Exposure to 2.45 GHz Radiation Triggers Changes in HSP-70, Glucocorticoid Receptors and GFAP Biomarkers in Rat Brain
Abstract
1. Introduction
2. Results
2.1. Heat Shock Protein (HSP) 70 Expression
2.1.1. HSP-70 in the Somatosensory Cortex
2.1.2. HSP-70 in the Limbic Cortex
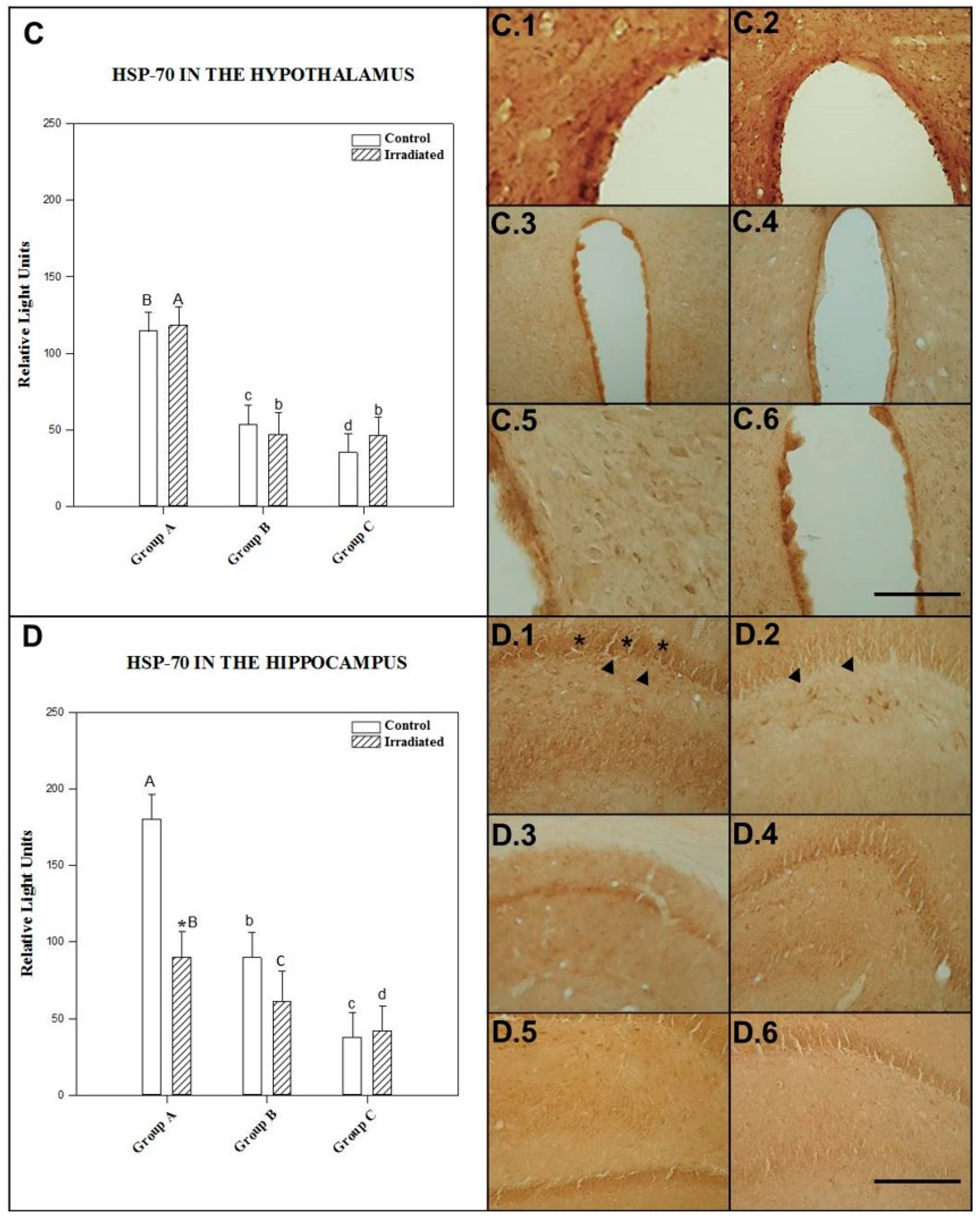
2.1.3. HSP-70 in the Hypothalamus
2.1.4. HSP-70 in the Hippocampus
2.2. Glucocorticoid Receptor (GCR) Expression
2.2.1. GCR in the Somatosensory Cortex
2.2.2. GCR in the Limbic Cortex
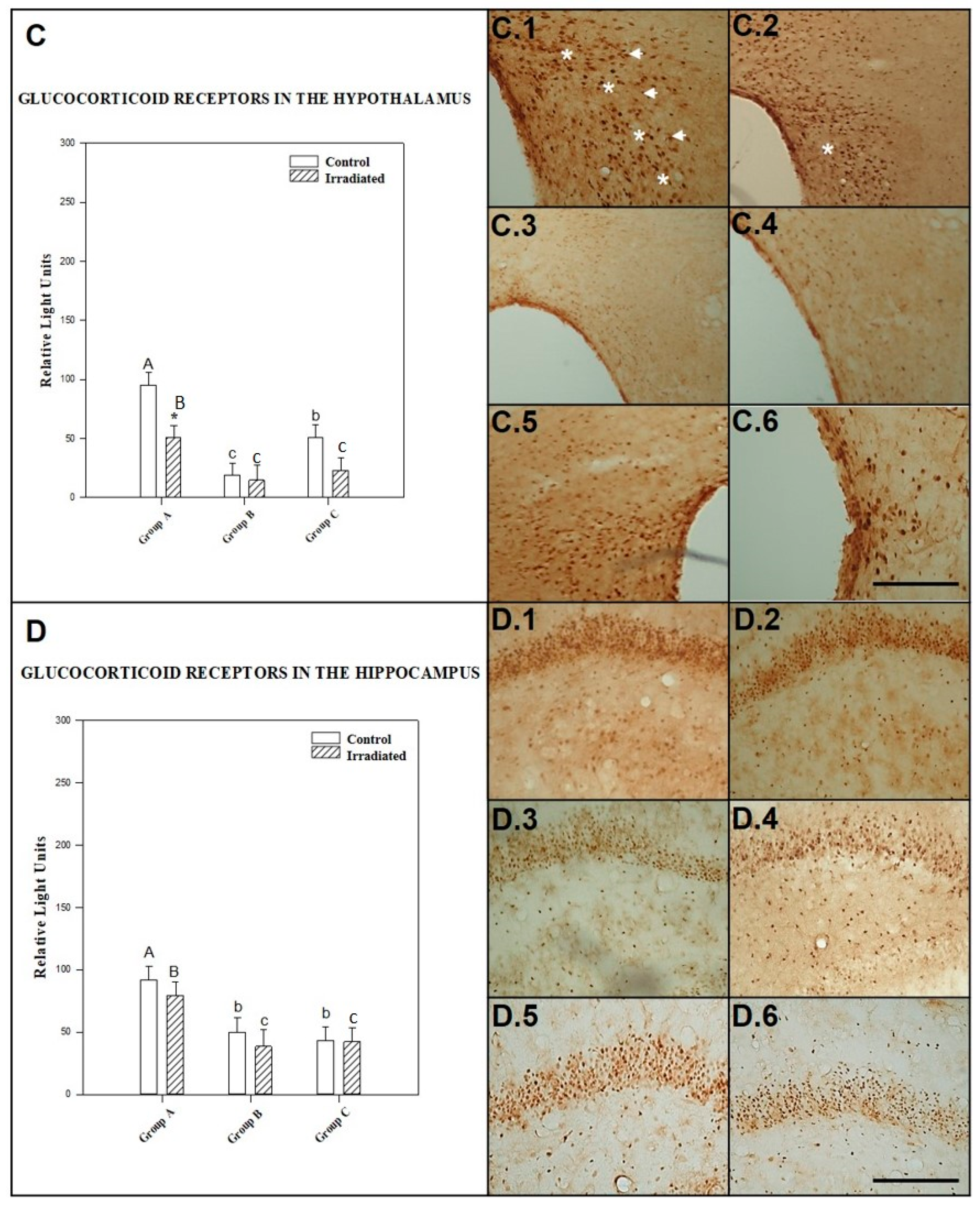
2.2.3. GCR in the Hypothalamus
2.2.4. GCR in the Hippocampus
2.3. Glial Fibrillary Acidic Protein (GFAP) Expression
2.3.1. GFAP in the Somatosensory Cortex
2.3.2. GFAP in the Limbic Cortex
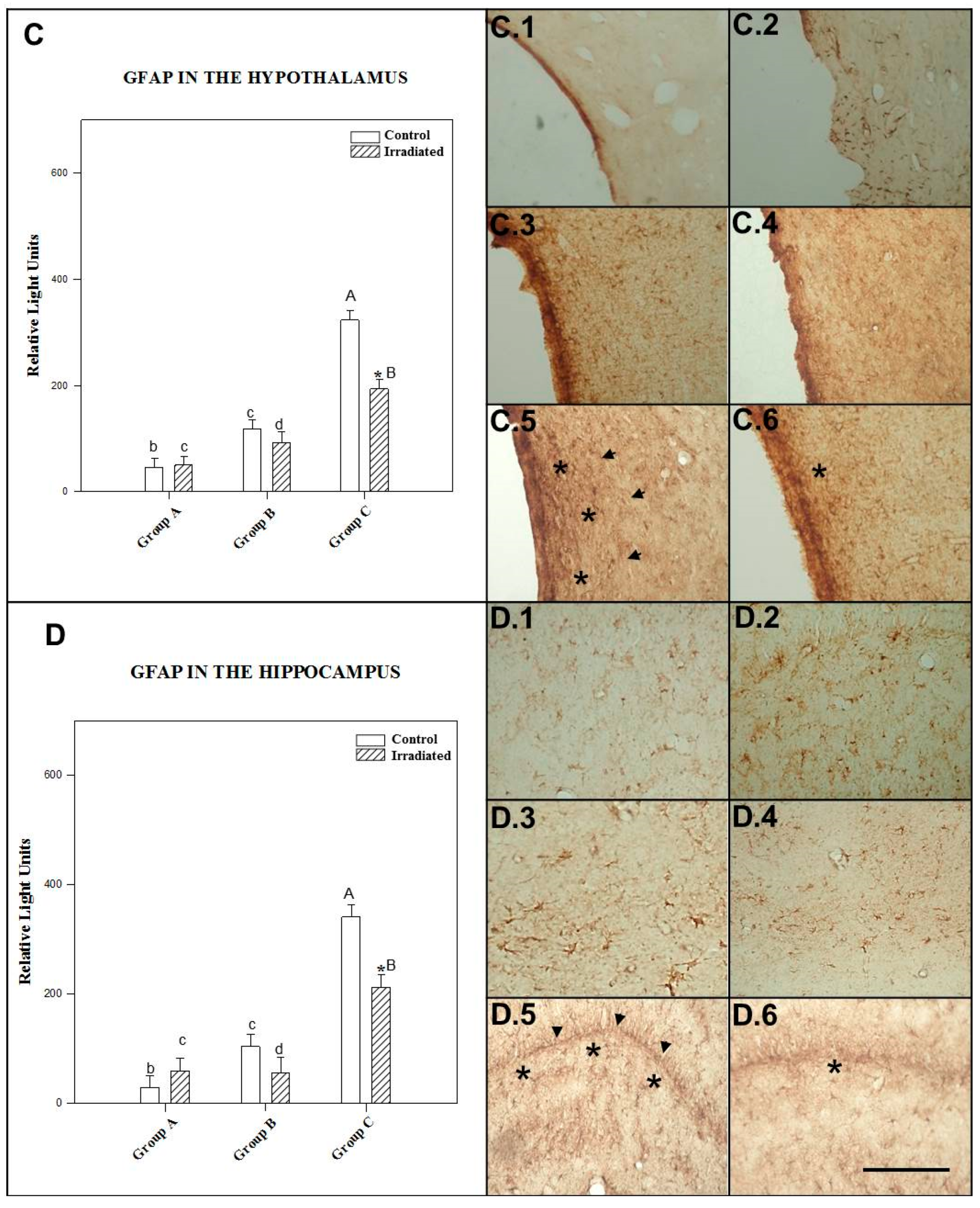
2.3.3. GFAP in the Hypothalamus
2.3.4. GFAP in the Hippocampus
3. Discussion
3.1. Influence and Temporal Evolution of 2.45 GHz Radiation on HSP-70 and GCR in Brain Tissues
3.2. The Influence and Temporal Evolution of 2.45 GHz Radiation on GFAP with the Decrease of GCR/HSP-70
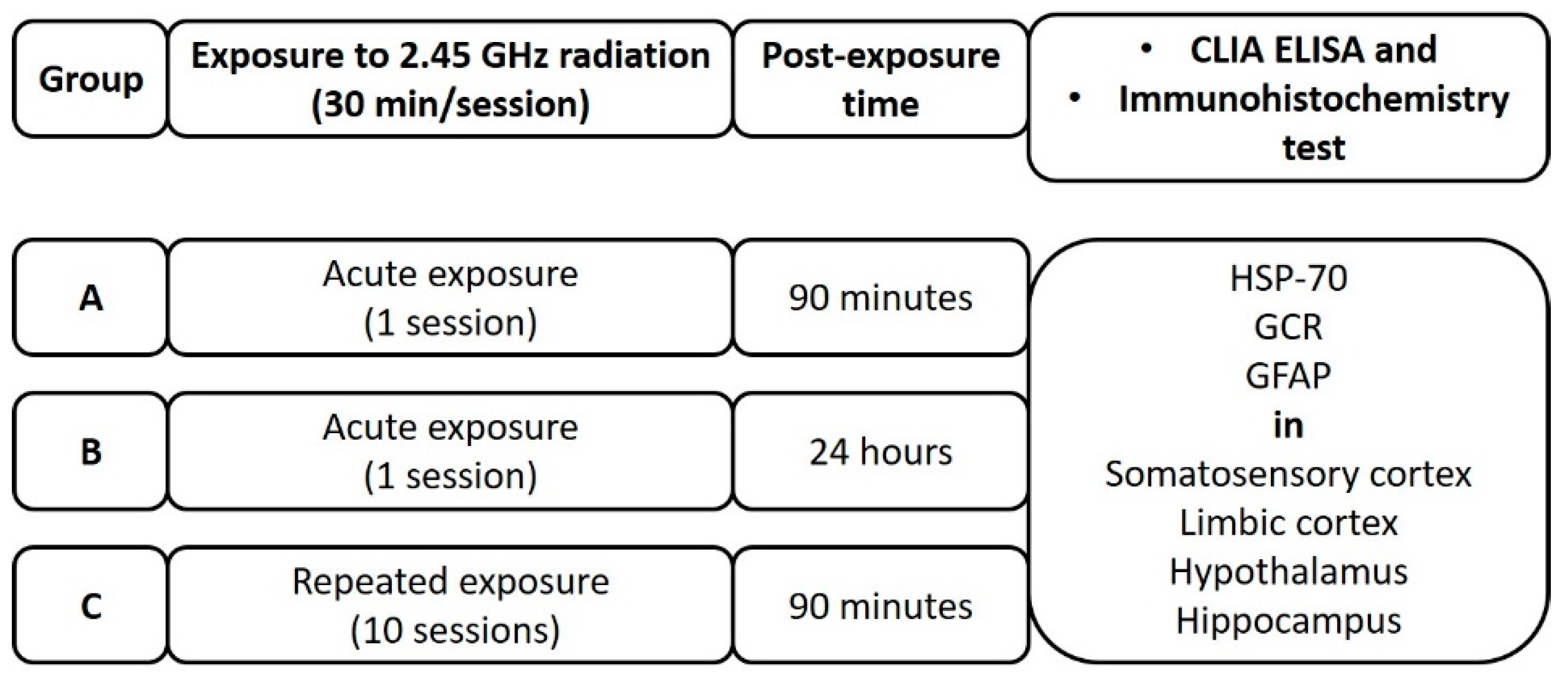
4. Material and Methods
4.1. Experimental Radiation System Protocol
4.2. Experimental Design
4.2.1. Tissue Extraction and Preparation of Cell Extracts for the ELISA Technique
Determination of Protein Concentration
Chemiluminescent Enzyme-Linked Immunosorbent Assay (CLIA ELISA)
