Localization and Dynamics of the Methionine Sulfoxide Reductases MsrB1 and MsrB2 in Beech Seeds
Abstract
1. Introduction
2. Results
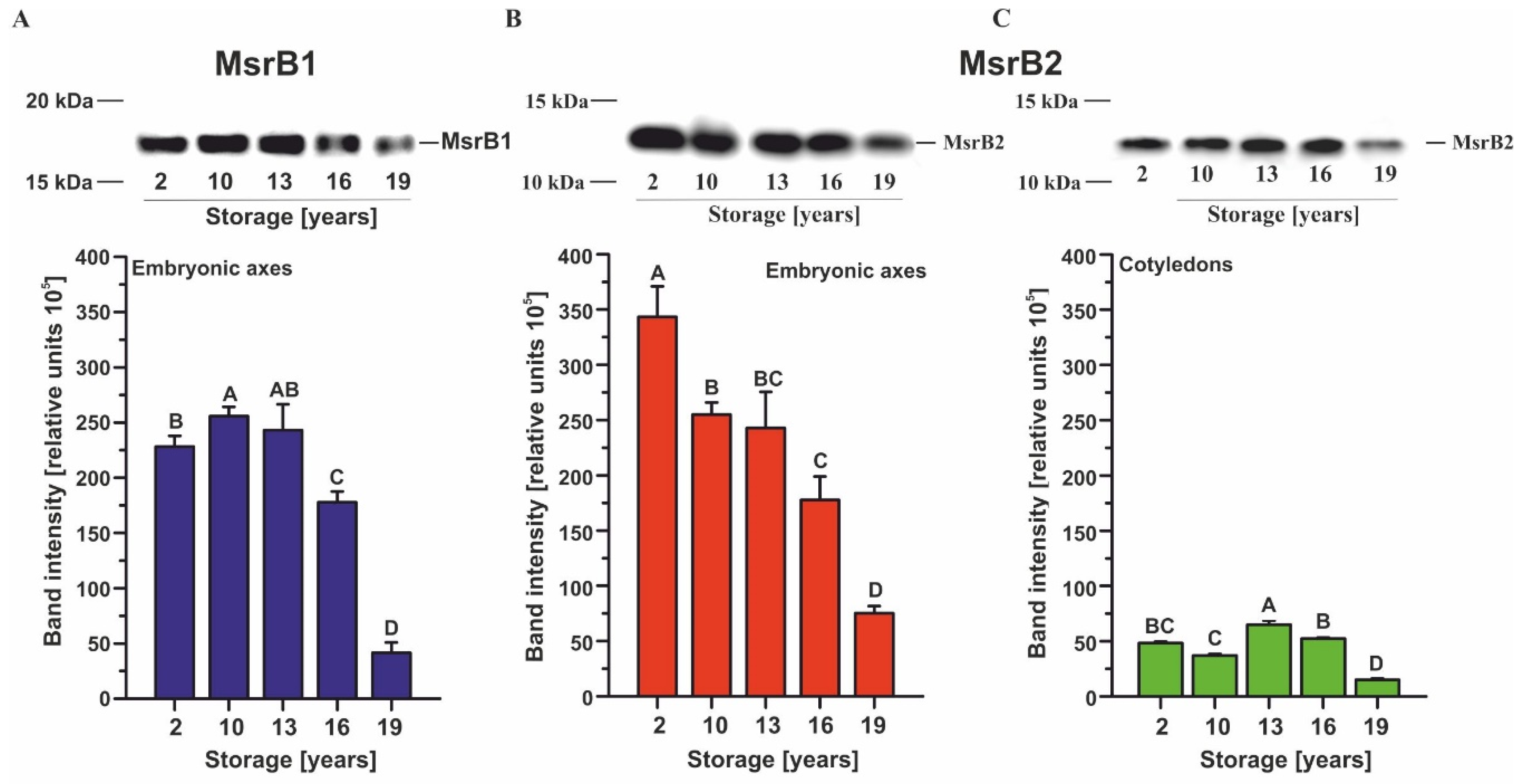
2.1. Immunodetection of MsrB1 and MsrB2
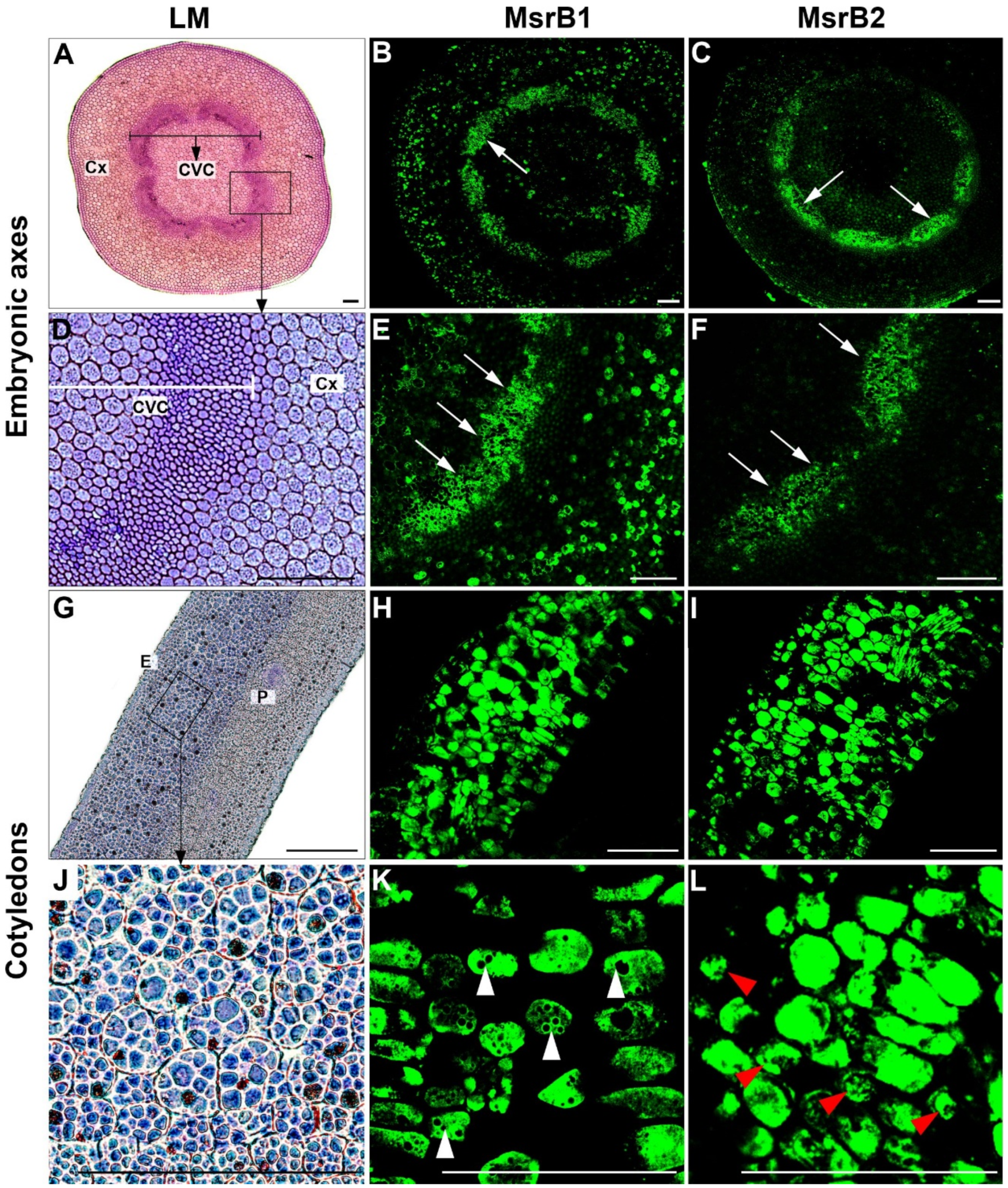
2.2. Tissue Localization of MsrB1 and MsrB2 in Beech Seeds
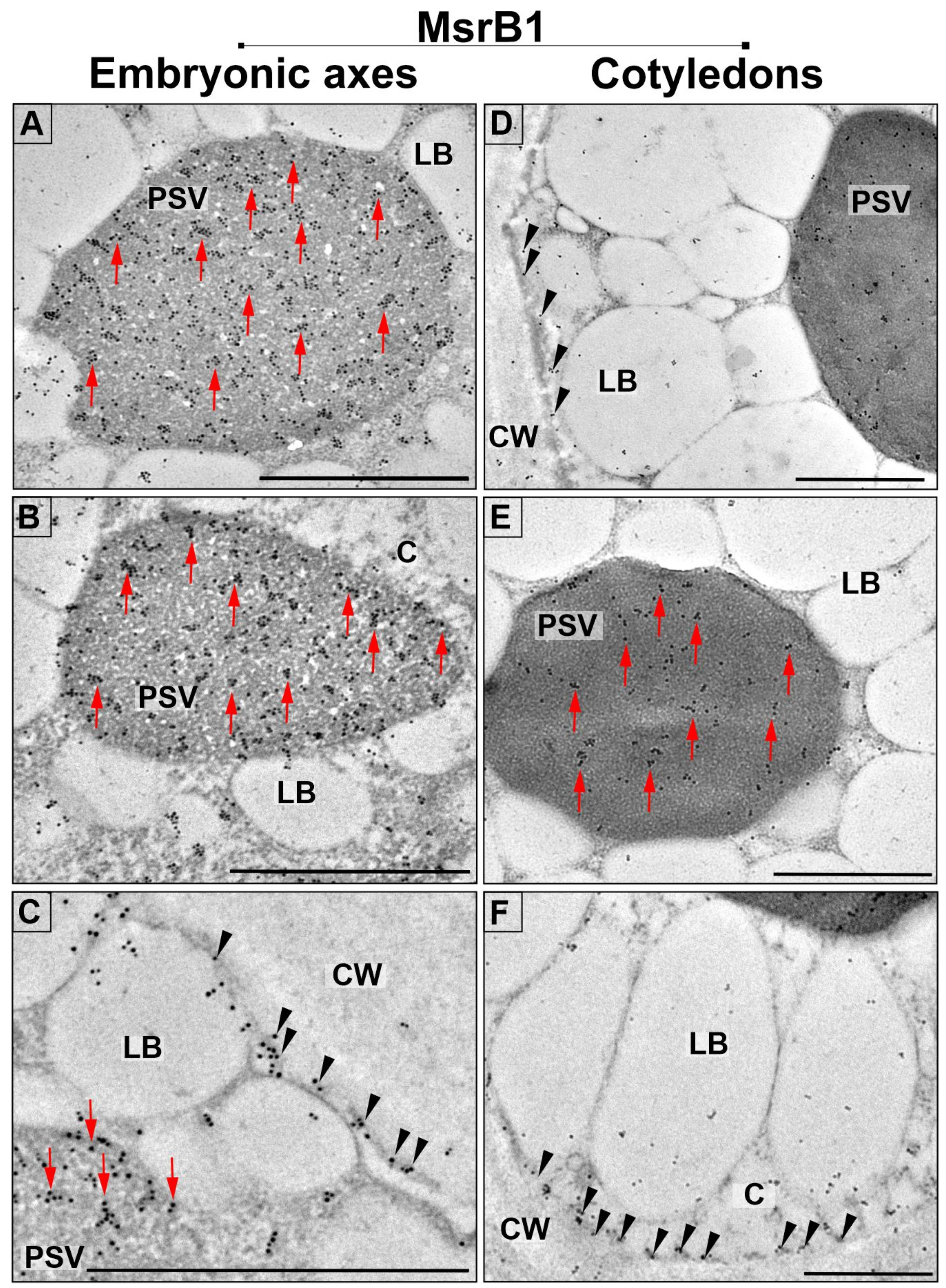
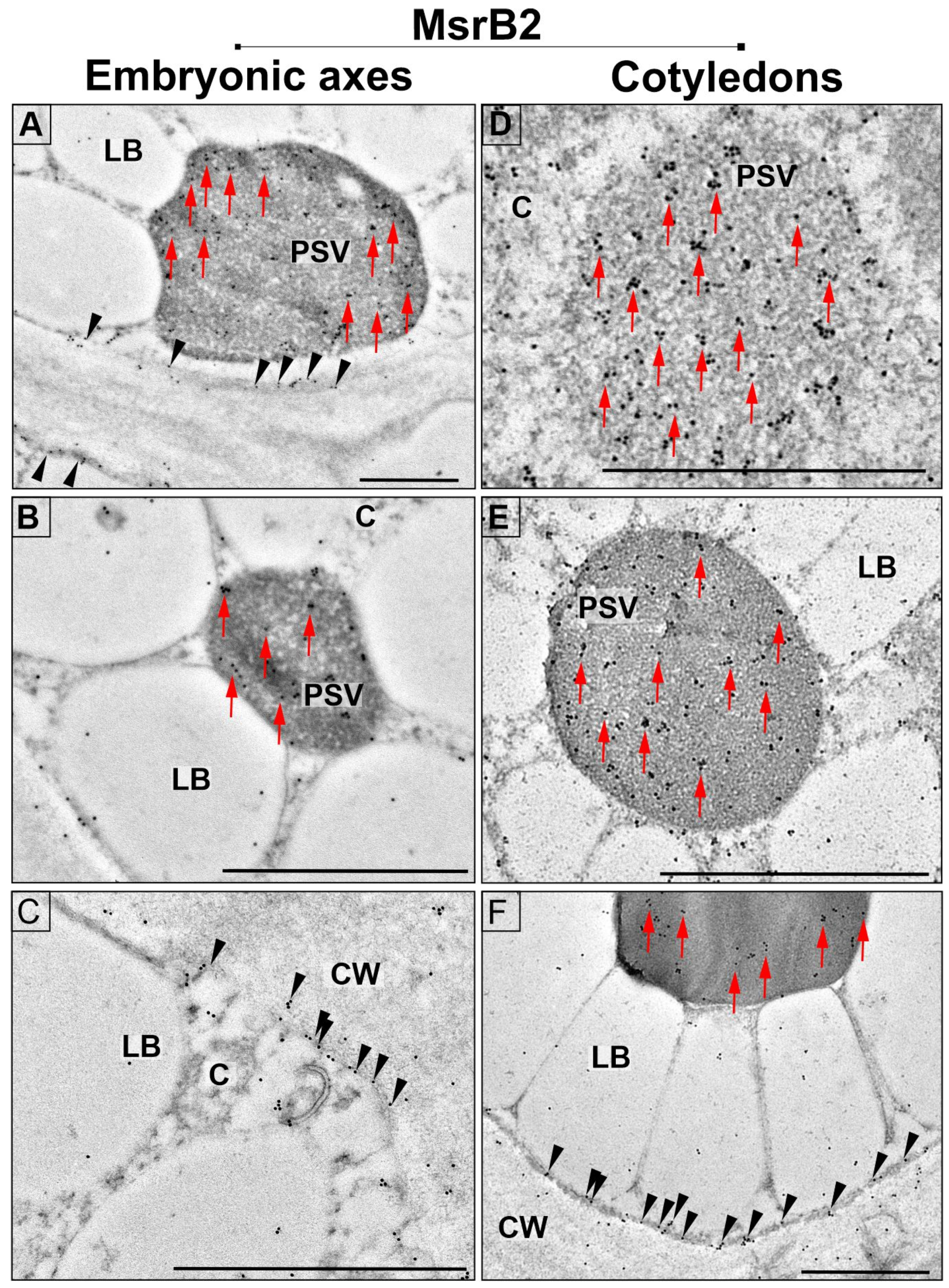
2.3. Subcellular Localization of MsrB1 and MsrB2 in Beech Seeds
2.4. Bioinformatics Analyses
2.4.1. Protein–Membrane Interactions
2.4.2. Protein–Nucleic Acid and Protein–Protein Interactions
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Protein Extraction and Western Blotting
4.3. Anatomical Studies
4.4. Immunolocalization of MsrB
4.4.1. Immunofluorescence
4.4.2. Immunocytochemistry
4.5. Bioinformatic Analyses
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cys | Cysteine |
| Grx | Glutaredoxin |
| HRP | Horseradish peroxidase |
| LBs | Lipid bodies |
| Met | Methionine |
| MetO | Methionine sulfoxide |
| Msr | Methionine sulfoxide reductase |
| PBS | Phosphate-buffered saline |
| PSV | Protein storage vacuoles |
| ROS | Reactive Oxygen Species |
| TSA | Tyramine Signal Amplification |
| Trx | Thioredoxin |
| WC | Water content |
References
- Brunet, J.; Fritz, Ö.; Richnau, G. Biodiversity in European beech forests-a review with recommendations for sustainable forest management. Ecol. Bull. 2010, 53, 77–94. [Google Scholar]
