From Obesity to Hippocampal Neurodegeneration: Pathogenesis and Non-Pharmacological Interventions
Abstract
1. Introduction
2. Gut Is a Potential Origin of Chronic, Low-Grade Inflammation in Obesity and Diabetes
3. From Inflammation in Adipose Tissue to Impaired Hippocampus Plasticity in Obese and Diabetic Conditions
4. Neuronal Mitochondria Are Involved in Obesity- or Diabetes-Induced Neuroinflammation and Hippocampal Impairment
5. Excessive Palmitate Consumption from Diet Triggers a Direct Neuroinflammatory Response in the Hippocampus
6. Obese and Diabetic Conditions Inhibit Hippocampal Feedback Control of the HPA Axis
7. High-Fats and Low-Carb Ketogenic Diet Elicits Neuroprotective Effects by Promoting Mitochondrial Dynamics and Reducing Oxidative Stress
8. SIRT1/SIRT3 Axis Potentiates the Pro-Cognitive Effects of Caloric Restriction and Intermittent Fasting
9. Physical Exercise-Induced Mediators on Promoting Metabolic and Synaptic Function in the Brain
9.1. Apelin
9.2. Irisin
9.3. Lactate
9.4. β-Hydroxybutyrate
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kant, A.K. Dietary patterns and health outcomes. J. Am. Diet. Assoc. 2004, 104, 615–635. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.; Shaw, J.; Group IDFETFC. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Eckel, R.H.; Alberti, K.G.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2010, 375, 181–183. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D. Dietary and policy priorities to reduce the global crises of obesity and diabetes. Nat. Food 2020, 1, 38–50. [Google Scholar] [CrossRef]
- Francis, H.M.; Stevenson, R.J. Higher reported saturated fat and refined sugar intake is associated with reduced hippocampal-dependent memory and sensitivity to interoceptive signals. Behav. Neurosci. 2011, 125, 943–955. [Google Scholar] [CrossRef]
- Gibson, E.L.; Barr, S.; Jeanes, Y.M. Habitual fat intake predicts memory function in younger women. Front. Hum. Neurosci. 2013, 7, 838. [Google Scholar] [CrossRef]
- Baym, C.L.; Khan, N.A.; Monti, J.M.; Raine, L.B.; Drollette, E.S.; Moore, R.D.; Scudder, M.R.; Kramer, A.F.; Hillman, C.H.; Cohen, N.J. Dietary lipids are differentially associated with hippocampal-dependent relational memory in prepubescent children. Am. J. Clin. Nutr. 2014, 99, 1026–1032. [Google Scholar] [CrossRef]
- Gardener, S.L.; Rainey-Smith, S.R.; Barnes, M.B.; Sohrabi, H.R.; Weinborn, M.; Lim, Y.Y.; Harrington, K.; Taddei, K.; Gu, Y.; Rembach, A.; et al. Dietary patterns and cognitive decline in an Australian study of ageing. Mol. Psychiatry 2015, 20, 860–866. [Google Scholar] [CrossRef]
- Brannigan, M.; Stevenson, R.J.; Francis, H. Thirst interoception and its relationship to a Western-style diet. Physiol. Behav. 2015, 139, 423–429. [Google Scholar] [CrossRef]
- Granic, A.; Davies, K.; Adamson, A.; Kirkwood, T.; Hill, T.R.; Siervo, M.; Mathers, J.C.; Jagger, C. Dietary Patterns High in Red Meat, Potato, Gravy, and Butter Are Associated with Poor Cognitive Functioning but Not with Rate of Cognitive Decline in Very Old Adults. J. Nutr. 2016, 146, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Attuquayefio, T.; Stevenson, R.J.; Boakes, R.A.; Oaten, M.J.; Yeomans, M.R.; Mahmut, M.; Francis, H.M. A high-fat high-sugar diet predicts poorer hippocampal-related memory and a reduced ability to suppress wanting under satiety. J. Exp. Psychol. Anim. Learn. Cogn. 2016, 42, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Attuquayefio, T.; Stevenson, R.J.; Oaten, M.J.; Francis, H.M. A four-day Western-style dietary intervention causes reductions in hippocampal-dependent learning and memory and interoceptive sensitivity. PLoS ONE 2017, 12, e0172645. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, R.J.; Francis, H.M.; Attuquayefio, T.; Gupta, D.; Yeomans, M.R.; Oaten, M.J.; Davidson, T. Hippocampal-dependent appetitive control is impaired by experimental exposure to a Western-style diet. R. Soc. Open Sci. 2020, 7, 191338. [Google Scholar] [CrossRef] [PubMed]
- Neth, B.J.; Craft, S. Insulin Resistance and Alzheimer’s Disease: Bioenergetic Linkages. Front. Aging Neurosci. 2017, 9, 345. [Google Scholar] [CrossRef]
- Ferreira, L.S.S.; Fernandes, C.S.; Vieira, M.N.N.; De Felice, F.G. Insulin Resistance in Alzheimer’s Disease. Front Neurosci. 2018, 12, 830. [Google Scholar] [CrossRef]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E.; et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [Google Scholar] [CrossRef]
- Hamer, J.A.; Testani, D.; Mansur, R.B.; Lee, Y.; Subramaniapillai, M.; McIntyre, R.S. Brain insulin resistance: A treatment target for cognitive impairment and anhedonia in depression. Exp. Neurol. 2019, 315, 1–8. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Yamaguchi, K.; Matsui, K.; Sano, T.; Kubota, T.; Hashimoto, T.; Mano, A.; Yamada, K.; Matsuo, Y.; Kubota, N. Differential effects of diet- and genetically-induced brain insulin resistance on amyloid pathology in a mouse model of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 15. [Google Scholar] [CrossRef]
- Vandal, M.; White, P.J.; Chevrier, G.; Tremblay, C.; St-Amour, I.; Planel, E.; Marette, A.; Calon, F. Age-dependent impairment of glucose tolerance in the 3xTg-AD mouse model of Alzheimer’s disease. FASEB J. 2015, 29, 4273–4284. [Google Scholar] [CrossRef]
- Macklin, L.; Griffith, C.M.; Cai, Y.; Rose, G.M.; Yan, X.X.; Patrylo, P.R. Glucose tolerance and insulin sensitivity are impaired in APP/PS1 transgenic mice prior to amyloid plaque pathogenesis and cognitive decline. Exp. Gerontol. 2017, 88, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Griffith, C.M.; Macklin, L.N.; Cai, Y.; Sharp, A.A.; Yan, X.X.; Reagan, L.P.; Strader, A.D.; Rose, G.M.; Patrylo, P.R. Impaired Glucose Tolerance and Reduced Plasma Insulin Precede Decreased AKT Phosphorylation and GLUT3 Translocation in the Hippocampus of Old 3xTg-AD Mice. J. Alzheimers Dis. 2019, 68, 809–837. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, R.; Tran, A.; Ishimwe, E.; Denner, L.; Dave, N.; Oddo, S.; Dineley, K.T. Central insulin dysregulation and energy dyshomeostasis in two mouse models of Alzheimer’s disease. Neurobiol. Aging 2017, 58, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kothari, V.; Luo, Y.; Tornabene, T.; O’Neill, A.M.; Greene, M.W.; Geetha, T.; Babu, J.R. High fat diet induces brain insulin resistance and cognitive impairment in mice. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 499–508. [Google Scholar] [CrossRef]
- Selles, M.C.; Fortuna, J.T.S.; Zappa-Villar, M.F.; de Faria, Y.P.R.; Souza, A.S.; Suemoto, C.K.; Leite, R.E.P.; Rodriguez, R.D.; Grinberg, L.T.; Reggiani, P.C.; et al. Adenovirus-Mediated Transduction of Insulin-Like Growth Factor 1 Protects Hippocampal Neurons from the Toxicity of Abeta Oligomers and Prevents Memory Loss in an Alzheimer Mouse Model. Mol. Neurobiol. 2020, 57, 1473–1483. [Google Scholar] [CrossRef]
- Rhea, E.M.; Raber, J.; Banks, W.A. ApoE and cerebral insulin: Trafficking, receptors, and resistance. Neurobiol. Dis. 2020, 137, 104755. [Google Scholar] [CrossRef]
- Holscher, C. Brain insulin resistance: Role in neurodegenerative disease and potential for targeting. Expert Opin. Investig. Drugs. 2020, 29, 333–348. [Google Scholar] [CrossRef]
- Horvath, A.; Salman, Z.; Quinlan, P.; Wallin, A.; Svensson, J. Patients with Alzheimer’s Disease Have Increased Levels of Insulin-like Growth Factor-I in Serum but not in Cerebrospinal Fluid. J. Alzheimers Dis. 2020, 75, 289–298. [Google Scholar] [CrossRef]
- Caberlotto, L.; Nguyen, T.P.; Lauria, M.; Priami, C.; Rimondini, R.; Maioli, S.; Cedazo-Minguez, A.; Sita, G.; Morroni, F.; Corsi, M.; et al. Cross-disease analysis of Alzheimer’s disease and type-2 Diabetes highlights the role of autophagy in the pathophysiology of two highly comorbid diseases. Sci. Rep. 2019, 9, 3965. [Google Scholar] [CrossRef]
- Desai, G.S.; Zheng, C.; Geetha, T.; Mathews, S.T.; White, B.D.; Huggins, K.W.; Zizza, C.A.; Broderick, T.L.; Babu, J.R. The pancreas-brain axis: Insight into disrupted mechanisms associating type 2 diabetes and Alzheimer’s disease. J. Alzheimers Dis. 2014, 42, 347–356. [Google Scholar] [CrossRef]
- De la Monte, S.M.; Wands, J.R. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J. Diabetes Sci. Technol. 2008, 2, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Arnoldussen, I.A.; Kiliaan, A.J.; Gustafson, D.R. Obesity and dementia: Adipokines interact with the brain. Eur. Neuropsychopharmacol. 2014, 24, 1982–1999. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Cheng, K.K.; Hoo, R.L.; Siu, P.M.; Yau, S.Y. The Novel Perspectives of Adipokines on Brain Health. Int. J. Mol. Sci. 2019, 20, 5638. [Google Scholar] [CrossRef] [PubMed]
- Danat, I.M.; Clifford, A.; Partridge, M.; Zhou, W.; Bakre, A.T.; Chen, A.; McFeeters, D.; Smith, T.; Wan, Y.; Copeland, J.; et al. Impacts of Overweight and Obesity in Older Age on the Risk of Dementia: A Systematic Literature Review and a Meta-Analysis. J. Alzheimers Dis. 2019, 70, S87–S99. [Google Scholar] [CrossRef]
