Local Epidermal Endocrine Estrogen Protects Human Melanocytes against Oxidative Stress, a Novel Insight into Vitiligo Pathology
Abstract
1. Introduction
2. Results
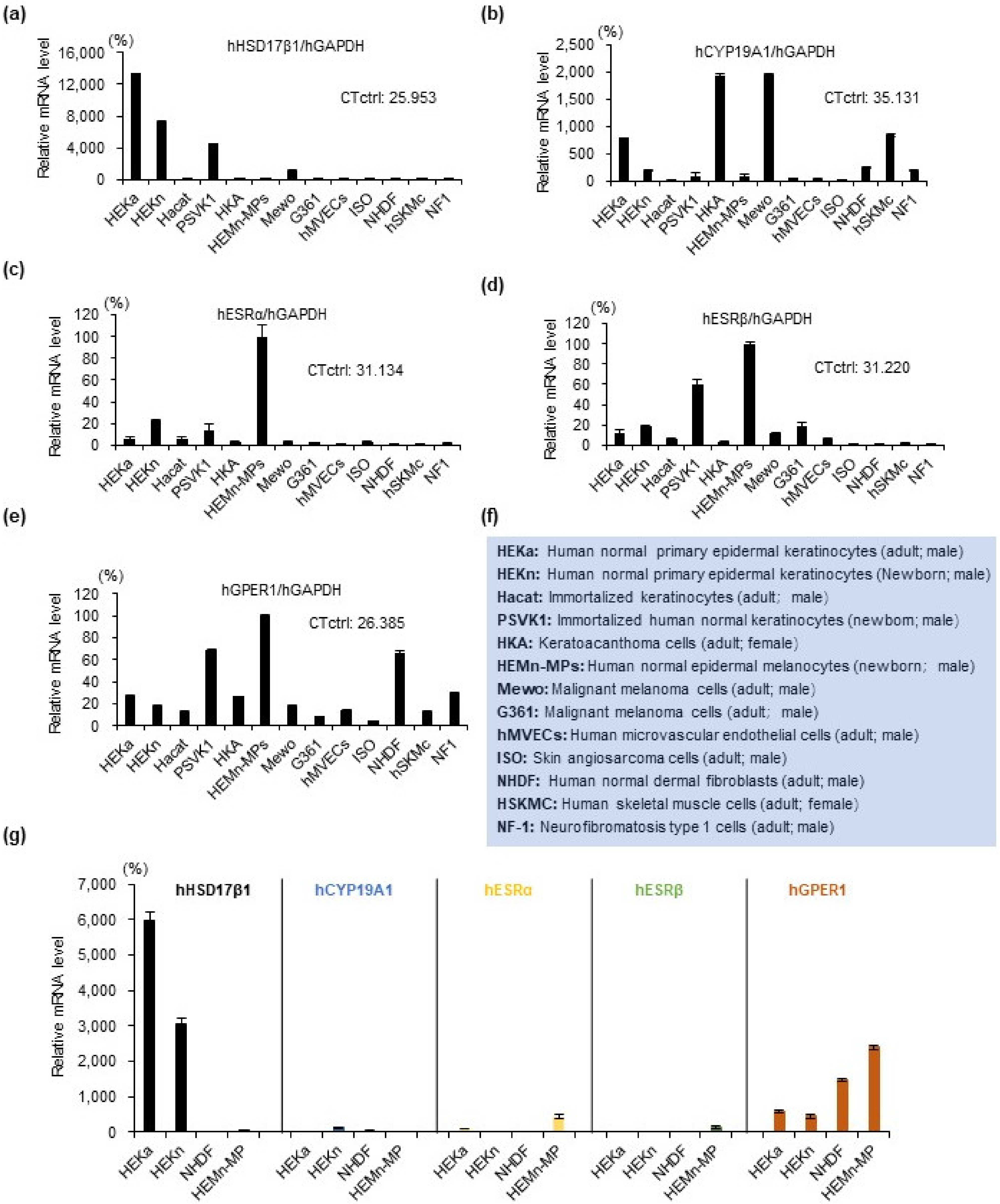
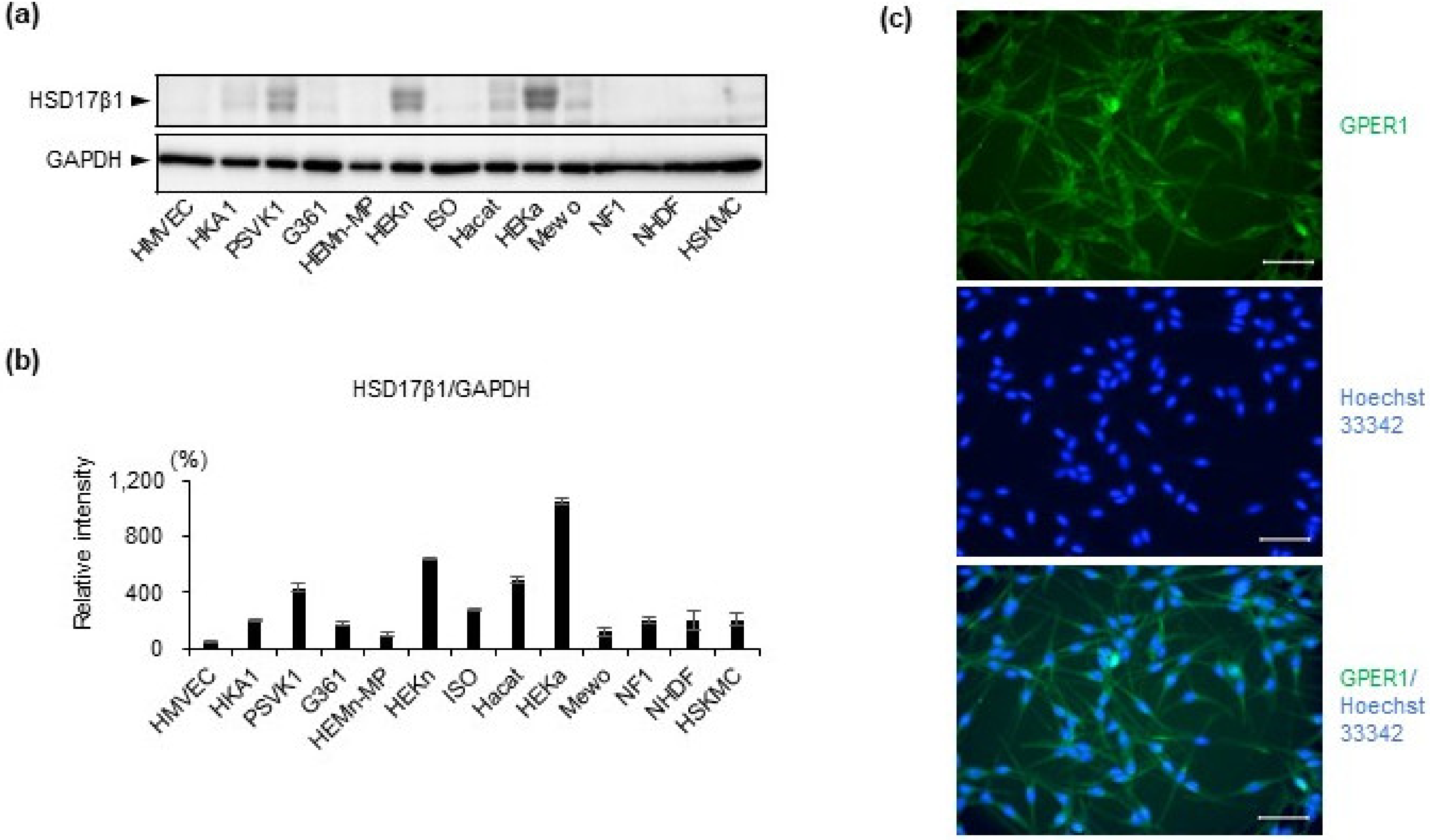
2.1. Estrogen Synthesis Enzyme HSD17β1 Is Expressed by Epidermal Keratinocytes and Estrogen Receptor GPER1 Is Expressed Mainly by Melanocytes
2.2. 17β Estradiol Does Not Increase Melanin Synthesis but Protects Melanocytes from Oxidative Stress-Induced Cytotoxicity
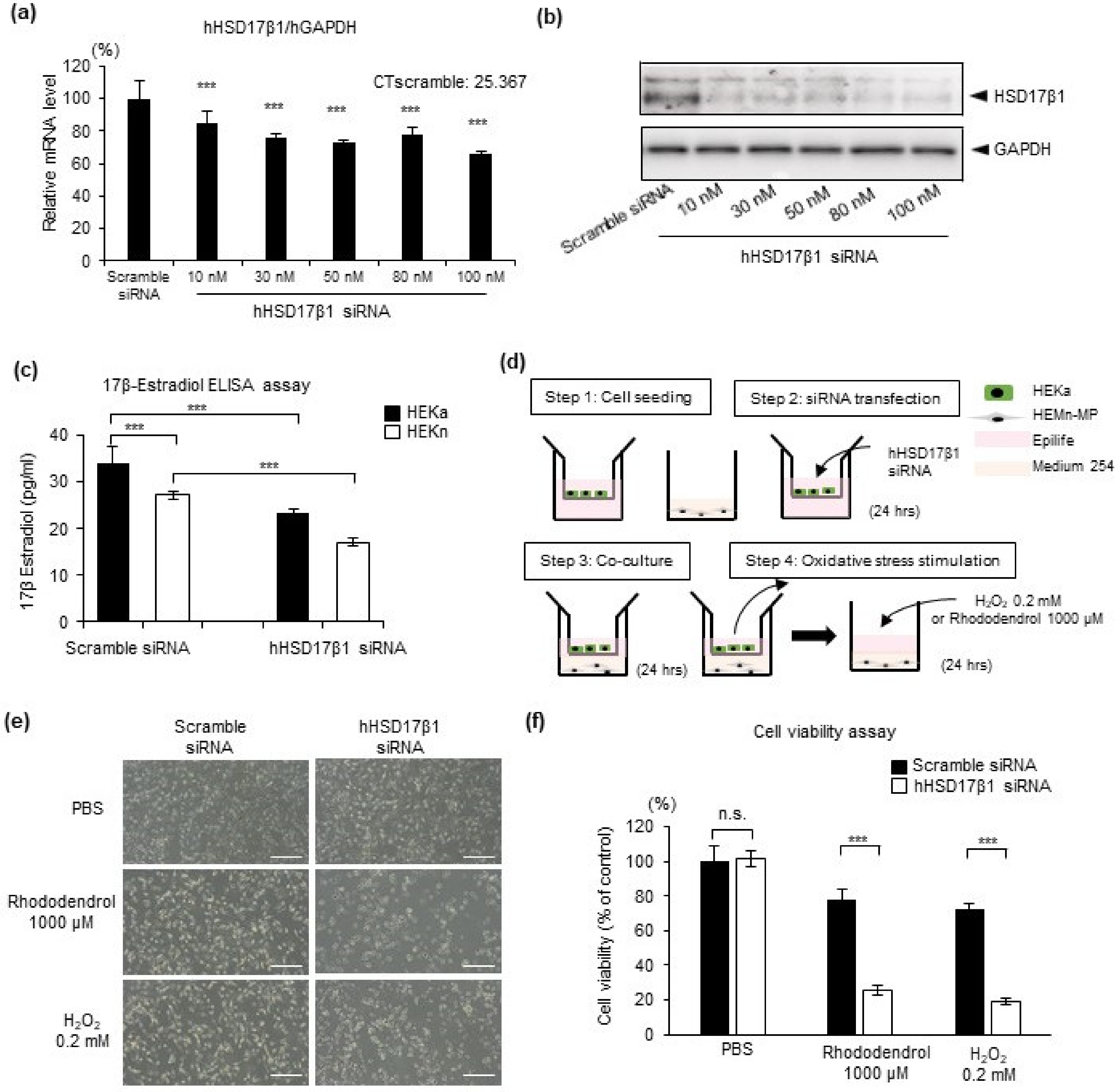
2.3. HSD17β1 Is Essential for Estradiol Synthesis in Keratinocytes and Keratinocyte-Derived Estradiol Attenuates Oxidative Stress-Induced Cell Death in Human Epidermal Melanocytes
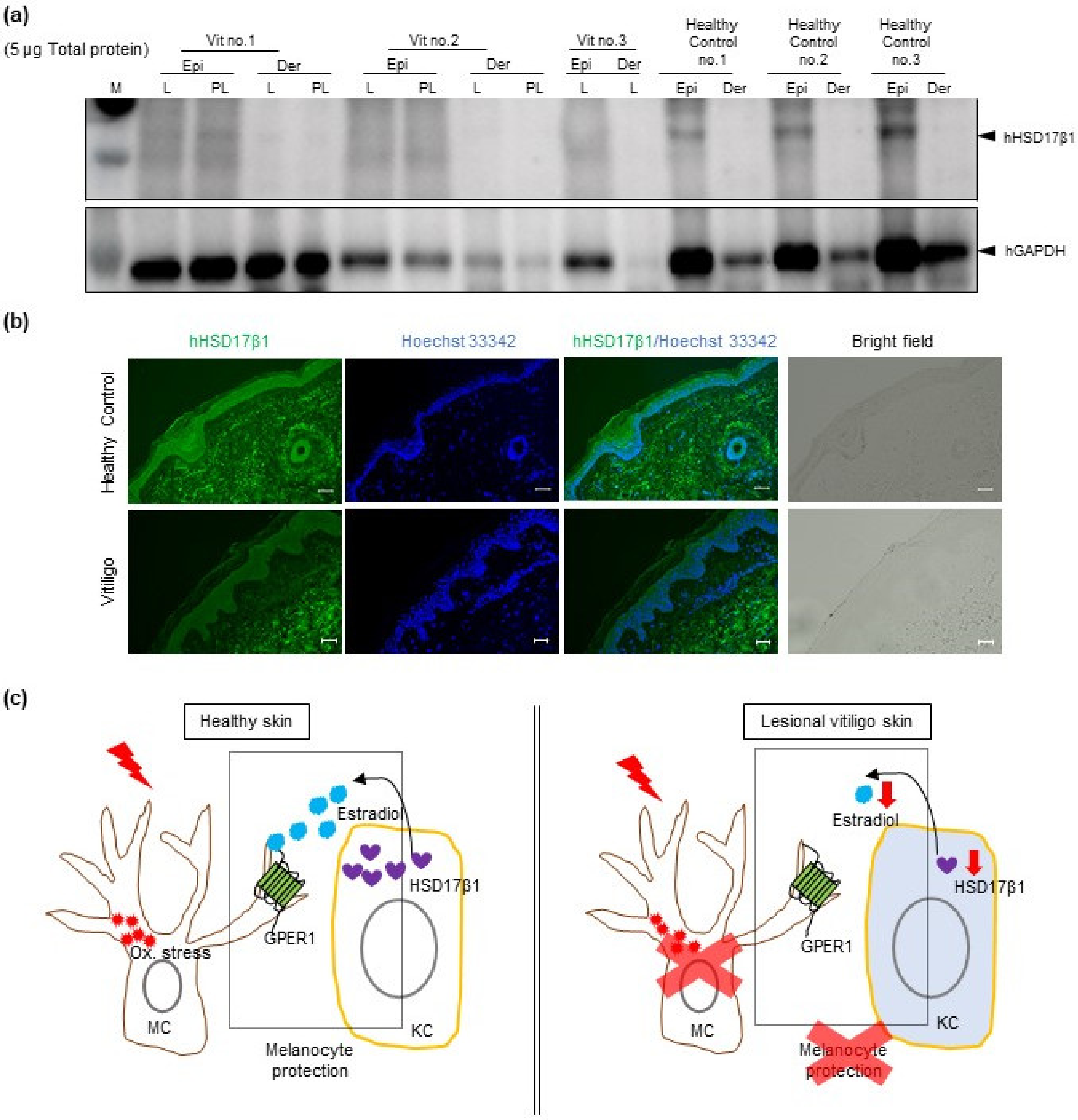
2.4. Reduced HSD17β1 Expression May Be Involved in the Loss of Melanocytes in Patients with Vitiligo
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture
4.2. Human Skin Specimens
4.3. Cell Treatment
4.4. RNA Isolation and Quantitative RT-PCR Analysis
| Primers used for real-time PCR | ||
|---|---|---|
| Gene Name | Sense | Antisense |
| hHSD17b1 | 5′-TTATTGCGCCAGCAAGTTCG-3′ | 5′-TTCTCCATGAAGGCGGTGTG-3′ |
| hCYP19A1 | 5′-CGGCCTTGTTCGTATGGTCA-3′ | 5′-CAGAAGGGTCAACACGTCCA-3′ |
| hESRα | 5′-GGAGAGGAGTTTGTGTGCCT-3′ | 5′-CCAGGACTCGGTGGATATGG3′ |
| hESRβ | 5′-CGTGACCGATGCTTTGGTTT-3′ | 5′-AGCAGATGTTCCATGCCCTT-3′ |