- Meller, S.; Frossard, E.; Luster, J. Phosphorus Allocation to Leaves of Beech Saplings Reacts to Soil Phosphorus Availability. Front. Plant. Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Leon-Lobos, P.; Ellis, R.H. Seed storage behaviour of Fagus sylvatica and Fagus crenata. Seed Sci. Res. 2002, 12, 31–37. [Google Scholar] [CrossRef]
- Ratajczak, E.; Staszak, A.; Wojciechowska, N.; Bagniewska-Zadworna, A.; Dietz, K. Regulation of thiol metabolism as a factor that influences the development and storage capacity of beech seeds. J. Plant. Physiol. 2019, 239, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Suszka, J.; Muller, C.; Bonnet-Masimbert, M.; Gordon, A. Seeds of Forest Broadleaves: From Harvest to Sowing; INRA: Paris, France, 1996. [Google Scholar]
- Pukacka, S.; Hoffmann, S.K.; Goslar, J.; Pukacki, P.M.; Wójkiewicz, E. Water and lipid relations in beech (Fagus sylvatica L.) seeds and its effect on storage behaviour. Biochim. Biophys. Acta 2003, 1621, 48–56. [Google Scholar] [CrossRef]
- Bailly, C. Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Kurek, K.; Plitta-Michalak, B.; Ratajczak, E. Reactive oxygen species as potential drivers of the seed aging process. Plants 2019, 8, 174. [Google Scholar] [CrossRef]
- Pukacka, S.; Ratajczak, E. Age-related biochemical changes during storage of beech (Fagus sylvatica L.) seeds. Seed Sci. Res. 2007, 17, 45–53. [Google Scholar] [CrossRef]
