Molecular Bases of Fruit Quality in Prunus Species: An Integrated Genomic, Transcriptomic, and Metabolic Review with a Breeding Perspective
Abstract
1. Introduction
2. Regulation and Signal Transduction during the Ripening Process
2.1. Ethylene
2.2. Abscisic Acid
2.3. Auxin
2.4. Gibberellins
2.5. Cytokinins
2.6. Jasmonates
3. Determination of Ripening Date
4. Fruit Color Development: Pigment Biosynthesis, Accumulation, and Degradation
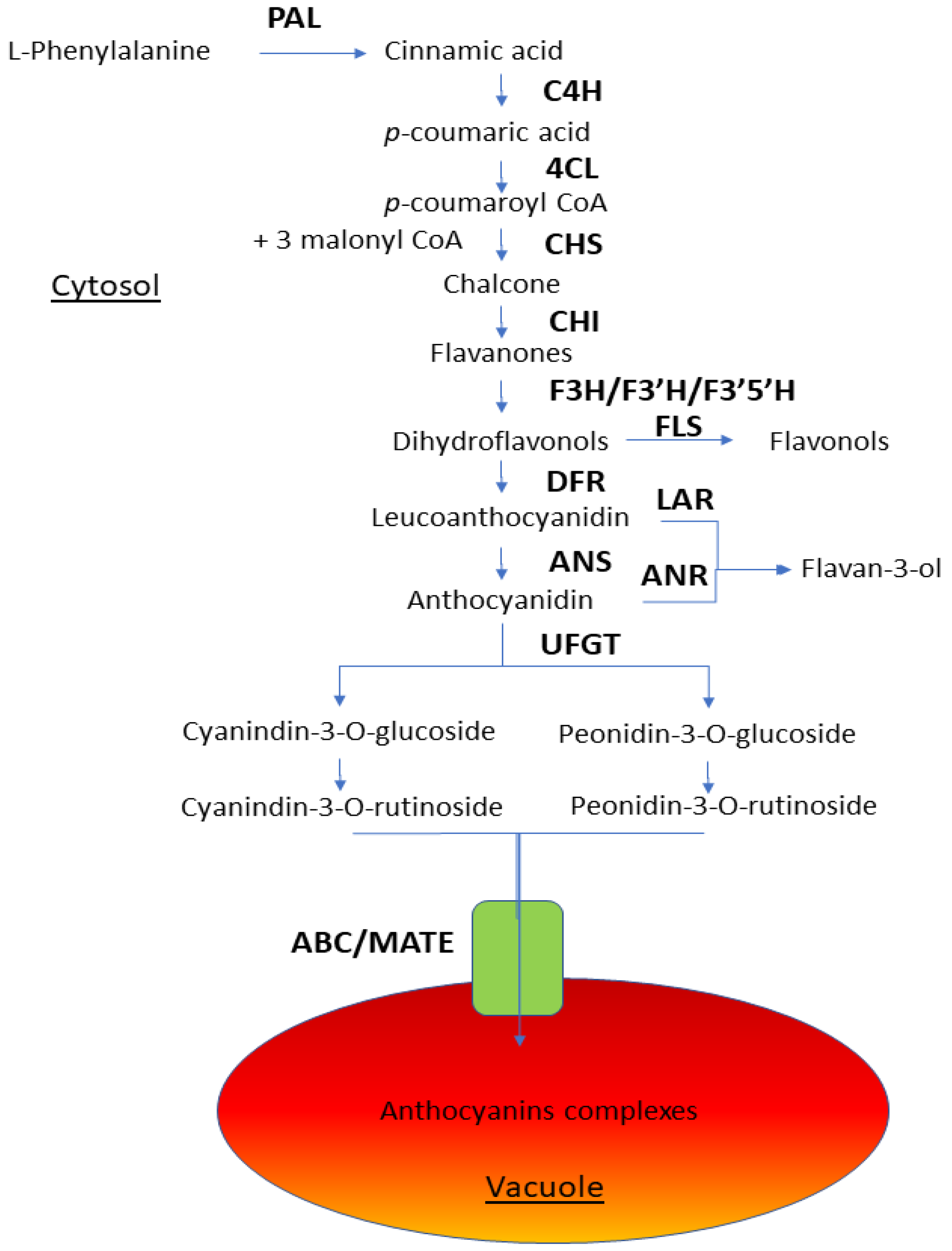
4.1. Anthocyanins
4.2. Flavonoids
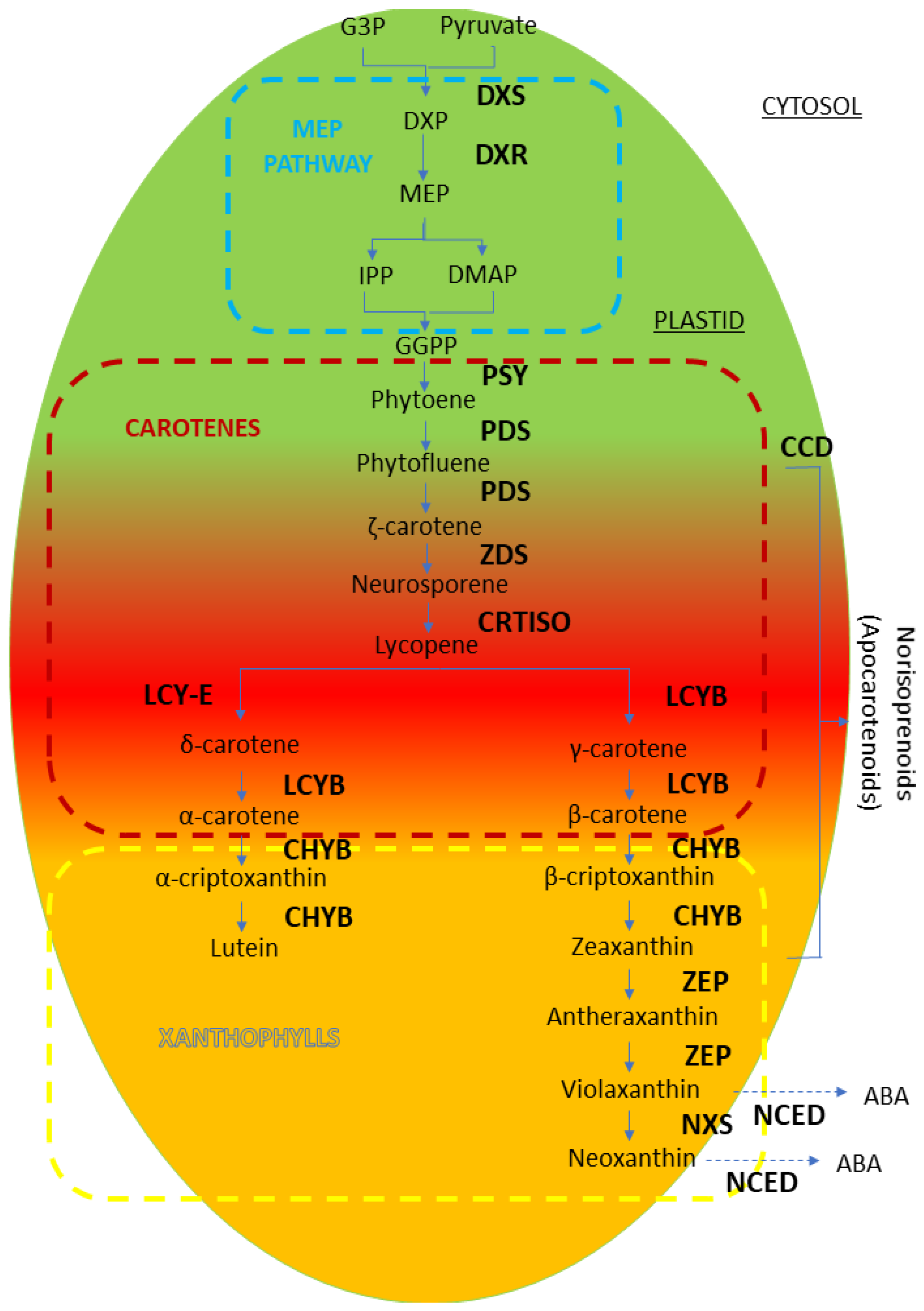
4.3. Carotenoids
4.4. Chlorophylls and Photosynthetic Apparatus
5. Biochemical Pathways Related to Flavor
5.1. Aroma
5.2. Taste
5.2.1. Soluble Solids
5.2.2. Acidity Loss
5.3. Cell Wall Degradation and Texture
6. Nutraceuticals and Antioxidant Compounds
7. Application of New Molecular Tools to Prunus Breeding and Selection for Fruit Quality
7.1. Genomics
7.2. Transcriptomics
7.3. Epigenetic Regulation
7.4. Marker-Assisted Selection for Superior Fruit Quality Genotypes
8. Conclusions: Challenges and New Opportunities
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Watada, A.E.; Herner, R.C.; Kader, A.A.; Romani, R.J.; Staby, G.L. Terminology for the description of developmental stages of horticultural crops. HortScience 1984, 19, 20–21. [Google Scholar]
- Brady, J.C. Fruit ripening. Ann. Rev. Plant Physiol. 1987, 38, 155–178. [Google Scholar] [CrossRef]
- Bonghi, C.; Manganaris, G.A. Systems biology approaches reveal new insights into the molecular mechanisms regulating flesh fruit quality. In Omics Technologies: Tools for Food Science; CRC Press: New York, NY, USA, 2012; 25p. [Google Scholar]
- Klee, H.J.; Giovannoni, J.J. Genetics and Control of Tomato Fruit Ripening and Quality Attributes. Ann. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Grimplet, J.; Romieu, C.; Audergon, J.M.; Marty, I.; Albagnac, G.; Lambert, P.; Terrier, N. Transcriptomic study of apricot fruit (Prunus armeniaca) ripening among 13 006 expressed sequence tags. Physiol. Plant. 2005, 125, 281–292. [Google Scholar] [CrossRef]
- Giovannoni, J.J. Genetic regulation of fruit development and ripening. Plant Cell 2004, 16, S170–S180. [Google Scholar] [CrossRef] [PubMed]
- Farinati, S.; Rasori, A.; Varotto, S.; Bonghi, C. Rosaceae Fruit Development, Ripening and Post-harvest: An Epigenetic Perspective. Front. Plant Sci. 2017, 8, 1247. [Google Scholar] [CrossRef] [PubMed]
- Lü, P.; Yu, S.; Zhu, N.; Chen, Y.-R.; Zhou, B.; Pan, Y.; Zhong, S. Genome encode analyses reveal the basis of convergent evolution of fleshy fruit ripening. Nat. Plants 2018, 4, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Osorio, S.; Scossa, F.; Fernie, A.R. Molecular regulation of fruit ripening. Front. Plant Sci. 2013, 4, 198. [Google Scholar] [CrossRef]
- Chalmers, D.J.; van den Ende, B. A reappraisal of the growth and development of peach fruit. Aust. J. Plant Physiol. 1975, 2, 623–634. [Google Scholar] [CrossRef]
- Biale, J.B. The postharvest biochemistry of tropical and subtropical fruits. Adv. Food Res. 1960, 10, 293–354. [Google Scholar]
- Jimenez, A.; Creissen, G.; Kular, B.; Firmin, J.; Robinson, S.; Verhoeyen, M.; Mullineaux, P. Changes in oxidative processes and components of the antioxidant system during tomato fruit ripening. Planta 2002, 214, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Prinsi, B.; Negri, A.S.; Fedeli, C.; Morgutti, S.; Negrini, N.; Cocucci, M.; Espen, L. Peach fruit ripening: A proteomic comparative analysis of the mesocarp of two cultivars with different flesh firmness at two ripening stages. Phytochemistry 2011, 72, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Zheng, H.; Zhang, Q.; Li, W. Profiling Taste and Aroma Compound Metabolism during Apricot Fruit Development and Ripening. Int. J. Mol. Sci. 2016, 17, 998. [Google Scholar] [CrossRef] [PubMed]
- Alba, R.; Payton, P.; Fei, Z.; Mcquinn, R.; Debbie, P.; Martin, G.B.; Tanksley, S.D.; Giovannoni, J.J. Transcriptome and Selected Metabolite Analyses Reveal Multiple Points of Ethylene Control during Tomato Fruit Development. Plant Cell 2005, 17, 2954–2965. [Google Scholar] [CrossRef] [PubMed]
- Infante, R.; Martínez-Gómez, P.; Predieri, S. Quality oriented fruit breeding: Peach [Prunus persica (L.) Batsch]. J. Food Agri. Environ. 2008, 6, 342–356. [Google Scholar]
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.M.; Vupputuri, S.; Myers, L.; Whelton, P.K. Fruit and vegetable intake and risk of cardiovascular disease in US adults: The first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am. J. Clin. Nutr. 2002, 76, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.L.; Hollman, P.C.H.; van de Putte, B. Content of potentially anticarcinogenic flavonoids of tea infusions, wines, and fruit juices. J. Agric. Food Chem. 1993, 41, 1242–1246. [Google Scholar] [CrossRef]
- Machlin, L.J. Critical assessment of the epidemiological data concerning the impact of antioxidant nutrients on cancer and cardiovascular disease. Crit. Rev. Food Sci. Nutr. 1995, 35, 41–49. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Bioavailability, Metabolic Effects, and Safety. Ann. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Van den Berg, H.; Faulks, R.; Granado, H.F.; Hirschberg, J.; Olmedilla, B.; Sandmann, G.; Stahl, W. The potential for the improvement of carotenoid levels in foods and the likely systemic effects. J. Sci. Food Agric. 2000, 80, 880–912. [Google Scholar] [CrossRef]
- Matas, A.J.; Gapper, N.E.; Chung, M.Y.; Giovannoni, J.J.; Rose, J.K. Biology and genetic engineering of fruit maturation for enhanced quality and shelf-life. Curr. Opin. Biotechnol. 2009, 20, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gómez, P. Editorial for Special Issue “Plant Genetics and Molecular Breeding”. Int. J. Mol. Sci. 2019, 20, 2659. [Google Scholar] [CrossRef] [PubMed]
- Bonghi, C.; Trainotti, L.; Botton, A.; Tadiello, A.; Rasori, A.; Ziliotto, F.; Ramina, A. A microarray approach to identify genes involved in seed-pericarp cross-talk and development in peach. BMC Plant Biol. 2011, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Khurana, A.; Sharma, A.K. Role of plant hormones and their interplay in development and ripening of fleshy fruits. J. Exp. Bot. 2014, 65, 4561–4575. [Google Scholar] [CrossRef]
- McAtee, P.; Karim, S.; Schaffer, R.; David, K. A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front. Plant Sci. 2013, 4, 79. [Google Scholar] [CrossRef]
- Wang, S.; Lin, H.; Saito, T.; Ohkawa, K.; Ohara, H.; Jia, H.; Kondo, S. Abscisic acid affects ethylene metabolism and carotenoid biosynthesis in Japanese apricot (Prunus mume Sieb. et Zucc.). Agri Gene 2019, 12, 100083. [Google Scholar] [CrossRef]
- Burg, S.P.; Burg, E.A. Ethylene action and ripening of fruits—Ethylene influences growth and development of plants and is the hormone which initiates fruit ripening. Science 1965, 148, 1190–1198. [Google Scholar] [CrossRef]
- Katz, E.; Lagunes, P.M.; Riov, J.; Weiss, D.; Goldschmidt, E.E. Molecular and physiological evidence suggests the existence of a system II-like pathway of ethylene production in non-climacteric Citrus fruit. Planta 2004, 219, 243–252. [Google Scholar] [CrossRef]