- Bowman, K.; Thambisetty, M.; Kuchel, G.A.; Ferrucci, L.; Melzer, D. Obesity and Longer Term Risks of Dementia in 65–74 Year Olds. Age Ageing 2019, 48, 367–373. [Google Scholar] [CrossRef]
- Lee, J.Y.; Han, K.; Han, E.; Kim, G.; Cho, H.; Kim, K.J.; Lee, B.W.; Kang, E.S.; Cha, B.; Brayne, C.; et al. Risk of Incident Dementia According to Metabolic Health and Obesity Status in Late Life: A Population-Based Cohort Study. J. Clin. Endocrinol. Metab. 2019, 104, 2942–2952. [Google Scholar] [CrossRef]
- Lee, A.T.C.; Richards, M.; Chan, W.C.; Chiu, H.F.K.; Lee, R.S.Y.; Lam, L.C.W. Higher dementia incidence in older adults with type 2 diabetes and large reduction in HbA1c. Age Ageing 2019, 48, 838–844. [Google Scholar] [CrossRef]
- Alsharif, A.A.; Wei, L.; Ma, T.; Man, K.K.C.; Lau, W.C.Y.; Brauer, R.; Almetwazi, M.; Howard, R.; Wong, I.C.K. Prevalence and Incidence of Dementia in People with Diabetes Mellitus. J. Alzheimers Dis. 2020, 75, 607–615. [Google Scholar] [CrossRef]
- Park, S.Y.; Cho, Y.R.; Kim, H.J.; Higashimori, T.; Danton, C.; Lee, M.K.; Dey, A.; Rothermel, B.; Kim, Y.; Kalinowski, A.; et al. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes 2005, 54, 3530–3540. [Google Scholar] [CrossRef]
- Koranyi, L.; James, D.; Mueckler, M.; Permutt, M.A. Glucose transporter levels in spontaneously obese (db/db) insulin-resistant mice. J. Clin. Investig. 1990, 85, 962–967. [Google Scholar] [CrossRef]
- Tomita, T.; Doull, V.; Pollock, H.G.; Krizsan, D. Pancreatic islets of obese hyperglycemic mice (ob/ob). Pancreas 1992, 7, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Beddow, S.A.; Samuel, V.T. Fasting hyperglycemia in the Goto-Kakizaki rat is dependent on corticosterone: A confounding variable in rodent models of type 2 diabetes. Dis. Models Mech. 2012, 5, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Boitard, C.; Etchamendy, N.; Sauvant, J.; Aubert, A.; Tronel, S.; Marighetto, A.; Laye, S.; Ferreira, G. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus 2012, 22, 2095–2100. [Google Scholar] [CrossRef] [PubMed]
- Stranahan, A.M.; Arumugam, T.V.; Cutler, R.G.; Lee, K.; Egan, J.M.; Mattson, M.P. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat. Neurosci. 2008, 11, 309–317. [Google Scholar] [CrossRef]
- Sharma, A.N.; Elased, K.M.; Garrett, T.L.; Lucot, J.B. Neurobehavioral deficits in db/db diabetic mice. Physiol. Behav. 2010, 101, 381–388. [Google Scholar] [CrossRef]
- Leuner, B.; Gould, E. Structural plasticity and hippocampal function. Annu. Rev. Psychol. 2010, 61, 111–140. [Google Scholar] [CrossRef]
- Moser, M.B.; Moser, E.I. Functional differentiation in the hippocampus. Hippocampus 1998, 8, 608–619. [Google Scholar] [CrossRef]
- Smith, M.A. Hippocampal vulnerability to stress and aging: Possible role of neurotrophic factors. Behav. Brain Res. 1996, 78, 25–36. [Google Scholar] [CrossRef]
- Miller, D.B.; O’Callaghan, J.P. Aging, stress and the hippocampus. Ageing Res. Rev. 2005, 4, 123–140. [Google Scholar] [CrossRef]
- Bettio, L.E.B.; Rajendran, L.; Gil-Mohapel, J. The effects of aging in the hippocampus and cognitive decline. Neurosci. Biobehav. Rev. 2017, 79, 66–86. [Google Scholar] [CrossRef]
- Ho, N.; Sommers, M.S.; Lucki, I. Effects of diabetes on hippocampal neurogenesis: Links to cognition and depression. Neurosci. Biobehav. Rev. 2013, 37, 1346–1362. [Google Scholar] [CrossRef] [PubMed]
- Cherbuin, N.; Sargent-Cox, K.; Fraser, M.; Sachdev, P.; Anstey, K.J. Being overweight is associated with hippocampal atrophy: The PATH Through Life Study. Int. J. Obes. 2015, 39, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Robison, L.S.; Albert, N.M.; Camargo, L.A.; Anderson, B.M.; Salinero, A.E.; Riccio, D.A.; Abi-Ghanem, C.; Gannon, O.J.; Zuloaga, K.L. High-Fat Diet-Induced Obesity Causes Sex-Specific Deficits in Adult Hippocampal Neurogenesis in Mice. eNeuro 2020, 7, ENEURO.0391-19.2019. [Google Scholar] [CrossRef]
- Ramos-Rodriguez, J.J.; Molina-Gil, S.; Ortiz-Barajas, O.; Jimenez-Palomares, M.; Perdomo, G.; Cozar-Castellano, I.; Lechuga-Sancho, A.M.; Garcia-Alloza, M. Central proliferation and neurogenesis is impaired in type 2 diabetes and prediabetes animal models. PLoS ONE 2014, 9, e89229. [Google Scholar] [CrossRef] [PubMed]
- Erion, J.R.; Wosiski-Kuhn, M.; Dey, A.; Hao, S.; Davis, C.L.; Pollock, N.K.; Stranahan, A.M. Obesity elicits interleukin 1-mediated deficits in hippocampal synaptic plasticity. J. Neurosci. 2014, 34, 2618–2631. [Google Scholar] [CrossRef] [PubMed]
- Bracke, A.; Domanska, G.; Bracke, K.; Harzsch, S.; van den Brandt, J.; Broker, B.; Bohlen, V.; Halbach, O. Obesity Impairs Mobility and Adult Hippocampal Neurogenesis. J. Exp. Neurosci. 2019, 13, 1179069519883580. [Google Scholar] [CrossRef]
- Porter, W.D.; Flatt, P.R.; Holscher, C.; Gault, V.A. Liraglutide improves hippocampal synaptic plasticity associated with increased expression of Mash1 in ob/ob mice. Int. J. Obes. 2013, 37, 678–684. [Google Scholar] [CrossRef]
- Yau, S.Y.; Lee, T.H.; Li, A.; Xu, A.; So, K.F. Adiponectin Mediates Running-Restored Hippocampal Neurogenesis in Streptozotocin-Induced Type 1 Diabetes in Mice. Front. Neurosci. 2018, 12, 679. [Google Scholar] [CrossRef]
- Yan, S.; Du, F.; Wu, L.; Zhang, Z.; Zhong, C.; Yu, Q.; Wang, Y.; Lue, L.; Walker, D.G.; Douglas, J.T.; et al. F1F0 ATP Synthase-Cyclophilin D Interaction Contributes to Diabetes-Induced Synaptic Dysfunction and Cognitive Decline. Diabetes 2016, 65, 3482–3494. [Google Scholar] [CrossRef]
- Li, X.H.; Xin, X.; Wang, Y.; Wu, J.Z.; Jin, Z.D.; Ma, L.N.; Nie, C.; Xiao, X.; Hu, Y.; Jin, M. Pentamethylquercetin protects against diabetes-related cognitive deficits in diabetic Goto-Kakizaki rats. J. Alzheimers Dis. 2013, 34, 755–767. [Google Scholar] [CrossRef]
- Yin, H.; Wang, W.; Yu, W.; Li, J.; Feng, N.; Wang, L.; Wang, X. Changes in Synaptic Plasticity and Glutamate Receptors in Type 2 Diabetic KK-Ay Mice. J. Alzheimers Dis. 2017, 57, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Nicola, Z.; Fabel, K.; Kempermann, G. Development of the adult neurogenic niche in the hippocampus of mice. Front. Neuroanat. 2015, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Beauquis, J.; Saravia, F.; Coulaud, J.; Roig, P.; Dardenne, M.; Homo-Delarche, F.; De Nicola, A. Prominently decreased hippocampal neurogenesis in a spontaneous model of type 1 diabetes, the nonobese diabetic mouse. Exp. Neurol. 2008, 210, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, A.; Mohapel, P.; Bouter, B.; Frielingsdorf, H.; Pizzo, D.; Brundin, P.; Erlanson-Albertsson, C. High-fat diet impairs hippocampal neurogenesis in male rats. Eur. J. Neurol. 2006, 13, 1385–1388. [Google Scholar] [CrossRef]
- Hwang, I.K.; Kim, I.Y.; Kim, D.W.; Yoo, K.Y.; Kim, Y.N.; Yi, S.S.; Won, M.; Lee, I.S.; Yoon, Y.S.; Seong, J.K. Strain-specific differences in cell proliferation and differentiation in the dentate gyrus of C57BL/6N and C3H/HeN mice fed a high fat diet. Brain Res. 2008, 1241, 1–6. [Google Scholar] [CrossRef]
- Yi, S.S.; Hwang, I.K.; Yoo, K.Y.; Park, O.K.; Yu, J.; Yan, B.; Kim, I.Y.; Kim, Y.N.; Pai, T.; Song, W.; et al. Effects of treadmill exercise on cell proliferation and differentiation in the subgranular zone of the dentate gyrus in a rat model of type II diabetes. Neurochem. Res. 2009, 34, 1039–1046. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Lee, K.; Martin, B.; Maudsley, S.; Golden, E.; Cutler, R.G.; Mattson, M.P. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus 2009, 19, 951–961. [Google Scholar] [CrossRef]
- Takeuchi, T.; Duszkiewicz, A.J.; Morris, R.G. The synaptic plasticity and memory hypothesis: Encoding, storage and persistence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130288. [Google Scholar] [CrossRef]
- Abraham, W.C.; Jones, O.D.; Glanzman, D.L. Is plasticity of synapses the mechanism of long-term memory storage? NPJ Sci. Learn. 2019, 4, 9. [Google Scholar] [CrossRef]
- Porter, D.W.; Kerr, B.D.; Flatt, P.R.; Holscher, C.; Gault, V.A. Four weeks administration of Liraglutide improves memory and learning as well as glycaemic control in mice with high fat dietary-induced obesity and insulin resistance. Diabetes Obes. Metab. 2010, 12, 891–899. [Google Scholar] [CrossRef]
- Gault, V.A.; Porter, W.D.; Flatt, P.R.; Holscher, C. Actions of exendin-4 therapy on cognitive function and hippocampal synaptic plasticity in mice fed a high-fat diet. Int. J. Obes. 2010, 34, 1341–1344. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Biessels, G.J.; Urban, I.J.; Gispen, W.H. Hippocampal synaptic plasticity in streptozotocin-diabetic rats: Impairment of long-term potentiation and facilitation of long-term depression. Neuroscience 1999, 90, 737–745. [Google Scholar] [CrossRef]