| hGPER1 | 5′-CTGCTTCTGTTTCGCGGATG-3′ | 5′-ACCCGGACAATGAGGGAGTA-3′ |
| hTYR | 5′-TGACTCCAATTAGCCAGTTCCT-3′ | 5′-GACAGCATTCCTTCTCCATCAG-3′ |
| hPMEL17 | 5′-CTATGTGCCTCTTGCTCATTCC-3′ | 5′-TGCTTGTTCCCTCCATCCA-3′ |
| hTYRP1 | 5′-CTCAATGGCGAGTGGTCTGT-3′ | 5′-TTCCAAGCACTGAGCGACAT-3′ |
| hGAPDH | 5′-GACAGTCAGCCGCATCTTCT-3′ | 5′-GCGCCCAATACGACCAAATC-3′ |
4.5. Melanin Content Assay
4.6. Cell Viability Assay
4.7. Western Blot Analysis
4.8. Immunofluorescence Staining of Cells
4.9. Fluorescent Immunohistochemistry Staining
4.10. RNA Interference
4.11. ELISA Assay
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denat, L.; Kadekaro, A.L.; Marrot, L.; Leachman, S.A.; Abdel-Malek, Z.A. Melanocytes as instigators and victims of oxidative stress. J. Investig. Dermatol. 2014, 134, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Taieb, A.; Picardo, M. Clinical practice. Vitiligo. N. Engl. J. Med. 2009, 360, 160–169. [Google Scholar]
- Picardo, M.; Dell’Anna, M.L.; Ezzedine, K.; Hamzavi, I.; Harris, J.E.; Parsad, D.; Taieb, A. Vitiligo. Nat. Rev. Dis. Primers 2015, 1, 15011. [Google Scholar] [CrossRef] [PubMed]
- Passi, S.; Grandinetti, M.; Maggio, F.; Stancato, A.; De Luca, C. Epidermal oxidative stress in vitiligo. Pigment Cell Res. 1998, 11, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Jimbow, K.; Chen, H.; Park, J.S.; Thomas, P.D. Increased sensitivity of melanocytes to oxidative stress and abnormal expression of tyrosinase-related protein in vitiligo. Br. J. Dermatol. 2001, 144, 55–65. [Google Scholar] [CrossRef]