- Liu, M.C.; Pirrello, J.; Chervin, C.; Roustan, J.P.; Bouzayen, M. Ethylene Control of Fruit Ripening: Revisiting the Complex Network of Transcriptional Regulation. Plant Physiol. 2015, 169, 2380–2390. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Rasori, A.; Bassi, D.; Geuna, F.; Ramina, A.; Tonutti, P.; Bonghi, C. Comparative transcript profiling of apricot (Prunus armeniaca L.) fruit development and on-tree ripening. Tree Genet. Gen. 2011, 7, 609–616. [Google Scholar] [CrossRef]
- Alexander, L.; Grierson, D. Ethylene biosynthesis and action in tomato: A model for climacteric fruit ripening. J. Exp. Bot. 2002, 53, 2039–2055. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, A.; Shemansky, J.M.; Chang, C.R.; Binder, B.M. History of Research on the Plant Hormone Ethylene. J. Plant Growth Regul. 2015, 34, 809–827. [Google Scholar] [CrossRef]
- Lin, Z.F.; Zhong, S.L.; Grierson, D. Recent advances in ethylene research. J. Exp. Bot. 2009, 60, 3311–3336. [Google Scholar] [CrossRef]
- Muñoz-Robredo, P.; Rubio, P.; Infante, R.; Campos-Vargas, R.; Manríquez, D.; González-Agüero, M.; Defilippi, B.G.G. Ethylene biosynthesis in apricot: Identification of a ripening-related 1-aminocyclopropane-1-carboxylic acid synthase (ACS) gene. Postharvest Biol. Technol. 2012, 63, 85–90. [Google Scholar] [CrossRef]
- Solano, R.; Ecker, J.R. Ethylene gas: Perception, signaling and response. Curr. Opin. Plant Biol. 1998, 1, 393–398. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, L.; Yuan, C.; Guan, J. Molecular characterization of ethylene-regulated anthocyanin biosynthesis in plums during fruit ripening. Plant Mol. Biol. Rep. 2015, 34, 777–785. [Google Scholar] [CrossRef]
- Rasori, A.; Ruperti, B.; Bonghi, C.; Tonutti, P.; Ramina, A. Characterization of two putative ethylene receptor genes expressed during peach fruit development and abscission. J. Exp. Bot. 2002, 53, 2333–2339. [Google Scholar] [CrossRef] [PubMed]
- Trainotti, L.; Tadiello, A.; Casadoro, G. The involvement of auxin in the ripening of climacteric fruits comes of age: The hormone plays a role of its own and has an intense interplay with ethylene in ripening peaches. J. Exp. Bot. 2007, 58, 3299–3308. [Google Scholar] [CrossRef]
- Chahine, H.; Gouble, B.; Audergon, J.M.; Souty, M.; Albagnac, G.; Jacquemin, G.; Hugues, M. Effect of ethylene on certain quality parameters of apricot fruit (Prunus armeniaca, L.) during maturation and postharvest evolution. Acta Hortic. 1999, 488, 577–584. [Google Scholar] [CrossRef]
- Fan, X.; Argenta, L.; Mattheis, J.P. Inhibition of ethylene action by 1-methylcyclopropene prolongs storage life of apricots. Postharvest Biol. Technol. 2000, 20, 135–142. [Google Scholar] [CrossRef]
- Marty, I.; Bureau, S.; Sarkissian, G.; Gouble, B.; Audergon, J.M.; Albagnac, G. Ethylene regulation of carotenoid accumulation and carotenogenic gene expression in colour-contrasted apricot varieties (Prunus armeniaca). J. Exp. Bot. 2005, 56, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Palou, L.; Crisosto, C.H. Postharvest Treatments to Reduce the Harmful Effects of Ethylene on Apricots. Acta Hortic. 2003, 599, 31–38. [Google Scholar] [CrossRef]
- Brady, C.J. Stone fruit. In Biochemistry of Fruit Ripening; Springer: Dordrecht, The Netherlands, 1993; pp. 379–404. [Google Scholar]
- Seo, M.; Koshiba, T. Complex regulation of ABA biosynthesis in plants. Trends Plant Sci. 2002, 7, 41–48. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Serrano, M.; Carbonell, A.; Burgos, L.; Riquelme, F.; Valero, D. Effects of postharvest putrescine treatment on extending shelf life and reducing mechanical damage in apricot. J. Food Sci. 2002, 67, 1706–1712. [Google Scholar] [CrossRef]
- Mei, Z.; Bing, Y.; Ping, L. The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J. Exp. Bot. 2009, 60, 1579–1588. [Google Scholar]
- Jackson, D.I.; Coombe, B.G. Gibberellin-like substances in the developing apricot fruit. Science 1966, 154, 277–278. [Google Scholar] [CrossRef]
- Webster, A.D.; Spencer, J.E. Fruit thinning plums and apricots. Plant Growth Reg. 2000, 31, 101–112. [Google Scholar] [CrossRef]
- Ghosh, S.; Halder, S. Effect of different kinds of gibberellin on temperate fruit crops: A review. Pharma Innov. J. 2018, 7, 315–319. [Google Scholar]
- Martínez-Romero, D.; Valero, D.; Serrano, M.; Burlo, F.; Carbonell, A.; Burgos, L.; Riquelme, F. Exogenous Polyamines and Gibberellic Acid Effects on Peach (Prunus persica L.) Storability Improvement. J. Food Sci. 2000, 65, 288–294. [Google Scholar]
- Southwick, S.M.; Yeager, J.T.; Weis, K.G. Use of gibberellins on ‘Patterson’ apricot (Prunus armeniaca) to reduce hand thinning and improve fruit size and firmness: Effects over three seasons. J. Hortic. Sci. 1997, 72, 645–652. [Google Scholar] [CrossRef]
- Looney, N.E. Improving fruit size, appearance, and other aspects of fruit crop quality with plant bioregulating. Acta Hortic. 1993, 329, 120–127. [Google Scholar] [CrossRef]
- Sakakibara, H. Cytokinins: Activity, Biosynthesis, and Translocation. Ann. Rev. Plant Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhang, T.; Zhao, C.; Zhi, J. Regulation of the expression of lipoxygenase genes in Prunus persica fruit ripening. Acta Physiol. Plant. 2011, 33, 1345–1352. [Google Scholar] [CrossRef]
- Ziosi, V.; Bonghi, C.; Bregoli, A.M.; Trainotti, L.; Biondi, L.; Sutthiwal, S.; Kondo, S.; Costa, G.; Torrigiani, P. Jasmonate-induced transcriptional changes suggest a negative interference with the ripening syndrome in peach fruit. J. Exp. Bot. 2008, 59, 563–573. [Google Scholar] [CrossRef]
- Brown, G.S.; Walker, T.D. Indicators of maturity in apricots using biplot multivariate analysis. J. Sci. Food Agric. 1990, 53, 321–331. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Bureau, S.; Renard, C.M.G.C.; Reich, M.; Ginies, C.; Audergon, J.M. Change in anthocyanin concentrations in red apricot fruits during ripening. Food Sci. Technol. 2009, 42, 372–377. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.; Espin, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agri. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Ruiz, D.; Egea, J.; Gil, M.I.; Tomás-Barberán, F.A. Characterization and quantitation of phenolic compounds in new apricot (Prunus armeniaca L.) varieties. J. Agri. Food Chem. 2005, 53, 9544–9552. [Google Scholar] [CrossRef]
- Petroni, K.; Tonelli, C. Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci. 2011, 181, 219–229. [Google Scholar] [CrossRef]
- Rahim, M.A.; Busatto, N.; Trainotti, L. Regulation of anthocyanin biosynthesis in peach fruits. Planta 2014, 240, 913–929. [Google Scholar] [CrossRef] [PubMed]
- Ravaglia, D.; Espley, R.V.; Henry-Kirk, R.A.; Andreotti, C.; Ziosi, V.; Hellens, R.P.; Allan, A.C. Transcriptional regulation of flavonoid biosynthesis in nectarine (Prunus persica) by a set of R2R3 MYB transcription factors. BMC Plant Biol. 2013, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- González, M.; Salazar, E.; Cabrera, S.; Olea, P.; Carrasco, B. Analysis of anthocyanin biosynthesis genes expression profiles in contrasting cultivars of Japanese plum (Prunus salicina L.) during fruit development. Gene Expr. Patterns 2016, 21, 54–62. [Google Scholar]
- Andreotti, C.; Ravaglia, D.; Ragaini, A.; Costa, G. Phenolic compounds in peach (Prunus persica) cultivars at harvest and during fruit maturation. Ann. Appl. Biol. 2008, 153, 11–23. [Google Scholar] [CrossRef]