4.2.2. Morphological Studies with Immunohistochemistry
Perfusion and Tissue Processing
Immunohistochemistry
4.2.3. Quantification and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arendash, G.W.; Mori, T.; Dorsey, M.; Gonzalez, R.; Tajiri, N.; Borlongan, C. Electromagnetic treatment to old Alzheimer’s mice reverses β-amyloid deposition, modifies cerebral blood flow, and provides selected cognitive benefit. PLoS ONE 2012, 7, e35751. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, H.; Teymouri, M.; Khademi, S. Radiofrequency radiation may help astronauts in space missions. J. Med. Hypotheses Ideas 2012, 6, 66–69. [Google Scholar] [CrossRef]
- Michaloliakos, A.; Rogalin, R.; Zhang, Y.; Psounis, K.; Caire, G. Performance modeling of next-generation WiFi networks. Comput. Netw. 2016, 105, 150–165. [Google Scholar] [CrossRef]
- Megha, K.; Deshmukh, P.S.; Banerjee, B.D.; Tripathi, A.K.; Ahmed, R.; Abegaonkar, M.P. Low intensity microwave radiation induced oxidative stress, inflammatory response and DNA damage in rat brain. Neurotoxicology 2015, 51, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, P.S.; Megha, K.; Nasare, N.; Banerjee, B.D.; Ahmed, R.S.; Abegaonkar, M.P.; Mediratta, P.K. Effect of Low Level Subchronic Microwave Radiation on Rat Brain. Biomed. Environ. Sci. 2016, 29, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Çelik, Ö.; Kahya, M.C.; Nazıroğlu, M. Oxidative stress of brain and liver is increased by Wi-Fi (2.45 GHz) exposure of rats during pregnancy and the development of newborns. J. Chem. Neuroanat. 2016, 75, 134–139. [Google Scholar] [CrossRef]
- Ritossa, F. A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia 1962, 18, 571–573. [Google Scholar] [CrossRef]
- Maroni, P.; Bendinelli, P.; Tiberio, L.; Rovetta, F.; Piccoletti, R.; Schiaffonati, L. In vivo heat-shock response in the brain: Signalling pathway and transcription factor activation. Brain Res. Mol. Brain Res. 2003, 119, 90–99. [Google Scholar] [CrossRef]
- Sun, X.C.; Xian, X.H.; Li, W.B.; Li, L.; Yan, C.Z.; Li, Q.J.; Zhang, M. Activation of p38 MAPK participates in brain ischemic tolerance induced by limb ischemic preconditioning by up-regulating HSP 70. Exp. Neurol. 2010, 224, 347–355. [Google Scholar] [CrossRef]
- Achanta, P.; Thompson, K.J.; Fuss, M.; Martinez, J.L. Gene expression changes in the rodent hippocampus following whole brain irradiation. Neurosci. Lett. 2007, 418, 143–148. [Google Scholar] [CrossRef]
- Ohtsuka, K.; Suzuki, T. Roles of molecular chaperones in the nervous system. Brain Res. Bull. 2000, 53, 141–146. [Google Scholar] [CrossRef]
