Tranexamic Acid Promotes Murine Bone Marrow-Derived Osteoblast Proliferation and Inhibits Osteoclast Formation In Vitro
Abstract
1. Introduction
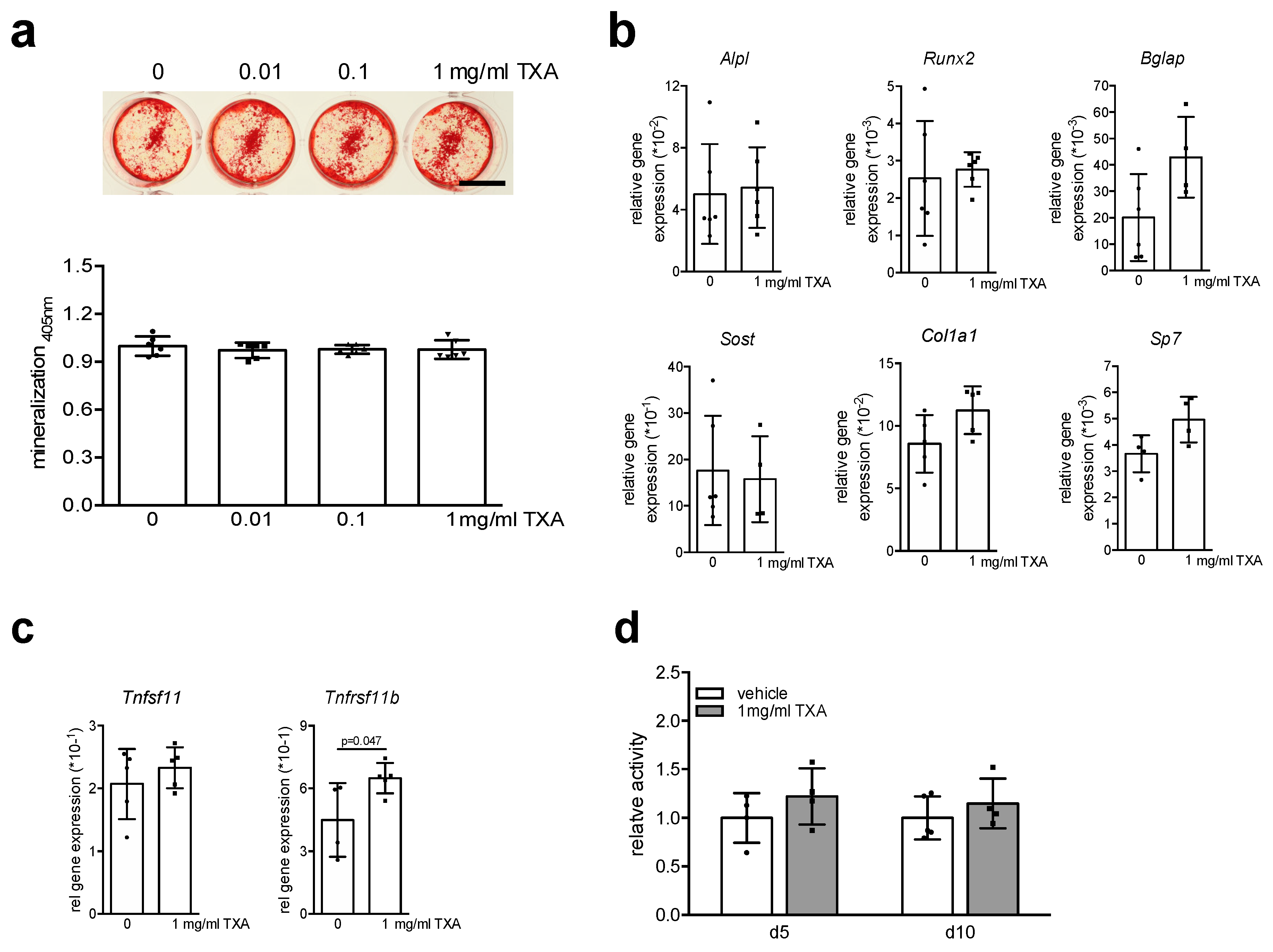
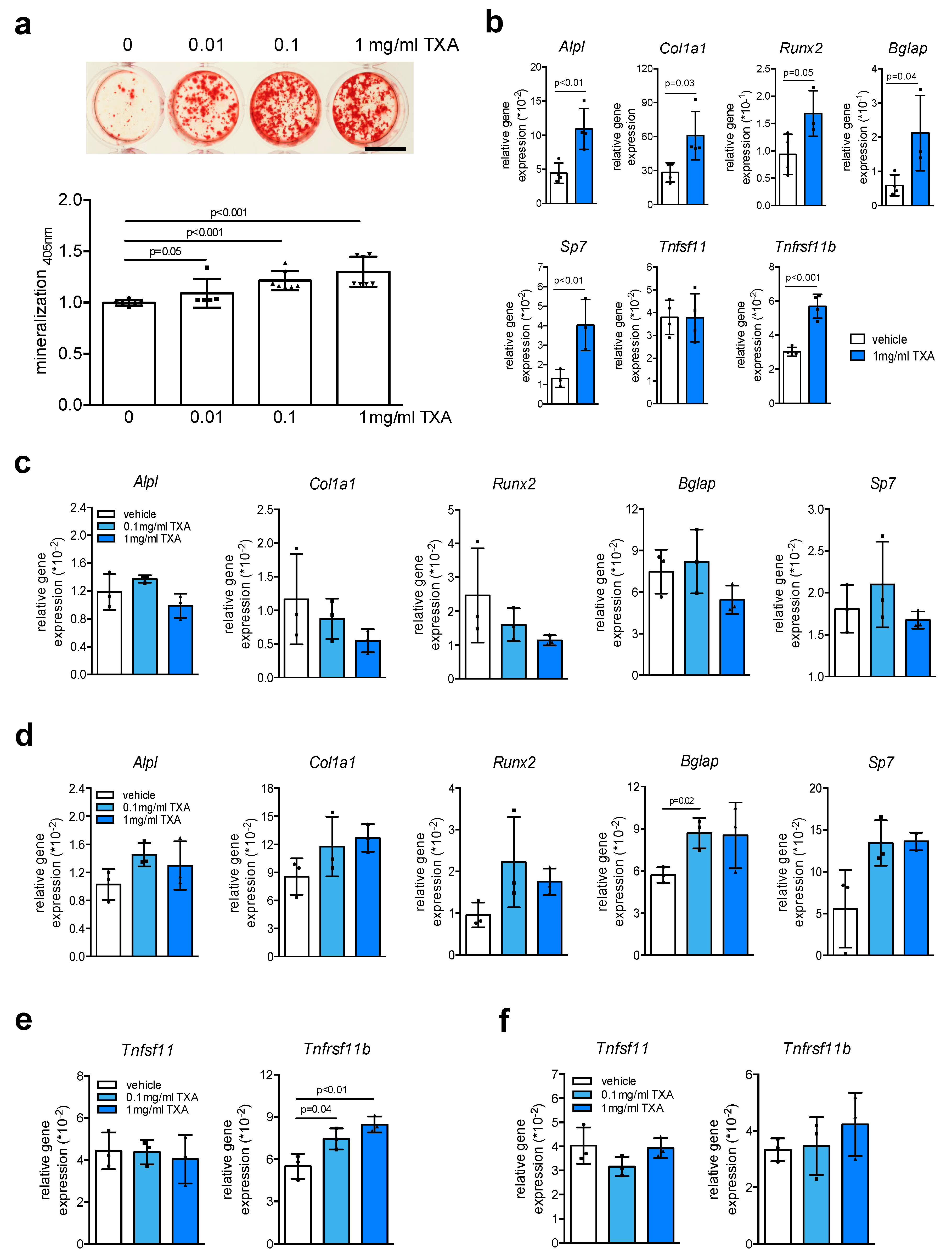
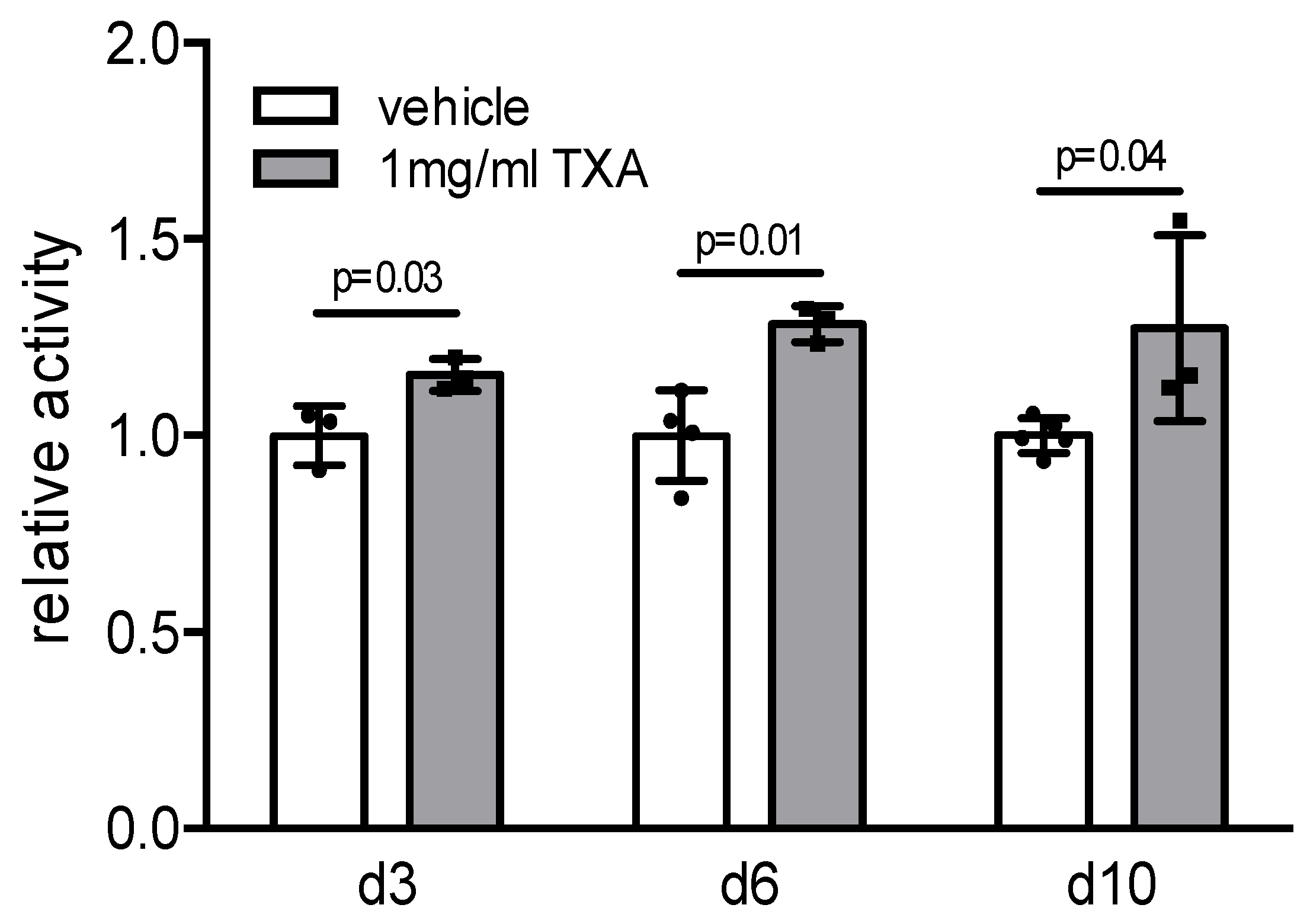
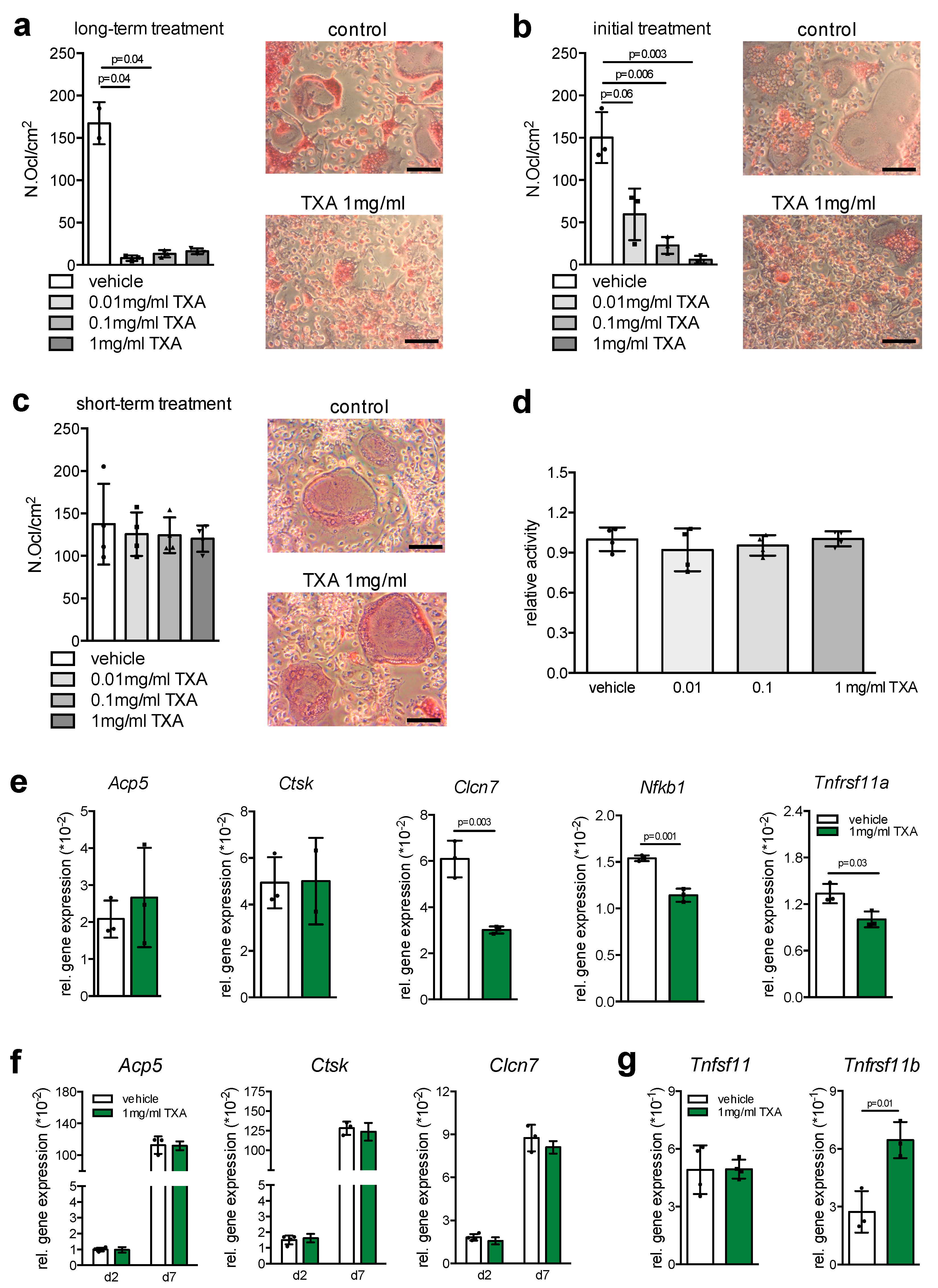
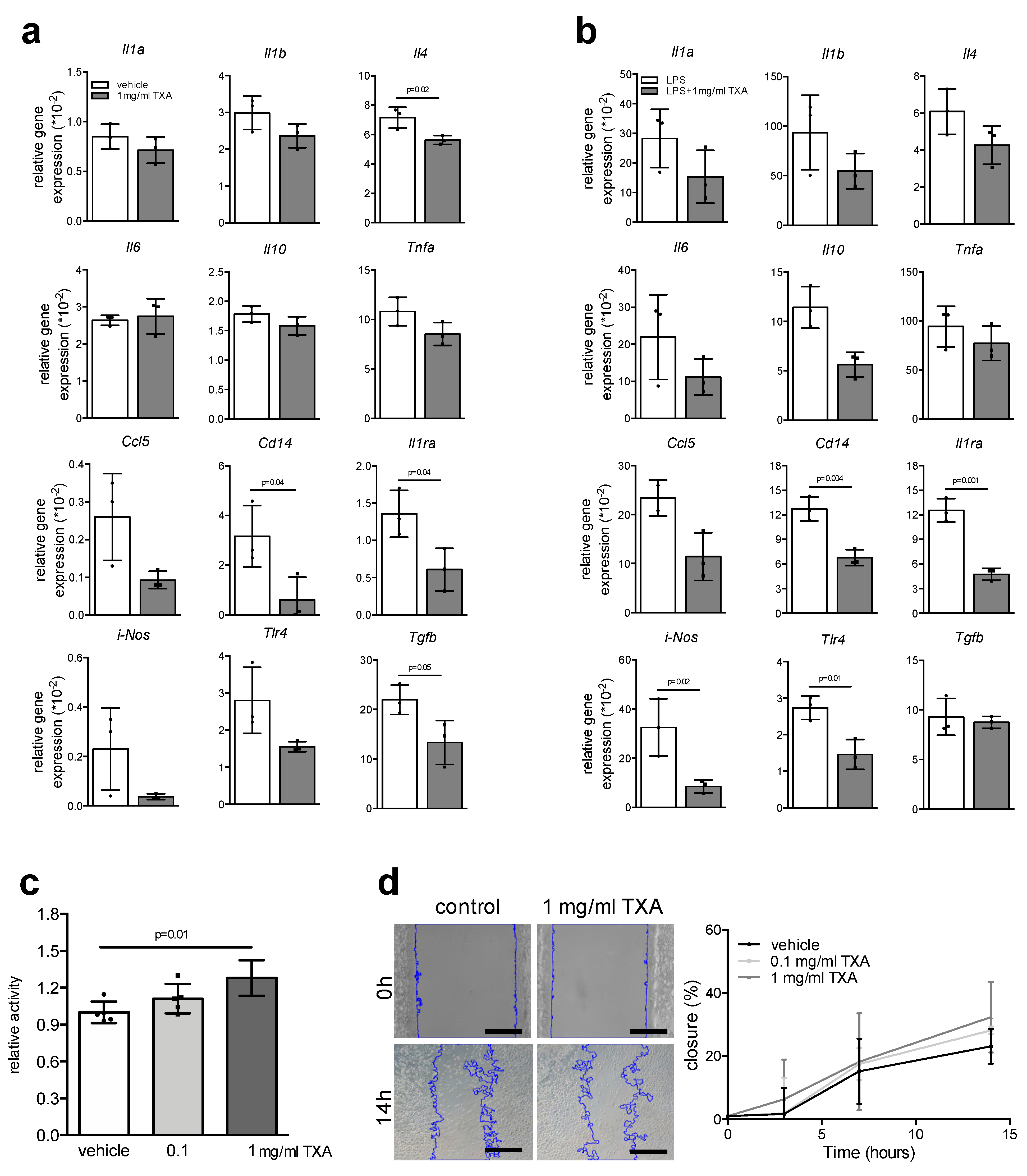
2. Results
3. Discussion
4. Materials and Methods
4.1. Primary Bone Cells
4.2. Expression Analysis
4.3. MTT Test
4.4. Migration Assay
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Acp5 | tartrate-resistant acid phosphatase |
| Alpl | alkaline phosphatase |
| Bglap | osteocalcin |
| Clcn7 | chloride channel 7 |
| Col1a1 | alpha-1 type I collagen |
| CRASH-2 | clinical randomization of an antifibrinolytic in significant hemorrhage 2 |
| Ctsk | cathepsin K |
| Il1ra | interleukin 1 receptor antagonist |
| iNos | NO synthase |
| LaGeSo | Landesamt für Gesundheit und Soziales |
| LPS | lipopolysaccharide |
| Nfkb1 | nuclear factor kappa B subunit 1 |
| Runx2 | runt-related transcription factor 2 |
| Sost | sclerostin |
| Sp7 | osterix |
| Tgfb | transforming growth factor beta |
| Tlr4 | toll-like receptor 4 |
| Tnfrsf11a | receptor activator of nf-κb |
| TXA | tranexamic acid |
References
