Polyamines Influence Mouse Sperm Channels Activity
Abstract
1. Introduction
2. Results
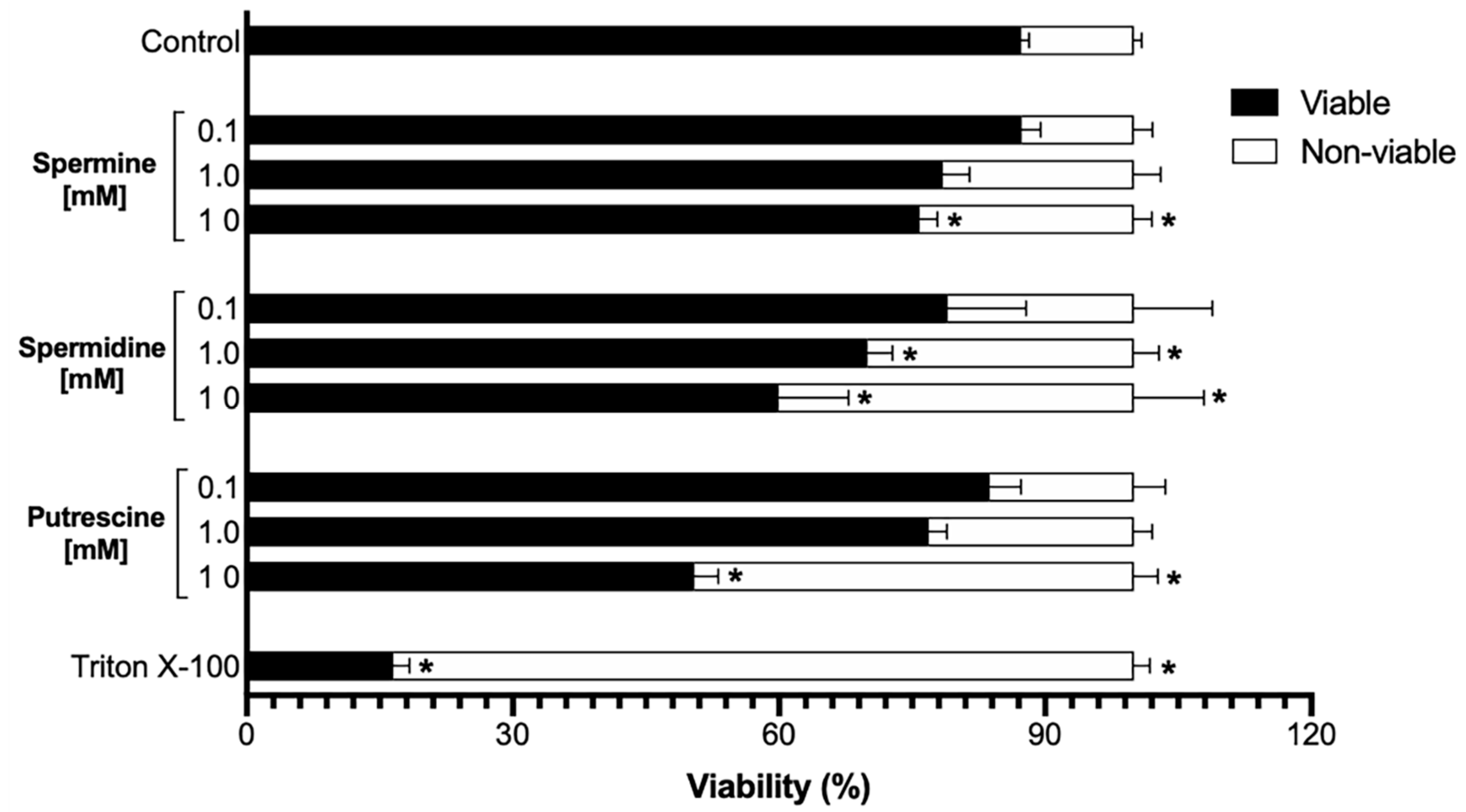
2.1. Sperm Viability Is Not Affected by Low Concentrations of Polyamines
2.2. Polyamines Decrease the [Cl−]i under Capacitation Conditions
2.3. Polyamines Increase the [Ca2+]i under Capacitation Conditions
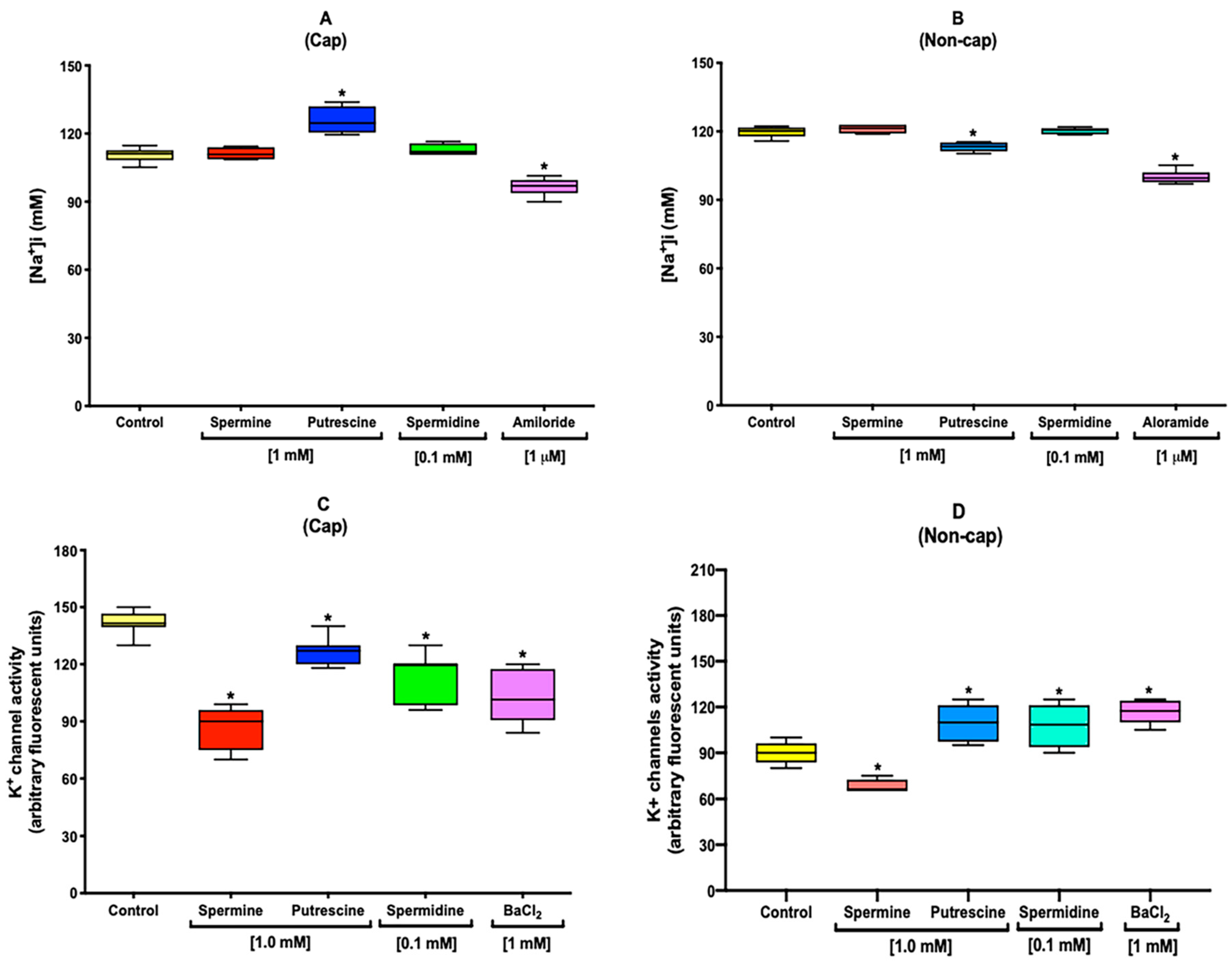
2.4. Polyamines Increase the [Na+]i under Capacitation Conditions
2.5. Polyamines Decrease the [K+]i under Capacitation Conditions
2.6. Polyamines Induce Changes in the Vm under Capacitation and Non-Capacitation Conditions
2.7. Polyamines Influence the Alkalization of pHi during Capacitation
2.8. Polyamines Decrease the ATP Level
2.9. In Silico Interaction of Polyamines with Sperm sAC
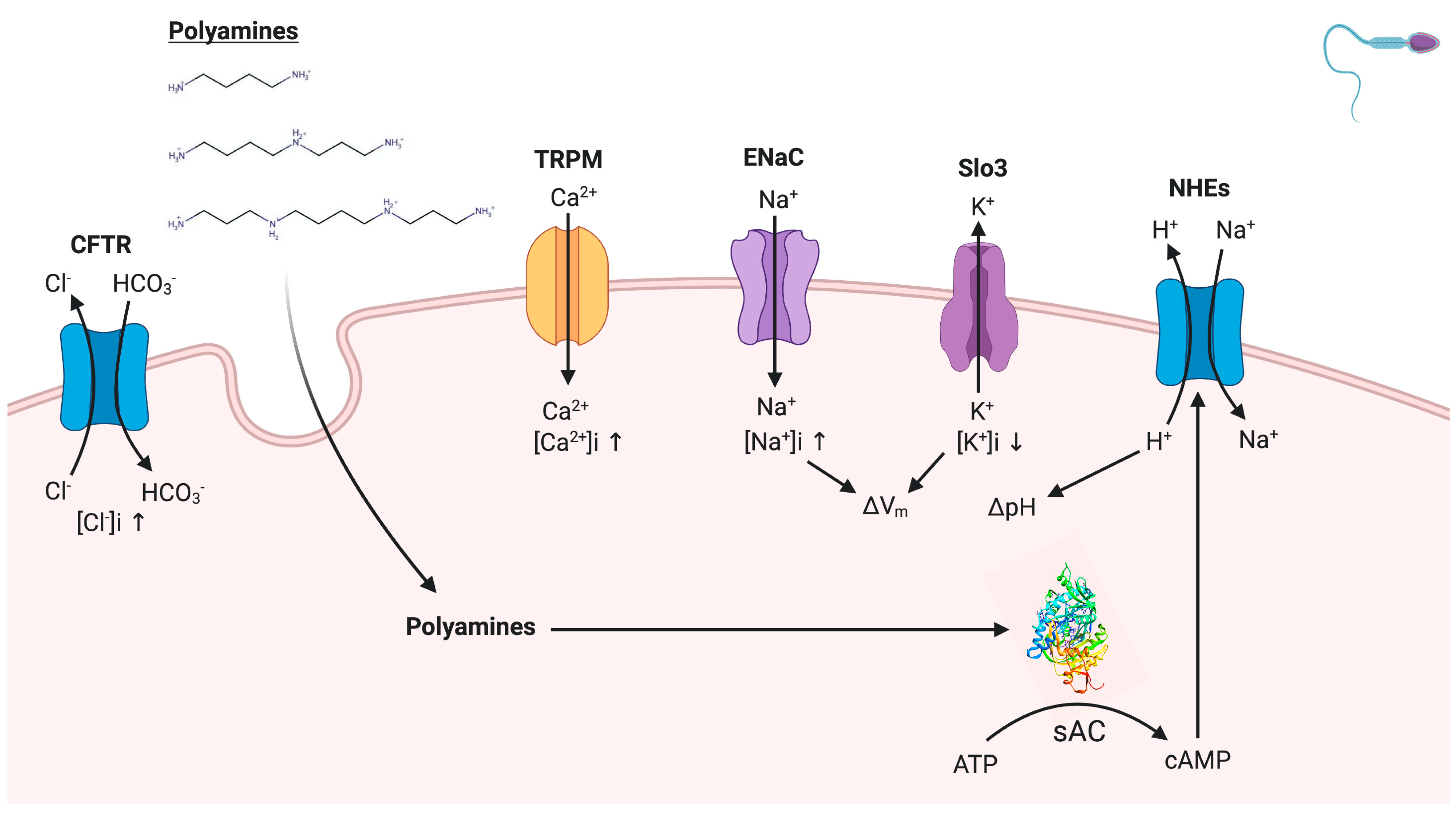
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Animal Procedures
4.3. Sperm Preparation
4.4. Evaluation of Sperm Viability
4.5. Intracellular Cl− Concentration Determination in Sperm Population
4.6. Intracellular Ca2+ Concentration Determination in Sperm Population
4.7. Intracellular Na+ Concentration Determination in Sperm Population
4.8. Intracellular K+ Concentration Determination in Sperm Population
4.9. Evaluation of Sperm Vm in Sperm Population
4.10. Quantification of Sperm pHi in Sperm Population
4.11. Quantification of Sperm ATP Level
4.12. In Silico Study of the Interaction between Polyamines and sAC
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| sAC | soluble adenylate cyclase |
| [Cl−]i | intracellular concentrations of chloride |
| [Ca2+]i | intracellular concentrations of calcium |
| [K+]i | intracellular concentrations of potassium |
| [Na+]i | intracellular concentrations of sodium |
| DIDS | 4,4′diisothiocyanatostilbene-2,2′-disulfonic acid disodium salt hydrate |
| Vm | membrane potential |
| cAMP | cyclic adenosine mono phosphate |
References