- Kalemba, E.M.; Pukacka, S. Carbonylated proteins accumulated as vitality decreases during long-term storage of beech (Fagus sylvatica L.) seeds. Trees 2014. [Google Scholar] [CrossRef]
- Rouhier, N.; Vieira Dos Santos, C.; Tarrago, L.; Rey, P. Plant methionine sulfoxide reductase A and B multigenic families. Photosyn. Res. 2006, 89, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Rey, P.; Tarrago, L. Physiological roles of plant methionine sulfoxide reductases in redox homeostasis and signaling. Antioxidants 2018, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-W.; Lee, S.-H.; Chieh, P.-S.; Lin, C.-S.; Wang, Y.-C.; Chan, M.-T. Arabidopsis root-abundant cytosolic methionine sulfoxide reductase B genes MsrB7 and MsrB8 are involved in tolerance to oxidative stress. Plant. Cell Physiol. 2012, 53, 1707–1719. [Google Scholar] [CrossRef] [PubMed]
- Brot, N.; Weissbach, L.; Werth, J.; Weissbach, H. Enzymatic reduction of protein-bound methionine sulfoxide. Proc. Natl. Acad. Sci. USA 1981, 78, 2155–2158. [Google Scholar] [CrossRef]
- Grimaud, R.; Ezraty, B.; Mitchell, J.K.; Lafitte, D.; Briand, C.; Derrick, P.J.; Barras, F. Repair of oxidized proteins. Identification of a new methionine sulfoxide reductase. J. Biol. Chem. 2001, 276, 48915–48920. [Google Scholar] [CrossRef]
- Lowther, W.T.; Weissbach, H.; Etienne, F.; Brot, N.; Matthews, B.W. The mirrored methionine sulfoxide reductases of Neisseria gonorrhoeae pilB. Nat. Struct. Biol. 2002, 9, 348–352. [Google Scholar] [CrossRef]