- He, Y.; Li, S.; Zhang, W.; Dai, W.; Cui, T.; Wang, G.; Gao, T.; Li, C. Dysregulated autophagy increased melanocyte sensitivity to H2O2-induced oxidative stress in vitiligo. Sci. Rep. 2017, 7, 42394. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Matteo, M.; Rollo, T.; De Rosario, F.; Greco, P.; Vendemiale, G.; Serviddio, G. Sex hormones modulate circulating antioxidant enzymes: Impact of estrogen therapy. Redox Biol. 2013, 1, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Strehlow, K.; Rotter, S.; Wassmann, S.; Adam, O.; Grohe, C.; Laufs, K.; Bohm, M.; Nickenig, G. Modulation of antioxidant enzyme expression and function by estrogen. Circ. Res. 2003, 93, 170–177. [Google Scholar] [CrossRef]
- Mann, V.; Huber, C.; Kogianni, G.; Collins, F.; Noble, B. The antioxidant effect of estrogen and Selective Estrogen Receptor Modulators in the inhibition of osteocyte apoptosis in vitro. Bone 2007, 40, 674–684. [Google Scholar] [CrossRef]
- Moosmann, B.; Behl, C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc. Natl. Acad. Sci. USA 1999, 96, 8867–8872. [Google Scholar] [CrossRef]