- Lefèvre, P.L.C.; Palin, M.-F.; Murphy, B.D. Polyamines on the Reproductive Landscape. Endocr. Rev. 2011, 32, 694–712. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.; Lutwak-Mann, C. Biochemistry of Spermatozoa: Chemical and Functional Correlations in Ejaculated Semen, Andrological Aspect. In Male Reproductive Function and Semen; Springer: New York, NY, USA, 1981; pp. 195–268. [Google Scholar]
- Williams, K. Interactions of polyamines with ion channels. Biochem. J. 1997, 325, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Persson, L. Polyamine homoeostasis. Essays Biochem. 2009, 46, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Björkgren, I.; Sipilä, P. The impact of epididymal proteins on sperm function. Reproduction 2019, 158, R155–R167. [Google Scholar] [CrossRef] [PubMed]
- Plant, T.M.; Zeleznik, A.; Albertini, D.F.; Goodman, R.L.; Herbison, A.; McCarthy, M.; Muglia, L.J.; Richards, J.S. Knobil and Neill’s Physiology of Reproduction: Two-Volume the Spermatozoon; Academic Press: New York, NY, USA, 2014; Volume 1, pp. 1–2550. [Google Scholar]
- Méndez, J.D. Polyamine Metabolism in Sperm Cell. Diabetes Complicat. 2018, 2, 1–3. [Google Scholar] [CrossRef]
- Melendrez, C.S.; Ruttle, J.L.; Hallford, D.M.; Chaudhry, P.S.; Casillas, E.R. Polyamines in ejaculated ram spermatozoa and their relationship with sperm motility. J. Androl. 1992, 13, 293–296. [Google Scholar]
- Bernardino, R.L.; Carrageta, D.F.; Sousa, M.; Alves, M.G.; Oliveira, P.F. pH and male fertility: Making sense on pH homeodynamics throughout the male reproductive tract. Cell. Mol. Life Sci. 2019, 76, 3783–3800. [Google Scholar] [CrossRef]
- Forsythe, I.D. Ion Channels: A physiological function for polyamines? Curr. Biol. 1995, 5, 1248–1251. [Google Scholar] [CrossRef][Green Version]
- Gallo, A.; Tosti, E. Ion currents involved in gamete physiology. Int. J. Dev. Biol. 2015, 59, 261–270. [Google Scholar] [CrossRef]
- Santi, C.; Orta, G.; Salkoff, L.; Visconti, P.E.; Darszon, A.; Treviño, C. K+ and Cl− Channels and Transporters in Sperm Function. Curr. Top. Dev. Biol. 2013, 102, 385–421. [Google Scholar] [CrossRef]