- Boschi-Muller, S.; Azza, S.; Sanglier-Cianferani, S.; Talfournier, F.; Van Dorsselear, A.; Branlant, G. A sulfenic acid enzyme intermediate is involved in the catalytic mechanism of peptide methionine sulfoxide reductase from Escherichia coli. J. Biol. Chem. 2000, 275, 35908–35913. [Google Scholar] [CrossRef]
- Tarrago, L.; Laugier, E.; Rey, P. Protein-repairing methionine sulfoxide reductases in photosynthetic organisms: Gene organization, reduction mechanisms, and physiological roles. Mol. Plant. 2009, 2, 202–217. [Google Scholar] [CrossRef]
- Vieira Dos Santos, C.V.; Cuiné, S.; Rouhier, N.; Rey, P. The Arabidopsis plastidic methionine sulfoxide reductase B proteins. sequence and activity characteristics, comparison of the expression with plastidic methionine sulfoxide reductase A, and induction by photooxidative stress. Plant. Physiol. 2005, 138, 909–922. [Google Scholar] [CrossRef]
- Sagher, D.; Brunell, D.; Brot, N.; Vallee, B.L.; Weissbach, H. Selenocompounds can serve as oxidoreductants with the methionine sulfoxide reductase enzymes. J. Biol. Chem. 2006, 281, 31184–31187. [Google Scholar] [CrossRef]
- Doney, R.C.; Thompson, J.F. The reduction of S-methyl-L-cysteine sulfoxide and L-methionine sulfoxide in turnip and bean leaves. Biochim. Biophys. Acta 1966, 124, 39–49. [Google Scholar] [CrossRef]