- Mori, K.; Sugaya, S.; Gemma, H. Decreased anthocyanin biosynthesis in grape berries grown under elevated night temperature condition. Sci. Hortic. 2005, 105, 319–330. [Google Scholar] [CrossRef]
- Leong, S.Y.; Oey, I. Effects of processing on anthocyanins, carotenoids and vitamin C in summer fruits and vegetables. Food Chem. 2012, 133, 1577–1587. [Google Scholar] [CrossRef]
- Xi, W.; Feng, J.; Liu, Y.; Zhang, S.; Zhao, G. The R2R3-MYB transcription factor PaMYB10 is involved in anthocyanin biosynthesis in apricots and determines red blushed skin. BMC Plant Biol. 2019, 19, 287. [Google Scholar] [CrossRef]
- Hu, B.; Zhao, J.; Lai, B.; Qin, Y.; Wang, H.; Hu, G. LcGST4 is an anthocyanin-related glutathione S-transferase gene in Litchi chinensis Sonn. Plant Cell Rep. 2016, 35, 831–843. [Google Scholar] [CrossRef]
- Kitamura, S.; Matsuda, F.; Tohge, T.; Yonekura-Sakakibara, K.; Yamazaki, M.; Saito, K.; Narumi, I. Metabolic profiling and cytological analysis of proanthocyanidins in immature seeds of Arabidopsis thaliana flavonoid accumulation mutants. Plant J. 2010, 624, 549–559. [Google Scholar] [CrossRef]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional control of flavonoid biosynthesis by MYB–bHLH–WDR complexes. Trends Plant Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Zhou, D.R.; Ye, X.F.; Jiang, C.C.; Pan, S.L. Identification of Candidate Anthocyanin-Related Genes by Transcriptomic Analysis of “Furongli” Plum (Prunus salicina Lindl.) during Fruit Ripening Using RNA-Seq. Front. Plant Sci. 2016, 7, 1338. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Chen, X.; Zong, X.; Shu, H.; Gao, D.; Liu, Q. Comparative transcriptome analysis of genes involved in anthocyanin biosynthesis in the red and yellow fruits of sweet cherry (Prunus avium L.). PLoS ONE 2015, 10, e0121164. [Google Scholar] [CrossRef] [PubMed]
- Ayour, J.; Sagar, M.; Alfeddy, M.N.; Taourirte, M.; Benichou, M. Evolution of pigments and their relationship with skin color based on ripening in fruits of different Moroccan genotypes of apricots (Prunus armeniaca L.). Sci. Hortic. 2016, 207, 168–175. [Google Scholar] [CrossRef]
- Ruiz, D.; Reich, M.; Bureau, S.; Renard, C.M.G.C.; Audergon, J.M. Application of reflectance colorimeter measurements and infrared spectroscopy methods to rapid and nondestructive evaluation of carotenoids content in apricot (Prunus armeniaca L.). J. Agric. Food Chem. 2008, 56, 4916–4922. [Google Scholar] [CrossRef]
- Ruiz, D.; Egea, J. Phenotypic diversity and relationships of fruit quality traits in apricot (Prunus armeniaca L.) germplasm. Euphytica 2007, 163, 143–158. [Google Scholar] [CrossRef]
- Walker, A.R.; Lee, E.; Bogs, J.; McDavid, D.J.; Thomas, M.R.; Robinson, S.P. White grapes arose through the mutation of two similar and adjacent regulatory genes. Plant J. 2007, 49, 772–885. [Google Scholar] [CrossRef]
- Jaakola, L.; Poole, M.; Jones, M.O.; Kamarainen-Karppinen, T.; Koskimaki, J.J.; Hohtola, A.; Seymour, G.B. A SQUAMOSA MADS Box Gene Involved in the Regulation of Anthocyanin Accumulation in Bilberry Fruits. Plant Physiol. 2010, 153, 1619–1629. [Google Scholar] [CrossRef]
- Takos, A.M.; Robinson, S.P.; Walker, A.R.L. Transcriptional regulation of the flavonoid pathway in the skin of dark-grown “Cripps” Red’ apples in response to sunlight. J. Hortic. Sci. Biotechnol. 2006, 81, 735–744. [Google Scholar] [CrossRef]
- Ubi, B.E.; Honda, C.; Bessho, H.; Kondo, S.; Wada, M.; Kobayashi, S.; Moriguchi, T. Expression analysis of anthocyanin biosynthetic genes in apple skin: Effect of UV-B and temperature. Plant Sci. 2006, 170, 571–578. [Google Scholar] [CrossRef]
- Povero, G.; Gonzali, S.; Bassolino, L.; Mazzucato, A.; Perata, P. Transcriptional analysis in high-anthocyanin tomatoes reveals synergistic effect of Aft and atv genes. J. Plant Physiol. 2011, 168, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Borovsky, Y.; Oren-Shamir, M.; Ovadia, R.; De Jong, W.; Paran, I. The A locus that controls anthocyanin accumulation in pepper encodes a MYB transcription factor homologous to Anthocyanin of Petunia. Appl. Genet. 2004, 109, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Xu, J.; Zhang, S.; Zhang, B.; Li, X.; Lin-Wang, K.; Chen, K.S.S. Coordinated regulation of anthocyanin biosynthesis in Chinese bayberry (Myrica rubra) fruit by a R2R3 MYB transcription factor. Planta 2010, 231, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Li, L. Carotenoid Metabolism: Biosynthesis, Regulation, and Beyond. J. Integr. Plant Biol. 2008, 50, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, D.; Egea, J.; Tomás-Barberán, F.A.; Gil, M.I. Carotenoids from new apricot (Prunus armeniaca L.) varieties and their relationship with flesh and skin color. J. Agric. Food Chem. 2005, 53, 6368–6374. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H.; Tokuda, H.; Satomi, Y.; Masuda, M.; Bu, P.; Onozuka, M.; Yano, M. Cancer prevention by carotenoids. Pure Appl. Chem. 1999, 71, 2273–2278. [Google Scholar] [CrossRef]
- Olson, J. Carotenoids and human health. Arch. Lat. Nutr. 1999, 49, 7–11. [Google Scholar]
- Stahl, W.; Ale-Agha, N.; Polidori, M. Non-antioxidant properties of carotenoids. J. Biol. Chem. 2002, 383, 553–558. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Paolini, M.; Abdel-Rahman, S.Z.; Sapone, A.; Pedulli, G.F.; Perocco, P.; Cantelli-Forti, G.; Legator, M.S. β-Carotene: A cancer chemopreventive agent or a co-carcinogen? Mutat. Res. Rev. Mutat. Res. 2003, 543, 195–200. [Google Scholar] [CrossRef]
- Voutilainen, S.; Nurmi, T.; Mursu, J.; Rissanen, T.H. Carotenoids and cardiovascular health. Am. J. Clin. Nutr. 2006, 83, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D. Carotenoids and cardiovascular disease: What research gaps remain? Curr. Opin. Lipidol. 2006, 17, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Brandi, F.; Bar, E.; Mourgues, F.; Horváth, G.; Turcsi, E.; Giuliano, G.; Rosati, C. Study of “Redhaven” peach and its white-fleshed mutant suggests a key role of CCD4 carotenoid dioxygenase in carotenoid and norisoprenoid volatile metabolism. BMC Plant Biol. 2011, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Cazzonelli, C.I.; Pogson, B.J. Source to sink: Regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 2010, 15, 266–274. [Google Scholar] [CrossRef]
- Kita, M.; Ban, Y.; Honda, C.; Yaegaki, H.; Moriguchi, T.; Kato, M.; Ikoma, Y. Carotenoid accumulation in Japanese apricot (Prunus mume Siebold & Zucc.): Molecular analysis of carotenogenic gene expression and ethylene regulation. J. Agric. Food Chem. 2007, 55, 3414–3420. [Google Scholar]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of Plant Volatiles: Nature’s Diversity and Ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef]
- Fraser, P.D.; Bramley, P.M. The biosynthesis and nutritional uses of carotenoids. Prog. Lipid Res. 2004, 43, 228–265. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef]
- Sandmann, G.; Romer, S.; Fraser, P.D. Understanding carotenoid metabolism as a necessity for genetic engineering of crop plants. Metab. Eng. 2006, 8, 291–302. [Google Scholar] [CrossRef]
- Liu, Y.; Roof, S.; Zhibiao, Y.; Barry, C.; van Tuinen, A.; Vrebalov, C.; Bowler, C.; Giovannoni, J. Manipulation of light signal transduction as a means of modifying fruit nutritional quality in tomato. Proc. Natl. Acad. Sci. USA 2004, 101, 9897–9902. [Google Scholar] [CrossRef]
