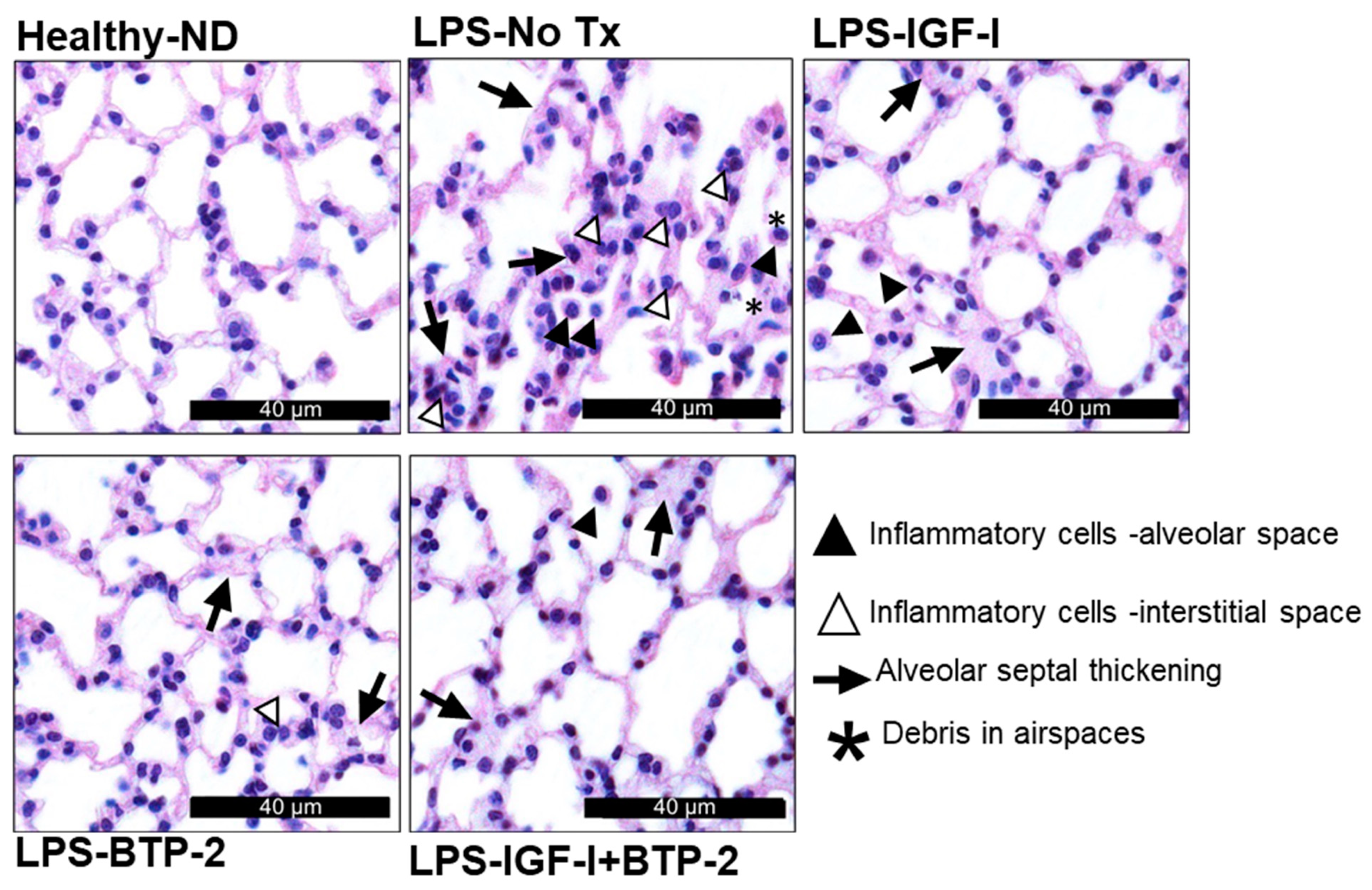
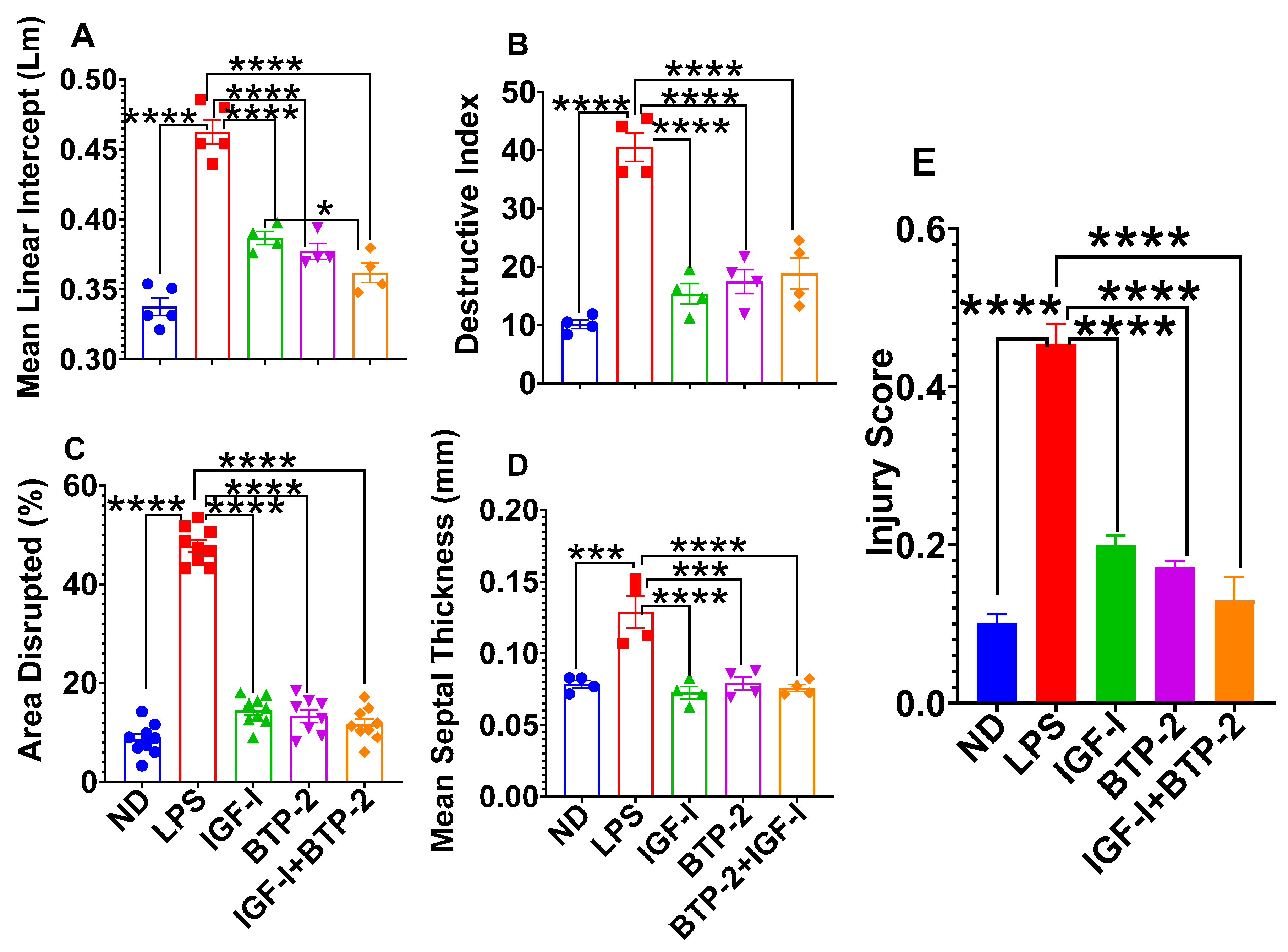
2.3. Lung Tissue Damage and Repair
Quantitative Lung Histology
3. Discussion
3.1. Assessment of Therapy
3.2. Inflammatory Cytokines Gene Expression
3.3. Vascular Integrity
3.4. Lung Injury/Tissue Repair
3.5. Potential Mechanisms for the Favorable Therapeutic Responses
3.6. Potential Clinical Relevance (Lung versus Kidney Responses)
4. Materials and Methods
4.1. Experimental Design
4.2. Animals
4.3. Acute Lung Injury Induced by LPS
4.4. Treatment
4.5. Measurements of mRNA Expression in the Lungs
4.6. Lung Immunocytochemistry
4.7. Lung Histology and Histologic Score
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hadjuk, B.; Pirozynski, M.; Pokojski, W.; Filipecki, S. Acute adult respiratory distress syndrome (ARDS): Progress in studies on its pathogenesis, diagnosis and treatment. Pol. Tyg. Lek. 1988, 43, 416–421. [Google Scholar]
- Johnson, E.R.; Matthay, M.A. Acute lung injury: Epidemiology, pathogenesis, and treatment. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 2063. [Google Scholar] [CrossRef] [PubMed]
- Hooper, W.C.; Mensah, G.A.; Haworth, S.G.; Black, S.M.; Garcia, J.G.N.; Langleben, D. Vascular endothelium summary statement V: Pulmonary hypertension and acute lung injury: Public health implications. Vasc. Pharmacol. 2007, 46, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Cross, L.J.; Matthay, M.A. Biomarkers in acute lung injury: Insights into the pathogenesis of acute lung injury. Crit. Care Clin. 2011, 27, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Grommes, J.; Soehnlein, O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011, 17, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Bai, C.; Wang, X. The value of the lipopolysaccharide-induced acute lung injury model in respiratory medicine. Expert Rev. Respir. Med. 2010, 4, 773–783. [Google Scholar] [CrossRef]
- Seehase, S.; Lauenstein, H.D.; Schlumbohm, C.; Switalla, S.; Neuhaus, V.; Forster, C.; Fieguth, H.G.; Pfennig, O.; Fuchs, E.; Kaup, F.J.; et al. LPS-induced lung inflammation in marmoset monkeys—An acute model for anti-inflammatory drug testing. PLoS ONE 2012, 7, e43709. [Google Scholar] [CrossRef]
- Li, C.H.; Tang, X.; Wasnik, S.; Wang, X.; Zhang, J.; Xu, Y.; Lau, K.W.; Nguyen, H.B.; Baylink, D.J. Mechanistic study of the cause of decreased blood 1,25-Dihydroxyvitamin D in sepsis. BMC Infect. Dis. 2019, 19, 1020. [Google Scholar] [CrossRef]
- Wang, Z.; Li, W.; Guo, Q.; Wang, Y.; Ma, L.; Zhang, X. Insulin-Like Growth Factor-1 Signaling in Lung Development and Inflammatory Lung Diseases. Biomed. Res. Int. 2018, 2018, 6057589. [Google Scholar] [CrossRef]