- Laugier, E.; Tarrago, L.; Santos, C.V.D.; Eymery, F.; Havaux, M.; Rey, P. Arabidopsis thaliana plastidial methionine sulfoxide reductases B, MSRBs, account for most leaf peptide MSR activity and are essential for growth under environmental constraints through a role in the preservation of photosystem antennae. Plant. J. 2010, 61, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Park, H.-M.; Chae, S.; Lee, T.-H.; Hwang, D.-J.; Oh, S.-D.; Park, J.-S.; Song, D.-G.; Pan, C.-H.; Choi, D.; et al. A pepper MSRB2 gene confers drought tolerance in rice through the protection of chloroplast-targeted genes. PLoS ONE 2014, 9, e90588. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-K.; Baek, K.-H.; Seong, E.S.; Joung, Y.H.; Choi, G.-J.; Park, J.M.; Cho, H.S.; Kim, E.A.; Lee, S.; Choi, D. CaMsrB2, pepper methionine sulfoxide reductase B2, is a novel defense regulator against oxidative stress and pathogen attack. Plant. Physiol. 2010, 154, 245–261. [Google Scholar] [CrossRef]
- Roy, S.; Nandi, A.K. Arabidopsis thaliana methionine sulfoxide reductase B8 influences stress-induced cell death and effector-triggered immunity. Plant. Mol. Biol. 2017, 93, 109–120. [Google Scholar] [CrossRef]
- Stolarska, E.; Bilska, K.; Wojciechowska, N.; Bagniewska-Zadworna, A.; Rey, P.; Kalemba, E.M. Integration of methionine sulfoxide reductases B1 and B2 and of their reduction systems in the redox network during the development of contrasted Norway maple and sycamore seeds. Under review.