- Trainotti, L.; Bonghi, C.; Ziliotto, F.; Zanin, D.; Rasori, A.; Casadoro, G.; Tonutti, P. The use of microarray μPEACH1.0 to investigate transcriptome changes during transition from pre-climacteric to climacteric phase in peach fruit. Plant Sci. 2006, 170, 606–613. [Google Scholar] [CrossRef]
- Kuai, B.; Chen, J.; Hörtensteiner, S. The biochemistry and molecular biology of chlorophyll breakdown. J. Exp. Bot. 2018, 69, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, M.V.; Lee, C.Y.; Liu, R.H. Antioxidant activity of fresh apples. Nature 2000, 405, 903–904. [Google Scholar] [CrossRef] [PubMed]
- Guyer, L.; Hofstetter, S.S.; Christ, B.; Lira, B.S.; Rossi, M.; Hörtensteiner, S. Different mechanisms are responsible for chlorophyll dephytylation during fruit ripening and leaf senescence in tomato. Plant Physiol. 2014, 166, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Ulrich, M.; Ongania, K.H.; Kräutler, B. Colorless Tetrapyrrolic Chlorophyll Catabolites Found in Ripening Fruit Are Effective Antioxidants. Angew. Chem. Int. Ed. 2007, 46, 8699–8702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Feng, C.; Li, W.; Qu, Z.; Zeng, M.; Xi, W. Transcriptional regulatory networks controlling taste and aroma quality of apricot (Prunus armeniaca L.) fruit during ripening. BMC Genom. 2019, 20, 45. [Google Scholar] [CrossRef]
- Sánchez, G.; Besada, C.; Badenes, M.L.; Monforte, A.J.; Granell, A. A Non-Targeted Approach Unravels the Volatile Network in Peach Fruit. PLoS ONE 2012, 7, e38992. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Manriquez, D.; Luengwilai, K.; Gonzalez-Aguero, M. Aroma Volatiles: Biosynthesis and Mechanisms of Modulation during Fruit Ripening. Adv. Bot. Res. 2009, 50, 1–37. [Google Scholar]
- Zhang, B.; Shen, J.Y.; Wei, W.; Xi, W.; Xu, C.J.; Ferguson, I.; Chen, K.S. Expression of Genes Associated with Aroma Formation Derived from the Fatty Acid Pathway during Peach Fruit Ripening. J. Agric. Food Chem. 2010, 26, 6157–6165. [Google Scholar] [CrossRef]
- Etienne, C.; Rothan, C.; Moing, A.; Plomion, C.; Bodénès, C.; Svanella-Dumas, L.; Dirlewanger, E. Candidate genes and QTLs for sugar and organic acid content in peach [ Prunus persica (L.) Batsch]. Appl. Genet. 2002, 105, 145–159. [Google Scholar] [CrossRef]
- Etienne, A.; Genard, M.; Lobit, P.; Mbeguie-A-Mbeguie, D.; Bugaud, C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J. Exp. Bot. 2013, 64, 1451–1469. [Google Scholar] [CrossRef] [PubMed]
- Tronconi, M.A.; Gerrard Wheeler, M.C.; Martinatto, A.; Zubimendi, J.P.; Andreo, C.S.; Drincovich, M.F. Allosteric substrate inhibition of Arabidopsis NAD-dependent malic enzyme 1 is released by fumarate. Phytochemistry 2015, 111, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Brummell, D.A.; Dal Cin, V.; Crisosto, C.H.; Labavitch, J.M. Cell wall metabolism during maturation, ripening and senescence of peach fruit. J. Exp. Bot. 2004, 55, 2029–2039. [Google Scholar] [CrossRef] [PubMed]
- Vicente, A.R.; Saladie, M.; Rose, J.K.C.; Labavitch, J.M. The linkage between cell wall metabolism and fruit softening: Looking to the future. J. Sci. Food Agric. 2007, 87, 1435–1448. [Google Scholar] [CrossRef]
- Hajnal, V.; Szalay, L.; Németh, S.; Ficzek, G.; Bujdosó, G.; Tóth, M. Changes in the fruit texture parameters and composition of apricot cultivars during ripening. Acta Aliment. 2012, 41, 73–82. [Google Scholar] [CrossRef]
- Li, D.; Lurie, S.; Hong-Wei, Z. Effect of 1-methylcyclopropene on ripening of Canino apricots and Royal Zee plums. Postharvest Biol. Technol. 2002, 24, 135–145. [Google Scholar]
- Mathooko, F.M.; Tsunashima, Y.; Owino, W.Z.O.; Kubo, Y.; Inaba, A. Regulation of genes encoding ethylene biosynthetic enzymes in peach (Prunus persica L.) fruit by carbon dioxide and 1-methylcyclopropene. Postharvest Biol. Technol. 2001, 21, 265–281. [Google Scholar] [CrossRef]
- Fan, X.; Shu, C.; Zhao, K.; Wang, X.; Cao, J.; Jiang, W. Regulation of apricot ripening and softening process during shelf life by post-storage treatments of exogenous ethylene and 1-methylcyclopropene. Sci. Hortic. 2018, 232, 63–70. [Google Scholar] [CrossRef]
- Pimentel, H.; Bray, N.L.; Puente, S.; Melsted, P.; Pachter, L. Differential analysis of RNA-seq incorporating quantification uncertainty. Nat. Methods 2017, 14, 687–699. [Google Scholar] [CrossRef]
- Harker, F.R.; Redgwell, R.J.; Hallett, I.C.; Murray, S.H.; Carter, G. Texture of Fresh Fruit. Hortic. Rev. 2010, 20, 121–224. [Google Scholar]
- Hampson, C.R.; Quamme, H.A.; Hall, J.W.; MacDonald, R.A.; King, M.C.; Cliff, M.A. Sensory evaluation as a selection tool in apple breeding. Euphytica 2000, 111, 79–90. [Google Scholar] [CrossRef]
- Kovacs, E.; Meresz, P.; Kristof, Z.; Nemeth-Szerdahelyi, E. Ripening and microstructure of apricot (Prunus Armeniaca L.). Acta Aliment. 2008, 37, 23–39. [Google Scholar] [CrossRef]
- Kovacs, E.; Nemeth-Szerdahelyi, E. β-Galactosidase activity and cell wall breakdown in apricots. J. Food Sci. 2002, 67, 2004–2008. [Google Scholar] [CrossRef]
- Hayama, H.; Tatsuki, M.; Ito, A.; Kashimura, Y. Ethylene and fruit softening in the stony hard mutation in peach. Postharvest Biol. Technol. 2006, 41, 16–21. [Google Scholar] [CrossRef]
- Luo, Z.; Xie, J.; Xu, T.; Zhang, L. Delay ripening of ‘Qingnai’ plum (Prunus salicina Lindl.) with 1-methylcyclopropene. Plant Sci. 2009, 177, 705–709. [Google Scholar] [CrossRef]
- Rose, J.K.C.; Braam, J.; Fry, S.C.; Nishitani, K. The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: Current perspectives and a new unifying nomenclature. Plant Cell Physiol. 2002, 43, 1421–1435. [Google Scholar] [CrossRef]
- Williams, L.E.; Lemoine, R.; Sauer, N. Sugar transporters in higher plants—A diversity of roles and complex regulation. Trends Plant Sci. 2000, 5, 283–290. [Google Scholar] [CrossRef]
- Giovannoni, J. Molecular Biology of fruit maturation and ripening. Ann. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 725–749. [Google Scholar] [CrossRef]
- Blanco-Portales, R.; Medina-Escobar, N.; Lopez-Raez, J.A.; Gonzalez-Reyes, J.A.; Villalba, J.M.; Moyano, E.; Caballero, J.L.; Muñoz-Blanco, J. Cloning, expression and immunolocalization pattern of a cinnamyl alcohol dehydrogenase gene from strawberry (Fragaria x ananassa cv. Chandler). J. Exp. Bot. 2002, 53, 1723–1734. [Google Scholar] [CrossRef]
- Femenia, A.; Sanchez, E.S.; Simal, S.; Rossello, C. Developmental and ripening-related effects on the cell wall of apricot (Prunus armeniaca) fruit. J. Sci. Food Agric. 1998, 77, 487–493. [Google Scholar] [CrossRef]
- Lahaye, M.; Falourd, X.; Quemener, B.; Devaux, M.F.; Audergon, J.M. Histological and cell wall polysaccharide chemical variability among apricot varieties. Food Sci. Technol. 2014, 58, 486–496. [Google Scholar] [CrossRef]
- Mbeguie-A-Mbeguie, D.; Gouble, B.; Gomez, R.M.; Audergon, J.M.; Albagnac, G.; Fils-Lycaon, B. Two expansin cDNAs from Prunus armeniaca expressed during fruit ripening are differently regulated by ethylene. Plant Physiol. Biochem. 2002, 40, 445–452. [Google Scholar] [CrossRef]
- Stanley, J.; Prakash, R.; Marshall, R.; Schröder, R. Effect of harvest maturity and cold storage on correlations between fruit properties during ripening of apricot (Prunus armeniaca). Postharvest Biol. Technol. 2013, 82, 39–50. [Google Scholar] [CrossRef]