- Rossaint, R.; Bouillon, B.; Cerny, V.; Coats, T.J.; Duranteau, J.; Fernandez-Mondejar, E.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Nardi, G.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fourth edition. Crit. Care 2016, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, P.M.; Levi, M. Prevention and treatment of major blood loss. N. Engl. J. Med. 2007, 356, 2301–2311. [Google Scholar] [CrossRef] [PubMed]
- Tengborn, L.; Blomback, M.; Berntorp, E. Tranexamic acid—An old drug still going strong and making a revival. Thromb. Res. 2015, 135, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Watts, G. Utako Okamoto. Lancet 2016, 387, 2286. [Google Scholar] [CrossRef]
- Levy, J.H.; Koster, A.; Quinones, Q.J.; Milling, T.J.; Key, N.S. Antifibrinolytic Therapy and Perioperative Considerations. Anesthesiology 2018, 128, 657–670. [Google Scholar] [CrossRef]
- The CRASH-2 Collaborators; Roberts, I.; Shakur, H.; Afolabi, A.; Brohi, K.; Coats, T.; Dewan, Y.; Gando, S.; Guyatt, G.; Hunt, B.J.; et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: An exploratory analysis of the CRASH-2 randomised controlled trial. Lancet 2011, 377, 1096–1101.e1–2. [Google Scholar] [PubMed]