- Musch, M.W.; Petrof, E.O.; Kojima, K.; Ren, H.; McKay, D.M.; Chang, E.B. Bacterial superantigen-treated intestinal epithelial cells upregulate heat shock proteins 25 and 72 and are resistant to oxidant cytotoxicity. Infect. Immun. 2004, 72, 3187–3194. [Google Scholar] [CrossRef][Green Version]
- Sinn, D.I.; Chu, K.; Lee, S.T.; Song, E.C.; Jung, K.H.; Kim, E.H.; Roh, J. Pharmacological induction of heat shock protein exerts neuroprotective effects in experimental intracerebral hemorrhage. Brain Res. 2007, 1135, 167–176. [Google Scholar] [CrossRef]
- Kim, N.; Kim, J.Y.; Yenari, M.A. Pharmacological induction of the 70-kDa heat shock protein protects against brain injury. Neuroscience 2015, 284, 912–919. [Google Scholar] [CrossRef]
- Lee, S.; Johnson, D.; Dunbar, K.; Dong, H.; Ge, X.; Kim, Y.C.; Gerber, H.L. 2.45 GHz radiofrequency fields alter gene expression in cultured human cells. FEBS Lett. 2005, 579, 4829–4836. [Google Scholar] [CrossRef]
- Cotgreave, I.A. Biological stress responses to radio frequency electromagnetic radiation: Are mobile phones really so (heat) shocking? Arch. Biochem. Biophys. 2005, 435, 227–240. [Google Scholar] [CrossRef]
- WHO International EMF Project. WHO Research Agenda for Radio Frequency Fields. Geneva Switzerland: WHO International EMF Project. 2006. Available online: https://www.who.int/peh-emf/research/rf_research_agenda_2006.pdf?ua=1 (accessed on 28 December 2020).
