The Involvement of Xanthone and (E)-Cinnamoyl Chromophores for the Design and Synthesis of Novel Sunscreening Agents
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Lipophilicity Evaluation
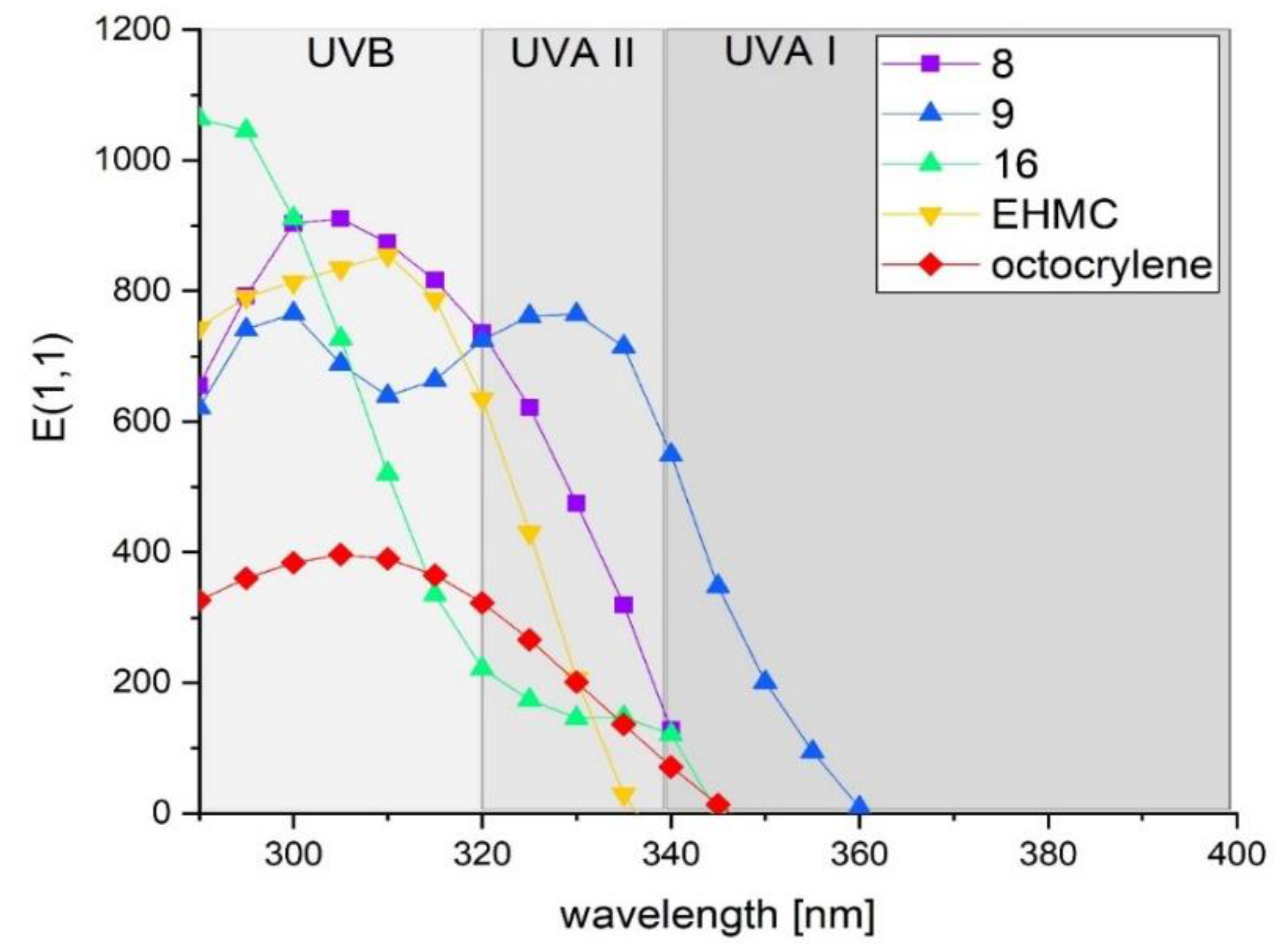
2.3. Ultraviolet Spectroscopic Properties
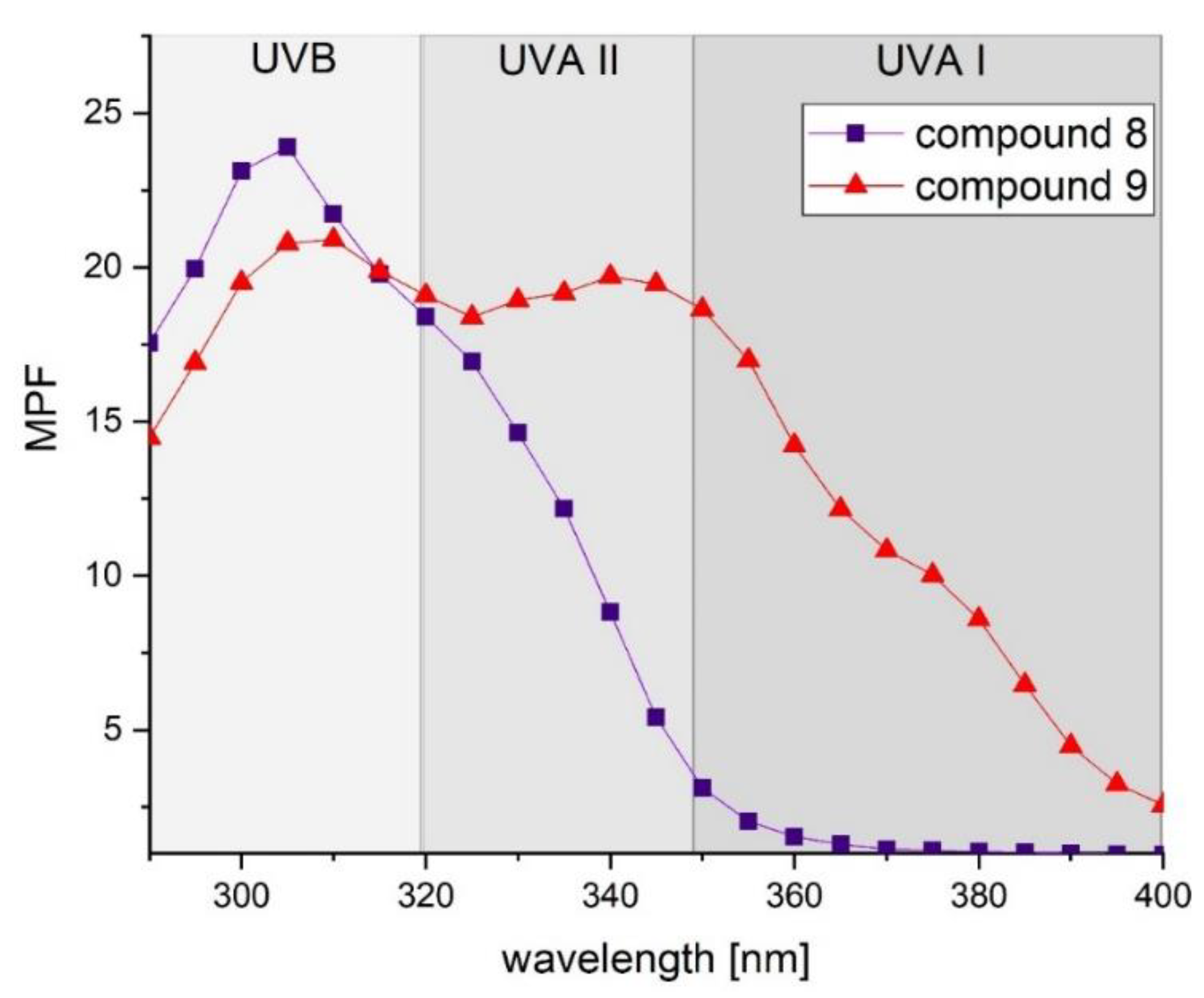
2.4. Photoprotective Activity
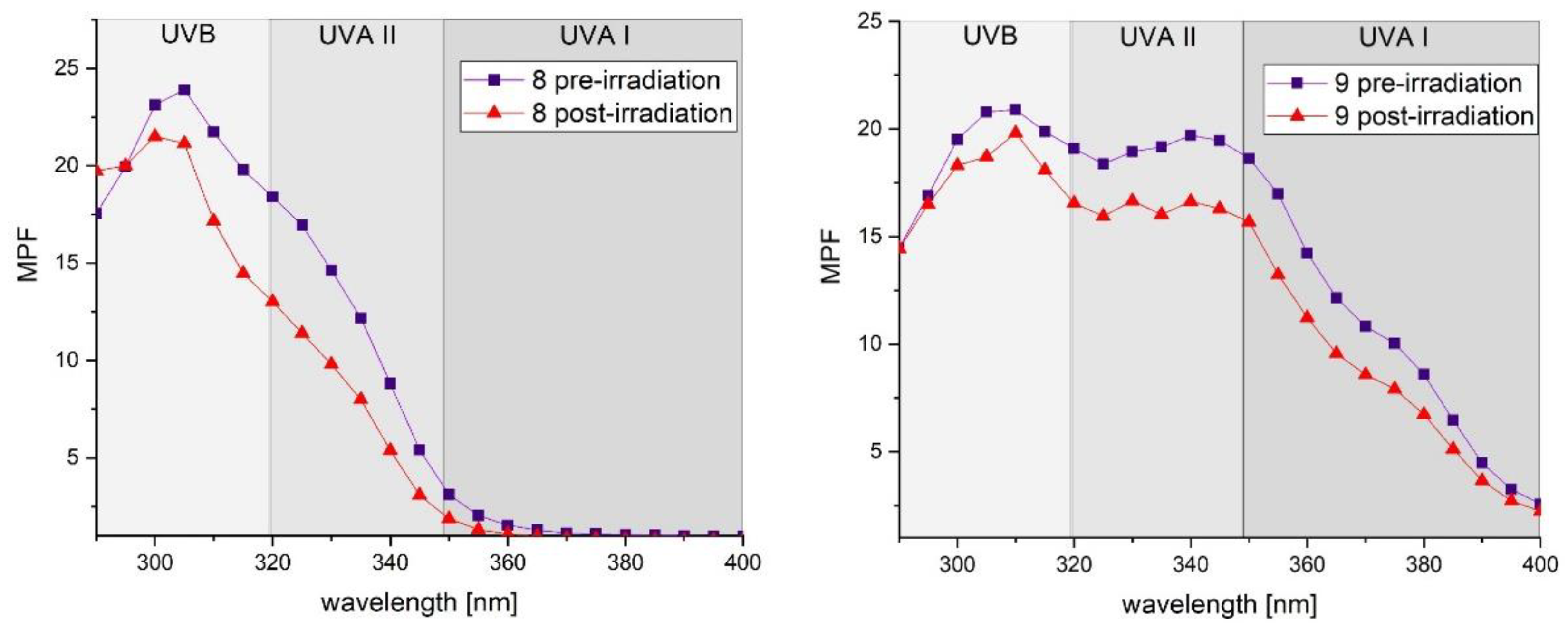
2.5. Photostability Studies
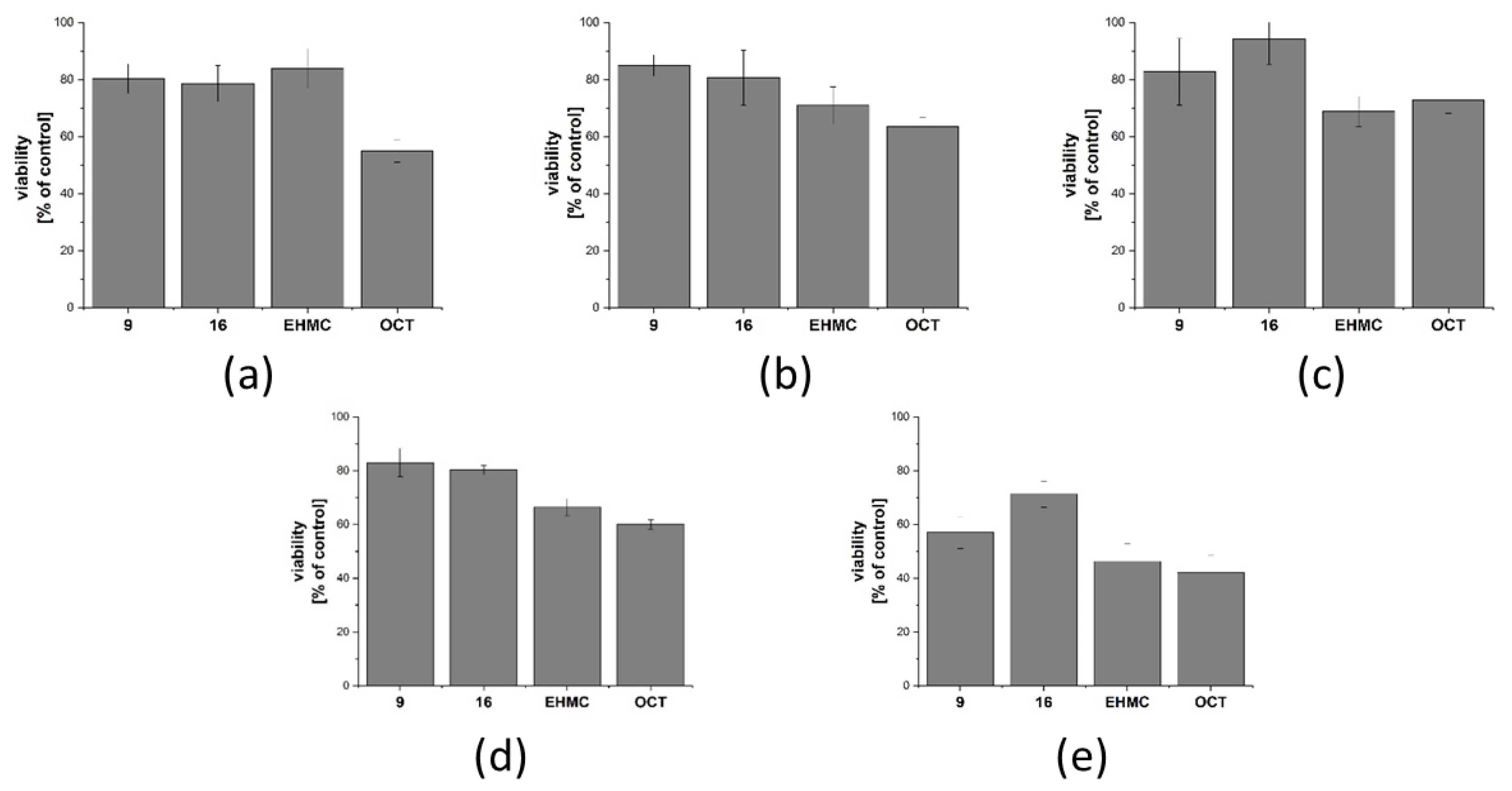
2.6. Safety Assessment
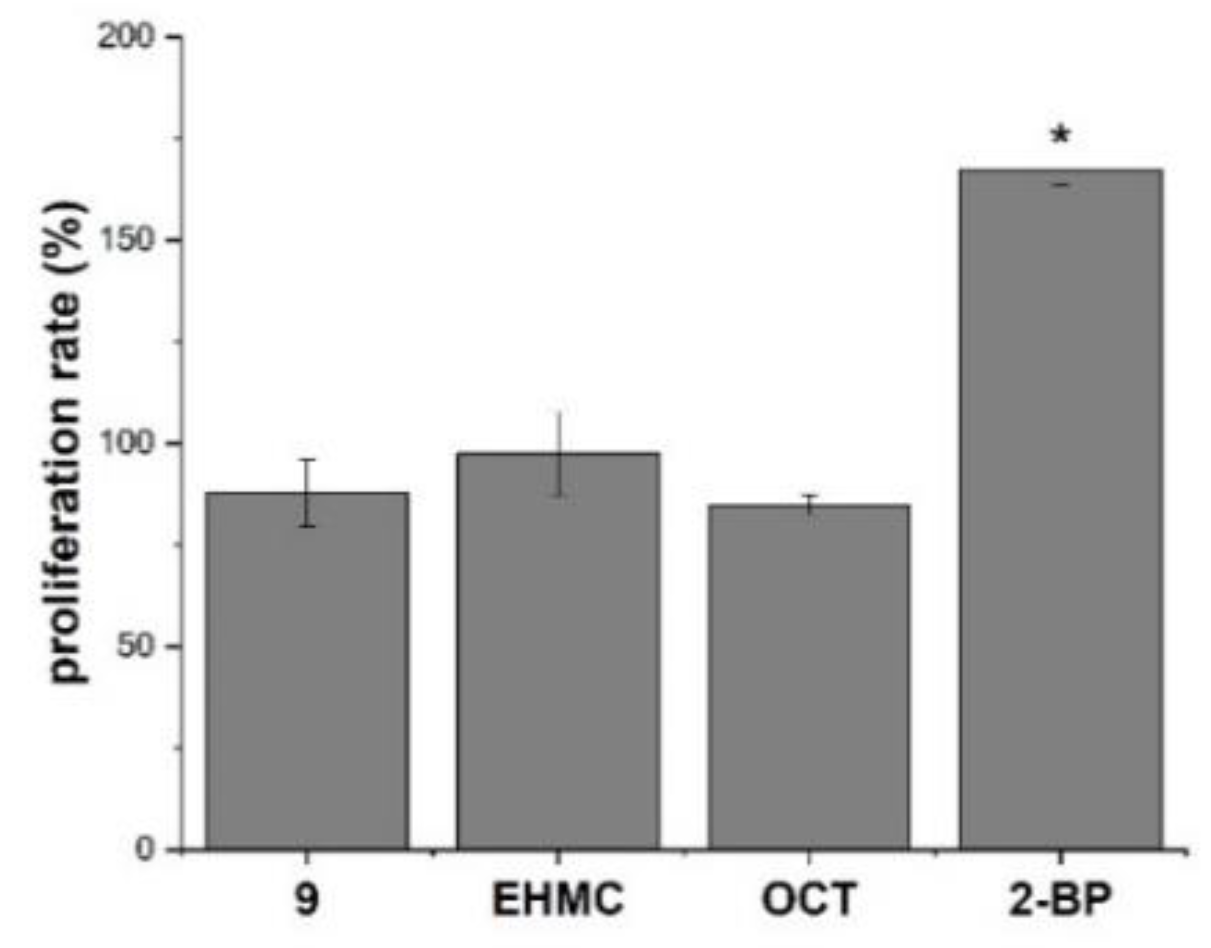
2.6.1. Cytotoxicity Assessment
2.6.2. In Vitro Effect on MCF-7 Cells—Preliminary Indirect Model of Estrogenic Activity
2.6.3. Mutagenic Activity
2.7. Structure-Activity Relationship Studies
3. Materials and Methods
3.1. Chemistry
3.1.1. Preparation of Substrates for Syntheses
3.1.2. Preparation of Compounds 1–13
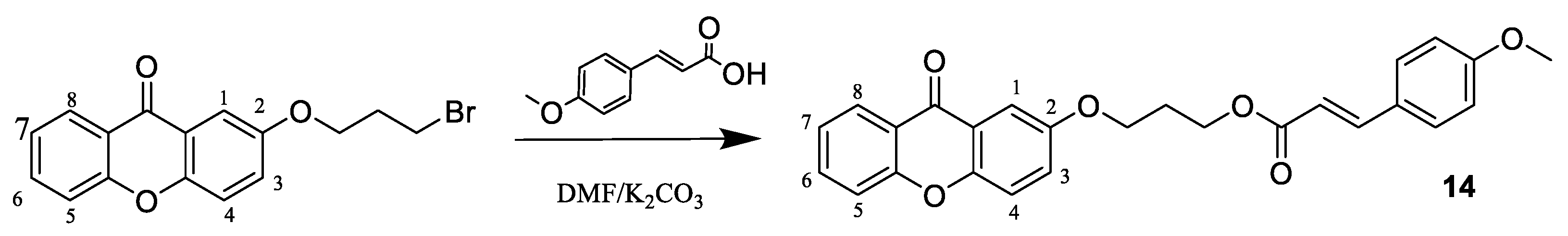
3.1.3. Preparation of Compound 14
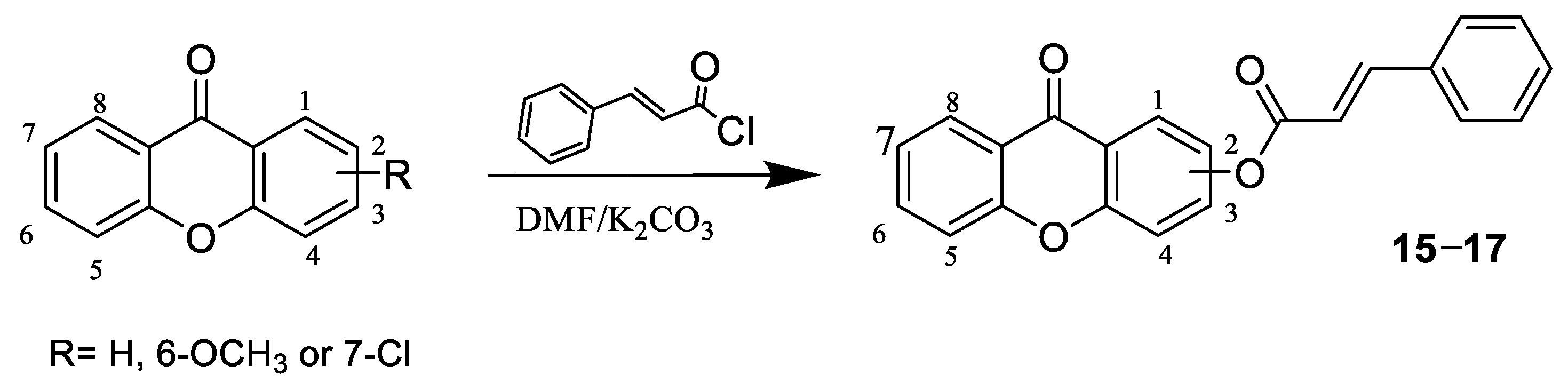
3.1.4. Preparation of Compounds 15–17
3.1.5. Physiochemical Properties of Tested Compounds
3.2. Evaluation of Lipophilicity Parameter RM0
3.3. Ultraviolet Spectroscopy
3.4. Photoprotective Activity
3.4.1. Preparation of Simple Cosmetic Formulations