- Dluzen, D.E. Neuroprotective effects of estrogen upon the nigrostriatal dopaminergic system. J. Neurocytol. 2000, 29, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Chiueh, C.; Lee, S.; Andoh, T.; Murphy, D. Induction of antioxidative and antiapoptotic thioredoxin supports neuroprotective hypothesis of estrogen. Endocrine 2003, 21, 27–31. [Google Scholar] [CrossRef]
- Gingerich, S.; Kim, G.L.; Chalmers, J.A.; Koletar, M.M.; Wang, X.; Wang, Y.; Belsham, D.D. Estrogen receptor alpha and G-protein coupled receptor 30 mediate the neuroprotective effects of 17beta-estradiol in novel murine hippocampal cell models. Neuroscience 2010, 170, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.B.; Zhang, N.; Guo, Y.Y.; Zhao, R.; Shi, T.Y.; Feng, S.F.; Wang, S.Q.; Yang, Q.; Li, X.Q.; Wu, Y.M.; et al. G-protein-coupled receptor 30 mediates rapid neuroprotective effects of estrogen via depression of NR2B-containing NMDA receptors. J. Neurosci. 2012, 32, 4887–4900. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Wang, R.; Tang, H.; Dong, Y.; Chan, A.; Sareddy, G.R.; Vadlamudi, R.K.; Brann, D.W. Brain-derived estrogen exerts anti-inflammatory and neuroprotective actions in the rat hippocampus. Mol. Cell. Endocrinol. 2014, 389, 84–91. [Google Scholar] [CrossRef]
- Bellei, B.; Pitisci, A.; Ottaviani, M.; Ludovici, M.; Cota, C.; Luzi, F.; Dell’Anna, M.L.; Picardo, M. Vitiligo: A possible model of degenerative diseases. PLoS ONE 2013, 8, e59782. [Google Scholar] [CrossRef]