- Yang, X.S.; He, G.L.; Hao, Y.T.; Xiao, Y.; Chen, C.H.; Zhang, G.B.; Yu, Z.P. Exposure to 2.45 GHz electromagnetic fields elicits an HSP-related stress response in rat hippocampus. Brain Res. Bull. 2012, 88, 371–378. [Google Scholar] [CrossRef]
- Wang, S.C.M.; Myers, S.; Dooms, C.; Capon, R.; Muscat, G.E. An ERRβ/γ agonist modulates GRα expression, and glucocorticoid responsive gene expression in skeletal muscle cells. Mol. Cell. Endocrinol. 2010, 315, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Oakley, R.H.; Cidlowski, J.A. Cellular processing of the glucocorticoid receptor gene and protein: New mechanisms for generating tissue-specific actions of glucocorticoids. J. Biol. Chem. 2011, 286, 3177–3184. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Van Heerikhuize, J.; Aronica, E.; Kawata, M.; Seress, L.; Joels, M.; Lucassen, P.J. Glucocorticoid receptor protein expression in human hippocampus; stability with age. Neurobiol. Aging 2013, 34, 1662–1673. [Google Scholar] [CrossRef] [PubMed]
- Saaltink, D.J.; Vreugdenhil, E. Stress, glucocorticoid receptors, and adult neurogenesis: A balance between excitation and inhibition? Cell. Mol. Life Sci. 2014, 71, 2499–2515. [Google Scholar] [CrossRef]
- Misa-Agustiño, M.J.; Leiro-Vidal, J.M.; Gomez-Amoza, J.L.; Jorge-Mora, M.T.; Jorge-Barreiro, F.J.; Salas-Sánchez, A.A.; López-Martín, E. EMF radiation at 2450 MHz triggers changes in the morphology and expression of heat shock proteins and glucocorticoid receptors in rat thymus. Life Sci. 2015, 127, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sarabdjitsingh, R.A.; Meijer, O.C.; de Kloet, E.R. Specificity of glucocorticoid receptor primary antibodies for analysis of receptor localization patterns in cultured cells and rat hippocampus. Brain Res. 2010, 1331, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Murphy-Royal, C.; Dupuis, J.; Groc, L.; Oliet, S.H. Astroglial glutamate transporters in the brain: Regulating neurotransmitter homeostasis and synaptic transmission. J. Neurosci. Res. 2017, 95, 2140–2151. [Google Scholar] [CrossRef] [PubMed]
- Nahirnyj, A.; Livne-Bar, I.; Guo, X.; Sivak, J.M. ROS detoxification and proinflammatory cytokines are linked by p38 MAPK signaling in a model of mature astrocyte activation. PLoS ONE 2013, 8, e83049. [Google Scholar] [CrossRef]
- Schiffer, D.; Giordana, M.T.; Migheli, A.; Giaccone, G.; Pezzotta, S.; Mauro, A. Glial fibrillary acidic protein and vimentin in the experimental glial reaction of the rat brain. Brain Res. 1986, 374, 110–118. [Google Scholar] [CrossRef]
- Eng, L.F. Glial fibrillary acidic protein (GFAP): The major protein of glial intermediate filaments in differentiated astrocytes. J. Neuroimmunol. 1985, 8, 203–214. [Google Scholar] [CrossRef]
- Eng, L.F. Regulation of glial intermediate filaments in astrogliosis. In The Biochemical Pathology of Astrocytes; Norenberg, M.D., Hertz, L., Schousboe, A., Eds.; Liss: New York, NY, USA, 1988; Volume 39, pp. 79–90. [Google Scholar]
- Eng, L.F.; Ghirnikar, R.S.; Lee, Y.L. Glial fibrillary acidic protein: GFAP thirty-one years (1969–2000). Neurochem. Res. 2000, 25, 1439–1451. [Google Scholar] [CrossRef]
- Reeves, S.A.; Helman, L.J.; Allison, A.; Israel, M.A. Molecular cloning and primary structure of human glial fibrillary protein. Proc. Natl. Acad. Sci. USA 1989, 86, 5178–5182. [Google Scholar] [CrossRef]
- Norton, W.T.; Aquino, D.A.; Hozumi, I.; Chiu, F.C.; Brosnan, C.F. Quantitative aspects of reactive gliosis: A review. Neurochem. Res. 1992, 17, 877–885. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Ammari, M.; Brillaud, E.; Gamez, C.; Lecomte, A.; Sakly, M.; Abdelmelek, H.; De Seze, R. Effect of a chronic GSM 900 MHz exposure on glia in the rat brain. Biomed. Pharmacother. 2008, 62, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Ammari, M.; Gamez, C.; Lecomte, A.; Sakly, M.; Abdelmelek, H.; De Seze, R. GFAP expression in the rat brain following sub-chronic exposure to a 900 MHz electromagnetic field signal. Int. J. Radiat. Biol. 2010, 86, 367–375. [Google Scholar] [CrossRef]
- Barthélémy, A.; Mouchard, A.; Bouji, M.; Blazy, K.; Puigsegur, R.; Villégier, A.S. Glial markers and emotional memory in rats following acute cerebral radiofrequency exposures. Environ. Sci. Pollut. Res. Int. 2016, 23, 25343–25355. [Google Scholar] [CrossRef] [PubMed]
- Franzellitti, S.; Valbonesi, P.; Contin, A.; Biondi, C.; Fabbri, E. HSP70 expression in human trophoblast cells exposed to different 1.8 GHz mobile phone signals. Radiat. Res. 2008, 17, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Gerner, C.; Haudek, V.; Schandl, U.; Bayer, E.; Gundacker, N.; Hutter, H.P.; Mosgoeller, W. Increased protein synthesis by cells exposed to a 1800-MHz radio-frequency mobile phone electromagnetic field, detected by proteome profiling. Int. Arch. Occup. Environ. Health 2010, 83, 691–702. [Google Scholar] [CrossRef]
- Brillaud, E.; Piotrowski, A.; De Seze, R. Effect of an acute 900MHz GSM exposure on glia in the rat brain: A time-dependent study. Toxicology 2007, 238, 23–33. [Google Scholar] [CrossRef]