- Beltrán, C.; Treviño, C.L.; Mata-Martínez, E.; Chávez, J.C.; Sánchez-Cárdenas, C.; Baker, M.; Darszon, A. Role of Ion Channels in the Sperm Acrosome Reaction. Transform. Facial Reg. Hum. Embryo 2016, 220, 35–69. [Google Scholar] [CrossRef]
- Rossato, M.; Balercia, G.; Lucarelli, G.; Foresta, C.; Mantero, F. Role of seminal osmolarity in the reduction of human sperm motility. Int. J. Androl. 2002, 25, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Krapf, D.; de la Vega-Beltrán, J.L.; Acevedo, J.J.; Darszon, A. Ion channels, phosphorylation and mamma-lian sperm capacitation. Asian J. Androl. 2011, 13, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Buffone, M.G.; Wertheimer, E.V.; Visconti, P.E.; Krapf, D. Central role of soluble adenylyl cyclase and cAMP in sperm physiology. Biochim. Biophys. Acta 2014, 1842 Pt B, 2610–2620. [Google Scholar] [CrossRef]
- Balbach, M.; Gervasi, M.G.; Hidalgo, D.M.E.; Visconti, P.; Levin, L.R.; Buck, J. Metabolic changes in mouse sperm during capacitation. Biol. Reprod. 2020, 103, 791–801. [Google Scholar] [CrossRef]
- Romero, F.; Nishigaki, T. Comparative genomic analysis suggests that the sperm-specific sodium/proton exchanger and soluble adenylyl cyclase are key regulators of CatSper among the Metazoa. Zool. Lett. 2019, 5, 1–12. [Google Scholar] [CrossRef]
- Halmekytö, M.; Hyttinen, J.M.; Sinervirta, R.; Utriainen, M.; Myöhänen, S.; Voipio, H.M.; Wahlfors, J.; Syrjänen, S.; Syrjänen, K.; Alhonen, L. Transgenic mice aberrantly expressing human ornithine decarboxylase gene. J. Biol. Chem. 1991, 266, 19746–19751. [Google Scholar]
- Hakovirta, H.; Keiski, A.; Toppari, J.; Halmekytö, M.; Alhonen, L.; Jänne, J.; Parvinen, M. Polyamines and regulation of spermatogenesis: Selective stimulation of late spermatogonia in transgenic mice overexpressing the human ornithine decarbox-ylase gene. Mol. Endocrinol. 1993, 7, 1430–1436. [Google Scholar]
- Morris, D.R. A new perspective on ornithine decarboxylase regulation: Prevention of polyamine toxicity is the overrid-ing theme. J. Cell Biochem. 1991, 46, 102–105. [Google Scholar] [CrossRef]