- Nelson, L.R.; Bulun, S.E. Estrogen production and action. J. Am. Acad. Dermatol. 2001, 45 Suppl. 3, S116–S124. [Google Scholar] [CrossRef]
- Zouboulis, C.C. The human skin as a hormone target and an endocrine gland. Hormones 2004, 3, 9–26. [Google Scholar] [CrossRef]
- Muto, M.; Furumoto, H.; Ohmura, A.; Asagami, C. Successful treatment of vitiligo with a sex steroid-thyroid hormone mixture. J. Dermatol. 1995, 22, 770–772. [Google Scholar] [CrossRef]
- Ichimiya, M. Immunohistochemical study of ACTH and alpha-MSH in vitiligo patients successfully treated with a sex steroid-thyroid hormone mixture. J. Dermatol. 1999, 26, 502–506. [Google Scholar] [CrossRef]
- Nagai, K.; Ichimiya, M.; Yokoyama, K.; Hamamoto, Y.; Muto, M. Successful treatment of non-segmental vitiligo: Systemic therapy with sex hormone-thyroid powder mixture. Horm. Res. 2000, 54, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Ranson, M.; Posen, S.; Mason, R.S. Human melanocytes as a target tissue for hormones: In vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J. Investig. Dermatol. 1988, 91, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.; Rubin, G.; Clyne, C.; Robertson, K.; O’Donnell, L.; Davis, S.; Jones, M. Local estrogen biosynthesis in males and females. Endocr. Relat. Cancer 1999, 6, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.; Rubin, G.; Clyne, C.; Robertson, K.; O’Donnell, L.; Jones, M.; Davis, S. The role of local estrogen biosynthesis in males and females. Trends Endocrinol. Metab. 2000, 11, 184–188. [Google Scholar] [CrossRef]