- Poirier, C.; Gorshkov, B.A.; Zemskova, M.A.; Bogatcheva, N.V.; Verin, A.D. TIMAP protects endothelial barrier from LPS-induced vascular leakage and is down-regulated by LPS. Respir. Physiol. Neurobiol. 2011, 179, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Conti, E.; Carrozza, C.; Capoluongo, E.; Volpe, M.; Crea, F.; Zuppi, C.; Andreotti, F. Insulin-like growth factor-1 as a vascular protective factor. Circulation 2004, 110, 2260–2265. [Google Scholar] [CrossRef]
- Wasnik, S.; Tang, X.; Bi, H.; Abdipour, A.; Carreon, E.E.; Sutjiadi, B.; Lyu, J.; Zhang, J.; Wilson, S.; Baylink, D.J. IGF-1 Deficiency Rescue and Intracellular Calcium Blockade Improves Survival and Corresponding Mechanisms in a Mouse Model of Acute Kidney Injury. Int. J. Mol. Sci. 2020, 21, 4095. [Google Scholar] [CrossRef]
- Bianda, T.; Hussain, M.A.; Glatz, Y.; Bouillon, R.; Froesch, E.R.; Schmid, C. Effects of short-term insulin-like growth factor-I or growth hormone treatment on bone turnover, renal phosphate reabsorption and 1,25 dihydroxyvitamin D3 production in healthy man. J. Intern. Med. 1997, 241, 143–150. [Google Scholar] [CrossRef]
- Novosyadlyy, R.; Kurshan, N.; Lann, D.; Vijayakumar, A.; Yakar, S.; LeRoith, D. Insulin-like growth factor-I protects cells from ER stress-induced apoptosis via enhancement of the adaptive capacity of endoplasmic reticulum. Cell Death Differ. 2008, 15, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, K.; Bezavada, L.; Escue, R.B.; Parthasarathi, K. Lipopolysaccharide induces endoplasmic store Ca2+-dependent inflammatory responses in lung microvessels. PLoS ONE 2013, 8, e63465. [Google Scholar] [CrossRef]
- Humbel, R.E. Insulin-Like Growth Factor-I and Factor-Ii. Eur. J. Biochem. 1990, 190, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Ohga, K.; Takezawa, R.; Yoshino, T.; Yamada, T.; Shimizu, Y.; Ishikawa, J. The suppressive effects of YM-58483/BTP-2, a store-operated Ca2+ entry blocker, on inflammatory mediator release in vitro and airway responses in vivo. Pulm. Pharmacol. Ther. 2008, 21, 360–369. [Google Scholar] [CrossRef]
- Ohga, K.; Takezawa, R.; Arakida, Y.; Shimizu, Y.; Ishikawa, J. Characterization of YM-58483/BTP2, a novel store-operated Ca2+ entry blocker, on T cell-mediated immune responses in vivo. Int. Immunopharmacol. 2008, 8, 1787–1792. [Google Scholar] [CrossRef]
- Smyth, J.T.; Hwang, S.Y.; Tomita, T.; DeHaven, W.I.; Mercer, J.C.; Putney, J.W. Activation and regulation of store-operated calcium entry. J. Cell Mol. Med. 2010, 14, 2337–2349. [Google Scholar] [CrossRef]