- The CRASH-2 Collaborators; Shakur, H.; Roberts, I.; Bautista, R.; Caballero, J.; Coats, T.; Dewan, Y.; El-Sayed, H.; Gogichaishvili, T.; Gupta, S.; et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): A randomised, placebo-controlled trial. Lancet 2010, 376, 23–32. [Google Scholar]
- Chen, Y.; Chen, Z.; Cui, S.; Li, Z.; Yuan, Z. Topical versus systemic tranexamic acid after total knee and hip arthroplasty: A meta-analysis of randomized controlled trials. Medicine 2016, 95, e4656. [Google Scholar] [CrossRef]
- Nishida, T.; Kinoshita, T.; Yamakawa, K. Tranexamic acid and trauma-induced coagulopathy. J. Intensive Care 2017, 5, 5. [Google Scholar] [CrossRef]
- Ambra, L.F.; de Girolamo, L.; Niu, W.; Phan, A.; Spector, M.; Gomoll, A.H. No effect of topical application of tranexamic acid on articular cartilage. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 931–935. [Google Scholar] [CrossRef]
- Jacob, B.; Kloss, N.; Bohle, S.; Kirschberg, J.; Zippelius, T.; Heinecke, M.; Matziolis, G.; Rohner, E. Tranexamic acid is toxic on human chondrocytes, in vitro. J. Orthop. 2020, 20, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, J.R.; Feltman, P.R.; Ritterman, S.A.; Ehrlich, M.G. Effects of Tranexamic Acid Cytotoxicity on In Vitro Chondrocytes. Am. J. Orthop. 2015, 44, E497–E502. [Google Scholar] [PubMed]