- Kamal, A.; Biessels, G.J.; Duis, S.E.; Gispen, W.H. Learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: Interaction of diabetes and ageing. Diabetologia 2000, 43, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Manschot, S.M.; Biessels, G.J.; Cameron, N.E.; Cotter, M.A.; Kamal, A.; Kappelle, L.J.; Gispen, W.H. Angiotensin converting enzyme inhibition partially prevents deficits in water maze performance, hippocampal synaptic plasticity and cerebral blood flow in streptozotocin-diabetic rats. Brain Res. 2003, 966, 274–282. [Google Scholar] [CrossRef]
- Jing, X.H.; Chen, S.L.; Shi, H.; Cai, H.; Jin, Z.G. Electroacupuncture restores learning and memory impairment induced by both diabetes mellitus and cerebral ischemia in rats. Neurosci. Lett. 2008, 443, 193–198. [Google Scholar] [CrossRef]
- Kamal, A.; Biessels, G.J.; Ramakers, G.M.; Hendrik Gispen, W. The effect of short duration streptozotocin-induced diabetes mellitus on the late phase and threshold of long-term potentiation induction in the rat. Brain Res. 2005, 1053, 126–130. [Google Scholar] [CrossRef]
- Artola, A.; Kamal, A.; Ramakers, G.M.; Gardoni, F.; Di Luca, M.; Biessels, G.J.; Cattabeni, F.; Gispen, W.H. Synaptic plasticity in the diabetic brain: Advanced aging? Prog. Brain Res. 2002, 138, 305–314. [Google Scholar]
- Katagiri, H.; Tanaka, K.; Manabe, T. Requirement of appropriate glutamate concentrations in the synaptic cleft for hippocampal LTP induction. Eur. J. Neurosci. 2001, 14, 547–553. [Google Scholar] [CrossRef]
- Brennan, B.P.; Hudson, J.I.; Jensen, J.E.; McCarthy, J.; Roberts, J.L.; Prescot, A.P.; Cohen, B.M.; Pope, H.G.; Renshaw, P.F.; Ongur, D. Rapid enhancement of glutamatergic neurotransmission in bipolar depression following treatment with riluzole. Neuropsychopharmacology 2010, 35, 834–846. [Google Scholar] [CrossRef]
- Lim, S.I.; Song, K.H.; Yoo, C.H.; Woo, D.C.; Choe, B.Y. High-fat diet-induced hyperglutamatergic activation of the hippocampus in mice: A proton magnetic resonance spectroscopy study at 9.4T. Neurochem. Int. 2018, 114, 10–17. [Google Scholar] [CrossRef]
- Lewerenz, J.; Maher, P. Chronic Glutamate Toxicity in Neurodegenerative Diseases-What is the Evidence? Front. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Passafaro, M.; Piech, V.; Sheng, M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat. Neurosci. 2001, 4, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, M.; Fusco, S.; Mainardi, M.; Scala, F.; Natale, F.; Lapenta, R.; Mattera, A.; Rinaudo, M.; Puma, D.D.L.; Ripoli, C. Brain insulin resistance impairs hippocampal synaptic plasticity and memory by increasing GluA1 palmitoylation through FoxO3a. Nat. Commun. 2017, 8, 2009. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhong, Z.; Wang, B.; Xia, X.; Yao, W.; Huang, L.; Wang, Y.; Ding, W. Early-life high-fat diet-induced obesity programs hippocampal development and cognitive functions via regulation of gut commensal Akkermansia muciniphila. Neuropsychopharmacology 2019, 44, 2054–2064. [Google Scholar] [CrossRef] [PubMed]
- Valladolid-Acebes, I.; Merino, B.; Principato, A.; Fole, A.; Barbas, C.; Lorenzo, M.P.; Garcia, A.; del Olmo, N.; Ruiz-Gayo, M.; Cano, V. High-fat diets induce changes in hippocampal glutamate metabolism and neurotransmission. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E396–E402. [Google Scholar] [CrossRef]
- Lizarbe, B.; Soares, A.F.; Larsson, S.; Duarte, J.M.N. Neurochemical Modifications in the Hippocampus, Cortex and Hypothalamus of Mice Exposed to Long-Term High-Fat Diet. Front. Neurosci. 2018, 12, 985. [Google Scholar] [CrossRef]
- Berge, L.I.; Riise, T. Comorbidity between Type 2 Diabetes and Depression in the Adult Population: Directions of the Association and Its Possible Pathophysiological Mechanisms. Int. J. Endocrinol. 2015, 2015, 164760. [Google Scholar] [CrossRef]
- Nikolic, M. Prevalence of comorbid depression and obesity in general practice. Br. J. Gen. Pract. 2015, 65, 451. [Google Scholar] [CrossRef][Green Version]
- Shpilberg, Y.; Beaudry, J.L.; D’Souza, A.; Campbell, J.E.; Peckett, A.; Riddell, M.C. A rodent model of rapid-onset diabetes induced by glucocorticoids and high-fat feeding. Dis. Models Mech. 2012, 5, 671–680. [Google Scholar] [CrossRef]
- Zemdegs, J.; Quesseveur, G.; Jarriault, D.; Penicaud, L.; Fioramonti, X.; Guiard, B.P. High-fat diet-induced metabolic disorders impairs 5-HT function and anxiety-like behavior in mice. Br. J. Pharmacol. 2016, 173, 2095–2110. [Google Scholar] [CrossRef]
- Hassan, A.M.; Mancano, G.; Kashofer, K.; Frohlich, E.E.; Matak, A.; Mayerhofer, R.; Reichmann, F.; Olivares, M.; Neyrinck, A.M.; Delzenne, N.M.; et al. High-fat diet induces depression-like behaviour in mice associated with changes in microbiome, neuropeptide Y, and brain metabolome. Nutr. Neurosci. 2019, 22, 877–893. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.M.; Mancano, G.; Kashofer, K.; Liebisch, G.; Farzi, A.; Zenz, G.; Claus, S.P.; Holzer, P. Anhedonia induced by high-fat diet in mice depends on gut microbiota and leptin. Nutr. Neurosci. 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Abe-Higuchi, N.; Uchida, S.; Yamagata, H.; Higuchi, F.; Hobara, T.; Hara, K.; Kobayashi, A.; Watanabe, Y. Hippocampal Sirtuin 1 Signaling Mediates Depression-like Behavior. Biol. Psychiatry 2016, 80, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, H.; Marchisella, F.; Ortega-Martinez, S.; Hollos, P.; Eerola, K.; Komulainen, E.; Kulesskaya, N.; Freemantle, E.; Fagerholm, V.; Savontous, E.; et al. JNK1 controls adult hippocampal neurogenesis and imposes cell-autonomous control of anxiety behaviour from the neurogenic niche. Mol. Psychiatry 2018, 23, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.Q.; Hu, Z.L.; Hu, Y.L.; Chen, X.; Wang, J.; Mao, L.; Lu, X.; Ni, M.; Chen, J.; Wang, F. Hippocampal CD39/ENTPD1 promotes mouse depression-like behavior through hydrolyzing extracellular ATP. EMBO Rep. 2020, 21, e47857. [Google Scholar] [CrossRef] [PubMed]
- Bavley, C.C.; Fischer, D.K.; Rizzo, B.K.; Rajadhyaksha, A.M. Cav1.2 channels mediate persistent chronic stress-induced behavioral deficits that are associated with prefrontal cortex activation of the p25/Cdk5-glucocorticoid receptor pathway. Neurobiol. Stress 2017, 7, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.; Lifschytz, T.; Ben-Ari, H.; Tatarskyy, P.; Merzel, T.K.; Lotan, A.; Lerer, B. Effect of chronic unpredictable stress on mice with developmental under-expression of the Ahi1 gene: Behavioral manifestations and neurobiological correlates. Transl. Psychiatry 2018, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Rudyk, C.; Dwyer, Z.; McNeill, J.; Salmaso, N.; Farmer, K.; Prowse, N.; Hayley, S. Chronic unpredictable stress influenced the behavioral but not the neurodegenerative impact of paraquat. Neurobiol. Stress 2019, 11, 100179. [Google Scholar] [CrossRef]
- Palumbo, M.C.; Dominguez, S.; Dong, H. Sex differences in hypothalamic-pituitary-adrenal axis regulation after chronic unpredictable stress. Brain Behav. 2020, 10, e01586. [Google Scholar] [CrossRef]
- Tannenbaum, B.M.; Brindley, D.N.; Tannenbaum, G.S.; Dallman, M.F.; McArthur, M.D.; Meaney, M.J. High-fat feeding alters both basal and stress-induced hypothalamic-pituitary-adrenal activity in the rat. Am. J. Physiol. 1997, 273, E1168–E1177. [Google Scholar] [CrossRef]
- Swierczynska, M.M.; Mateska, I.; Peitzsch, M.; Bornstein, S.R.; Chavakis, T.; Eisenhofer, G.; Lamounier-Zepter, V.; Eaton, S. Changes in morphology and function of adrenal cortex in mice fed a high-fat diet. Int. J. Obes. 2015, 39, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Sobesky, J.L.; D’Angelo, H.M.; Weber, M.D.; Anderson, N.D.; Frank, M.G.; Watkins, L.R.; Maier, S.F.; Barrientos, R.M. Glucocorticoids Mediate Short-Term High-Fat Diet Induction of Neuroinflammatory Priming, the NLRP3 Inflammasome, and the Danger Signal HMGB1. eNeuro 2016, 3, ENEURO.0113-16.2016. [Google Scholar] [CrossRef] [PubMed]
- Boudaba, C.; Szabo, K.; Tasker, J.G. Physiological mapping of local inhibitory inputs to the hypothalamic paraventricular nucleus. J. Neurosci. 1996, 16, 7151–7160. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; Dolgas, C.M.; Carlson, S.L. Ventral subiculum regulates hypothalamo-pituitary-adrenocortical and behavioural responses to cognitive stressors. Neuroscience 1998, 86, 449–459. [Google Scholar] [CrossRef]
- Herman, J.P.; Tasker, J.G.; Ziegler, D.R.; Cullinan, W.E. Local circuit regulation of paraventricular nucleus stress integration: Glutamate-GABA connections. Pharmacol. Biochem. Behav. 2002, 71, 457–468. [Google Scholar] [CrossRef]
- Yau, S.Y.; Lau, B.W.; Tong, J.B.; Wong, R.; Ching, Y.P.; Qiu, G.; Tang, S.; Lee, T.M.C.; So, K. Hippocampal neurogenesis and dendritic plasticity support running-improved spatial learning and depression-like behaviour in stressed rats. PLoS ONE 2011, 6, e24263. [Google Scholar] [CrossRef]
- Yau, S.Y.; Lau, B.W.; Zhang, E.D.; Lee, J.C.; Li, A.; Lee, T.M.; Ching, Y.; Xu, A.; So, K. Effects of voluntary running on plasma levels of neurotrophins, hippocampal cell proliferation and learning and memory in stressed rats. Neuroscience 2012, 222, 289–301. [Google Scholar] [CrossRef]