- Simpson, E.R. Sources of estrogen and their importance. J. Steroid. Biochem. Mol. Biol. 2003, 86, 225–230. [Google Scholar] [CrossRef]
- Anderson, D.; Schmid, T.E.; Baumgartner, A.; Cemeli-Carratala, E.; Brinkworth, M.H.; Wood, J.M. Oestrogenic compounds and oxidative stress (in human sperm and lymphocytes in the Comet assay). Mutat. Res. 2003, 544, 173–178. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Chiuchiarelli, G.; Cemeli, E.; Elwary, S.M.; Gillbro, J.M.; Spencer, J.D.; Rokos, H.; Panske, A.; Chavan, B.; Wood, J.M.; et al. Estrogens can contribute to hydrogen peroxide generation and quinone-mediated DNA damage in peripheral blood lymphocytes from patients with vitiligo. J. Investig. Dermatol. 2006, 126, 1036–1042. [Google Scholar] [CrossRef]
- Yang, F.; Yang, L.; Wataya-Kaneda, M.; Yoshimura, T.; Tanemura, A.; Katayama, I. Uncoupling of ER/Mitochondrial Oxidative Stress in mTORC1 Hyperactivation-Associated Skin Hypopigmentation. J. Investig. Dermatol. 2018, 138, 669–678. [Google Scholar] [CrossRef]