3.4.2. In Vitro Photoprotection Study
3.5. Photostability Study
3.6. In Vitro Viability Assessment
3.7. Estrogenic Activity
3.8. Mutagenicity Assay Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maier, T.; Korting, H.C. Sunscreens—Which and what for? Skin Pharmacol. Physiol. 2005, 18, 253–262. [Google Scholar] [CrossRef]
- Kim, S.; Choi, K. Occurrences, toxicities, and ecological risks of benzophenone-3, a common component of organic sunscreen products: A mini-review. Environ. Int. 2014, 70, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Fabbrocini, G.; Triassi, M.; Mauriello, M.C.; Torre, G.; Annunziata, M.C.; de Vita, V.; Pastore, F.; D’Arco, V.; Monfrecola, G. Epidemiology of skin cancer: Role of some environmental factors. Cancers 2010, 2, 1980–1989. [Google Scholar] [CrossRef] [PubMed]
- Durrer, S.; Maerkel, K.; Schlumpf, M.; Lichtensteiger, W. Estrogen target gene regulation and coactivator expression in rat uterus after developmental exposure to the ultraviolet filter 4-methylbenzylidene camphor. Endocrinology 2005, 146, 2130–2139. [Google Scholar] [CrossRef]
- Schlumpf, M.; Kypke, K.; Vökt, C.C.; Birchler, M.; Durrer, S.; Faass, O.; Ehnes, C.; Fuetsch, M.; Gaille, C.; Henseler, M.; et al. Endocrine active UV filters: Developmental toxicity and exposure through breast milk. Chimia 2008, 62, 345–351. [Google Scholar] [CrossRef]
- Gilbert, E.; Pirot, F.; Bertholle, V.; Roussel, L.; Falson, F.; Padois, K. Commonly used UV filter toxicity on biological functions: Review of last decade studies. Int. J. Cosmet. Sci. 2013, 35, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Herzog, B.; Hueglin, D.; Osterwalder, U. New Sunscreen Actives. In Sunscreens. Regulations and Commercial Development, 3rd ed.; Shaath, N.A., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2005; pp. 291–320. [Google Scholar] [CrossRef]
- Baker, L.A.; Clark, S.L.; Habershon, S.; Stavros, V.G. Ultrafast Transient Absorption Spectroscopy of the Sunscreen Constituent Ethylhexyl Triazone. J. Phys. Chem. Lett. 2017, 8, 2113–2118. [Google Scholar] [CrossRef]
- Bino, A.; Baldisserotto, A.; Scalambra, E.; Dissette, V.; Vedaldi, D.E.; Salvador, A.; Durini, E.; Manfredini, S. Design, synthesis and biological evaluation of novel hydroxy-phenyl-1H-benzimidazoles as radical scavengers and UV-protective agents. J. Enzym. Inhib. Med. Chem. 2017, 32, 527–537. [Google Scholar] [CrossRef]