- Wojciechowska, N.; Alipour, S.; Stolarska, E.; Bilska, K.; Rey, P.; Kalemba, E.M. Peptide-bound methionine sulfoxide (MetO) Levels and MsrB2 abundance are differentially regulated during the desiccation phase in contrasted Acer seeds. Antioxidants 2020, 9, 391. [Google Scholar] [CrossRef]
- Châtelain, E.; Satour, P.; Laugier, E.; Vu, B.L.; Payet, N.; Rey, P.; Montrichard, F. Evidence for participation of the methionine sulfoxide reductase repair system in plant seed longevity. Proc. Natl. Acad. Sci. USA 2013, 110, 3633–3638. [Google Scholar] [CrossRef]
- Jiang, G.; Xiao, L.; Yan, H.; Zhang, D.; Wu, F.; Liu, X.; Su, X.; Dong, X.; Wang, J.; Duan, X.; et al. Redox regulation of methionine in calmodulin affects the activity levels of senescence-related transcription factors in litchi. Biochim. Biophys. Acta BBA 2017, 1861, 1140–1151. [Google Scholar] [CrossRef]
- Jiang, G.; Wu, F.; Li, Z.; Li, T.; Gupta, V.K.; Duan, X.; Jiang, Y. Sulfoxidation regulation of Musa acuminata calmodulin (MaCaM) influences the functions of MaCaM-binding proteins. Plant. Cell Physiol. 2018, 59, 1214–1224. [Google Scholar] [CrossRef]
- Zimmermann, P.; Hirsch-Hoffmann, M.; Hennig, L.; Gruissem, W. GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant. Physiol. 2004, 136, 2621–2632. [Google Scholar] [CrossRef]
- Chen, Y.; Shanmugam, S.K.; Dalbey, R.E. The principles of protein targeting and transport across cell membranes. Protein J. 2019, 38, 236–248. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, M.; Bonatti, S. Mechanisms controlling the activity of localization signal sequences. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-12-809633-8. [Google Scholar]
- Guo, X.; Wu, Y.; Wang, Y.; Chen, Y.; Chu, C. OsMSRA4.1 and OsMSRB1.1, two rice plastidial methionine sulfoxide reductases, are involved in abiotic stress responses. Planta 2009, 230, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wen, L.; Gao, X.; Jin, C.; Xue, Y.; Yao, X. CSS-Palm 2.0: An updated software for palmitoylation sites prediction. Protein Eng. Des. Sel. 2008, 21, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, M.-J.; Moskovitz, J.; Salamini, F.; Bartels, D. Reverse genetic approaches in plants and yeast suggest a role for novel, evolutionarily conserved, selenoprotein-related genes in oxidative stress defense. Mol. Gen. Genom. 2002, 267, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, B.J.W.; Costa, M.C.D.; Maia, J.; Bentsink, L.; Ligterink, W.; Hilhorst, H.W.M. Acquisition and loss of desiccation tolerance in seeds: From experimental model to biological relevance. Planta 2015, 241, 563–577. [Google Scholar] [CrossRef]
- Sánchez, J.; Nikolau, B.J.; Stumpf, P.K. Reduction of N-acetyl methionine sulfoxide in plants. Plant. Physiol. 1983, 73, 619–623. [Google Scholar] [CrossRef]
- Laugier, E.; Tarrago, L.; Courteille, A.; Innocenti, G.; Eymery, F.; Rumeau, D.; Issakidis-Bourguet, E.; Rey, P. Involvement of thioredoxin y2 in the preservation of leaf methionine sulfoxide reductase capacity and growth under high light. Plant. Cell Environ. 2013, 36, 670–682. [Google Scholar] [CrossRef]
- Ratajczak, E.; Kalemba, E.M.; Pukacka, S. Age-related changes in protein metabolism of beech (Fagus sylvatica L.) seeds during alleviation of dormancy and in the early stage of germination. Plant. Physiol. Biochem. 2015, 94, 114–121. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Parihar, P.; Mishra, R.K.; Tripathi, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M. Reactive oxygen species (ROS): Beneficial companions of plants’ developmental processes. Front. Plant. Sci. 2016, 7. [Google Scholar] [CrossRef]