- Yau, S.Y.; Li, A.; Zhang, E.D.; Christie, B.R.; Xu, A.; Lee, T.M.; So, K. Sustained running in rats administered corticosterone prevents the development of depressive behaviors and enhances hippocampal neurogenesis and synaptic plasticity without increasing neurotrophic factor levels. Cell Transpl. 2014, 23, 481–492. [Google Scholar] [CrossRef]
- Gainey, S.J.; Kwakwa, K.A.; Bray, J.K.; Pillote, M.M.; Tir, V.L.; Towers, A.E.; Freund, G.G. Short-Term High-Fat Diet (HFD) Induced Anxiety-Like Behaviors and Cognitive Impairment Are Improved with Treatment by Glyburide. Front. Behav. Neurosci. 2016, 10, 156. [Google Scholar] [CrossRef]
- Wang, Z.; Ge, Q.; Wu, Y.; Zhang, J.; Gu, Q.; Han, J. Impairment of Long-term Memory by a Short-term High-fat Diet via Hippocampal Oxidative Stress and Alterations in Synaptic Plasticity. Neuroscience 2020, 424, 24–33. [Google Scholar] [CrossRef]
- McLean, F.H.; Grant, C.; Morris, A.C.; Horgan, G.W.; Polanski, A.J.; Allan, K.; Campbell, F.M.; Langston, R.F.; Williams, L.M. Rapid and reversible impairment of episodic memory by a high-fat diet in mice. Sci. Rep. 2018, 8, 11976. [Google Scholar] [CrossRef] [PubMed]
- Sims-Robinson, C.; Bakeman, A.; Bruno, E.; Jackson, S.; Glasser, R.; Murphy, G.G.; Feldman, E.L. Dietary Reversal Ameliorates Short- and Long-Term Memory Deficits Induced by High-fat Diet Early in Life. PLoS ONE 2016, 11, e0163883. [Google Scholar] [CrossRef] [PubMed]
- Avena, N.M. Examining the addictive-like properties of binge eating using an animal model of sugar dependence. Exp. Clin. Psychopharmacol. 2007, 15, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, S.; Patrone, A.; Reynaldo, M.; Perello, M. Acute high fat diet consumption activates the mesolimbic circuit and requires orexin signaling in a mouse model. PLoS ONE 2014, 9, e87478. [Google Scholar] [CrossRef]
- Bodnar, H.; Denyko, B.; Waenke, P.; Ball, K.T. Vulnerability to diet-induced obesity is associated with greater food priming-induced reinstatement of palatable food seeking. Physiol. Behav. 2020, 213, 112730. [Google Scholar] [CrossRef]
- Kumar, M.; Chail, M. Sucrose and saccharin differentially modulate depression and anxiety-like behavior in diabetic mice: Exposures and withdrawal effects. Psychopharmacology 2019, 236, 3095–3110. [Google Scholar] [CrossRef]
- Razzoli, M.; Sanghez, V.; Bartolomucci, A. Chronic subordination stress induces hyperphagia and disrupts eating behavior in mice modeling binge-eating-like disorder. Front. Nutr. 2015, 1, 30. [Google Scholar] [CrossRef]
- Sefton, C.; Harno, E.; Davies, A.; Small, H.; Allen, T.J.; Wray, J.R.; Lawrence, C.B.; Coll, A.P.; White, A. Elevated Hypothalamic Glucocorticoid Levels Are Associated with Obesity and Hyperphagia in Male Mice. Endocrinology 2016, 157, 4257–4265. [Google Scholar] [CrossRef]
- Razzoli, M.; Pearson, C.; Crow, S.; Bartolomucci, A. Stress, overeating, and obesity: Insights from human studies and preclinical models. Neurosci. Biobehav. Rev. 2017, 76 (Pt A), 154–162. [Google Scholar] [CrossRef]
- Henderson, Y.O.; Smith, G.P.; Parent, M.B. Hippocampal neurons inhibit meal onset. Hippocampus 2013, 23, 100–107. [Google Scholar] [CrossRef]
- Henderson, Y.O.; Nalloor, R.; Vazdarjanova, A.; Parent, M.B. Sweet orosensation induces Arc expression in dorsal hippocampal CA1 neurons in an experience-dependent manner. Hippocampus 2016, 26, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Parent, M.B. Cognitive control of meal onset and meal size: Role of dorsal hippocampal-dependent episodic memory. Physiol. Behav. 2016, 162, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Davidson, T.L.; Chan, K.; Jarrard, L.E.; Kanoski, S.E.; Clegg, D.J.; Benoit, S.C. Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus 2009, 19, 235–252. [Google Scholar] [CrossRef]
- Sweeney, P.; Yang, Y. An excitatory ventral hippocampus to lateral septum circuit that suppresses feeding. Nat. Commun. 2015, 6, 10188. [Google Scholar] [CrossRef] [PubMed]
- Kanoski, S.E.; Hayes, M.R.; Greenwald, H.S.; Fortin, S.M.; Gianessi, C.A.; Gilbert, J.R.; Grill, H.J. Hippocampal leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology 2011, 36, 1859–1870. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.M.; Hahn, J.D.; Konanur, V.R.; Lam, A.; Kanoski, S.E. Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology 2015, 40, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Hannapel, R.C.; Henderson, Y.H.; Nalloor, R.; Vazdarjanova, A.; Parent, M.B. Ventral hippocampal neurons inhibit postprandial energy intake. Hippocampus 2017, 27, 274–284. [Google Scholar] [CrossRef]
- Morin, J.P.; Rodriguez-Duran, L.F.; Guzman-Ramos, K.; Perez-Cruz, C.; Ferreira, G.; Diaz-Cintra, S.; Pacheco-Lopez, G. Palatable Hyper-Caloric Foods Impact on Neuronal Plasticity. Front. Behav. Neurosci. 2017, 11, 19. [Google Scholar] [CrossRef]
- Davidson, T.L.; Jones, S.; Roy, M.; Stevenson, R.J. The Cognitive Control of Eating and Body Weight: It’s More Than What You “Think”. Front. Psychol. 2019, 10, 62. [Google Scholar] [CrossRef]
- Yau, S.Y.; Li, A.; Hoo, R.L.; Ching, Y.P.; Christie, B.R.; Lee, T.M.; Xu, A.; So, K. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc. Natl. Acad. Sci. USA 2014, 111, 15810–15815. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, X.; Lu, X.Y. Adiponectin Exerts Neurotrophic Effects on Dendritic Arborization, Spinogenesis, and Neurogenesis of the Dentate Gyrus of Male Mice. Endocrinology 2016, 157, 2853–2869. [Google Scholar] [CrossRef] [PubMed]
- Bloemer, J.; Pinky, P.D.; Smith, W.D.; Bhattacharya, D.; Chauhan, A.; Govindarajulu, M.; Hong, H.; Dhanasekaran, M.; Judd, R.; Amin, R.H.; et al. Adiponectin Knockout Mice Display Cognitive and Synaptic Deficits. Front. Endocrinol. 2019, 10, 819. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liang, Y.; Chen, K.; Yau, S.Y.; Sun, X.; Cheng, K.K.; Xu, A.; So, K.; Li, A. Potential Involvement of Adiponectin Signaling in Regulating Physical Exercise-Elicited Hippocampal Neurogenesis and Dendritic Morphology in Stressed Mice. Front. Cell. Neurosci. 2020, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Aou, S.; Oomura, Y.; Hori, N.; Fukunaga, K.; Hori, T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience 2002, 113, 607–615. [Google Scholar] [CrossRef]
- Winocur, G.; Greenwood, C.E.; Piroli, G.G.; Grillo, C.A.; Reznikov, L.R.; Reagan, L.P.; McEwen, B.S. Memory impairment in obese Zucker rats: An investigation of cognitive function in an animal model of insulin resistance and obesity. Behav. Neurosci. 2005, 119, 1389–1395. [Google Scholar] [CrossRef]
- Oomura, Y.; Hori, N.; Shiraishi, T.; Fukunaga, K.; Takeda, H.; Tsuji, M.; Matsumiya, T.; Ishibashi, M.; Aou, S.; Li, X.L.; et al. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides 2006, 27, 2738–2749. [Google Scholar] [CrossRef]
- Yook, J.S.; Rakwal, R.; Shibato, J.; Takahashi, K.; Koizumi, H.; Shima, T.; Ikemoto, M.J.; Oharomari, L.K.; McEwen, B.S.; Soya, H. Leptin in hippocampus mediates benefits of mild exercise by an antioxidant on neurogenesis and memory. Proc. Natl. Acad. Sci. USA 2019, 116, 10988–10993. [Google Scholar] [CrossRef]
- Naranjo, V.; Contreras, A.; Merino, B.; Plaza, A.; Lorenzo, M.P.; Garcia-Caceres, C.; Chowen, J.A.; Ruiz-Gayo, M.; del Olmo, N. Specific Deletion of the Astrocyte Leptin Receptor Induces Changes in Hippocampus Glutamate Metabolism, Synaptic Transmission and Plasticity. Neuroscience 2019, 447, 182–190. [Google Scholar] [CrossRef]
- Dinel, A.L.; Andre, C.; Aubert, A.; Ferreira, G.; Laye, S.; Castanon, N. Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome. PLoS ONE 2011, 6, e24325. [Google Scholar] [CrossRef]
- Boitard, C.; Cavaroc, A.; Sauvant, J.; Aubert, A.; Castanon, N.; Laye, S.; Ferreira, G. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav. Immun. 2014, 40, 9–17. [Google Scholar] [CrossRef]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.A.; Pellme, F.; Hammarstedt, A.; Sandqvist, M.; Brekke, H.; Caidahl, K.; Forsberg, M.; Volkmann, R.; Carvalho, E.; Funahashi, T.; et al. A novel cellular marker of insulin resistance and early atherosclerosis in humans is related to impaired fat cell differentiation and low adiponectin. FASEB J. 2003, 17, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guo, M.; Zhang, D.; Cheng, S.Y.; Liu, M.; Ding, J.; Scherer, P.E.; Liu, F.; Lu, X. Adiponectin is critical in determining susceptibility to depressive behaviors and has antidepressant-like activity. Proc. Natl. Acad. Sci. USA 2012, 109, 12248–12253. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.C.; Cheng, O.Y.; Jian, M.; Kwan, J.S.; Ho, P.W.; Cheng, K.K.; Yeung, P.K.K.; Zhou, L.L.; Hoo, R.L.; Chung, S.K.; et al. Chronic adiponectin deficiency leads to Alzheimer’s disease-like cognitive impairments and pathologies through AMPK inactivation and cerebral insulin resistance in aged mice. Mol. Neurodegener. 2016, 11, 71. [Google Scholar] [CrossRef]
- Guo, M.; Li, C.; Lei, Y.; Xu, S.; Zhao, D.; Lu, X.Y. Role of the adipose PPARgamma-adiponectin axis in susceptibility to stress and depression/anxiety-related behaviors. Mol. Psychiatry 2017, 22, 1056–1068. [Google Scholar] [CrossRef] [PubMed]