- Saul, S.; Gibhardt, C.S.; Schmidt, B.; Lis, A.; Pasieka, B.; Conrad, D.; Jung, P.; Gaupp, R.; Wonnenberg, B.; Diler, E.; et al. A calcium-redox feedback loop controls human monocyte immune responses: The role of ORAI Ca2+ channels. Sci. Signal. 2016, 9, ra26. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.Y.; Veckman, V.; Limmer, K.; David, M. Phospholipase Cgamma-2 and intracellular calcium are required for lipopolysaccharide-induced Toll-like receptor 4 (TLR4) endocytosis and interferon regulatory factor 3 (IRF3) activation. J. Biol. Chem. 2012, 287, 3704–3709. [Google Scholar] [CrossRef]
- Rahman, S.; Rahman, T. Unveiling some FDA-approved drugs as inhibitors of the store-operated Ca2+ entry pathway. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Zhang, W.; Qi, Z.; Wang, Y. BTP2, a Store-Operated Calcium Channel Inhibitor, Attenuates Lung Ischemia-Reperfusion Injury in Rats. Inflammation 2017, 40, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Guler, H.P.; Zapf, J.; Schmid, C.; Froesch, E.R. Insulin-like growth factors I and II in healthy man. Estimations of half-lives and production rates. Acta. Endocrinol. 1989, 121, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Baylink, D.J.; Mohan, S. Insulin-like growth factor-binding proteins in serum and other biological fluids: Regulation and functions. Endocr. Rev. 1997, 18, 801–831. [Google Scholar] [CrossRef]
- Allard, J.B.; Duan, C.M. IGF-Binding Proteins: Why Do They Exist and Why Are There So Many? Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef]
- Woodall, S.M.; Breier, B.H.; O’Sullivan, U.; Gluckman, P.D. The effect of the frequency of subcutaneous insulin-like growth factor-1 administration on weight gain in growth hormone deficient mice. Horm. Metab. Res. 1991, 23, 581–584. [Google Scholar] [CrossRef]
- Wang, P.; Han, X.; Mo, B.; Huang, G.; Wang, C. LPS enhances TLR4 expression and IFNgamma production via the TLR4/IRAK/NFkappaB signaling pathway in rat pulmonary arterial smooth muscle cells. Mol. Med. Rep. 2017, 16, 3111–3116. [Google Scholar] [CrossRef]
- Guzman, R.; Valente, E.G.; Pretorius, J.; Pacheco, E.; Qi, M.Y.; Bennett, B.D.; Fong, D.H.; Lin, F.F.; Bi, V.; McBride, H.J. Expression of ORAI1, a Plasma Membrane Resident Subunit of the CRAC Channel, in Rodent and Non-rodent Species. J. Histochem. Cytochem. 2014, 62, 864–878. [Google Scholar] [CrossRef] [PubMed]