- Marzec-Schmidt, K.; Wojciechowska, N.; Nemeczek, K.; Ludwików, A.; Mucha, J.; Bagniewska-Zadworna, A. Allies or Enemies: The role of reactive oxygen species in developmental processes of black cottonwood (Populus trichocarpa). Antioxidants 2020, 9, 199. [Google Scholar] [CrossRef]
- Pukacka, S.; Ratajczak, E. Production and scavenging of reactive oxygen species in Fagus sylvatica seeds during storage at varied temperature and humidity. J. Plant. Physiol. 2005, 162, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, J.-M.; Rogers, J.C. Sorting of proteins to vacuoles in plant cells. In Protein Trafficking in Plant. Cells; Soll, J., Ed.; Springer: Dordrecht, The Netherlands, 1998; pp. 127–144. ISBN 978-94-011-5298-3. [Google Scholar]
- Ishida, H.; Izumi, M.; Wada, S.; Makino, A. Roles of autophagy in chloroplast recycling. Biochim. Biophys. Acta 2014, 1837, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Izumi, M.; Nakamura, S.; Li, N. Autophagic Turnover of chloroplasts: Its roles and regulatory mechanisms in response to sugar starvation. Front. Plant. Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Penfield, S.; Graham, S.; Graham, I.A. Storage reserve mobilization in germinating oilseeds: Arabidopsis as a model system. Biochem. Soc. Trans. 2005, 33, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Eliášová, K.; Pešek, B.; Vondráková, Z. Storage compounds, ABA and fumarase in Fagus sylvatica embryos during stratification. Dendrobiology 2015, 74, 25–32. [Google Scholar] [CrossRef]
- Kalemba, E.M.; Bagniewska-Zadworna, A.; Suszka, J.; Pukacka, S. Dehydration sensitivity at the early seedling establishment stages of the european beech (Fagus sylvatica L.). Forests 2019, 10, 900. [Google Scholar] [CrossRef]
- Gao, J.; Yin, D.H.; Yao, Y.; Sun, H.; Qin, Z.; Schöneich, C.; Williams, T.D.; Squier, T.C. Loss of conformational stability in calmodulin upon methionine oxidation. Biophys. J. 1998, 74, 1115–1134. [Google Scholar] [CrossRef]
- El-Maarouf-Bouteau, H.; Meimoun, P.; Job, C.; Job, D.; Bailly, C. Role of protein and mRNA oxidation in seed dormancy and germination. Front. Plant. Sci. 2013, 4, 77. [Google Scholar] [CrossRef]
- Walters, C. Orthodoxy, recalcitrance and in-between: Describing variation in seed storage characteristics using threshold responses to water loss. Planta 2015, 242, 397–406. [Google Scholar] [CrossRef]
- Furt, F.; Simon-Plas, F.; Mongrand, S. Lipids of the Plant Plasma Membrane. In The Plant Plasma Membrane; Springer: Berlin/Heidelberg, Germany, 2010; Volume 19. [Google Scholar]
- Pöyry, S.; Vattulainen, I. Role of charged lipids in membrane structures-Insight given by simulations. Biochim. Biophys. Acta 2016, 1858, 2322–2333. [Google Scholar] [CrossRef]
- Guan, X.; Fierke, C.A. Understanding protein palmitoylation: Biological significance and enzymology. Sci. China Chem. 2011, 54, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Cole, N.B.; Lim, J.C.; Zhao, H.; Levine, R.L. Dual sites of protein initiation control the localization and myristoylation of methionine sulfoxide reductase A. J. Biol. Chem. 2016, 291, 22338. [Google Scholar] [CrossRef] [PubMed]
- Kibinza, S.; Vinel, D.; Côme, D.; Bailly, C.; Corbineau, F. Sunflower seed deterioration as related to moisture content during ageing, energy metabolism and active oxygen species scavenging. Physiol. Plant. 2006, 128, 496–506. [Google Scholar] [CrossRef]