- Rege, S.D.; Geetha, T.; Broderick, T.L.; Babu, J.R. Can Diet and Physical Activity Limit Alzheimer’s Disease Risk? Curr. Alzheimer Res. 2017, 14, 76–93. [Google Scholar] [CrossRef]
- Heiston, E.M.; Eichner, N.Z.; Gilbertson, N.M.; Malin, S.K. Exercise improves adiposopathy, insulin sensitivity and metabolic syndrome severity independent of intensity. Exp. Physiol. 2020, 105, 632–640. [Google Scholar] [CrossRef]
- Dyer, A.H.; Briggs, R.; Mockler, D.; Gibney, J.; Kennelly, S.P. Non-pharmacological interventions for cognition in patients with Type 2 diabetes mellitus: A systematic review. QJM 2020, 113, 155–161. [Google Scholar] [CrossRef]
- Srikanth, V.; Sinclair, A.J.; Hill-Briggs, F.; Moran, C.; Biessels, G.J. Type 2 diabetes and cognitive dysfunction-towards effective management of both comorbidities. Lancet Diabetes Endocrinol. 2020, 8, 535–545. [Google Scholar] [CrossRef]
- Pereira, S.S.; Alvarez-Leite, J.I. Low-Grade Inflammation, Obesity, and Diabetes. Curr. Obes. Rep. 2014, 3, 422–431. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Ji, Y.; Sakata, Y.; Tso, P. Nutrient-induced inflammation in the intestine. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 315–321. [Google Scholar] [CrossRef]
- Hersoug, L.G.; Moller, P.; Loft, S. Role of microbiota-derived lipopolysaccharide in adipose tissue inflammation, adipocyte size and pyroptosis during obesity. Nutr. Res. Rev. 2018, 31, 153–163. [Google Scholar] [CrossRef]
- Griffiths, E.A.; Duffy, L.C.; Schanbacher, F.L.; Qiao, H.; Dryja, D.; Leavens, A.; Rossman, J.; Rich, G.; Dirienzo, D.; Ogra, P.L. In vivo effects of bifidobacteria and lactoferrin on gut endotoxin concentration and mucosal immunity in Balb/c mice. Dig. Dis. Sci. 2004, 49, 579–589. [Google Scholar] [CrossRef]
- Tsukumo, D.M.; Carvalho-Filho, M.A.; Carvalheira, J.B.; Prada, P.O.; Hirabara, S.M.; Schenka, A.A.; Araujo, E.P.; Vassallo, J.; Curi, R.; Velloso, L.A.; et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 2007, 56, 1986–1998. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Tesar, B.M.; Walker, W.E.; Goldstein, D.R. Dual signaling of MyD88 and TRIF is critical for maximal TLR4-induced dendritic cell maturation. J. Immunol. 2008, 181, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Akhter, N.; Hasan, A.; Shenouda, S.; Wilson, A.; Kochumon, S.; Ali, S.; Tuomilehto, J.; Sindhu, S.; Ahmad, R. TLR4/MyD88 -mediated CCL2 production by lipopolysaccharide (endotoxin): Implications for metabolic inflammation. J. Diabetes Metab. Disord. 2018, 17, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Piao, W.; Ru, L.W.; Piepenbrink, K.H.; Sundberg, E.J.; Vogel, S.N.; Toshchakov, V.Y. Recruitment of TLR adapter TRIF to TLR4 signaling complex is mediated by the second helical region of TRIF TIR domain. Proc. Natl. Acad. Sci. USA 2013, 110, 19036–19041. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; Hunter, D.; Huber, R.; Lemieux, J.; Slaymaker, S.; Vaddi, K.; Charo, I.; Leibel, R.L.; Ferrante, A.W., Jr. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Investig. 2006, 116, 115–124. [Google Scholar] [CrossRef]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef]
- Kamei, N.; Tobe, K.; Suzuki, R.; Ohsugi, M.; Watanabe, T.; Kubota, N.; Ohtsuka-Kowatari, N.; Kumagai, K.; Sakamoto, K.; Kobayashi, M.; et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J. Biol. Chem. 2006, 281, 26602–26614. [Google Scholar] [CrossRef]
- Caesar, R.; Tremaroli, V.; Kovatcheva-Datchary, P.; Cani, P.D.; Backhed, F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015, 22, 658–668. [Google Scholar] [CrossRef]
- Bruce-Keller, A.J.; Salbaum, J.M.; Luo, M.; Blanchard Et Taylor, C.M.; Welsh, D.A.; Berthoud, H. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol. Psychiatry 2015, 77, 607–615. [Google Scholar] [CrossRef]
- D’Amato, A.; Di Cesare Mannelli, L.; Lucarini, E.; Man, A.L.; Le Gall, G.; Branca, J.J.V.; Ghelardini, C.; Amedei, A.; Bertelli, E.; Regoli, M.; et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome 2020, 8, 140. [Google Scholar] [CrossRef]
- Kundu, P.; Lee, H.U.; Garcia-Perez, I.; Tay, E.X.Y.; Kim, H.; Faylon, L.E.; Martin, K.A.; Purbojati, R.; Drautz-Moses, D.I.; Ghosh, S.; et al. Neurogenesis and prolongevity signaling in young germ-free mice transplanted with the gut microbiota of old mice. Sci. Transl. Med. 2019, 11, eaau4760. [Google Scholar] [CrossRef] [PubMed]
- Suarez, A.N.; Hsu, T.M.; Liu, C.M.; Noble, E.E.; Cortella, A.M.; Nakamoto, E.M.; Hahn, J.D.; de Lartigue, G.; Kanoski, S.E. Gut vagal sensory signaling regulates hippocampus function through multi-order pathways. Nat. Commun. 2018, 9, 2181. [Google Scholar] [CrossRef] [PubMed]
- Burhans, M.S.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus. Compr. Physiol. 2018, 9, 1–58. [Google Scholar] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Castoldi, A.; Naffah de Souza, C.; Camara, N.O.; Moraes-Vieira, P.M. The Macrophage Switch in Obesity Development. Front. Immunol. 2015, 6, 637. [Google Scholar] [CrossRef]
- Guo, D.H.; Yamamoto, M.; Hernandez, C.M.; Khodadadi, H.; Baban, B.; Stranahan, A.M. Visceral adipose NLRP3 impairs cognition in obesity via IL-1R1 on CX3CR1+ cells. J. Clin. Investig. 2020, 130, 1961–1976. [Google Scholar] [CrossRef]
- Terauchi, Y.; Matsui, J.; Kamon, J.; Yamauchi, T.; Kubota, N.; Komeda, K.; Aizawa, S.; Akanuma, Y.; Tomita, M.; Kadowaki, T. Increased serum leptin protects from adiposity despite the increased glucose uptake in white adipose tissue in mice lacking p85alpha phosphoinositide 3-kinase. Diabetes 2004, 53, 2261–2270. [Google Scholar] [CrossRef]
- Koch, A.; Weiskirchen, R.; Zimmermann, H.W.; Sanson, E.; Trautwein, C.; Tacke, F. Relevance of serum leptin and leptin-receptor concentrations in critically ill patients. Mediat. Inflamm. 2010, 2010, 473540. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q.; Zhang, L.; Li, C.; Jiang, H. Expression of ghrelin and leptin during the development of type 2 diabetes mellitus in a rat model. Mol. Med. Rep. 2013, 7, 223–228. [Google Scholar] [CrossRef][Green Version]
- Paglialunga, S.; Ludzki, A.; Root-McCaig, J.; Holloway, G.P. In adipose tissue, increased mitochondrial emission of reactive oxygen species is important for short-term high-fat diet-induced insulin resistance in mice. Diabetologia 2015, 58, 1071–1080. [Google Scholar] [CrossRef]
- Franchi, L.; Eigenbrod, T.; Munoz-Planillo, R.; Nunez, G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 2009, 10, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Vandanmagsar, B.; Youm, Y.H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Pulskens, W.P.; Sadler, J.J.; Butter, L.M.; Teske, G.J.; Ulland, T.K.; Eisenbarth, S.C.; Florquin, S.; Flavell, R.A.; Leemans, J.C.; et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc. Natl. Acad. Sci. USA 2009, 106, 20388–20393. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Huang, R.; Han, J.; Cai, R.; Guo, D.; Lin, H.; Wang, J.; Wang, S. Increased plasma Interleukin-1beta level is associated with memory deficits in type 2 diabetic patients with mild cognitive impairment. Psychoneuroendocrinology 2018, 96, 148–154. [Google Scholar] [CrossRef]
- Ouchi, N.; Walsh, K. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta 2007, 380, 24–30. [Google Scholar] [CrossRef]
- Ouchi, N.; Kihara, S.; Arita, Y.; Maeda, K.; Kuriyama, H.; Okamoto, Y.; Hotta, K.; Nishida, M.; Takahashi, M.; Nakamura, T.; et al. Novel modulator for endothelial adhesion molecules: Adipocyte-derived plasma protein adiponectin. Circulation 1999, 100, 2473–2476. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef]
- Arnold, S.E.; Lucki, I.; Brookshire, B.R.; Carlson, G.C.; Browne, C.A.; Kazi, H.; Bang, S.; Choi, B.; Chen, Y.; McMullen, M.F.; et al. High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol. Dis. 2014, 67, 79–87. [Google Scholar] [CrossRef]
- Qi, Y.; Takahashi, N.; Hileman, S.M.; Patel, H.R.; Berg, A.H.; Pajvani, U.B.; Scherer, P.E.; Ahima, R.S. Adiponectin acts in the brain to decrease body weight. Nat. Med. 2004, 10, 524–529. [Google Scholar] [CrossRef]
- Nicolas, S.; Cazareth, J.; Zarif, H.; Guyon, A.; Heurteaux, C.; Chabry, J.; Petit-Paitel, A. Globular Adiponectin Limits Microglia Pro-Inflammatory Phenotype through an AdipoR1/NF-kappaB Signaling Pathway. Front. Cell. Neurosci. 2017, 11, 352. [Google Scholar] [CrossRef]
- Jeon, B.T.; Jeong, E.A.; Shin, H.J.; Lee, Y.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Roh, G.S. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes 2012, 61, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Dey, A.; Yu, X.; Stranahan, A.M. Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain Behav. Immun. 2016, 51, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Cope, E.C.; LaMarca, E.A.; Monari, P.K.; Olson, L.B.; Martinez, S.; Zych, A.D.; Katchur, N.J.; Gould, E. Microglia Play an Active Role in Obesity-Associated Cognitive Decline. J. Neurosci. 2018, 38, 8889–8904. [Google Scholar] [CrossRef] [PubMed]