- He, L.P.; Hewavitharana, T.; Soboloff, J.; Spassova, M.A.; Gill, D.L. A functional link between store-operated and TRPC channels revealed by the 3,5-bis(trifluoromethyl)pyrazole derivative, BTP2. J. Biol. Chem. 2005, 280, 10997–11006. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Erxleben, C.; Abramowitz, J.; Flockerzi, V.; Zhu, M.X.; Armstrong, D.L.; Birnbaumer, L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc. Natl. Acad. Sci. USA 2008, 105, 2895–2900. [Google Scholar] [CrossRef]
- Wu, Y.L.; Xie, J.; An, S.W.; Oliver, N.; Barrezueta, N.X.; Lin, M.H.; Birnbaumer, L.; Huang, C.L. Inhibition of TRPC6 channels ameliorates renal fibrosis and contributes to renal protection by soluble klotho. Kidney Int. 2017, 91, 830–841. [Google Scholar] [CrossRef]
- Kuper, C.; Beck, F.X.; Neuhofer, W. Toll-like receptor 4 activates NF-kappaB and MAP kinase pathways to regulate expression of proinflammatory COX-2 in renal medullary collecting duct cells. Am. J. Physiol. Renal. Physiol. 2012, 302, F38–F46. [Google Scholar] [CrossRef]
- Delcroix, V.; Mauduit, O.; Tessier, N.; Montillaud, A.; Lesluyes, T.; Ducret, T.; Chibon, F.; Van Coppenolle, F.; Ducreux, S.; Vacher, P. The Role of the Anti-Aging Protein Klotho in IGF-1 Signaling and Reticular Calcium Leak: Impact on the Chemosensitivity of Dedifferentiated Liposarcomas. Cancers 2018, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1 beta secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.L.; Xu, S.X.; Zhou, H.; Liu, H.Z.; Jiang, W.; Hao, J.; Hu, Z.M. IL-1 beta induces apoptosis and autophagy via mitochondria pathway in human degenerative nucleus pulposus cells. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Song, X.; He, X.; Li, X.; Qian, Y. The roles and functional mechanisms of interleukin-17 family cytokines in mucosal immunity. Cell Mol. Immunol. 2016, 13, 418–431. [Google Scholar] [CrossRef]
- Lee, W.H.; Seo, D.; Lim, S.G.; Suk, K. Reverse Signaling of Tumor Necrosis Factor Superfamily Proteins in Macrophages and Microglia: Superfamily Portrait in the Neuroimmune Interface. Front. Immunol. 2019, 10, 262. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-alpha signaling in macrophages. Crit. Rev. Eukaryot. Gene Express 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Denson, L.A.; Menon, R.K.; Shaufl, A.; Bajwa, H.S.; Williams, C.R.; Karpen, S.J. TNF-alpha downregulates murine hepatic growth hormone receptor expression by inhibiting Sp1 and Sp3 binding. J. Clin. Investig 2001, 107, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. bioRxiv 2020, 184, 149–168.e17. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.O. Vascular endothelial growth factors and vascular permeability. Cardiovasc. Res. 2010, 87, 262–271. [Google Scholar] [CrossRef]