- López-Martín, E.; Bregains, J.; Relova-Quinteiro, J.L.; Cadarso-Suárez, C.; Jorge-Barreiro, F.J.; Ares-Pena, F.J. The action of pulse-modulated GSM radiation increases regional changes in brain activity and c-Fos expression in cortical and subcortical areas in a rat model of picrotoxin-induced seizure proneness. J. Neurosci. Res. 2009, 87, 1484–1499. [Google Scholar] [CrossRef]
- Carballo-Quintás, M.; Martinez-Silva, I.; Cadarso-Suarez, C.; Alvarez-Folgueiras, M.; Ares-Pena, F.J.; Lopez-Martín, E. A study of biomarkers after acute exposure to GSM radiation at 900 MHz in the picrotoxin model of rat brain. NeuroToxicology 2011, 32, 478–494. [Google Scholar] [CrossRef]
- Jorge-Mora, T.; Alvarez Folgueiras, M.; Leiro-Vidal, J.M.; Jorge-Barreiro, F.J.; Ares-Pena, F.J.; López-Martin, E. Exposure to 2.45 GHz microwave radiation provokes cerebral changes in induction of HSP-90 α/β heat shock protein in rat. Prog. Electromagn. Res. 2010, 100, 351–379. [Google Scholar] [CrossRef]
- Chao, H.M.; Choo, P.H.; McEwen, B.S. Glucocorticoid and mineralocorticoid receptor mRNA expression in rat brain. Neuroendocrinology 1989, 50, 365–371. [Google Scholar] [CrossRef]
- Filipović, D.; Gavrilović, L.; Dronjak, S.; Radojčić, M.B. Brain glucocorticoid receptor and heat shock protein 70 levels in rats exposed to acute, chronic or combined stress. Neuropsychobiology 2005, 51, 107–114. [Google Scholar] [CrossRef]
- Cullinan, W.E.; Herman, J.P.; Battaglia, D.F.; Akil, H.; Watson, S.J. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 1995, 64, 477–505. [Google Scholar] [CrossRef]
- Hoppenrath, K.; Funke, K. Time-course of changes in neuronal activity markers following iTBS-TMS of the rat neocortex. Neurosci. Lett. 2013, 536, 19–23. [Google Scholar] [CrossRef]
- Girotti, M.; Pace, T.W.; Gaylord, R.I.; Rubin, B.A.; Herman, J.P.; Spencer, R.L. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience 2006, 138, 1067–1081. [Google Scholar] [CrossRef]
- Misa-Agustiño, M.J.; Leiro, J.M.; Jorge-Mora, M.T.; Rodríguez-González, J.A.; Jorge Barreiro, F.J.; Ares-Pena, F.J.; López-Martín, E. Electromagnetic fields at 2.45 GHz trigger changes in heat shock proteins 90 and 70 without altering apoptotic activity in rat thyroid gland. Biol. Open 2012, 1, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, E.; Condello, S.; Currò, M.; Ferlazzo, N.; Caccamo, D.; Magazù, S.; Ientile, R. Modulation of heat shock protein response in SH-SY5Y by mobile phone microwaves. World J. Biol. Chem. 2012, 263, 34–40. [Google Scholar] [CrossRef]
- De Kloet, E.R.; Joëls, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Xu, H.; Liang, M.; Huang, J.H.; He, X. Association between reduced expression of hippocampal glucocorticoid receptors and cognitive dysfunction in a rat model of traumatic brain injury due to lateral head acceleration. Neurosci. Lett. 2013, 533, 50–54. [Google Scholar] [CrossRef] [PubMed]
- French, P.W.; Penny, R.; Laurence, J.A.; McKenzie, D.R. Mobile phones, heat shock proteins and cancer. Differentiation 2001, 67, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Filipović, D.; Gavrilović, L.; Dronjak, S.; Radojčić, M.B. The effect of repeated physical exercise on hippocampus and brain cortex in stressed rats. Ann. N. Y. Acad. Sci. 2007, 1096, 207–219. [Google Scholar] [CrossRef]
- Valbonesi, P.; Franzellitti, S.; Bersani, F.; Contin, A.; Fabbri, E. Effects of the exposure to intermittent 1.8 GHz radio frequency electromagnetic fields on HSP70 expression and MAPK signaling pathways in PC12 cells. Int. J. Radiat. Biol. 2014, 90, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Fukudo, S.; Abe, K.; Itoyama, Y.; Mochizuki, S.; Sawai, T.; Hongo, M. Psychophysiological stress induces heat shock cognate protein 70 messenger RNA in the hippocampus of rats. Neuroscience 1999, 91, 1205–1208. [Google Scholar] [CrossRef]
- López-Furelos, A.; Leiro-Vidal, J.M.; Salas-Sánchez, A.Á.; Ares-Pena, F.J.; López-Martín, M.E. Evidence of cellular stress and caspase-3 resulting from a combined two-frequency signal in the cerebrum and cerebellum of Sprague-Dawley rats. Oncotarget 2016, 7, 64674. [Google Scholar] [CrossRef] [PubMed]
- Kirschke, E.; Goswami, D.; Southworth, D.; Griffin, P.R.; Agard, D.A. Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell 2014, 157, 1685–1697. [Google Scholar] [CrossRef] [PubMed]
- Coveñas, R.; de León, M.; Cintra, A.; Bjelke, B.; Gustafsson, J.Å.; Fuxe, K. Coexistence of c-Fos and glucocorticoid receptor immunoreactivities in the CRF immunoreactive neurons of the paraventricular hypothalamic nucleus of the rat after acute immobilization stress. Neurosci. Lett. 1993, 149, 149–152. [Google Scholar] [CrossRef]
- Zelena, D.; Domokos, Á.; Jain, S.K.; Jankord, R.; Filaretova, L. The stimuli specific role of vasopressin in the hypothalamus–pituitary–adrenal axis response to stress. J. Endocrinol. 2009, 202, 263–278. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Molodtsova, I.D.; Medvedev, D.S.; Poliakova, V.O.; Lin’kova, N.S.; Gurko, G.I. Influence of millimeter-wave electromagnetic emission on Nitric Oxid synthesis during vessel endothelium aging in vitro. Adv. Gerontol. 2014, 28, 68–71. [Google Scholar]