- Rolvien, T.; Amling, M. Bone biology in the elderly: Clinical importance for fracture treatment. Innov. Surg. Sci. 2016, 1, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Schlickewei, C.W.; Kleinertz, H.; Thiesen, D.M.; Mader, K.; Priemel, M.; Frosch, K.H.; Keller, J. Current and Future Concepts for the Treatment of Impaired Fracture Healing. Int. J. Mol. Sci. 2019, 20, 5805. [Google Scholar] [CrossRef] [PubMed]
- Schlundt, C.; El Khassawna, T.; Serra, A.; Dienelt, A.; Wendler, S.; Schell, H.; van Rooijen, N.; Radbruch, A.; Lucius, R.; Hartmann, S.; et al. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone 2018, 106, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Sinder, B.P.; Pettit, A.R.; McCauley, L.K. Macrophages: Their Emerging Roles in Bone. J. Bone Miner. Res. 2015, 30, 2140–2149. [Google Scholar] [CrossRef]
- Cuellar, J.M.; Yoo, A.; Tovar, N.; Coelho, P.G.; Jimbo, R.; Vandeweghe, S.; Kirsch, T.; Quirno, M.; Errico, T.J. The effects of Amicar and TXA on lumbar spine fusion in an animal model. Spine 2014, 39, E1132–E1137. [Google Scholar] [CrossRef]
- Demol, J.; Eyckmans, J.; Roberts, S.J.; Luyten, F.P.; Van Oosterwyck, H. Does tranexamic acid stabilised fibrin support the osteogenic differentiation of human periosteum derived cells? Eur. Cells Mater. 2011, 21, 272–285. [Google Scholar] [CrossRef]