- Castro-Sanchez, S.; Garcia-Yague, A.J.; Kugler, S.; Lastres-Becker, I. CX3CR1-deficient microglia shows impaired signalling of the transcription factor NRF2: Implications in tauopathies. Redox Biol. 2019, 22, 101118. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Trushina, E.; Morland, C.; Prigione, A.; Casadesus, G.; Andrews, Z.B.; Beal, M.F.; Bergersen, L.H.; Brinton, R.D.; de la Monte, S.; et al. Brain energy rescue: An emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2020, 19, 609–633. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, S.; Buzkova, J.; Muniandy, M.; Kaksonen, R.; Ollikainen, M.; Ismail, K.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Vuolteenaho, K.; et al. Impaired Mitochondrial Biogenesis in Adipose Tissue in Acquired Obesity. Diabetes 2015, 64, 3135–3145. [Google Scholar] [CrossRef]
- Bournat, J.C.; Brown, C.W. Mitochondrial dysfunction in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 446–452. [Google Scholar] [CrossRef]
- Zorzano, A.; Liesa, M.; Palacin, M. Role of mitochondrial dynamics proteins in the pathophysiology of obesity and type 2 diabetes. Int. J. Biochem. Cell Biol. 2009, 41, 1846–1854. [Google Scholar] [CrossRef]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef]
- Ruiz-Ramirez, A.; Chavez-Salgado, M.; Peneda-Flores, J.A.; Zapata, E.; Masso, F.; El-Hafidi, M. High-sucrose diet increases ROS generation, FFA accumulation, UCP2 level, and proton leak in liver mitochondria. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1198–E1207. [Google Scholar] [CrossRef]
- Massaad, C.A.; Klann, E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid. Redox Signal 2011, 14, 2013–2054. [Google Scholar] [CrossRef] [PubMed]
- Ritz, P.; Berrut, G. Mitochondrial function, energy expenditure, aging and insulin resistance. Diabetes Metab. 2005, 31, 5S67–5S73. [Google Scholar] [CrossRef]
- Zhou, Y.; Lian, S.; Zhang, J.; Lin, D.; Huang, C.; Liu, L.; Chen, Z. Mitochondrial Perturbation Contributing to Cognitive Decline in Streptozotocin-Induced Type 1 Diabetic Rats. Cell. Physiol. Biochem. 2018, 46, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.; St-Pierre, J. PGC1alpha and mitochondrial metabolism--emerging concepts and relevance in ageing and neurodegenerative disorders. J. Cell. Sci. 2012, 125 (Pt 21), 4963–4971. [Google Scholar] [CrossRef]
- Petrov, D.; Pedros, I.; Artiach, G.; Sureda, F.X.; Barroso, E.; Pallas, M.; Casadesus, G.; Beas-Zarate, C.; Carro, E.; Ferrer, I.; et al. High-fat diet-induced deregulation of hippocampal insulin signaling and mitochondrial homeostasis deficiences contribute to Alzheimer disease pathology in rodents. Biochim. Biophys. Acta 2015, 1852, 1687–1699. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Wan, R.; Yang, J.L.; Kamimura, N.; Son, T.G.; Ouyang, X.; Luo, Y.; Okun, E.; Mattson, M.P. Involvement of PGC-1alpha in the formation and maintenance of neuronal dendritic spines. Nat. Commun. 2012, 3, 1250. [Google Scholar] [CrossRef]
- Erturk, A.; Wang, Y.; Sheng, M. Local pruning of dendrites and spines by caspase-3-dependent and proteasome-limited mechanisms. J. Neurosci. 2014, 34, 1672–1688. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Zhang, J.; Ma, L.; Gu, J.; Ho, G. Contribution of neural cell death to depressive phenotypes of streptozotocin-induced diabetic mice. Dis. Models Mech. 2014, 7, 723–730. [Google Scholar] [CrossRef]
- He, X.; Sun, J.; Huang, X. Expression of caspase-3, Bax and Bcl-2 in hippocampus of rats with diabetes and subarachnoid hemorrhage. Exp. Ther. Med. 2018, 15, 873–877. [Google Scholar] [CrossRef]
- Suzuki, J.; Denning, D.P.; Imanishi, E.; Horvitz, H.R.; Nagata, S. Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science 2013, 341, 403–406. [Google Scholar] [CrossRef]
- Segawa, K.; Kurata, S.; Yanagihashi, Y.; Brummelkamp, T.R.; Matsuda, F.; Nagata, S. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science 2014, 344, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Fuller, A.D.; Van Eldik, L.J. MFG-E8 regulates microglial phagocytosis of apoptotic neurons. J. Neuroimmune Pharmacol. 2008, 3, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Hu, Q.K.; Chen, L.; Liu, S.Y.; Su, S.; Tao, H.; Zhang, L.; Sun, T.; He, L. HSPB8 Promotes the Fusion of Autophagosome and Lysosome during Autophagy in Diabetic Neurons. Int. J. Med. Sci. 2017, 14, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Sangwung, P.; Petersen, K.F.; Shulman, G.I.; Knowles, J.W. Mitochondrial Dysfunction, Insulin Resistance, and Potential Genetic Implications. Endocrinology 2020, 161, bqaa017. [Google Scholar] [CrossRef] [PubMed]
- Iwabu, M.; Yamauchi, T.; Okada-Iwabu, M.; Sato, K.; Nakagawa, T.; Funata, M.; Yamaguchi, M.; Namiki, S.; Nakayama, R.; Tabata, M.; et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature 2010, 464, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Pepin, M.E.; Koentges, C.; Pfeil, K.; Gollmer, J.; Kersting, S.; Wiese, S.; Hoffman, M.M.; Odening, K.; Muehlen, C.v.; Diehl, P.; et al. Dysregulation of the Mitochondrial Proteome Occurs in Mice Lacking Adiponectin Receptor 1. Front. Endocrinol. 2019, 10, 872. [Google Scholar] [CrossRef]
- Piccinin, E.; Villani, G.; Moschetta, A. Metabolic aspects in NAFLD, NASH and hepatocellular carcinoma: The role of PGC1 coactivators. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 160–174. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Wang, J.G.; Liu, C.L.; Yan, H.J. AdipoRon improves cognitive dysfunction of Alzheimer’s disease and rescues impaired neural stem cell proliferation through AdipoR1/AMPK pathway. Exp. Neurol. 2020, 327, 113249. [Google Scholar] [CrossRef]
- Kim, M.W.; Abid, N.B.; Jo, M.H.; Jo, M.G.; Yoon, G.H.; Kim, M.O. Suppression of adiponectin receptor 1 promotes memory dysfunction and Alzheimer’s disease-like pathologies. Sci. Rep. 2017, 7, 12435. [Google Scholar] [CrossRef]
- Quehenberger, O.; Armando, A.M.; Brown, A.H.; Milne, S.B.; Myers, D.S.; Merrill, A.H.; Bandyopadhyay, S.; Jones, K.N.; Kelly, S.; Shaner, R.L. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 2010, 51, 3299–3305. [Google Scholar] [CrossRef]
- Pilitsis, J.G.; Diaz, F.G.; Wellwood, J.M.; Oregan, M.H.; Fairfax, M.R.; Phillis, J.W.; Coplin, W.M. Quantification of free fatty acids in human cerebrospinal fluid. Neurochem. Res. 2001, 26, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Karmi, A.; Iozzo, P.; Viljanen, A.; Hirvonen, J.; Fielding, B.A.; Virtanen, K.; Oikonen, V.; Kemppainen, J.; Viljanen, T.; Guiducci, L.; et al. Increased brain fatty acid uptake in metabolic syndrome. Diabetes 2010, 59, 2171–2177. [Google Scholar] [CrossRef] [PubMed]
- Melo, H.M.; Seixas da Silva, G.D.S.; Sant’Ana, M.R.; Teixeira, C.V.L.; Clarke, J.R.; Miya Coreixas, V.S.; de Melo, B.C.; Fortuna, J.T.S.; Forny-Germano, L.; Ledo, J.H.; et al. Palmitate Is Increased in the Cerebrospinal Fluid of Humans with Obesity and Induces Memory Impairment in Mice via Pro-inflammatory TNF-alpha. Cell Rep. 2020, 30, 2180–2194. [Google Scholar] [CrossRef] [PubMed]
- Vinuesa, A.; Bentivegna, M.; Calfa, G.; Filipello, F.; Pomilio, C.; Bonaventura, M.M.; Lux-Lantos, V.; Matzkin, M.E.; Gregosa, A.; Presa, J.; et al. Early Exposure to a High-Fat Diet Impacts on Hippocampal Plasticity: Implication of Microglia-Derived Exosome-like Extracellular Vesicles. Mol. Neurobiol. 2019, 56, 5075–5094. [Google Scholar] [CrossRef]
- Steffensen, C.; Dekkers, O.M.; Lyhne, J.; Pedersen, B.G.; Rasmussen, F.; Rungby, J.; Poulsen, P.L.; Jorgensen, J.O.L. Hypercortisolism in Newly Diagnosed Type 2 Diabetes: A Prospective Study of 384 Newly Diagnosed Patients. Horm. Metab. Res. 2019, 51, 62–68. [Google Scholar] [CrossRef]
- Buchenauer, T.; Behrendt, P.; Bode, F.J.; Horn, R.; Brabant, G.; Stephan, M.; Nave, H. Diet-induced obesity alters behavior as well as serum levels of corticosterone in F344 rats. Physiol. Behav. 2009, 98, 563–569. [Google Scholar] [CrossRef]
- Wosiski-Kuhn, M.; Erion, J.R.; Gomez-Sanchez, E.P.; Gomez-Sanchez, C.E.; Stranahan, A.M. Glucocorticoid receptor activation impairs hippocampal plasticity by suppressing BDNF expression in obese mice. Psychoneuroendocrinology 2014, 42, 165–177. [Google Scholar] [CrossRef]
- Oster, H.; Challet, E.; Ott, V.; Arvat, E.; de Kloet, E.R.; Dijk, D.J.; Lightman, S.; Vgontzas, A.; van Cauter, E. The Functional and Clinical Significance of the 24-Hour Rhythm of Circulating Glucocorticoids. Endocr. Rev. 2017, 38, 3–45. [Google Scholar] [CrossRef]
- Herman, J.P.; Schafer, M.K.; Young, E.A.; Thompson, R.; Douglass, J.; Akil, H.; Watson, S.J. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J. Neurosci. 1989, 9, 3072–3082. [Google Scholar] [CrossRef]
- Song, Y.; Meng, Q.X.; Wu, K.; Hua, R.; Song, Z.J.; Song, Y.; Qin, X.; Cao, J.; Zhang, Y. Disinhibition of PVN-projecting GABAergic neurons in AV region in BNST participates in visceral hypersensitivity in rats. Psychoneuroendocrinology 2020, 117, 104690. [Google Scholar] [CrossRef]