- Uemura, T.; Gerner, E.W. Polyamine Transport Systems in Mammalian Cells and Tissues. Methods Mol. Biol. 2011, 720, 339–348. [Google Scholar] [CrossRef]
- Hernández-González, E.O.; Treviño, C.L.; Castellano, L.E.; De La Vega-Beltrán, J.L.; Ocampo, A.Y.; Wertheimer, E.; Visconti, P.E.; Darszon, A. Involvement of Cystic Fibrosis Transmembrane Conductance Regulator in Mouse Sperm Capacitation. J. Biol. Chem. 2007, 282, 24397–24406. [Google Scholar] [CrossRef] [PubMed]
- Yefimova, M.; Bourmeyster, N.; Becq, F.; Burel, A.; Lavault, M.-T.; Jouve, G.; Veau, S.; Pimentel, C.; Jégou, B.; Ravel, C. Update on the cellular and molecular aspects of cystic fibrosis transmembrane conductance regulator (CFTR) and male fertility. Morphologie 2019, 103, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Grasemann, H.; Shehnaz, D.; Enomoto, M.; Leadley, M.; Belik, J.; Ratjen, F. L-ornithine derived polyamines in cystic fibro-sis airways. PLoS ONE 2012, 7, e46618. [Google Scholar] [CrossRef] [PubMed]
- Lou, D.Y.; Dominguez, I.; Toselli, P.; Landesman-Bollag, E.; O’Brien, C.; Seldin, D.C. The Alpha Catalytic Subunit of Protein Kinase CK2 Is Required for Mouse Embryonic Development. Mol. Cell. Biol. 2007, 28, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Farinha, C.M.; Swiatecka-Urban, A.; Brautigan, D.L.; Jordan, P. Regulatory Crosstalk by Protein Kinases on CFTR Traf-ficking and Activity. Front. Chem. 2016, 4, 1. [Google Scholar] [CrossRef]
- Miller, M.R.; Mansell, S.A.; Meyers, S.A.; Lishko, P.V. Flagellar ion channels of sperm: Similarities and differences be-tween species. Cell Calcium 2015, 58, 105–113. [Google Scholar] [CrossRef]
- Vyklicka, L.; Lishko, P.V. Dissecting the signaling pathways involved in the function of sperm flagellum. Curr. Opin. Cell Biol. 2020, 63, 154–161. [Google Scholar] [CrossRef]
- Castellano, L.E.; Treviño, C.L.; Rodríguez, D.; Serrano, C.J.; Pacheco, J.; Tsutsumi, V.; Felix, R.; Darszon, A. Transient re-ceptor potential (TRPC) channels in human sperm: Expression, cellular localization and involvement in the regulation of flagel-lar motility. FEBS Lett. 2003, 541, 69–74. [Google Scholar] [CrossRef]