- Popiół, J.; Gunia-Krzyżak, A.; Piska, K.; Żelaszczyk, D.; Koczurkiewicz, P.; Słoczyńska, K.; Wójcik-Pszczoła, K.; Krupa, A.; Kryczyk-Poprawa, A.; Żesławska, E.; et al. Discovery of Novel UV-Filters with Favorable Safety Profiles in the 5-Arylideneimidazolidine-2,4-dione Derivatives Group. Molecules 2019, 24, 2321. [Google Scholar] [CrossRef]
- Gunia-Krzyżak, A.; Słoczyńska, K.; Popiół, J.; Koczurkiewicz, P.; Marona, H.; Pękala, E. Cinnamic acid derivatives in cosmetics: Current use and future prospects. Int. J. Cosmet. Sci. 2018, 40, 356–366. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off. J. Eur. Union 2009, 50, 59–209. [Google Scholar]
- Stiefel, C.; Schwack, W. Photoprotection in changing times—UV filter efficacy and safety, sensitization processes and regulatory aspects. Int. J. Cosmet. Sci. 2015, 37, 2–30. [Google Scholar] [CrossRef] [PubMed]
- Shaath, N.A. On the theory of ultraviolet absorption by sunscreen chemicals. J. Soc. Cosmet. Chem. 1987, 82, 193–207. Available online: https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=7659827 (accessed on 5 August 2020).