- Bates, D.O.; Harper, S.J. Regulation of vascular permeability by vascular endothelial growth factors. Vascul. Pharmacol. 2002, 39, 225–237. [Google Scholar] [CrossRef]
- Skalli, O.; Pelte, M.F.; Peclet, M.C.; Gabbiani, G.; Gugliotta, P.; Bussolati, G.; Ravazzola, M.; Orci, L. Alpha-smooth muscle actin, a differentiation marker of smooth muscle cells, is present in microfilamentous bundles of pericytes. J. Histochem. Cytochem. 1989, 37, 315–321. [Google Scholar] [CrossRef]
- Alarcon-Martinez, L.; Yilmaz-Ozcan, S.; Yemisci, M.; Schallek, J.; Kilic, K.; Can, A.; Di Polo, A.; Dalkara, T. Capillary pericytes express alpha-smooth muscle actin, which requires prevention of filamentous-actin depolymerization for detection. Elife 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Song, S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol 2005, 7, 452–464. [Google Scholar] [CrossRef]
- Toyonaga, T.; Matsuura, M.; Mori, K.; Honzawa, Y.; Minami, N.; Yamada, S.; Kobayashi, T.; Hibi, T.; Nakase, H. Lipocalin 2 prevents intestinal inflammation by enhancing phagocytic bacterial clearance in macrophages. Sci. Rep. 2016, 6, 35014. [Google Scholar] [CrossRef]
- Pan, T.L.; Nielsen, L.D.; Allen, M.J.; Shannon, K.M.; Shannon, J.M.; Selman, M.; Mason, R.J. Serum SP-D is a marker of lung injury in rats. Am. J. Physiol-Lung C 2002, 282, L824–L832. [Google Scholar] [CrossRef]
- Haimovitz-Friedman, A.; Cordon-Cardo, C.; Bayoumy, S.; Garzotto, M.; McLoughlin, M.; Gallily, R.; Edwards, C.K., 3rd; Schuchman, E.H.; Fuks, Z.; Kolesnick, R. Lipopolysaccharide induces disseminated endothelial apoptosis requiring ceramide generation. J. Exp. Med. 1997, 186, 1831–1841. [Google Scholar] [CrossRef]
- Kawasaki, M.; Kuwano, K.; Hagimoto, N.; Matsuba, T.; Kunitake, R.; Tanaka, T.; Maeyama, T.; Hara, N. Protection from lethal apoptosis in lipopolysaccharide-induced acute lung injury in mice by a caspase inhibitor. Am. J. Pathol. 2000, 157, 597–603. [Google Scholar] [CrossRef]
- Munshi, N.; Fernandis, A.Z.; Cherla, R.P.; Park, I.W.; Ganju, R.K. Lipopolysaccharide-induced apoptosis of endothelial cells and its inhibition by vascular endothelial growth factor. J. Immunol. 2002, 168, 5860–5866. [Google Scholar] [CrossRef]
- Didiasova, M.; Zakrzewicz, D.; Magdolen, V.; Nagaraj, C.; Balint, Z.; Rohde, M.; Preissner, K.T.; Wygrecka, M. STIM1/ORAI1-mediated Ca2+ Influx Regulates Enolase-1 Exteriorization. J. Biol. Chem. 2015, 290, 11983–11999. [Google Scholar] [CrossRef]
- Penna, A.; Demuro, A.; Yeromin, A.V.; Zhang, S.L.; Safrina, O.; Parker, I.; Cahalan, M.D. The CRAC channel consists of a tetramer formed by Stim-induced dimerization of Orai dimers. Nature 2008, 456, 116–120. [Google Scholar] [CrossRef]