- Escoffier, J.; Krapf, D.; Navarrete, F.; Darszon, A.; Visconti, P.E. Flow cytometry analysis reveals a decrease in intracellu-lar sodium during sperm capacitation. J. Cell Sci. 2012, 125 Pt 2, 473–485. [Google Scholar] [CrossRef]
- Puga Molina, L.C.; Pinto, N.A.; Torres, N.I.; González-Cota, A.L.; Luque, G.M.; Balestrini, P.A.; Romarowski, A.; Krapf, D.; Santi, C.M.; Treviño, C.L.; et al. CFTR/ENaC-dependent regulation of membrane potential during human sperm capacitation is initiated by bicarbonate uptake through NBC. J. Biol. Chem. 2018, 293, 9924–9936. [Google Scholar] [CrossRef]
- Brown, S.G.; Publicover, S.J.; Barratt, C.L.R.; Da Silva, S.J.M. Human sperm ion channel (dys)function: Implications for fertilization. Hum. Reprod. Updat. 2019, 25, 758–776. [Google Scholar] [CrossRef] [PubMed]
- Santi, C.M.; Martínez-López, P.; De La Vega-Beltrán, J.L.; Butler, A.; Alisio, A.; Darszon, A.; Salkoff, L. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 2010, 584, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Suma, A.; Granata, D.; Thomson, A.S.; Carnevale, V.; Rothberg, B.S. Polyamine blockade and binding energetics in the MthK potassium channel. J. Gen. Physiol. 2020, 152, 7. [Google Scholar] [CrossRef]
- Ritagliati, C.; Baro Graf, C.; Stival, C.; Krapf, D. Regulation mechanisms and implications of sperm membrane hyperpo-larization. Mech. Dev. 2018, 154, 33–43. [Google Scholar] [CrossRef]
- Hernández-González, E.O.; Sosnik, J.; Edwards, J.; Acevedo, J.J.; Mendoza-Lujambio, I.; López-González, I.; Demarco, I.; Wertheimer, E.; Darszon, A.; Visconti, P.E. Sodium and epithelial sodium channels participate in the regulation of the capacita-tion-associated hyperpolarization in mouse sperm. J. Biol. Chem. 2006, 281, 5623–5633. [Google Scholar] [CrossRef] [PubMed]