- Heck, A.L.; Handa, R.J. Sex differences in the hypothalamic-pituitary-adrenal axis’ response to stress: An important role for gonadal hormones. Neuropsychopharmacology 2019, 44, 45–58. [Google Scholar] [CrossRef]
- Salmaso, N.; Stevens, H.E.; McNeill, J.; El Sayed, M.; Ren, Q.; Maragnoli, M.E.; Vaccarino, F.M. Fibroblast growth factor 2 modulates hypothalamic pituitary axis activity and anxiety behavior through glucocorticoid receptors. Biol. Psychiatry 2016, 80, 479–489. [Google Scholar] [CrossRef]
- Chen, D.Y.; Bambah-Mukku, D.; Pollonini, G.; Alberini, C.M. Glucocorticoid receptors recruit the CaMKII [alpha]-BDNF-CREB pathways to mediate memory consolidation. Nat. Neurosci. 2012, 15, 1707–1714. [Google Scholar] [CrossRef]
- Moraitis, A.G.; Block, T.; Nguyen, D.; Belanoff, J.K. The role of glucocorticoid receptors in metabolic syndrome and psychiatric illness. J. Steroid Biochem. Mol. Biol. 2017, 165, 114–120. [Google Scholar] [CrossRef]
- Oakley, R.H.; Cidlowski, J.A. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Maskey, D.; Kim, H.J.; Kim, H.G.; Kim, M.J. Calcium-binding proteins and GFAP immunoreactivity alterations in murine hippocampus after 1 month of exposure to 835MHz radiofrequency at SAR values of 1.6 and 4.0 W/kg. Neurosci. Lett. 2012, 506, 292–296. [Google Scholar] [CrossRef]
- Nichols, N.R.; Day, J.R.; Laping, N.J.; Johnson, S.A.; Finch, C.E. GFAP mRNA increases with age in rat and human brain. Neurobiol. Aging 1993, 14, 421–429. [Google Scholar] [CrossRef]
- Nichols, N.R.; Finch, C.E.; Nelson, J.F. Food restriction delays the age-related increase in GFAP mRNA in rat hypothalamus. Neurobiol. Aging 1998, 16, 105–110. [Google Scholar] [CrossRef]
- Bouji, M.; Lecomte, A.; Hode, Y.; De Seze, R.; Villégier, A.S. Effects of 900 MHz radiofrequency on corticosterone, emotional memory and neuroinflammation in middle-aged rats. Exp. Gerontol. 2012, 47, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Court-Kowalski, S.; Finnie, J.W.; Manavis, J.; Blumbergs, P.C.; Helps, S.C.; Vink, R. Effect of long-term (2 years) exposure of mouse brains to global system for mobile communication (GSM) radiofrequency fields on astrocytic immunoreactivity. Bioelectromagnetics 2015, 36, 245–250. [Google Scholar] [CrossRef]
- Chisholm, N.C.; Sohrabji, F. Astrocytic response to cerebral ischemia is influenced by sex differences and impaired by aging. Neurobiol. Dis. 2016, 85, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Nichols, N.R.; Agolley, D.; Zieba, M.; Bye, N. Glucocorticoid regulation of glial responses during hippocampal neurodegeneration and regeneration. Brain Res. Brain Res. Rev. 2005, 48, 287–301. [Google Scholar] [CrossRef]
- Vielkind, U.; Walencewicz, A.; Levine, J.M.; Bohn, M.C. Type II glucocorticoid receptors are expressed in oligodendrocytes and astrocytes. J. Neurosci. Res. 1990, 27, 360–373. [Google Scholar] [CrossRef]
- Unemura, K.; Kume, T.; Kondo, M.; Maeda, Y.; Izumi, Y.; Akaike, A. Glucocorticoids decrease astrocyte numbers by reducing glucocorticoid receptor expression in vitro and in vivo. J. Pharmacol. Sci. 2012, 119, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Crossin, K.L.; Tai, M.H.; Krushel, L.A.; Mauro, V.P.; Edelman, G.M. Glucocorticoid receptor pathways are involved in the inhibition of astrocytes proliferation. Proc. Natl. Acad. Sci. USA 1997, 94, 2687–2692. [Google Scholar] [CrossRef] [PubMed]
- Nichols, N.R.; Osterburg, H.H.; Masters, J.N.; Millar, S.L.; Finch, C.E. Messenger RNA for glial fibrillary acidic protein is decreased in rat brain following acute and chronic corticosterone treatment. Brain Res. Mol. Brain Res. 1990, 7, 1–7. [Google Scholar] [CrossRef]
- Owens-Grillo, J.K.; Hoffmann, K.; Hutchison, K.A.; Yem, A.W.; Deibel, M.R.; Handschumacher, R.E.; Pratt, W.B. The cyclosporin A-binding immunophilin CyP-40 and the FK506-binding immunophilin hsp56 bind to a common site on hsp90 and exist in independent cytosolic heterocomplexes with the untransformed glucocorticoid receptor. J. Biol. Chem. 1995, 270, 20479–20484. [Google Scholar] [CrossRef]
- Beere, H.M.; Green, D.R. Stress management–heat shock protein-70 and the regulation of apoptosis. Trends Cell Biol. 2001, 11, 6–10. [Google Scholar] [CrossRef]
- Multhoff, G.; Pockley, A.G.; Schmid, T.E.; Schilling, D. The role of heat shock protein 70 (Hsp70) in radiation-induced immunomodulation. Cancer Lett. 2015, 368, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Swanson, R.A. Astrocytes and brain injury. J. Cereb. Blood Flow. Metab. 2003, 23, 137–149. [Google Scholar] [CrossRef]
- Xu, J.; Drew, P.D. 9-Cis-retinoic acid suppresses inflammatory responses of microglia and astrocytes. J. Neuroimmunol. 2006, 171, 135–144. [Google Scholar] [CrossRef] [PubMed]
- SPEAG, Schmid & Partner Engineering AG. Reference Manual for the SEMCAD Simulation Platform for Electromagnetic Compatibility, Antenna Design and Dosimetry. Available online: https://speag.swiss/products/semcad/solutions (accessed on 28 December 2020).
- Directive 2010/63/UE of European Parliament and the Council 22 de September (2010). Concerning the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 276, 33–79.