- Shaath, N.A. Ultraviolet filters. Photochem. Photobiol. Sci. 2010, 9, 464–469. [Google Scholar] [CrossRef]
- Hanson, K.M.; Narayanan, S.; Nichols, V.M.; Bardeen, C.J. Photochemical degradation of the UV filter octyl methoxycinnamate in solution and in aggregates. Photochem. Photobiol. Sci. 2015, 14, 1607–1616. [Google Scholar] [CrossRef]
- Cidade, H.; Rocha, V.; Palmeira, A.; Marques, C.; Tiritan, M.E.; Ferreira, H.; Lobo, J.S.; Almeida, I.F.; Sousa, M.E.; Pinto, M. In silico and in vitro antioxidant and cytotoxicity evaluation of oxygenated xanthone derivatives. Arab. J. Chem. 2020, 13, 17–26. [Google Scholar] [CrossRef]
- Klesiewicz, K.; Karczewska, E.; Budak, A.; Marona, H.; Szkaradek, N. Anti-Helicobacter pylori activity of some newly synthesized derivatives of xanthone. J. Antibiot. 2016, 69, 825–834. [Google Scholar] [CrossRef]
- Gobbi, S.; Rampa, A.; Bisi, A.; Belluti, F.; Valenti, P.; Caputo, A.; Zampiron, A.; Carrara, M. Synthesis and antitumor activity of new derivatives of xanthen-9-one-4-acetic acid. J. Med. Chem. 2002, 45, 4931–4939. [Google Scholar] [CrossRef]
- Szkaradek, N.; Sypniewski, D.; Żelaszczyk, D.; Gałka, S.; Borzdziłowska, P.; Marona, H.; Bednarek, I. Influence of New Synthetic Xanthones on the Proliferation and Migration Potential of Cancer Cell Lines In Vitro. Anticancer Agents Med. Chem. 2019, 19, 1949–1965. [Google Scholar] [CrossRef]
- Żelaszczyk, D.; Lipkowska, A.; Szkaradek, N.; Słoczyńska, K.; Gunia-Krzyżak, A.; Librowski, T.; Marona, H. Synthesis and preliminary anti-inflammatory evaluation of xanthone derivatives. Heterocycl. Commun. 2018, 24, 231–236. [Google Scholar] [CrossRef]
- Telang, M.; Dhulap, S.; Mandhare, A.; Hirwani, R. Therapeutic and cosmetic applications of mangiferin: A patent review. Expert Opin. Ther. Pat. 2013, 23, 1561–1580. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Song, J.H.; Youn, U.J.; Hyun, J.W.; Jeong, W.S.; Lee, M.Y.; Choi, H.J.; Lee, H.K.; Chae, S. Inhibition of UVB-induced wrinkle formation and MMP-9 expression by mangiferin isolated from Anemarrhena asphodeloides. Eur. J. Pharmacol. 2012, 689, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, C.M.; Gaspar, L.R. Mangiferin and naringenin affect the photostability and phototoxicity of sunscreens containing avobenzone. J. Photochem. Photobiol. B Biol. 2015, 151, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Żelaszczyk, D.; Jakubczyk, M.; Pytka, K.; Rapacz, A.; Walczak, M.; Janiszewska, P.; Pańczyk, K.; Żmudzki, P.; Słoczyńska, K.; Marona, H.; et al. Design, synthesis and evaluation of activity and pharmacokinetic profile of new derivatives of xanthone and piperazine in the central nervous system. Bioorg. Med. Chem. Lett. 2019, 29, 126679. [Google Scholar] [CrossRef] [PubMed]
- Marona, H.; Eckstein, M.; Krupinska, J.; Mazur, J.; Piotrowicz, J.; Cebo, B. Synthesis and some biological properties of 2-xanthonylalkyl- (alkoxy) carboxylic acids. Pol. J. Pharmacol. Pharm. 1986, 38, 107–114. [Google Scholar] [PubMed]
- Eckstein, M.; Marona, H. Preparation and properties of some 2-mercaptomethylxanthones. Pol. J. Chem. 1981, 12, 1281–1285. [Google Scholar] [CrossRef]
- Davies, J.S.H.; Lamb, F.; Suschitzky, H. 367. Studies in the xanthone series. Part II. Preparation and reactions of 1-formyl-2-hydroxyxanthone. J. Chem. Soc. 1958, 1790. [Google Scholar] [CrossRef]
- Lin, C.-N.; Liou, S.-S.; Ko, F.-N.; Teng, C.-M. γ-pyrone compounds. IV: Synthesis and antiplatelet effects of mono- and dioxygenated xanthones and xanthonoxypropanolamine. J. Pharm. Sci. 1993, 82, 11–16. [Google Scholar] [CrossRef]
- Guerre, C.; Thull, U.; Gaillard, P.; Carrupt, P.A.; Testa, B.; Fernandes, E.; Silva, F.; Pinto, M.; Pinto, M.M.M.; Wolfender, J.L.; et al. Natural and synthetic xanthones as monoamine oxidase inhibitors: Biological assay and 3D-QSAR. Helv. Chim. Acta 2001, 84, 552–570. [Google Scholar] [CrossRef]
- Marona, H.; Szkaradek, N.; Kubacka, M.; Bednarski, M.; Filipek, B.; Cegla, M.; Szneler, E. Synthesis and Evaluation of Some Xanthone Derivatives for Anti-Arrhythmic, Hypotensive Properties and Their Affinity for Adrenergic Receptors. Arch. Pharm. 2008, 341, 90–98. [Google Scholar] [CrossRef]
- Marona, H.; Szkaradek, N.; Karczewska, E.; Trojanowska, D.; Budak, A.; Bober, P.; Przepiórka, W.; Cegla, M.; Szneler, E. Antifungal and antibacterial activity of the newly synthesized 2-xanthone derivatives. Arch. Pharm. 2009, 342, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Boyce, C.B.C.; Milborrow, B.V. A Simple Assessment of Partition Data for Correlating Structure and Biological Activity Using Thin-Layer Chromatography. Nature 1965, 208, 537–539. [Google Scholar] [CrossRef]
- Pękala, E.; Marona, H. Estimating the lipophilicity of a number of 2-amino-1-cyclohexanol derivatives exhibiting anticonvulsant activity. Biomed. Chromatogr. 2009, 23, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Molinspiration. Available online: https://www.molinspiration.com/cgi-bin/properties (accessed on 27 August 2020).