- De La Vega-Beltran, J.L.; Sánchez-Cárdenas, C.; Krapf, D.; Hernandez-González, E.O.; Wertheimer, E.; Treviño, C.L.; Visconti, P.E.; Darszon, A. Mouse Sperm Membrane Potential Hyperpolarization Is Necessary and Sufficient to Prepare Sperm for the Acrosome Reaction. J. Biol. Chem. 2012, 287, 44384–44393. [Google Scholar] [CrossRef]
- Wang, D.; Hu, J.; Bobulescu, I.A.; Quill, T.A.; McLeroy, P.; Moe, O.W.; Garbers, D.L. A sperm-specific Na+/H+ exchanger (sNHE) is critical for expression and in vivo bicarbonate regulation of the soluble adenylyl cyclase (sAC). Proc. Natl. Acad. Sci. USA 2007, 104, 9325–9330. [Google Scholar] [CrossRef]
- Nishigaki, T.; José, O.; González-Cota, A.L.; Romero, F.; Treviño, C.L.; Darszon, A. Intracellular pH in sperm physiology. Biochem. Biophys. Res. Commun. 2014, 450, 1149–1158. [Google Scholar] [CrossRef]
- Martins, A.D.; Bernardino, R.L.; Neuhaus-Oliveira, A.; Sousa, M.; Sá, R.; Alves, M.G.; Oliveira, P.F. Physiology of Na+/H+ Exchangers in the Male Reproductive Tract: Relevance for Male Fertility. Biol. Reprod. 2014, 91, 11. [Google Scholar] [CrossRef]
- Hess, K.C.; Jones, B.H.; Marquez, B.; Chen, Y.; Ord, T.S.; Kamenetsky, M.; Miyamoto, C.; Zippin, J.H.; Kopf, G.S.; Suarez, S.S.; et al. The “Soluble” Adenylyl Cyclase in Sperm Mediates Multiple Signaling Events Required for Fertilization. Dev. Cell 2005, 9, 249–259. [Google Scholar] [CrossRef]
- Shah, G.V.; Sheth, A.R.; Mugatwala, P.P.; Rao, S.S. Effect of spermine on adenyl cyclase activity of spermatozoa. Experientia 1975, 31, 631–632. [Google Scholar] [CrossRef]
- Casillas, E.R.; Elder, C.M.; Hoskins, D.D. Adenylate cyclase activity of bovine spermatozoa during maturation in the epididymis and the activation of sperm particulate adenylate cyclase by GTP and polyamines. J. Reprod. Fertil. 1980, 59, 297–302. [Google Scholar] [CrossRef]
- Shah, G.V.; Sheth, A.R. Inhibition of phosphodiesterase activity of human spermatozoa by spermine. Experientia 1978, 34, 980–981. [Google Scholar] [CrossRef]
- Méndez, J.D.; Hernández, M.P. Effect of L-arginine and polyamines on sperm motility. Ginecol. Obstet. Mex. 1993, 61, 229–234. [Google Scholar]