- Zeng, H.; He, X.C.; Tuo, Q.H.; Liao, D.F.; Zhang, G.Q.; Chen, J.X. LPS causes pericyte loss and microvascular dysfunction via disruption of Sirt3/angiopoietins/Tie-2 and HIF-2 alpha/Notch3 pathways. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Sogkas, G.; Rau, E.; Atschekzei, F.; Syed, S.N.; Schmidt, R.E. The Pyrazole Derivative BTP2 Attenuates IgG Immune Complex-induced Inflammation. Inflammation 2018, 41, 42–49. [Google Scholar] [CrossRef]
- Tauseef, M.; Knezevic, N.; Chava, K.R.; Smith, M.; Sukriti, S.; Gianaris, N.; Obukhov, A.G.; Vogel, S.M.; Schraufnagel, D.E.; Dietrich, A.; et al. TLR4 activation of TRPC6-dependent calcium signaling mediates endotoxin-induced lung vascular permeability and inflammation. J. Exp. Med. 2012, 209, 1953–1968. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.F.; Chen, Q.Z.; Yang, C.T.; Fu, Z.D.; Zhao, S.T.; Chen, Y.; Li, S.N.; Liao, L.; Zhou, Y.B.; Huang, J.R.; et al. TRPC6 contributes to LPS-induced inflammation through ERK1/2 and p38 pathways in bronchial epithelial cells. Am. J. Physiol. Physiol. 2018, 314, C278–C288. [Google Scholar] [CrossRef] [PubMed]
- Valdes, J.A.; Flores, S.; Fuentes, E.N.; Osorio-Fuentealba, C.; Jaimovich, E.; Molina, A. IGF-1 induces IP3 -dependent calcium signal involved in the regulation of myostatin gene expression mediated by NFAT during myoblast differentiation. J. Cell Physiol. 2013, 228, 1452–1463. [Google Scholar] [CrossRef]
- Lai, D.; Wan, M.; Wu, J.; Preston-Hurlburt, P.; Kushwaha, R.; Grundstrom, T.; Imbalzano, A.N.; Chi, T. Induction of TLR4-target genes entails calcium/calmodulin-dependent regulation of chromatin remodeling. Proc. Natl. Acad. Sci. USA 2009, 106, 1169–1174. [Google Scholar] [CrossRef]
- Li, S.; Ma, F.; Yokota, T.; Garcia, G., Jr.; Palermo, A.; Wang, Y.; Farrell, C.; Wang, Y.C.; Wu, R.; Zhou, Z.; et al. Metabolic reprogramming and epigenetic changes of vital organs in SARS-CoV-2 induced systemic toxicity. JCI Insight 2020. [Google Scholar] [CrossRef]
- Kothapalli, C.R.; Ramamurthi, A. Benefits of concurrent delivery of hyaluronan and IGF-1 cues to regeneration of crosslinked elastin matrices by adult rat vascular cells. J. Tissue Eng. Regen. Med. 2008, 2, 106–116. [Google Scholar] [CrossRef]
- Imrie, H.; Viswambharan, H.; Sukumar, P.; Abbas, A.; Cubbon, R.M.; Yuldasheva, N.; Gage, M.; Smith, J.; Galloway, S.; Skromna, A.; et al. Novel role of the IGF-1 receptor in endothelial function and repair: Studies in endothelium-targeted IGF-1 receptor transgenic mice. Diabetes 2012, 61, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yin, C.; Wang, J.; Yang, M.; Ma, H.; Jin, G.; Song, M.; Hu, Z.; Shen, H.; Hang, D. Pre-diagnostic circulating concentrations of insulin-like growth factor-1 and risk of COVID-19 mortality: Results from UK Biobank. Eur. J. Epidemiol. 2021. [Google Scholar] [CrossRef]