- Herman, J.P. Regulation of adrenocorticosteroid receptor mRNA expression in the central nervous system. Cell Mol. Neurobiol. 1993, 13, 349–372. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.L.; Pariante, C.M.; Jamel, S.; Thomas, S.A. Central nervous system (CNS) delivery of glucocorticoids is fine-tuned by saturable transporters at the blood-CNS barriers and nonbarrier regions. Endocrinology 2010, 151, 5294–5305. [Google Scholar] [CrossRef] [PubMed]
- Boyle, M.P.; Kolber, B.J.; Vogt, S.K.; Wozniak, D.F.; Muglia, L.J. Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J. Neurosci. 2006, 26, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Furay, A.R.; Bruestle, A.E.; Herman, J.P. The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology 2008, 149, 5482–5490. [Google Scholar] [CrossRef]
- Check, J.H.; Wilson, C.; Cohen, R.; Sarumi, M. Evidence that Mifepristone, a progesterone receptor antagonist, can cross the blood brain barrier and provide palliative benefits for glioblastoma multiforme grade IV. Anticancer Res. 2014, 34, 2385–2388. [Google Scholar]
- Brummelte, S.; Galea, L.A. Chronic high corticosterone reduces neurogenesis in the dentate gyrus of adult male and female rats. Neuroscience 2010, 168, 680–690. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Wang, Z. Chronic corticosterone exposure reduces hippocampal astrocyte structural plasticity and induces hippocampal atrophy in mice. Neurosci. Lett. 2015, 592, 76–81. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.; Jung, J.W.; Kim, B.C.; Ryu, J.H.; Kim, D.H. Glucocorticoid- and long-term stress-induced aberrant synaptic plasticity are mediated by activation of the glucocorticoid receptor. Arch. Pharm. Res. 2015, 38, 1204–1212. [Google Scholar] [CrossRef]
- Khazen, T.; Hatoum, O.A.; Ferreira, G.; Maroun, M. Acute exposure to a high-fat diet in juvenile male rats disrupts hippocampal-dependent memory and plasticity through glucocorticoids. Sci. Rep. 2019, 9, 12270. [Google Scholar] [CrossRef]
- Michel, V.; Peinnequin, A.; Alonso, A.; Buguet, A.; Cespuglio, R.; Canini, F. Effect of glucocorticoid depletion on heat-induced Hsp70, IL-1beta and TNF-alpha gene expression. Brain Res. 2007, 1164, 63–71. [Google Scholar] [CrossRef]
- Vilela, F.C.; Antunes-Rodrigues, J.; Elias, L.L.; Giusti-Paiva, A. Corticosterone synthesis inhibitor metyrapone preserves changes in maternal behavior and neuroendocrine responses during immunological challenge in lactating rats. Neuroendocrinology 2013, 97, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Hao, S.; Erion, J.R.; Wosiski-Kuhn, M.; Stranahan, A.M. Glucocorticoid sensitization of microglia in a genetic mouse model of obesity and diabetes. J. Neuroimmunol. 2014, 269, 20–27. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stranahan, A.M.; Arumugam, T.V.; Mattson, M.P. Lowering corticosterone levels reinstates hippocampal brain-derived neurotropic factor and Trkb expression without influencing deficits in hypothalamic brain-derived neurotropic factor expression in leptin receptor-deficient mice. Neuroendocrinology 2011, 93, 58–64. [Google Scholar] [CrossRef]
- Binder, D.K.; Scharfman, H.E. Brain-derived neurotrophic factor. Growth Factors 2004, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.V.; Bothwell, M.A.; Ross, A.H.; Koprowski, H.; Lanahan, A.A.; Buck, C.R.; Sehgal, A. Gene transfer and molecular cloning of the human NGF receptor. Science 1986, 232, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Zagrebelsky, M.; Holz, A.; Dechant, G.; Barde, Y.A.; Bonhoeffer, T.; Korte, M. The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons. J. Neurosci. 2005, 25, 9989–9999. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A. Ketogenic diet for obesity: Friend or foe? Int. J. Environ. Res. Public Health 2014, 11, 2092–2107. [Google Scholar] [CrossRef] [PubMed]
- Ulamek-Koziol, M.; Czuczwar, S.J.; Januszewski, S.; Pluta, R. Ketogenic Diet and Epilepsy. Nutrients 2019, 11, 2510. [Google Scholar] [CrossRef]
- Manninen, A.H. Metabolic effects of the very-low-carbohydrate diets: Misunderstood “villains” of human metabolism. J. Int. Soc. Sports Nutr. 2004, 1, 7–11. [Google Scholar] [CrossRef]
- Dhatariya, K.K.; Glaser, N.S.; Codner, E.; Umpierrez, G.E. Diabetic ketoacidosis. Nat. Rev. Dis. Primers 2020, 6, 40. [Google Scholar] [CrossRef]
- Cahill, G.F., Jr.; Herrera, M.G.; Morgan, A.P.; Soeldner, J.S.; Steinke, J.; Levy, P.L.; Reichard, G.A.; Kipnis, D.M. Hormone-fuel interrelationships during fasting. J. Clin. Investig. 1966, 45, 1751–1769. [Google Scholar] [CrossRef] [PubMed]
- Adrogue, H.J.; Wilson, H.; Boyd, A.E., 3rd; Suki, W.N.; Eknoyan, G. Plasma acid-base patterns in diabetic ketoacidosis. N. Engl. J. Med. 1982, 307, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Kanikarla-Marie, P.; Jain, S.K. Hyperketonemia and ketosis increase the risk of complications in type 1 diabetes. Free Radic. Biol. Med. 2016, 95, 268–277. [Google Scholar] [CrossRef]
- Veech, R.L.; Chance, B.; Kashiwaya, Y.; Lardy, H.A.; Cahill, G.F., Jr. Ketone bodies, potential therapeutic uses. IUBMB Life 2001, 51, 241–247. [Google Scholar] [PubMed]
- Neal, E.G.; Chaffe, H.; Schwartz, R.H.; Lawson, M.S.; Edwards, N.; Fitzsimmons, G.; Whitney, A.; Cross, J.H. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia 2009, 50, 1109–1117. [Google Scholar] [CrossRef]
- Achanta, L.B.; Rae, C.D. beta-Hydroxybutyrate in the Brain: One Molecule, Multiple Mechanisms. Neurochem. Res. 2017, 42, 35–49. [Google Scholar] [CrossRef]
- Goldberg, E.L.; Shchukina, I.; Asher, J.L.; Sidorov, S.; Artyomov, M.N.; Dixit, V.D. Ketogenesis activates metabolically protective gammadelta T cells in visceral adipose tissue. Nat. Metab. 2020, 2, 50–61. [Google Scholar] [CrossRef]
- Asrih, M.; Altirriba, J.; Rohner-Jeanrenaud, F.; Jornayvaz, F.R. Ketogenic Diet Impairs FGF21 Signaling and Promotes Differential Inflammatory Responses in the Liver and White Adipose Tissue. PLoS ONE 2015, 10, e0126364. [Google Scholar] [CrossRef]
- Newman, J.C.; Covarrubias, A.J.; Zhao, M.; Yu, X.; Gut, P.; Ng, C.P.; Huang, Y.; Haldar, S.; Verdin, E. Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice. Cell Metab. 2017, 26, 547–557. [Google Scholar] [CrossRef]
- Elamin, M.; Ruskin, D.N.; Masino, S.A.; Sacchetti, P. Ketogenic Diet Modulates NAD(+)-Dependent Enzymes and Reduces DNA Damage in Hippocampus. Front. Cell. Neurosci. 2018, 12, 263. [Google Scholar] [CrossRef]
- Hasan-Olive, M.M.; Lauritzen, K.H.; Ali, M.; Rasmussen, L.J.; Storm-Mathisen, J.; Bergersen, L.H. A Ketogenic Diet Improves Mitochondrial Biogenesis and Bioenergetics via the PGC1alpha-SIRT3-UCP2 Axis. Neurochem. Res. 2019, 44, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Belenky, P.; Bogan, K.L.; Brenner, C. NAD+ metabolism in health and disease. Trends Biochem. Sci. 2007, 32, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.M.; Kong, X.; Moncada, E.; Chen, Y.; Imamura, H.; Wang, P.; Berns, M.W.; Yokomori, K.; Digman, M.A. NAD+ consumption by PARP1 in response to DNA damage triggers metabolic shift critical for damaged cell survival. Mol. Biol. Cell 2019, 30, 2584–2597. [Google Scholar] [CrossRef] [PubMed]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Suuronen, T.; Ojala, J.; Kaarniranta, K.; Salminen, A. Antagonistic crosstalk between NF-kappaB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal 2013, 25, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.Y.; Miller, C.; Bitterman, K.J.; Wall, N.R.; Hekking, B.; Kessler, B.; Howitz, K.T.; Gorospe, M.; de Cabo, R.; Sinclair, D.A. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 2004, 305, 390–392. [Google Scholar] [CrossRef]
- Kim, D.; Nguyen, M.D.; Dobbin, M.M.; Fischer, A.; Sananbenesi, F.; Rodgers, J.T.; Delalle, I.; Baur, J.A.; Sui, G.; Armour, S.M.; et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007, 26, 3169–3179. [Google Scholar] [CrossRef]
- Khan, R.S.; Fonseca-Kelly, Z.; Callinan, C.; Zuo, L.; Sachdeva, M.M.; Shindler, K.S. SIRT1 activating compounds reduce oxidative stress and prevent cell death in neuronal cells. Front. Cell. Neurosci. 2012, 6, 63. [Google Scholar] [CrossRef]
- Lauritzen, K.H.; Hasan-Olive, M.M.; Regnell, C.E.; Kleppa, L.; Scheibye-Knudsen, M.; Gjedde, A.; Klungland, A.; Bohr, V.A.; Storm-Mathisen, J.; Bergersen, L.H. A ketogenic diet accelerates neurodegeneration in mice with induced mitochondrial DNA toxicity in the forebrain. Neurobiol. Aging 2016, 48, 34–47. [Google Scholar] [CrossRef]
- Kane, A.E.; Sinclair, D.A. Sirtuins and NAD(+) in the Development and Treatment of Metabolic and Cardiovascular Diseases. Circ. Res. 2018, 123, 868–885. [Google Scholar] [CrossRef]
- Vaziri, H.; Dessain, S.K.; Ng Eaton, E.; Imai, S.I.; Frye, R.A.; Pandita, T.K.; Guarente, L.; Weinberg, R.A. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 2001, 107, 149–159. [Google Scholar] [CrossRef]