- Morales, M.E.; Rico, G.; Bravo, C.; Tapia, R.; Alvarez, C.; Méndez, J.D. Progressive motility increase caused by L-arginine and polyamines in sperm from patients with idiopathic and diabetic asthenozoospermia. Ginecol. Obstet. Mex. 2003, 71, 297–303. [Google Scholar] [PubMed]
- Cordero-Martínez, J.; Flores-Alonso, J.C.; Aguirre-Alvarado, C.; Oviedo, N.; Alcántara-Farfán, V.; García-Pérez, C.A.; Bermúdez-Ruiz, K.F.; Jiménez-Gutiérrez, G.E.I.; Rodriguez-Paez, L.; De Anda, N.A.O. Influence of Echeveria gibbiflora DC aqueous crude extract on mouse sperm energy metabolism and calcium-dependent channels. J. Ethnopharmacol. 2019, 248, 112321. [Google Scholar] [CrossRef]
- Cordero-Martínez, J.; Reyes-Miguel, T.; Rodríguez-Páez, L.; Garduño-Siciliano, L.; Maldonado-García, D.; Roa-Espitia, A.L.; Hernández-González, E.O. TMEM16A inhibition impedes capacitation and acquisition of hyperactivated motility in guinea pig sperm. J. Cell. Biochem. 2018, 119, 5944–5959. [Google Scholar] [CrossRef]
- Wang, L.-W.; Chen, L.-X.; Liu, S.-W.; Li, Y.; Zou, L.-L.; Guan, Y.-T.; Peng, S.; Zheng, L.-X.; Deng, S.-M.; Zhu, L.-Y. Chloride channels are involved in sperm motility and are downregulated in spermatozoa from patients with asthenozoospermia. Asian J. Androl. 2017, 19, 418–424. [Google Scholar] [CrossRef]
- Li, K.; Xue, Y.; Chen, A.; Jiang, Y.; Xie, H.; Shi, Q.; Zhang, S.; Ni, Y. Heat Shock Protein 90 Has Roles in Intracellular Calcium Homeostasis, Protein Tyrosine Phosphorylation Regulation, and Progesterone-Responsive Sperm Function in Human Sperm. PLoS ONE 2014, 9, e115841. [Google Scholar] [CrossRef]
- Vílchez, M.C.; Morini, M.; Peñaranda, D.S.; Gallego, V.; Asturiano, J.F.; Pérez, L. Sodium affects the sperm motility in the European eel. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 198, 51–58. [Google Scholar] [CrossRef]
- Beacham, D.W.; Blackmer, T.; Grady, M.O.; Hanson, G.T. Cell-Based Potassium Ion Channel Screening Using the FluxOR™ Assay. J. Biomol. Screen. 2010, 15, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.K. The Effect of Curcumin on Intracellular pH (pHi), Membrane Hyperpolarization and Sperm Motility. J. Reprod. Infertil. 2014, 15, 62–70. [Google Scholar] [PubMed]