- Pukacka, S.; Ratajczak, E. Factors influencing the storability of Fagus sylvatica L. seeds after release from dormancy. Plant. Growth Regul. 2014, 72, 17–27. [Google Scholar] [CrossRef][Green Version]
- Bewley, J.D.; Bradford, K.; Hilhorst, H.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-4692-7. [Google Scholar]
- Sun, X.; Sun, M.; Jia, B.; Qin, Z.; Yang, K.; Chen, C.; Yu, Q.; Zhu, Y. A Glycine soja methionine sulfoxide reductase B5a interacts with the Ca(2+) /CAM-binding kinase GsCBRLK and activates ROS signaling under carbonate alkaline stress. Plant. J. 2016, 86, 514–529. [Google Scholar] [CrossRef] [PubMed]
- Tarrago, L.; Laugier, E.; Zaffagnini, M.; Marchand, C.; Le Maréchal, P.; Rouhier, N.; Lemaire, S.D.; Rey, P. Regeneration mechanisms of Arabidopsis thaliana methionine sulfoxide reductases b by glutaredoxins and thioredoxins. J. Biol. Chem. 2009, 284, 18963–18971. [Google Scholar] [CrossRef] [PubMed]
- Couturier, J.; Vignols, F.; Jacquot, J.-P.; Rouhier, N. Glutathione- and glutaredoxin-dependent reduction of methionine sulfoxide reductase A. FEBS Lett. 2012, 586, 3894–3899. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wojciechowska, N.; Marzec-Schmidt, K.; Kalemba, E.M.; Zarzyńska-Nowak, A.; Jagodziński, A.M.; Bagniewska-Zadworna, A. Autophagy counteracts instantaneous cell death during seasonal senescence of the fine roots and leaves in Populus trichocarpa. BMC Plant. Biol 2018, 18, 260. [Google Scholar] [CrossRef]
- Wojciechowska, N.; Smugarzewska, I.; Marzec-Schmidt, K.; Zarzyńska-Nowak, A.; Bagniewska-Zadworna, A. Occurrence of autophagy during pioneer root and stem development in Populus trichocarpa. Planta 2019, 250, 1789–1801. [Google Scholar] [CrossRef]
- Gofman, Y.; Haliloglu, T.; Ben-Tal, N. Monte Carlo simulations of peptide–membrane interactions with the MCPep web server†. Nucleic. Acids Res. 2012, 40, W358–W363. [Google Scholar] [CrossRef] [PubMed]
- Käll, L.; Krogh, A.; Sonnhammer, E.L.L. Advantages of combined transmembrane topology and signal peptide prediction—The Phobius web server. Nucleic. Acids Res. 2007, 35, W429–W432. [Google Scholar] [CrossRef] [PubMed]
- Ofran, Y.; Rost, B. ISIS: Interaction sites identified from sequence. Bioinformatics 2007, 23, e13–e16. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein–protein association networks, made broadly accessible. Nucleic. Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojciechowska, N.; Bagniewska-Zadworna, A.; Minicka, J.; Michalak, K.M.; Kalemba, E.M. Localization and Dynamics of the Methionine Sulfoxide Reductases MsrB1 and MsrB2 in Beech Seeds. Int. J. Mol. Sci. 2021, 22, 402. https://doi.org/10.3390/ijms22010402
Wojciechowska N, Bagniewska-Zadworna A, Minicka J, Michalak KM, Kalemba EM. Localization and Dynamics of the Methionine Sulfoxide Reductases MsrB1 and MsrB2 in Beech Seeds. International Journal of Molecular Sciences. 2021; 22(1):402. https://doi.org/10.3390/ijms22010402
Chicago/Turabian StyleWojciechowska, Natalia, Agnieszka Bagniewska-Zadworna, Julia Minicka, Kornel M. Michalak, and Ewa M. Kalemba. 2021. "Localization and Dynamics of the Methionine Sulfoxide Reductases MsrB1 and MsrB2 in Beech Seeds" International Journal of Molecular Sciences 22, no. 1: 402. https://doi.org/10.3390/ijms22010402
APA StyleWojciechowska, N., Bagniewska-Zadworna, A., Minicka, J., Michalak, K. M., & Kalemba, E. M. (2021). Localization and Dynamics of the Methionine Sulfoxide Reductases MsrB1 and MsrB2 in Beech Seeds. International Journal of Molecular Sciences, 22(1), 402. https://doi.org/10.3390/ijms22010402