- Kara, F.M.; Verma, K.; Xavier, S.; PhD, T.K.; Errico, T.J. Epsilon Aminocaproic and Transexamic Acid Decrease Osteoblast Differentiation and Mineralization In Vitro: E-Poster #53. Spine J. Meet. Abstr. 2009, 10, 191. [Google Scholar]
- Georgiev, G.P.; Tanchev, P.P.; Zheleva, Z.; Kinov, P. Comparison of topical and intravenous administration of tranexamic acid for blood loss control during total joint replacement: Review of literature. J. Orthop. Translat. 2018, 13, 7–12. [Google Scholar] [CrossRef]
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Catala-Lehnen, P.; Huebner, A.K.; Jeschke, A.; Heckt, T.; Lueth, A.; Krause, M.; Koehne, T.; Albers, J.; Schulze, J.; et al. Calcitonin controls bone formation by inhibiting the release of sphingosine 1-phosphate from osteoclasts. Nat. Commun. 2014, 5, 5215. [Google Scholar] [CrossRef] [PubMed]
- Vi, L.; Baht, G.S.; Whetstone, H.; Ng, A.; Wei, Q.; Poon, R.; Mylvaganam, S.; Grynpas, M.; Alman, B.A. Macrophages promote osteoblastic differentiation in-vivo: Implications in fracture repair and bone homeostasis. J. Bone Miner. Res. 2015, 30, 1090–1102. [Google Scholar] [CrossRef] [PubMed]