- Trainotti, L.; Zanin, D.; Casadoro, G. A cell wall-oriented genomic approach reveals a new and unexpected complexity of the softening in peaches. J. Exp. Bot. 2003, 54, 1821–1832. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Fratianni, F.; Ombra, M.N.; D’Acierno, A.; Cipriano, L.; Nazzaro, F. Apricots: Biochemistry and functional properties. Curr. Opin. Food Sci. 2018, 19, 23–29. [Google Scholar] [CrossRef]
- Steinmetz, K.A.; Potter, J.D. Vegetables, Fruit, and Cancer Prevention: A Review. J. Am. Diet. Assoc. 1996, 96, 1027–1039. [Google Scholar] [CrossRef]
- Van Duyn, M.A.S.; Pivonka, E. Overview of the Health Benefits of Fruit and Vegetable Consumption for the Dietetics Professional: Selected Literature. J. Am. Diet. Assoc. 2000, 100, 1511–1521. [Google Scholar] [CrossRef]
- Katayama, S.; Ogawa, H.; Nakamura, S. Apricot Carotenoids Possess Potent Anti-amyloidogenic Activity in Vitro. J. Agri. Food Chem. 2011, 59, 12691–12696. [Google Scholar] [CrossRef]
- Mangels, A.R.; Holden, J.M.; Beecher, G.R.; Forman, M.R.; Lanza, E. Carotenoid content of fruits and vegetables: An evaluation of analytic data. J. Am. Diet. Assoc. 1993, 93, 284–296. [Google Scholar] [CrossRef]
- Curl, A.L. Carotenoids of apricots. Food Res. 1960, 25, 190–196. [Google Scholar] [CrossRef]
- Yuan, J.M.; Stram, D.O.; Arakawa, K.; Lee, H.P.; Yu, M.C. Dietary cryptoxanthin and reduced risk of lung cancer: The Singapore Chinese Health Study. Cancer Epidemiol. Biomark. Prev. 2003, 12, 890–898. [Google Scholar]
- Yamaguchi, M. Role of carotenoid β-cryptoxanthin in bone homeostasis. J. Biomed. Sci. 2012, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.J.; Symmons, D.P.; Lunt, M.; Welch, A.; Bingham, S.A.; Day, N.E.; Silman, A.J. Dietary β-cryptoxanthin and inflammatory polyarthritis: Results from a population-based prospective study. Am. J. Clin. Nutr. 2005, 82, 451–455. [Google Scholar] [CrossRef]
- Müller, H. Determination of the carotenoid content in selected vegetables and fruit by HPLC and photodiode array detection. Eur. Food Res. Technol. 1997, 204, 88–94. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N.; Skrzypczak-Jankun, E.; Morgan, M.R.A.; Williamson, G. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Song, W.; Derito, C.M.; Liu, M.K.; He, X.; Dong, M.; Liu, R.H. Cellular antioxidant activity of common vegetables. J. Agric. Food Chem. 2010, 58, 6621–6629. [Google Scholar] [CrossRef]
- Sochor, J.; Zitka, O.; Skutkova, H.; Pavlik, D.; Babula, P.; Krska, B.; Kizek, R. Content of Phenolic Compounds and Antioxidant Capacity in Fruits of Apricot Genotypes. Molecules 2010, 15, 6285–6305. [Google Scholar] [CrossRef]
- Dragovic-Uzelac, V.; Delonga, K.; Levaj, B.; Djakovic, S.; Pospisil, J. Phenolic profiles of raw apricots, pumpkins, and their purees in the evaluation of apricot nectar and jam authenticity. J. Agric. Food Chem. 2005, 53, 4836–4842. [Google Scholar] [CrossRef]
- Marlett, J.A.; Vollendorf, N.W. Dietary fiber content and composition of different forms of fruits. Food Chem. 1994, 51, 39–44. [Google Scholar] [CrossRef]
- Jacobs, L.R. Fiber and colon cancer. Gastroenterol. Clin. N. Am. 1988, 17, 747–760. [Google Scholar]
- Potter, J.D. The Epidemiology of Fiber and Colorectal Cancer. Diet. Fiber 1990, 2, 431–445. [Google Scholar]
- Trock, B.; Lanza, E.; Greenwald, P. Dietary Fiber, Vegetables, and Colon Cancer: Critical Review and Meta-analyses of the Epidemiologic Evidence. J. Natl. Cancer Inst. 1990, 82, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Génard, M.; Bertin, N.; Borel, C.; Bussières, P.; Gautier, H.; Habib, R.; Léchaudel, M.; LeComte, A.; Lescourret, F.; Lobit, P.; et al. Towards a virtual fruit focusing on quality: Modelling features and potential uses. J. Exp. Bot. 2007, 58, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.; Rubio, M.; Trainotti, L.; Verde, I.; Bonghi, C.; Martínez-Gómez, P. Prunus transcription factors: Breeding perspectives. Front. Plant Sci. 2015, 6, 443. [Google Scholar] [CrossRef]
- Verde, I.; The International Peach Genome Initiative; Abbott, A.G.; Scalabrin, S.; Jung, S.; Shu, S.; Marroni, F.; Zhebentyayeva, T.; Dettori, M.T.; Grimwood, J.; et al. The high-quality draft of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nat. Genet. 2013, 45, 487–494. [Google Scholar] [CrossRef]
- Verde, I.; Jenkins, J.; Dondini, L.; Micali, S.; Pagliarani, G.; Vendramin, E.; Paris, R.; Aramini, V.; Gazza, L.; Rossini, L.; et al. The Peach v2.0 release: High-resolution linkage mapping and deep resequencing improve chromosome-scale assembly and contiguity. BMC Genom. 2017, 18, 225. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, W.; Sun, L.; Zhao, F.; Huang, B.; Yang, W.; Tao, Y.; Wang, J.; Yuan, Z.; Fan, G.; et al. The genome of Prunus mume. Nat. Commum. 2012, 3, 1318. [Google Scholar] [CrossRef]
- Shirasawa, K.; Isuzugawa, K.; Ikenaga, M.; Saito, Y.; Yamamoto, T.; Hirakawa, H.; Isobe, S. The genome sequence of sweet cherry (Prunus avium) for use in genomics-assisted breeding. DNA Res. 2017, 24, 499–508. [Google Scholar] [CrossRef]
- Shirasawa, K.; Esumi, T.; Hirakawa, H.; Tanaka, H.; Itai, A.; Ghelfi, A.; Nagasaki, H.; Isobe, S. Phased genome sequence of an interspecific hybrid flowering cherry, ‘Somei-Yoshino’ (Cerasus × yedoensis). DNA Res. 2019, 26, 379–389. [Google Scholar] [CrossRef]
- Zhebentyayeva, T.; Shankar, V.; Scorza, R.; Callahan, A.; Ravelonandro, M.; Castro, S.; DeJong, T.; Saski, C.A.; Dardick, C. Genetic characterization of worldwide Prunus domestica (plum) germplasm using sequence-based genotyping. Hortic. Res. 2019, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pérez, R.; Pavan, S.; Mazzeo, R.; Moldovan, C.; Cigliano, R.A.; Del Cueto, J.; Ricciardi, F.; Lotti, C.; Dicenta, F.; López-Marqués, R.; et al. Mutation of a bHLH transcription factor allowed almond domestication. Science 2019, 364, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Alioto, T.; Alexiou, K.; Bardil, A.; Barteri, F.; Castanera, R.; Cruz, F.; Dhingra, A.; Duval, H.; Fernández i Martí, Á.; Frias, L. Transposons played a major role in the diversification between the closely related almond (Prunus dulcis) and peach (P. persica) genomes: Results from the almond genome sequence. Plant J. 2020, 101, 455–472. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Zhang, J.; Wang, S.; Yang, L.; Luo, Y.; Gao, S.; Zhang, M.; Wu, S.; Hu, S.; Wang, Y. The apricot (Prunus armeniaca L.) genome elucidates Rosaceae evolution and beta-carotenoid synthesis. Hortic. Res. 2019, 6, 128. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, Q.; Liu, N.; Zhang, Y.; Xu, M.; Zhang, Y.; Ma, X.; Liu, W. Genetic diversity of Chines plum (Prunus salicina) based on whole-genome resequencing. bioRxiv 2020. [Google Scholar] [CrossRef]
- Martínez-Gómez, P.; Sánchez-Pérez, R.; Rubio, M. Clarifying omics concepts, challenges and opportunities for Prunus breeding in the post-genomic era. Omics J. Integr. Biol. 2012, 16, 268–283. [Google Scholar] [CrossRef]
- Salazar, J.A.; Ruiz, D.; Campoy, J.A.; Sánchez-Pérez, R.; Crisosto, C.H.; Martínez-García, P.J.; Blenda, A.; Jung, S.; Main, D.; Martínez-Gómez, P.; et al. Quantitative Trait Loci (QTL) and Mendelian Trait Loci (MTL) analysis in Prunus: A breeding perspective and beyond. Plant Mol. Biol. Rep. 2014, 32, 1–18. [Google Scholar] [CrossRef]