- Lee, C.K.; Uchida, T.; Kitagawa, K.; Yagi, A.; Kim, N.; Goto, S. Relationship between Lipophilicity and Skin Permeability of Various Drugs from an Ethanol/Water/Lauric Acid System. Biol. Pharm. Bull. 1994, 17, 1421–1424. [Google Scholar] [CrossRef]
- European Commission; Scientific Committee on Consumer Safety. The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation. 9th Revision. 2015. Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_190.pdf (accessed on 6 August 2020).
- Determination of Sunscreen UVA Photoprotection in Vitro; ISO 24443:2012; International Organization for Standardization: London, UK, 2012.
- Couteau, C.; Pommier, M.; Paparis, E.; Coiffard, L.J.M. Study of the efficacy of 18 sun filters authorized in European Union tested in vitro. Die Pharm. Int. J. Pharm. Sci. 2007, 62, 449–452. [Google Scholar] [CrossRef]
- Couteau, C.; Paparis, E.; Chauvet, C.; Coiffard, L. Tris-biphenyl triazine, a new ultraviolet filter studied in terms of photoprotective efficacy. Int. J. Pharm. 2015, 487, 120–123. [Google Scholar] [CrossRef]
- The Comission of the European Communities. Commission Recommendation of 22 September 2006 on the efficacy of sunscreen products and the claims made relating thereto. Off. J. Eur. Union. 2006, 265, 39–43. Available online: https://eur-lex.europa.eu/legal-content/IT/TXT/PDF/?uri=CELEX:32006H0647&from=EN (accessed on 1 September 2020).
- Ferrero, L.; Pissavini, M.; Dehais, A.; Marguerie, S.; Zastrow, L. Importance of substrate roughness for in vitro sun protection assessment. Int. J. Cosmet. Sci. 2007, 29, 59. [Google Scholar] [CrossRef]
- Vielhaber, G.; Grether-Beck, S.; Koch, O.; Johncock, W.; Krutmann, J. Sunscreens with an absorption maximum of ≥360 nm provide optimal protection against UVA1-induced expression of matrix metalloproteinase-1, interleukin-1, and interleukin-6 in human dermal fibroblasts. Photochem. Photobiol. Sci. 2006, 5, 275–282. [Google Scholar] [CrossRef]
- Hojerová, J.; Medovcíková, A.; Mikula, M. Photoprotective efficacy and photostability of fifteen sunscreen products having the same label SPF subjected to natural sunlight. Int. J. Pharm. 2011, 408, 27–38. [Google Scholar] [CrossRef]
- Garoli, D.; Pelizzo, M.G.; Bernardini, B.; Nicolosi, P.; Alaibac, M. Sunscreen tests: Correspondence between in vitro data and values reported by the manufacturers. J. Dermatol. Sci. 2008, 52, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; El-Boury, S.; Paparis, E.; Sébille-Rivain, V.; Coiffard, L.J.M. In vitro UV-A protection factor (PF-UVA) of organic and inorganic sunscreens. Pharm. Dev. Technol. 2009, 14, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.R.; Maia Campos, P.M.B.G. Evaluation of the photostability of different UV filter combinations in a sunscreen. Int. J. Pharm. 2006, 307, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Schlumpf, M.; Cotton, B.; Conscience, M.; Haller, V.; Steinmann, B.; Lichtensteiger, W. In vitro and in vivo estrogenicity of UV screens. Environ. Health Perspect. 2001, 109, 239–244. [Google Scholar] [CrossRef]
- Ullmann, F.; Kipper, H. Ueber Methoxy-chlor-benzoësäure. Berichte Der Dtsch. Chem. Gesellschaft. 1905, 38, 2120–2126. [Google Scholar] [CrossRef][Green Version]
- Pickert, M.; Frahm, A.W. Substituted Xanthones as Antimycobacterial agents, Part 1: Synthesis and Assignment of 1H/13C NMR Chemical Shifts. Arch. Pharm. 1998, 331, 177–192. [Google Scholar] [CrossRef]
- Julia, M.; Tchernoff, G. 3,6-Disubstituted xanthones. Bull. Soc. Chim. Fr. 1952, 128, 546–549. [Google Scholar]
- Eckstein, M.; Marona, H.; Mazur, J. Synthesis and some biological properties of substituted xanthone-2-carboxylic acids. Pol. J. Pharmacol. Pharm. 1983, 35, 159–167. [Google Scholar]
- Marona, H.; Pękala, E.; Filipek, B.; Maciąg, D.; Szneler, E. Pharmacological properties of some aminoalkanolic derivatives of xanthone. Die Pharm. 2001, 56, 567–572. [Google Scholar]
- de Oliveira, C.A.; Peres, D.D.; Rugno, C.M.; Kojima, M.; Pinto, C.S.A.O.; Consiglieri, V.O.; Keneko, T.M.; Rosado, C.; Mota, J.; Velasco, M.V.R.; et al. Functional photostability and cutaneous compatibility of bioactive UVA sun care products. J. Photochem. Photobiol. B Biol. 2015, 148, 154–159. [Google Scholar] [CrossRef]
- Organisation for Economic Cooperation and Development Test Guideline No. 471: Bacterial Reverse Mutation Test. Available online: https://www.oecd.org/chemicalsafety/risk-assessment/1948418.pdf (accessed on 3 September 2020).