- Yang, L.; Serada, S.; Fujimoto, M.; Terao, M.; Kotobuki, Y.; Kitaba, S.; Matsui, S.; Kudo, A.; Naka, T.; Murota, H.; et al. Periostin facilitates skin sclerosis via PI3K/Akt dependent mechanism in a mouse model of scleroderma. PLoS ONE 2012, 7, e41994. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, A.; Yang, L.; Kuroda, Y.; Guo, J.; Teng, L.; Tsuruta, D.; Katayama, I. Local Epidermal Endocrine Estrogen Protects Human Melanocytes against Oxidative Stress, a Novel Insight into Vitiligo Pathology. Int. J. Mol. Sci. 2021, 22, 269. https://doi.org/10.3390/ijms22010269
Yamamoto A, Yang L, Kuroda Y, Guo J, Teng L, Tsuruta D, Katayama I. Local Epidermal Endocrine Estrogen Protects Human Melanocytes against Oxidative Stress, a Novel Insight into Vitiligo Pathology. International Journal of Molecular Sciences. 2021; 22(1):269. https://doi.org/10.3390/ijms22010269
Chicago/Turabian StyleYamamoto, Asako, Lingli Yang, Yasutaka Kuroda, Jiao Guo, Lanting Teng, Daisuke Tsuruta, and Ichiro Katayama. 2021. "Local Epidermal Endocrine Estrogen Protects Human Melanocytes against Oxidative Stress, a Novel Insight into Vitiligo Pathology" International Journal of Molecular Sciences 22, no. 1: 269. https://doi.org/10.3390/ijms22010269
APA StyleYamamoto, A., Yang, L., Kuroda, Y., Guo, J., Teng, L., Tsuruta, D., & Katayama, I. (2021). Local Epidermal Endocrine Estrogen Protects Human Melanocytes against Oxidative Stress, a Novel Insight into Vitiligo Pathology. International Journal of Molecular Sciences, 22(1), 269. https://doi.org/10.3390/ijms22010269