- Salazar, J.A.; Pacheco, I.; Shinya, P.; Zapata, P.; Silva, C.; Ruiz, D.; Martínez-Gómez, P.; Infante, R. Genotyping by Sequencing for SNP-Based Linkage Analysis and Identification of QTLs Linked to Fruit Quality Traits in Japanese Plum (Prunus salicina Lindl.). Front. Plant Sci. 2017, 8, 476. [Google Scholar] [CrossRef]
- García-Gómez, B.E.; Salazar, J.A.; Dondini, L.; Martínez-Gómez, P.; Ruiz, D. Identification of QTLs linked to fruit quality traits in apricot (Prunus armeniaca L.) and biological validation through gene expression analysis using qPCR. Mol. Breed. 2019, 39, 28. [Google Scholar] [CrossRef]
- García-Gómez, B.; Razi, M.; Salazar, J.A.; Prudencio, A.S.; Ruiz, D.; Dondini, L.; Martínez-Gómez, P. Comparative Analysis of SSR Markers Developed in Exon, Intron, and Intergenic Regions and Distributed in Regions Controlling Fruit Quality Traits in Prunus Species: Genetic Diversity and Association Studies. Plant Mol. Biol. Rep. 2018, 36, 23–35. [Google Scholar] [CrossRef]
- Salazar, J.A.; Ruiz, D.; Egea, J.; Martínez-Gómez, P. Transmission of fruit quality traits in apricot (Prunus armeniaca L.) and analysis of linked quantitative trait loci (QTLs) using simple sequence repeat (SSR) markers. Plant Mol. Biol. Rep. 2013, 31, 1506–1517. [Google Scholar] [CrossRef]
- Salazar, J.A.; Ruiz, D.; Campoy, J.A.; Tartarini, S.; Dondini, L.; Martínez-Gómez, P. Inheritance of reproductive phenology traits and related QTL identification in apricot. Tree Genet. Genomes 2016, 12, 71. [Google Scholar] [CrossRef]
- Socquet-Juglard, D.; Christen, D.; Devènes, G.; Gessler, C.; Duffy, B.; Patocchi, A. Mapping Architectural, Phenological, and Fruit Quality QTLs in Apricot. Plant Mol. Biol. Rep. 2013, 31, 387–397. [Google Scholar] [CrossRef]
- Sánchez-Pérez, R.; Howad, W.; Dicenta, F.; Arús, P.; Martínez-Gómez, P. Mapping major genes and quantitative trait loci controlling agronomic traits in almond. Plant Breed. 2007, 126, 310–318. [Google Scholar] [CrossRef]
- Dirlewanger, E.; Cosson, P.; Boudehri, K.; Renaud, C.; Capdeville, G.; Tauzin, Y.; Laigret, F.; Moing, A. Development of a second-generation genetic linkage map for peach [Prunus persica (L.) Batsch] and characterization of morphological traits affecting flower and fruit. Tree Genet. Genomes 2006, 3, 1–13. [Google Scholar] [CrossRef]
- Eduardo, I.; Pacheco, I.; Chietera, G.; Bassi, D.; Pozzi, C.; Vecchietti, A.; Rossini, L. QTL analysis of fruit quality traits in two peach intraspecific populations and importance of maturity date pleiotropic effect. Tree Genet. Genomes 2011, 7, 323–335. [Google Scholar] [CrossRef]
- Fan, S.; Bielenberg, D.G.; Zhebentyayeva, T.N.; Reighard, G.L.; Okie, W.R.; Holland, D.; Abbott, A.G. Mapping quantitative trait loci associated with chilling requirement, heat requirement and bloom date in peach (Prunus persica). New Phytol. 2010, 185, 917–930. [Google Scholar] [CrossRef]
- Fresnedo-Ramírez, J.; Bink, M.C.A.M.; van de Weg, E.; Famula, T.R.; Crisosto, C.H.; Frett, T.J.; Gradziel, T.M. QTL mapping of pomological traits in peach and related species breeding germplasm. Mol. Breed. 2015, 35, 166. [Google Scholar] [CrossRef]
- Sánchez, G.; Martínez, J.; Romeu, J.; García, J.; Monforte, A.J.; Badenes, M.L.; Granell, A. The peach volatilome modularity is reflected at the genetic and environmental response levels in a QTL mapping population. BMC Plant Biol. 2014, 14, 137. [Google Scholar] [CrossRef]
- Salazar, J.A.; Pacheco, I.; Zapata, P.; Shinya, P.; Ruiz, D.; Martínez-Gómez, P.; Infante, R. Identification of loci controlling phenology, fruit quality and post-harvest quantitative parameters in Japanese plum (Prunus salicina Lindl.). Postharvest Biol. Technol. 2020, 169, 111292. [Google Scholar] [CrossRef]
- Nunez-Lillo, G.; Balladares, C.; Pavez, C.; Urra, C.; Sanhueza, D.; Vendramin, E.; Dettori, M.T.; Arus, P.; Verde, I.; Blanco-Herrera, F. High-density genetic map and QTL analysis of soluble solid content, maturity date, and mealiness in peach using genotyping by sequencing. Sci. Hortic. 2019, 257, 108734. [Google Scholar] [CrossRef]
- Calle, A.; Wunsch, A. Multiple-population QTL mapping of maturity and fruit-quality traits reveals LG4 region as a breeding target in sweet cherry (Prunus avium L.). Hortic. Res. 2020, 7, 127. [Google Scholar] [CrossRef]
- Rawandoozi, Z.J.; Hartmann, T.P.; Carpenedo, S.; Gasic, K.; Linge, C.D.; Cai, L.C.; Van de Weg, E.; Byrne, D.H. Identification and characterization of QTLs for fruit quality traits in peach through a multi-family approach. BMC Genom. 2020, 21, 522. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Xu, Z.; Zhang, S.Y.; Wang, X.J.; Ma, X.F.; Zheng, J.C.; Xing, L.B.; Zhang, D.; Ma, J.J.; Han, M.Y. Construction of a high-density SNP-based genetic map and identification of fruit-related QTLs and candidate genes in peach [Prunus persica (L.) Batsch]. BMC Plant Biol. 2020, 20, 438. [Google Scholar] [CrossRef] [PubMed]
- Dirlewanger, E.; Graziano, E.; Joobeur, T.; Garriga-Caldere, F.; Cosson, P.; Howad, W.; Arus, P. Comparative mapping and marker-assisted selection in Rosaceae fruit crops. Proc. Natl. Acad. Sci. USA 2004, 101, 9891–9896. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yamaguchi, M.; Hayashi, T. An integrated genetic linkage map of peach by SSR, STS, AFLP and RAPD. J. Jpn. Soc. Hortic. Sci. 2005, 74, 204–213. [Google Scholar] [CrossRef]
- Dirlewanger, E.; Moing, A.; Rothan, C.; Svanella, L.; Pronier, V.; Guye, A.; Monet, R. Mapping QTLs controlling fruit quality in peach (Prunus persica (L.) Batsch). Appl. Genet. 1999, 98, 18–31. [Google Scholar] [CrossRef]
- Dirlewanger, E.; Quero-García, J.; Le Dantec, L.; Lambert, P.; Ruiz, D.; Dondini, L.; Arús, P. Comparison of the genetic determinism of two key phenological traits, flowering and maturity dates, in three Prunus species: Peach, apricot and sweet cherry. Heredity 2012, 109, 280–292. [Google Scholar] [CrossRef]
- Shulaev, V.; Korban, S.; Sosinski, B.; Abbott, A.G.; Aldwinckle, H.; Folta, K.M.; Dandekar, A.M. Multiple models for Rosaceae genomics. Plant Physiol. 2008, 147, 985–1003. [Google Scholar] [CrossRef]
- Abdelghafar, A.; Linge, C.D.; Okie, W.R.; Gasic, K. Mapping QTLs for phytochemical compounds and fruit quality in peach. Mol. Breed. 2020, 40, 32. [Google Scholar] [CrossRef]
- Martínez-Gómez, P.; Crisosto, C.; Bonghi, C.; Rubio, M. New approaches to Prunus transcriptome analysis. Genetica 2011, 139, 755–769. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Ziliotto, F.; Rasori, A.; Bonghi, C.; Ramina, A.; Banfi, R.; Tonutti, P. Gene expression profile during apricot fruit growth, using a peach microarray. Acta Hortic. 2009, 817, 113–118. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Ziliotto, F.; Rasori, A.; Bonghi, C.K.; Ramina, A.; Tonutti, P. A Comparative Transcriptomic Approach to Elucidate Common and Divergent Mechanisms Involved in Apricot and Peach Fruit Development and Ripening. Acta Hortic. 2010, 862, 577–581. [Google Scholar] [CrossRef]