- Flückiger-Isler, S.B.; Braun, K.; Gervais, V.; Hasler-Nguyen, N.; Reimann, R.; Van Gompel, J.; Wunderlich, H.; Engelhardt, G. Assessment of the performance of the Ames II assay: A collaborative study with 19 coded compounds. Mutat. Res. 2004, 558, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Flückiger-Isler, S.; Kamber, M. Direct comparison of the Ames microplate format (MPF) test in liquid medium with the standard Ames pre-incubation assay on agar plates by use of equivocal to weakly positive test compounds. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012, 747, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Xenometrix. Available online: https://www.xenometrix.ch (accessed on 3 September 2020).
- Umbuzeiro Gde, A.; Rech, C.; Correia, S.; Bergamasco, A.; Cardenette, G.; Flückiger-Isler, S.; Kamber, M. Comparison of the Salmonella/microsome microsuspension assay with the new microplate fluctuation protocol for testing the mutagenicity of environmental samples. Environ. Mol. Mutagen. 2009, 405, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Reifferscheid, G.; Maes, H.M.; Allner, B.; Badurova, J.; Belkin, S.; Bluhm, K.; Brauer, F.; Bressling, J.; Domeneghetti, S.; Elad, T.; et al. International round-robin study on the Ames fluctuation test. Environ. Mol. Mutagen. 2012, 53, 185–197. [Google Scholar] [CrossRef]
- Smith, K.E.C.; Heringa, M.B.; Uytewaal, M.; Mayer, P. The dosing determines mutagenicity of hydrophobic compounds in the Ames II assay with metabolic transformation: Passive dosing versus solvent spiking. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2013, 750, 12–18. [Google Scholar] [CrossRef]

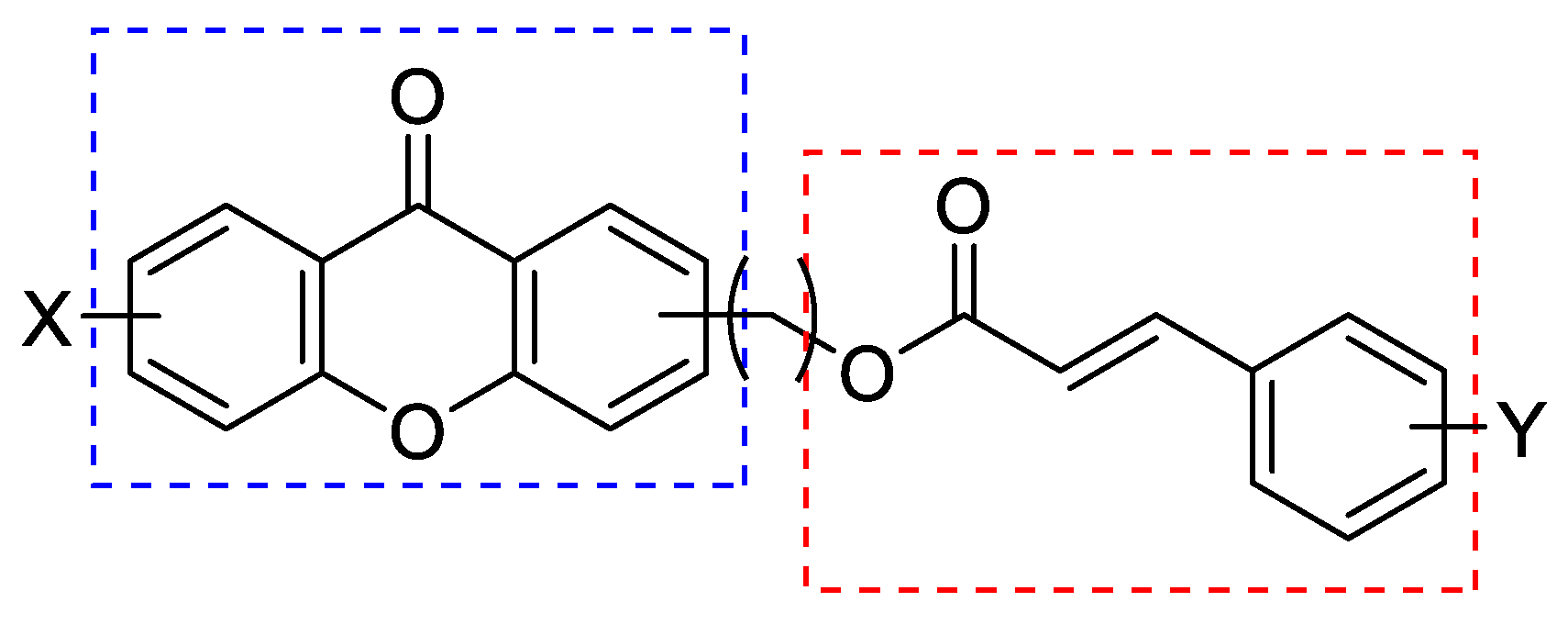

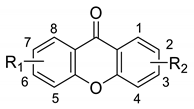
| Compound | R1 | Position of R2 | R2 | miLogP * | RM0 ** |
|---|---|---|---|---|---|
| 1 | H | 4- | 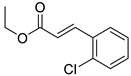 | 6.16 | 5.02 |
| 2 | H | 4- |  | 6.38 | 5.28 |
| 3 | 6-OCH3 | 4- | 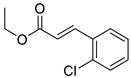 | 6.19 | 5.66 |
| 4 | 6-OCH3 | 4- | 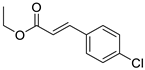 | 6.42 | 5.06 |
| 5 | 6-OCH3 | 4- | 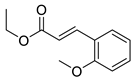 | 5.57 | 5.10 |
| 6 | 6-OCH3 | 4- | 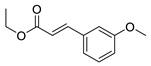 | 5.77 | 4.96 |
| 7 | 6-OCH3 | 4- |  | 5.79 | 5.06 |
| 8 | 6-OCH3 | 2- | 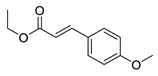 | 5.82 | 6.15 |
| 9 | 6-OCH3 | 2- |  | 5.62 | 4.64 |
| 10 | 6-OCH3 | 2- | 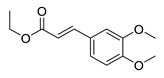 | 5.41 | 4.53 |
| 11 | 6-OCH3 | 2- | 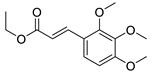 | 5.39 | 4.50 |
| 12 | 6-OCH3 | 2- | 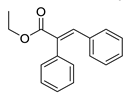 | 7.10 | 5.62 |
| 13 | 7-Cl | 2- | 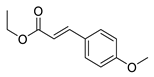 | 6.44 | 4.85 |
| 14 | H | 2- |  | 6.14 | 5.18 |
| 15 | H | 2- | 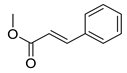 | 5.24 | 4.19 |
| 16 | H | 3- | 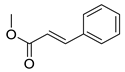 | 5.24 | 4.54 |
| 17 | H | 4- |  | 5.21 | 4.25 |
| Compound | Absorption Range (nm) | λmax (nm) | εmax (M−1 cm−1) | E(1,1) (λmax) (g−1 L cm−1) | ‹E1,1›mean |
|---|---|---|---|---|---|
| 1 | 290–352 | 290 | 15,311 | 392 | 69 |
| 2 | 290–351 | 290 | 25,688 | 657 | 133 |
| 3 | 290–342 | 290 | 27,690 | 658 | 168 |
| 4 | 290–341 | 296 | 36,895 | 948 | 202 |
| 5 | 290–350 | 290 | 24,118 | 580 | 221 |