- Schoenecker, J.; Mignemi, N.; Stutz, C.; Liu, Q.; Edwards, J.; Lynch, C.; Holt, G.; Schwartz, H.; Mencio, G.; Hamm, H. 2010 Young Investigator Award winner: Therapeutic aprotinin stimulates osteoblast proliferation but inhibits differentiation and bone matrix mineralization. Spine 2010, 35, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Daci, E.; Everts, V.; Torrekens, S.; Van Herck, E.; Tigchelaar-Gutterr, W.; Bouillon, R.; Carmeliet, G. Increased bone formation in mice lacking plasminogen activators. J. Bone Miner. Res. 2003, 18, 1167–1176. [Google Scholar] [CrossRef]
- Furlan, F.; Galbiati, C.; Jorgensen, N.R.; Jensen, J.E.; Mrak, E.; Rubinacci, A.; Talotta, F.; Verde, P.; Blasi, F. Urokinase plasminogen activator receptor affects bone homeostasis by regulating osteoblast and osteoclast function. J. Bone Miner. Res. 2007, 22, 1387–1396. [Google Scholar] [CrossRef]
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T. RANKL biology: Bone metabolism, the immune system, and beyond. Inflamm. Regen. 2020, 40, 2. [Google Scholar] [CrossRef]
- Michalski, M.N.; McCauley, L.K. Macrophages and skeletal health. Pharmacol. Ther. 2017, 174, 43–54. [Google Scholar] [CrossRef]