- Conesa, A.; Madrigal, P.; Tarazona, S.; Gomez-Cabrero, D.; Cervera, A.; McPherson, A.; Mortazavi, A. A survey of best practices for RNA-seq data analysis. Genome Biol. 2016, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Richter, R.A.; Jung, Y.; Zhu, Q.; Li, R.W. Web-based bioinformatics workflows for end-to-end RNA-seq data computation and analysis in agricultural animal species. BMC Genom. 2016, 17, 761. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Li, L.; Tang, Y.; Qi, W.; Liu, X.; Qiao, L.; Wang, W.; Jia, X. A label-free quantitative proteomic investigation reveals stage-responsive ripening genes in apricot fruits. J. Hortic. Sci. Biotechnol. 2017, 92, 261–269. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Zhang, Q.; Xu, J.; Liu, W.; Dong, W. Comparative transcriptome profiling and morphology provide insights into endocarp cleaving of apricot cultivar (Prunus armeniaca L.). BMC Plant Biol. 2017, 17, 72. [Google Scholar] [CrossRef]
- García-Gómez, B.E.; Ruiz, D.; Salazar, J.A.; Rubio, M.; Martínez-García, P.J.; Martínez-Gómez, P. Analysis of Metabolites and Gene Expression Changes Relative to Apricot (Prunus armeniaca L.) Fruit Quality during Development and Ripening. Front. Plant Sci. 2020, 11, 1269. [Google Scholar]
- Pan, H.F.; Sheng, Y.; Gao, Z.H.; Chen, H.L.; Qi, Y.J.; Yi, X.K.; Zhang, J.Y. Transcriptome analysis of peach (Prunus persica L. Batsch) during the late stage of fruit ripening. Genet. Mol. Res. 2016, 15, 15049335. [Google Scholar] [CrossRef]
- Pan, L.; Zeng, W.; Niu, L.; Lu, Z.; Liu, H.; Cui, G.; Wang, Z. PpYUC11, a strong candidate gene for the stony hard phenotype in peach (Prunus persica L. Batsch), participates in IAA biosynthesis during fruit ripening. J. Exp. Bot. 2015, 66, 7031–7044. [Google Scholar] [CrossRef]
- Sanhueza, D.; Vizoso, P.; Balic, I.; Campos-Vargas, R.; Meneses, C. Transcriptomic analysis of fruit stored under cold conditions using controlled atmosphere in Prunus persica cv. “Red Pearl”. Front. Plant Sci. 2015, 6, 788. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, S.; Gu, C.; Zhou, Y.; Zhou, H.; Ma, J.; Han, Y. Deep RNA-Seq uncovers the peach transcriptome landscape. Plant Mol. Biol. 2013, 83, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Gao, L.; Gao, J.; Xu, Y.; Liu, H.; Cao, X.; Chen, K. Genome-wide identification, expression patterns, and functional analysis of UDP glycosyltransferase family in peach (Prunus persica L. Batsch). Front. Plant Sci. 2017, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wang, G.; Tan, J.; Zheng, J.; Zhang, X.; Xu, F.; Liao, Y. Identification of candidate genes involved in anthocyanin accumulation using Illmuina-based RNA-seq in peach skin. Sci. Hortic. 2019, 250, 184–198. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, X.X.; Zhang, Z.; Gao, Z.H. Identification of differentially expressed genes associated with flower color in peach using genome-wide transcriptional analysis. Genet. Mol. Res. 2015, 14, 4724–4739. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Saha, P.; Farcuh, M.; Li, B.; Sadka, A.; Blumwald, E. RNA-Seq Analysis of Spatiotemporal Gene Expression Patterns During Fruit Development Revealed Reference Genes for Transcript Normalization in Plums. Plant Mol. Biol. Rep. 2015, 33, 1634–1649. [Google Scholar] [CrossRef]
- González, M.; Maldonado, J.; Salazar, E.; Silva, H.; Carrasco, B. De novo transcriptome assembly of “Angeleno” and “Lamoon” Japanese plum cultivars (Prunus salicina). Genom. Data 2016, 9, 35–36. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Hao, R.; Cheng, T.; Pan, H.; Yang, W.; Wang, J.; Zhang, Q. Genome-Wide Analysis of the AP2/ERF Gene Family in Prunus mume. Plant Mol. Biol. Rep. 2013, 31, 741–750. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Q.; Sun, L.; Du, D.; Cheng, T.; Pan, H.; Wang, J. Genome-wide identification, characterisation and expression analysis of the MADS-box gene family in Prunus mume. Mol. Genet. Genom. 2014, 289, 903–920. [Google Scholar] [CrossRef]
- Alkio, M.; Jonas, U.; Declercq, M.; Van Nocker, S.; Knoche, M. Transcriptional dynamics of the developing sweet cherry (Prunus avium L.) fruit: Sequencing, annotation and expression profiling of exocarp-Associated genes. Hortic. Res. 2014, 1, 11. [Google Scholar] [CrossRef]
- Carrasco-Valenzuela, T.; Munoz-Espinoza, C.; Riveros, A.; Pedreschi, R.; Arus, P.; Campos-Vargas, R.; Meneses, C. Expression QTL (eQTLs) Analyses Reveal Candidate Genes Associated With Fruit Flesh Softening Rate in Peach [Prunus persica (L.) Batsch]. Front. Plant Sci. 2019, 10, 1581. [Google Scholar] [CrossRef]
- Huang, Y.F.; Vialet, S.; Guiraud, J.L.; Torregrosa, L.; Bertrand, Y.; Cheynier, V.; Terrier, N. A negative MYB regulator of proanthocyanidin accumulation, identified through expression quantitative locus mapping in the grape berry. New Phytol. 2014, 201, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Omura, M.; Shimada, T.; Fujii, H.; Endo, T.; Shimizu, T.; Ikoma, Y. Expression Quantitative Trait Loci Analysis of Carotenoid Metabolism-related Genes in Citrus. J. Jpn. Soc. Hortic. Sci. 2014, 83, 32–43. [Google Scholar] [CrossRef]
- Shang, X.L.; Zhang, J.P.; Ma, Y.H.; Wang, L.R. Preliminary identification of candidate genes associated with the peach fruit sorbitol content based on comparative transcriptome analysis. Sci. Hortic. 2020, 263, 109151. [Google Scholar] [CrossRef]
- Saze, H. Epigenetic memory transmission through mitosis and meiosis in plants. Semin. Cell Dev. Biol. 2008, 19, 527–536. [Google Scholar] [CrossRef]
- Feng, S.; Jacobsen, S.E. Epigenetic modifications in plants: An evolutionary perspective. Curr. Opin. Plant Biol. 2011, 14, 179–186. [Google Scholar] [CrossRef]
- Pascual, J.; Cañal, M.J.; Correia, B.; Escandon, M.; Hasbún, R.; Meijón, M.; Pinto, G.; Valledor, L. Can epigenetics help forest plants to adapt to climate change? In Epigenetics in Plants of Agronomic Importance: Fundamentals and Applications; Álvarez-Venegas, R., De la Peña, C., Casas-Mollano, J.A., Eds.; Springer: Cham, Switzerland, 2014; pp. 125–146. [Google Scholar]
- Van Gurp, T.; Wagemaker, N.; Wouters, B.; Vergeer, P.; Ouborg, J.N.; Verhoeven, K.J. epiGBS: A reference-free reduced representation bisulfite sequencing technique. Nat. Methods 2014, 13, 322–328. [Google Scholar] [CrossRef]
- Cheng, J.; Wei, G.; Zhou, H.; Gu, C.; Vimolmangkang, S.; Liao, L.; Han, Y. Unraveling the mechanism underlying the glycosylation and methylation of anthocyanins in peach. Plant Physiol. 2014, 166, 1044–1058. [Google Scholar] [CrossRef]
- Luby, J.J.; Shaw, D.V. Does marker-assisted selection make dollars and sense in a fruit breeding program? HortScience 2001, 36, 872–879. [Google Scholar] [CrossRef]
- Druka, A.; Potokina, E.; Luo, Z.; Jiang, N.; Chen, X.; Kearsey, M.; Waugh, R. Expression quantitative trait loci analysis in plants. Plant Biotechnol. J. 2010, 8, 10–27. [Google Scholar] [CrossRef]
- Aranzana, M.J.; Decroocq, V.; Dirlewanger, E.; Eduardo, I.; Gao, Z.S.; Gasic, K.; Arús, P. Prunus genetics and applications after de novo genome sequencing: Achievements and prospects. Hortic. Res. 2019, 6, 58. [Google Scholar] [CrossRef]
- Pirona, R.; Eduardo, I.; Pacheco, I.; Da Silva Linge, C.; Miculan, M.; Verde, I.; Rossini, L. Fine mapping and identification of a candidate gene for a major locus controlling maturity date in peach. BMC Plant Biol. 2013, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Fresnedo-Ramírez, J.; Frett, T.J.; Sandefur, P.J.; Salgado-Rojas, A.; Clark, J.R.; Gasic, K.; Peace, C.P.; Anderson, N.; Hartmann, T.P.; Byrne, D.; et al. QTL mapping and breeding value estimation through pedigree-based analysis of fruit size and weight in four diverse peach breeding programs. Tree Genet. Genomes 2016, 12, 25. [Google Scholar] [CrossRef]