| 6 | 290–342 | 290 | 25,749 | 619 | 168 |
| 7 | 290–346 | 300 | 39,002 | 937 | 342 |
| 8 | 290–348 | 303 | 38,128 | 916 | 314 |
| 9 | 290–369 | 299, 329 | 34,534, 34,177 | 774, 766 | 360 |
| 10 | 290–355 | 301, 326 | 31,005, 30,469 | 694, 682 | 307 |
| 11 | 290–354 | 304 | 33,330 | 898 | 326 |
| 12 | 290–340 | 299 | 21,409 | 463 | 112 |
| 13 | 290–359 | 312 | 26,590 | 633 | 243 |
| 14 | 290–375 | 300 | 28,360 | 659 | 214 |
| 15 | 290–355 | 290 | 38,152 | 1115 | 188 |
| 16 | 290–345 | 290 | 36,410 | 1064 | 235 |
| 17 | 290–351 | 290 | 20,100 | 587 | 120 |
| 4-MBC | 290–328 | 300 | 22,053 | 867 | 221 |
| OCT | 290–347 | 306 | 14,330 | 396 | 141 |
| EHMC | 290–337 | 309 | 24,907 | 858 | 266 |
| BMDM | 290–391 | 357 | 35,939 | 1158 | 520 |
| Compound | SPFin vitro ± SD | UVA PF ± SD | λc (nm) | UVA/UVB Ratio |
|---|---|---|---|---|
| 8 | 14.69 ± 2.25 | 4.92 ± 0.78 | 345 | 0.329 |
| 9 | 19.69 ± 0.46 | 12.64 ± 0.32 | 381 | 0.830 |
| Compound | % of Initial SPFin vitro | % of Initial UVA PF | λc(nm) | UVA/UVB Ratio | ||
|---|---|---|---|---|---|---|
| Pre-Irradiation | Post-Irradiation | Pre-Irradiation | Post-Irradiation | |||
| 8 | 76.05 | 67.20 | 345 | 335 | 0.339 | 0.201 |
| 9 | 89.03 | 82.75 | 381 | 380 | 0.830 | 0.784 |
| Positive Wells per Microplate | |||||
|---|---|---|---|---|---|
| Salmonella typhimurium | |||||
| TA98 | TA100 | TA1535 | TA1537 | ||
| Compound | Concentration (mM) | FIB * | |||
| 8 | 0.1 | 0.5 | 0.1 | 0.7 | 0.7 |
| 0.2 | 1.1 | 1.2 | 0.4 | 1.0 | |
| 0.5 | 1.0 | 1.3 | 0.3 | 0.7 | |
| 9 | 0.1 | 0.4 | 0.8 | 0.1 | 1.0 |
| 0.2 | 0.2 | 0.7 | 0.8 | 0.3 | |
| 0.5 | 0.5 | 0.8 | 0.7 | 1.0 | |
| Positive control ** | 6.4 | 3.4 | 14.7 | 48.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popiół, J.; Gunia-Krzyżak, A.; Słoczyńska, K.; Koczurkiewicz-Adamczyk, P.; Piska, K.; Wójcik-Pszczoła, K.; Żelaszczyk, D.; Krupa, A.; Żmudzki, P.; Marona, H.; et al. The Involvement of Xanthone and (E)-Cinnamoyl Chromophores for the Design and Synthesis of Novel Sunscreening Agents. Int. J. Mol. Sci. 2021, 22, 34. https://doi.org/10.3390/ijms22010034
Popiół J, Gunia-Krzyżak A, Słoczyńska K, Koczurkiewicz-Adamczyk P, Piska K, Wójcik-Pszczoła K, Żelaszczyk D, Krupa A, Żmudzki P, Marona H, et al. The Involvement of Xanthone and (E)-Cinnamoyl Chromophores for the Design and Synthesis of Novel Sunscreening Agents. International Journal of Molecular Sciences. 2021; 22(1):34. https://doi.org/10.3390/ijms22010034
Chicago/Turabian StylePopiół, Justyna, Agnieszka Gunia-Krzyżak, Karolina Słoczyńska, Paulina Koczurkiewicz-Adamczyk, Kamil Piska, Katarzyna Wójcik-Pszczoła, Dorota Żelaszczyk, Anna Krupa, Paweł Żmudzki, Henryk Marona, and et al. 2021. "The Involvement of Xanthone and (E)-Cinnamoyl Chromophores for the Design and Synthesis of Novel Sunscreening Agents" International Journal of Molecular Sciences 22, no. 1: 34. https://doi.org/10.3390/ijms22010034
APA StylePopiół, J., Gunia-Krzyżak, A., Słoczyńska, K., Koczurkiewicz-Adamczyk, P., Piska, K., Wójcik-Pszczoła, K., Żelaszczyk, D., Krupa, A., Żmudzki, P., Marona, H., & Pękala, E. (2021). The Involvement of Xanthone and (E)-Cinnamoyl Chromophores for the Design and Synthesis of Novel Sunscreening Agents. International Journal of Molecular Sciences, 22(1), 34. https://doi.org/10.3390/ijms22010034