- Jimenez, J.J.; Iribarren, J.L.; Lorente, L.; Rodriguez, J.M.; Hernandez, D.; Nassar, I.; Perez, R.; Brouard, M.; Milena, A.; Martinez, R.; et al. Tranexamic acid attenuates inflammatory response in cardiopulmonary bypass surgery through blockade of fibrinolysis: A case control study followed by a randomized double-blind controlled trial. Crit. Care 2007, 11, R117. [Google Scholar] [CrossRef]
- Busuttil, S.J.; Ploplis, V.A.; Castellino, F.J.; Tang, L.; Eaton, J.W.; Plow, E.F. A central role for plasminogen in the inflammatory response to biomaterials. J. Thromb. Haemost. 2004, 2, 1798–1805. [Google Scholar] [CrossRef]
- Picetti, R.; Shakur-Still, H.; Medcalf, R.L.; Standing, J.F.; Roberts, I. What concentration of tranexamic acid is needed to inhibit fibrinolysis? A systematic review of pharmacodynamics studies. Blood Coagul. Fibrinolysis 2019, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Raisz, L.G.; Trummel, C.L.; Holick, M.F.; DeLuca, H.F. 1,25-dihydroxycholecalciferol: A potent stimulator of bone resorption in tissue culture. Science 1972, 175, 768–769. [Google Scholar] [CrossRef] [PubMed]
- Andersson, L.; Nilsoon, I.M.; Colleen, S.; Granstrand, B.; Melander, B. Role of urokinase and tissue activator in sustaining bleeding and the management thereof with EACA and AMCA. Ann. N. Y. Acad. Sci. 1968, 146, 642–658. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranowsky, A.; Appelt, J.; Tseneva, K.; Jiang, S.; Jahn, D.; Tsitsilonis, S.; Frosch, K.