- Calle, A.; Balas, F.; Cai, L.C.; Iezzoni, A.; Lopez-Corrales, M.; Serradilla, M.J.; Wunsch, A. Fruit size and firmness QTL alleles of breeding interest identified in a sweet cherry ‘Ambrunes’ x ‘Sweetheart’ population. Mol. Breed. 2020, 40, 86. [Google Scholar] [CrossRef]
- López-Girona, E.; Zhang, Y.; Eduardo, I.; Mora, J.R.H.; Alexiou, K.G.; Arús, P.; Aranzana, M.J. A deletion affecting an LRR-RLK gene co-segregates with the fruit flat shape trait in peach. Sci. Rep. 2017, 7, 6714. [Google Scholar] [CrossRef]
- Falchi, R.; Vendramin, E.; Zanon, L.; Scalabrin, S.; Cipriani, G.; Verde, I.; Morgante, M. Three distinct mutational mechanisms acting on a single gene underpin the origin of yellow flesh in peach. Plant J. 2013, 76, 175–187. [Google Scholar] [CrossRef]
- Bretó, M.P.; Cantín, C.M.; Iglesias, I.; Arús, P.; Eduardo, I. Mapping a major gene for red skin color suppression (highlighter) in peach. Euphytica 2017, 213, 14. [Google Scholar] [CrossRef]
- Frett, T.J.; Reighard, G.L.; Okie, W.R.; Gasic, K. Mapping quantitative trait loci associated with blush in peach [Prunus persica (L.) Batsch]. Tree Genet. Genomes 2014, 10, 367–381. [Google Scholar] [CrossRef]
- Boudehri, K.; Bendahmane, A.; Cardinet, G.; Troadec, C.; Moing, A.; Dirlewanger, E. Phenotypic and fine genetic characterization of the D locus controlling fruit acidity in peach. BMC Plant Biol. 2009, 9, 59. [Google Scholar] [CrossRef]
- Lester, D.R.; Sherman, W.B.; Atwell, B.J. Endopolygalacturonase and the Melting Flesh (M) Locus in Peach. J. Am. Soc. Hortic. Sci. 1996, 121, 231–235. [Google Scholar] [CrossRef]
- Peace, C.P.; Crisosto, C.H.; Gradziel, T.M. Endopolygalacturonase: A candidate gene for Freestone and Melting Flesh in peach. Mol. Breed. 2005, 16, 21–31. [Google Scholar] [CrossRef]
- Vendramin, E.; Pea, G.; Dondini, L.; Pacheco, I.; Dettori, M.T.; Gazza, L.; Rossini, L. A unique mutation in a MYB gene cosegregates with the nectarine phenotype in peach. PLoS ONE 2014, 9, e90574. [Google Scholar] [CrossRef] [PubMed]
- Eduardo, I.; Picañol, R.; Rojas, E.; Batlle, I.; Howad, W.; Aranzana, M.J.; Arús, P. Mapping of a major gene for the slow ripening character in peach: Co-location with the maturity date gene and development of a candidate gene-based diagnostic marker for its selection. Euphytica 2015, 205, 627–636. [Google Scholar] [CrossRef]
- Eduardo, I.; Chietera, G.; Pirona, R.; Pacheco, I.; Troggio, M.; Banchi, E.; Pozzi, C. Genetic dissection of aroma volatile compounds from the essential oil of peach fruit: QTL analysis and identification of candidate genes using dense SNP maps. Tree Genet. Genomes 2013, 9, 189–204. [Google Scholar] [CrossRef]
- Gonzalez-Aguero, M.; Troncoso, S.; Gudenschwager, O.; Campos-Vargas, R.; Moya-Leon, M.A.; Defilippi, B.G. Differential expression levels of aroma-related genes during ripening of apricot (Prunus armeniaca L.). Plant Physiol. Biochem. 2009, 47, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.N.; Zhang, Q.Y.; Li, W.H.; Zhang, S.K.; Xi, W.P. Identification of key genes and regulators associated with carotenoid metabolism in apricot (Prunus armeniaca) fruit using weighted gene coexpression network analysis. BMC Genom. 2019, 20, 876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Q.; Sun, X.; Du, X.; Liu, W.; Dong, W. Differential expression of genes encoding phenylpropanoid enzymes in an apricot cultivar (Prunus armeniaca L.) with cleavable endocarp. Trees 2019, 33, 1695–1710. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Pedryc, A.; Papp, N.; Abrankó, L.; Stefanovits-Bányai, É.; Hegedus, A. Molecular genetics of the flavonoid biosynthesis in two apricot genotypes. Acta Hortic. 2012, 966, 107–112. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, F.; Zhao, Y.; Shi, L.; Zhu, X. Cloning and expression analysis of polygalacturonase and pectin methylesterase genes during softening in apricot (Prunus armeniaca L.) fruit. Sci. Hortic. 2019, 256, 108607. [Google Scholar] [CrossRef]
- Leida, C.; Ríos, G.; Soriano, J.M.; Pérez, B.; Llácer, G.; Crisosto, C.H.; Badenes, M.L. Identification and genetic characterization of an ethylene-dependent polygalacturonase from apricot fruit. Postharvest Biol. Technol. 2011, 62, 26–34. [Google Scholar] [CrossRef]
- Li, X.Y.; Korir, N.K.; Liu, L.L.; Shangguan, L.F.; Wang, Y.Z.; Han, J.; Fang, J.G. Microarray analysis of differentially expressed genes engaged in fruit development between Prunus mume and Prunus armeniaca. J. Plant Physiol. 2012, 169, 1776–1788. [Google Scholar] [CrossRef]


| Changes | Character | Events |
|---|---|---|
| Biochemical | Color | Chlorophyll degradation |
| Dismantling of photosynthetic apparatus | ||
| Biosynthesis of anthocyanins Accumulation of carotenoids | ||
| Texture | Solubilization of pectin and cellulose | |
| Starch hydrolysis | ||
| Changes in protein content | ||
| Hydration of cell walls | ||
| Cell wall enzyme activity | ||
| Flavor and aroma | Biosynthesis, accumulation and degradation of organic acids | |
| Biosynthesis, accumulation and degradation of sugars | ||
| Acidity loss | ||
| Production of volatile organic compounds (VOCs) | ||
| Alcohol ester synthesis | ||
| Metabolic | Control of pathways | Increase in respiration ratio |
| Ethylene biosynthesis | ||
| Changes in the metabolism of starch and organic acids | ||
| Altered regulation of existing metabolic pathways | ||
| Molecular | Gene expression | Ripening-specific messanger RNA (mRNA) synthesis |
| Small and interference RNA appearance | ||
| Disappearance of mRNAs DNA demethylation | ||
| Protein expression | Synthesis of de novo ripening-specific proteins | |
| Disappearance of proteins | ||
| Biosynthesis of allergenic compounds | ||
| Increase pathogen susceptibility |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Gómez, B.E.; Salazar, J.A.; Nicolás-Almansa, M.; Razi, M.; Rubio, M.; Ruiz, D.; Martínez-Gómez, P. Molecular Bases of Fruit Quality in Prunus Species: An Integrated Genomic, Transcriptomic, and Metabolic Review with a Breeding Perspective. Int. J. Mol. Sci. 2021, 22, 333. https://doi.org/10.3390/ijms22010333
García-Gómez BE, Salazar JA, Nicolás-Almansa M, Razi M, Rubio M, Ruiz D, Martínez-Gómez P. Molecular Bases of Fruit Quality in Prunus Species: An Integrated Genomic, Transcriptomic, and Metabolic Review with a Breeding Perspective. International Journal of Molecular Sciences. 2021; 22(1):333. https://doi.org/10.3390/ijms22010333
Chicago/Turabian StyleGarcía-Gómez, Beatriz E., Juan A. Salazar, María Nicolás-Almansa, Mitra Razi, Manuel Rubio, David Ruiz, and Pedro Martínez-Gómez. 2021. "Molecular Bases of Fruit Quality in Prunus Species: An Integrated Genomic, Transcriptomic, and Metabolic Review with a Breeding Perspective" International Journal of Molecular Sciences 22, no. 1: 333. https://doi.org/10.3390/ijms22010333
APA StyleGarcía-Gómez, B. E., Salazar, J. A., Nicolás-Almansa, M., Razi, M., Rubio, M., Ruiz, D., & Martínez-Gómez, P. (2021). Molecular Bases of Fruit Quality in Prunus Species: An Integrated Genomic, Transcriptomic, and Metabolic Review with a Breeding Perspective. International Journal of Molecular Sciences, 22(1), 333. https://doi.org/10.3390/ijms22010333