- Bhattacharjee, R.; Goswami, S.; Dey, S.; Gangoda, M.; Brothag, C.; Eisa, A.; Woodgett, J.; Phiel, C.; Kline, D.; Vijayara-ghavan, S. Isoform-specific requirement for GSK3α in sperm for male fertility. Biol. Reprod. 2018, 99, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Kleinboelting, S.; Ramos-Espiritu, L.; Buck, H.; Colis, L.; Heuvel, J.V.D.; Glickman, J.F.; Levin, L.R.; Buck, J.; Steegborn, C. Bithionol Potently Inhibits Human Soluble Adenylyl Cyclase through Binding to the Allosteric Activator Site. J. Biol. Chem. 2016, 291, 9776–9784. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and Auto-DockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Páez, L.; Aguirre-Alvarado, C.; Oviedo, N.; Alcántara-Farfán, V.; Lara-Ramírez, E.E.; Jimenez-Gutierrez, G.E.; Cordero-Martínez, J. Polyamines Influence Mouse Sperm Channels Activity. Int. J. Mol. Sci. 2021, 22, 441. https://doi.org/10.3390/ijms22010441
Rodríguez-Páez L, Aguirre-Alvarado C, Oviedo N, Alcántara-Farfán V, Lara-Ramírez EE, Jimenez-Gutierrez GE, Cordero-Martínez J. Polyamines Influence Mouse Sperm Channels Activity. International Journal of Molecular Sciences. 2021; 22(1):441. https://doi.org/10.3390/ijms22010441
Chicago/Turabian StyleRodríguez-Páez, Lorena, Charmina Aguirre-Alvarado, Norma Oviedo, Verónica Alcántara-Farfán, Edgar E. Lara-Ramírez, Guadalupe Elizabeth Jimenez-Gutierrez, and Joaquín Cordero-Martínez. 2021. "Polyamines Influence Mouse Sperm Channels Activity" International Journal of Molecular Sciences 22, no. 1: 441. https://doi.org/10.3390/ijms22010441
APA StyleRodríguez-Páez, L., Aguirre-Alvarado, C., Oviedo, N., Alcántara-Farfán, V., Lara-Ramírez, E. E., Jimenez-Gutierrez, G. E., & Cordero-Martínez, J. (2021). Polyamines Influence Mouse Sperm Channels Activity. International Journal of Molecular Sciences, 22(1), 441. https://doi.org/10.3390/ijms22010441