-H.; Keller, J. Tranexamic Acid Promotes Murine Bone Marrow-Derived Osteoblast Proliferation and Inhibits Osteoclast Formation In Vitro. Int. J. Mol. Sci. 2021, 22, 449. https://doi.org/10.3390/ijms22010449
Baranowsky A, Appelt J, Tseneva K, Jiang S, Jahn D, Tsitsilonis S, Frosch K-H, Keller J. Tranexamic Acid Promotes Murine Bone Marrow-Derived Osteoblast Proliferation and Inhibits Osteoclast Formation In Vitro. International Journal of Molecular Sciences. 2021; 22(1):449. https://doi.org/10.3390/ijms22010449
Chicago/Turabian StyleBaranowsky, Anke, Jessika Appelt, Kristina Tseneva, Shan Jiang, Denise Jahn, Serafeim Tsitsilonis, Karl-Heinz Frosch, and Johannes Keller. 2021. "Tranexamic Acid Promotes Murine Bone Marrow-Derived Osteoblast Proliferation and Inhibits Osteoclast Formation In Vitro" International Journal of Molecular Sciences 22, no. 1: 449. https://doi.org/10.3390/ijms22010449
APA StyleBaranowsky, A., Appelt, J., Tseneva, K., Jiang, S., Jahn, D., Tsitsilonis, S., Frosch, K.-H., & Keller, J. (2021). Tranexamic Acid Promotes Murine Bone Marrow-Derived Osteoblast Proliferation and Inhibits Osteoclast Formation In Vitro. International Journal of Molecular Sciences, 22(1), 449. https://doi.org/10.3390/ijms22010449