- Trevillyan, J.M.; Chiou, X.G.; Chen, Y.W.; Ballaron, S.J.; Sheets, M.P.; Smith, M.L.; Wiedeman, P.E.; Warrior, U.; Wilkins, J.; Gubbins, E.J.; et al. Potent inhibition of NFAT activation and T cell cytokine production by novel low molecular weight pyrazole compounds. J. Biol. Chem. 2001, 276, 48118–48126. [Google Scholar] [CrossRef] [PubMed]
- Fintini, D.; Brufani, C.; Cappa, M. Profile of mecasermin for the long-term treatment of growth failure in children and adolescents with severe primary IGF-1 deficiency. Ther. Clin. Risk Manag. 2009, 5, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Aeffner, F.; Bolon, B.; Davis, I.C. Mouse Models of Acute Respiratory Distress Syndrome: A Review of Analytical Approaches, Pathologic Features, and Common Measurements. Toxicol. Pathol. 2015, 43, 1074–1092. [Google Scholar] [CrossRef]
- Bailey-Downs, L.C.; Mitschelen, M.; Sosnowska, D.; Toth, P.; Pinto, J.T.; Ballabh, P.; Valcarcel-Ares, M.N.; Farley, J.; Koller, A.; Henthorn, J.C.; et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: A novel model of vascular aging. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 313–329. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munoz, K.; Wasnik, S.; Abdipour, A.; Bi, H.; Wilson, S.M.; Tang, X.; Ghahramanpouri, M.; Baylink, D.J. The Effects of Insulin-Like Growth Factor I and BTP-2 on Acute Lung Injury. Int. J. Mol. Sci. 2021, 22, 5244. https://doi.org/10.3390/ijms22105244
Munoz K, Wasnik S, Abdipour A, Bi H, Wilson SM, Tang X, Ghahramanpouri M, Baylink DJ. The Effects of Insulin-Like Growth Factor I and BTP-2 on Acute Lung Injury. International Journal of Molecular Sciences. 2021; 22(10):5244. https://doi.org/10.3390/ijms22105244
Chicago/Turabian StyleMunoz, Kevin, Samiksha Wasnik, Amir Abdipour, Hongzheng Bi, Sean M. Wilson, Xiaolei Tang, Mahdis Ghahramanpouri, and David J. Baylink. 2021. "The Effects of Insulin-Like Growth Factor I and BTP-2 on Acute Lung Injury" International Journal of Molecular Sciences 22, no. 10: 5244. https://doi.org/10.3390/ijms22105244
APA StyleMunoz, K., Wasnik, S., Abdipour, A., Bi, H., Wilson, S. M., Tang, X., Ghahramanpouri, M., & Baylink, D. J. (2021). The Effects of Insulin-Like Growth Factor I and BTP-2 on Acute Lung Injury. International Journal of Molecular Sciences, 22(10), 5244. https://doi.org/10.3390/ijms22105244