| Single Exposure (after 1.5 h) | Single Exposure (after 24 h) | Repeated Exposure | ||
|---|---|---|---|---|
| SMS CORTEX | NO RAD | 129 ± 16 b,c | 42 ± 11 a | 29 ± 5 a |
| RAD | 112 ± 18 b,c | 37 ± 9 a | 39 ± 6 a | |
| L. CORTEX | NO RAD | 123 ± 16 b,c | 34 ± 2 a | 26 ± 5 a |
| RAD | 99 ± 18 b,c | 64 ± 13 a | 66 ± 22 *,a | |
| HIPOTHA | NO RAD | 114 ± 20 b,c | 53 ± 7 a,c | 34 ± 11 a,b |
| RAD | 117 ± 15 b,c | 46 ± 14 a | 45 ± 5 a | |
| HIPPCAM | NO RAD | 179 ± 30 b,c | 89 ± 17 a,c | 37 ± 7 a,b |
| RAD | 90 ± 8 *,c | 60 ± 20 | 41 ± 6 a |
| Single Exposure (after 1.5 h) | Single Exposure (after 24 h) | Repeated Exposure | ||
|---|---|---|---|---|
| SMS CORTEX | NO RAD | 89 ± 24 c | 45 ± 10 | 35 ± 6 a |
| RAD | 47 ± 14 *,c | 62 ± 17 | 30 ± 4 a | |
| L. CORTEX | NO RAD | 174 ± 7 b,c | 233 ± 7 a,c | 115 ± 8 a,b |
| RAD | 90 ± 7 *,b,c | 141 ± 9 *a | 167 ± 9 *a | |
| HIPOTHA | NO RAD | 95 ± 16 b,c | 18 ± 10 a,c | 50 ± 11 a,b |
| RAD | 50 ± 8 * | 14 ± 9 | 23 ± 4 | |
| HIPPCAM | NO RAD | 91 ± 15 b,c | 49 ± 11 a | 42 ± 7 a |
| RAD | 78 ± 14 b | 38 ± 13 a | 41 ± 4 a |
| Single Exposure (after 1.5 h) | Single Exposure (after 24 h) | Repeated Exposure | ||
|---|---|---|---|---|
| SMS CORTEX | NO RAD | 30 ± 2 b | 54 ± 5 a | 29 ± 5 |
| RAD | 28 ± 3 b | 48 ± 10 a | 39 ± 6 | |
| L. CORTEX | NO RAD | 35 ± 3 b,c | 53 ± 7 a,c | 260 ± 27 a,b |
| RAD | 42 ± 5 c | 57 ± 5 c | 175 ± 20 *,b | |
| HIPOTHA | NO RAD | 44 ± 6 b,c | 117 ± 14 a,c | 32 ± 3 a,b |
| RAD | 49 ± 4 b,c | 91 ± 11 a,c | 19 ± 10 *,a,b | |
| HIPPCAM | NO RAD | 27 ± 4 b,c | 103 ± 13 a,c | 340 ± 50 a,b |
| RAD | 59 ± 4 *,c | 55 ± 4 *,c | 211 ± 15 a,b |
| Experimental Measurement of Specific Absorption Rate by FDTD | |||||||
|---|---|---|---|---|---|---|---|
| WE [g] | PTR [W] | E [V/m] | Time Elapsed until Perfusion [h] | SARE IN BRAIN | SARE IN BODY | ||
| Mean [W/kg] | Peak 1 g | Mean [W/kg] | Peak 1 g | ||||
| 227.10 | 3.00 | 40.28 | Single exposure 1.5 | 0.069420 | 0.085499 | 0.040445 | 0.199947 |
| 219.90 | Single exposure 24 | 0.071551 | 0.088124 | 0.041687 | 0.206086 | ||
| 279.20 | Repeated exposure 1.5 | 0.07509 | 0.09249 | 0.04375 | 0.21629 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Othman, H.; López-Furelos, A.; Leiro-Vidal, J.M.; Ammari, M.; Sakly, M.; Abdelmelek, H.; Salas-Sánchez, A.Á.; Ares-Pena, F.; López-Martín, E. Exposure to 2.45 GHz Radiation Triggers Changes in HSP-70, Glucocorticoid Receptors and GFAP Biomarkers in Rat Brain. Int. J. Mol. Sci. 2021, 22, 5103. https://doi.org/10.3390/ijms22105103
Othman H, López-Furelos A, Leiro-Vidal JM, Ammari M, Sakly M, Abdelmelek H, Salas-Sánchez AÁ, Ares-Pena F, López-Martín E. Exposure to 2.45 GHz Radiation Triggers Changes in HSP-70, Glucocorticoid Receptors and GFAP Biomarkers in Rat Brain. International Journal of Molecular Sciences. 2021; 22(10):5103. https://doi.org/10.3390/ijms22105103
Chicago/Turabian StyleOthman, Haifa, Alberto López-Furelos, José Manuel Leiro-Vidal, Mohamed Ammari, Mohsen Sakly, Hafedh Abdelmelek, Aarón Ángel Salas-Sánchez, Francisco Ares-Pena, and Elena López-Martín. 2021. "Exposure to 2.45 GHz Radiation Triggers Changes in HSP-70, Glucocorticoid Receptors and GFAP Biomarkers in Rat Brain" International Journal of Molecular Sciences 22, no. 10: 5103. https://doi.org/10.3390/ijms22105103
APA StyleOthman, H., López-Furelos, A., Leiro-Vidal, J. M., Ammari, M., Sakly, M., Abdelmelek, H., Salas-Sánchez, A. Á., Ares-Pena, F., & López-Martín, E. (2021). Exposure to 2.45 GHz Radiation Triggers Changes in HSP-70, Glucocorticoid Receptors and GFAP Biomarkers in Rat Brain. International Journal of Molecular Sciences, 22(10), 5103. https://doi.org/10.3390/ijms22105103