- Mouchiroud, L.; Houtkooper, R.H.; Moullan, N.; Katsyuba, E.; Ryu, D.; Canto, C.; Mottis, A.; Jo, Y.; Viswanathan, M.; Schoonjans, K.; et al. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell 2013, 154, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Rajamohan, S.B.; Pillai, V.B.; Gupta, M.; Sundaresan, N.R.; Birukov, K.G.; Samant, S.; Hottiger, M.O.; Gupta, M.P. SIRT1 promotes cell survival under stress by deacetylation-dependent deactivation of poly(ADP-ribose) polymerase 1. Mol. Cell Biol. 2009, 29, 4116–4129. [Google Scholar] [CrossRef] [PubMed]
- Hirschey, M.D.; Shimazu, T.; Goetzman, E.; Jing, E.; Schwer, B.; Lombard, D.B.; Grueter, C.A.; Harris, C.; Biddinger, S.; Ilkayeva, O.R.; et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 2010, 464, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Nogueiras, R.; Habegger, K.M.; Chaudhary, N.; Finan, B.; Banks, A.S.; Dietrich, M.O.; Horvath, T.L.; Sinclair, D.A.; Pfluger, P.T.; Tschop, M.H. Sirtuin 1 and sirtuin 3: Physiological modulators of metabolism. Physiol. Rev. 2012, 92, 1479–1514. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, I.; Pellegrini, L.; D’Aquila, P.; Saladini, S.; Lococo, E.; Polletta, L.; Vernucci, E.; Foglio, E.; Coppola, S.; Sansone, L.; et al. SIRT1-SIRT3 Axis Regulates Cellular Response to Oxidative Stress and Etoposide. J. Cell. Physiol. 2017, 232, 1835–1844. [Google Scholar] [CrossRef]
- Chen, D.; Steele, A.D.; Lindquist, S.; Guarente, L. Increase in activity during calorie restriction requires Sirt1. Science 2005, 310, 1641. [Google Scholar] [CrossRef]
- Bordone, L.; Cohen, D.; Robinson, A.; Motta, M.C.; van Veen, E.; Czopik, A.; Steele, A.D.; Crowe, H.; Marmor, S.; Luo, J. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 2007, 6, 759–767. [Google Scholar] [CrossRef]
- Schenk, S.; McCurdy, C.E.; Philp, A.; Chen, M.Z.; Holliday, M.J.; Bandyopadhyay, G.K.; Osborn, O.; Baar, K.; Olefsky, J.M. Sirt1 enhances skeletal muscle insulin sensitivity in mice during caloric restriction. J. Clin. Investig. 2011, 121, 4281–4288. [Google Scholar] [CrossRef]
- Chen, D.; Bruno, J.; Easlon, E.; Lin, S.J.; Cheng, H.L.; Alt, F.W.; Guarente, L. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008, 22, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- Heyward, F.D.; Gilliam, D.; Coleman, M.A.; Gavin, C.F.; Wang, J.; Kaas, G.; Trieu, R.; Lewis, J.; Moulden, J.; Sweatt, J.D. Obesity Weighs down Memory through a Mechanism Involving the Neuroepigenetic Dysregulation of Sirt1. J. Neurosci. 2016, 36, 1324–1335. [Google Scholar] [CrossRef] [PubMed]
- Fusco, S.; Ripoli, C.; Podda, M.V.; Ranieri, S.C.; Leone, L.; Toietta, G.; McBurney, M.W.; Schutz, G.; Riccio, A.; Grassi, C.; et al. A role for neuronal cAMP responsive-element binding (CREB)-1 in brain responses to calorie restriction. Proc. Natl. Acad. Sci. USA 2012, 109, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Seok, S.; Yau, P.; Li, X.; Kemper, B.; Kemper, J.K. Obesity and aging diminish sirtuin 1 (SIRT1)-mediated deacetylation of SIRT3, leading to hyperacetylation and decreased activity and stability of SIRT3. J. Biol. Chem. 2017, 292, 17312–17323. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, A.; Li, Y.-J.; Yang, Y.; Kishimoto, Y.; Zhang, S.; Wang, Y.; Wan, R.; Raefsky, S.M.; Lu, D.; et al. SIRT3 mediates hippocampal synaptic adaptations to intermittent fasting and ameliorates deficits in APP mutant mice. Nat. Commun. 2019, 10, 1886. [Google Scholar] [CrossRef]
- Fu, J.; Jin, J.; Cichewicz, R.H.; Hageman, S.A.; Ellis, T.K.; Xiang, L.; Peng, Q.; Jiang, M.; Arbez, N.; Hotaling, K.; et al. Trans-(-)-epsilon-Viniferin increases mitochondrial sirtuin 3 (SIRT3), activates AMP-activated protein kinase (AMPK), and protects cells in models of Huntington Disease. J. Biol. Chem. 2012, 287, 24460–24472. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, X.; Zhang, H.; Shi, R.; Hui, Y.; Jin, X.; Zhang, W.; Wang, L.; Wang, Q.; Wang, D.; et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat. Commun. 2020, 11, 855. [Google Scholar] [CrossRef]
- Aleidi, S.; Issa, A.; Bustanji, H.; Khalil, M.; Bustanji, Y. Adiponectin serum levels correlate with insulin resistance in type 2 diabetic patients. Saudi Pharm. J. 2015, 23, 250–256. [Google Scholar] [CrossRef]
- Gariballa, S.; Alkaabi, J.; Yasin, J.; Al Essa, A. Total adiponectin in overweight and obese subjects and its response to visceral fat loss. BMC Endocr. Disord. 2019, 19, 55. [Google Scholar] [CrossRef]
- Gauthier, M.S.; Couturier, K.; Latour, J.G.; Lavoie, J.M. Concurrent exercise prevents high-fat-diet-induced macrovesicular hepatic steatosis. J. Appl. Physiol. 2003, 94, 2127–2134. [Google Scholar] [CrossRef]
- Gauthier, M.S.; Couturier, K.; Charbonneau, A.; Lavoie, J.M. Effects of introducing physical training in the course of a 16-week high-fat diet regimen on hepatic steatosis, adipose tissue fat accumulation, and plasma lipid profile. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.L.; Jeon, J.Y.; Liu, F.F.; Maratos-Flier, E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E586–E594. [Google Scholar] [CrossRef] [PubMed]
- Maesako, M.; Uemura, K.; Kubota, M.; Kuzuya, A.; Sasaki, K.; Hayashida, N.; Asada-Utsugi, M.; Watanabe, K.; Uemura, M.; Kihara, T.; et al. Exercise is more effective than diet control in preventing high fat diet-induced beta-amyloid deposition and memory deficit in amyloid precursor protein transgenic mice. J. Biol. Chem. 2012, 287, 23024–23033. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Jung, S.Y.; Kim, K.; Kim, C.J. Treadmill exercise ameliorates Alzheimer disease-associated memory loss through the Wnt signaling pathway in the streptozotocin-induced diabetic rats. J. Exerc. Rehabil. 2016, 12, 276–283. [Google Scholar] [CrossRef]
- Kim, T.W.; Choi, H.H.; Chung, Y.R. Treadmill exercise alleviates impairment of cognitive function by enhancing hippocampal neuroplasticity in the high-fat diet-induced obese mice. J. Exerc. Rehabil. 2016, 12, 156–162. [Google Scholar] [CrossRef]
- Klein, C.; Jonas, W.; Iggena, D.; Empl, L.; Rivalan, M.; Wiedmer, P.; Spranger, J.; Hellweg, R.; Winter, Y.; Steiner, B. Exercise prevents high-fat diet-induced impairment of flexible memory expression in the water maze and modulates adult hippocampal neurogenesis in mice. Neurobiol. Learn Mem. 2016, 131, 26–35. [Google Scholar] [CrossRef]
- De Senna, P.N.; Bagatini, P.B.; Galland, F.; Bobermin, L.; do Nascimento, P.S.; Nardin, P.; Tramontina, A.C.; Gonccalves, C.A.; Achaval, M.; Xavier, L.L. Physical exercise reverses spatial memory deficit and induces hippocampal astrocyte plasticity in diabetic rats. Brain Res. 2017, 1655, 242–251. [Google Scholar] [CrossRef]
- Shima, T.; Matsui, T.; Jesmin, S.; Okamoto, M.; Soya, M.; Inoue, K.; Liu, Y.; Torres-Aleman, I.; McEwen, B.S.; Soya, H. Moderate exercise ameliorates dysregulated hippocampal glycometabolism and memory function in a rat model of type 2 diabetes. Diabetologia 2017, 60, 597–606. [Google Scholar] [CrossRef]
- Park, H.S.; Cho, H.S.; Kim, T.W. Physical exercise promotes memory capability by enhancing hippocampal mitochondrial functions and inhibiting apoptosis in obesity-induced insulin resistance by high fat diet. Metab. Brain Dis. 2018, 33, 283–292. [Google Scholar] [CrossRef]
- Mehta, B.K.; Singh, K.K.; Banerjee, S. Effect of exercise on type 2 diabetes-associated cognitive impairment in rats. Int. J. Neurosci. 2019, 129, 252–263. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, J.; Liu, Y.; Li, J.; Liu, B.; Li, M.; Lou, S. Aerobic Exercise Improves Synaptic-Related Proteins of Diabetic Rats by Inhibiting FOXO1/NF-kappaB/NLRP3 Inflammatory Signaling Pathway and Ameliorating PI3K/Akt Insulin Signaling Pathway. J. Mol. Neurosci. 2019, 69, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.C.; Grabowska, W.A.; Chun, Y.; Risacher, S.L.; Philip, V.M.; Saykin, A.J.; Rizzo, S.J.S.; Howell, G.R. Exercise prevents obesity-induced cognitive decline and white matter damage in mice. Neurobiol. Aging 2019, 80, 154–172. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Baek, K.W.; Yu, H.S.; Ko, I.G.; Hwang, L.; Park, J.J. High-intensity exercise improves cognitive function and hippocampal brain-derived neurotrophic factor expression in obese mice maintained on high-fat diet. J. Exerc. Rehabil. 2020, 16, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhai, X.; Li, H.; Ji, L. Depression-like behaviors in mice subjected to co-treatment of high-fat diet and corticosterone are ameliorated by AICAR and exercise. J. Affect Disord. 2014, 156, 171–177. [Google Scholar] [CrossRef]
- Cordeira, J.; Monahan, D. Voluntary wheel running reduces weight gain in mice by decreasing high-fat food consumption. Physiol. Behav. 2019, 207, 1–6. [Google Scholar] [CrossRef]
- Liang, N.C.; Bello, N.T.; Moran, T.H. Wheel running reduces high-fat diet intake, preference and mu-opioid agonist stimulated intake. Behav. Brain Res. 2015, 284, 1–10. [Google Scholar] [CrossRef]
- Moody, L.; Liang, J.; Choi, P.P.; Moran, T.H.; Liang, N.C. Wheel running decreases palatable diet preference in Sprague-Dawley rats. Physiol. Behav. 2015, 150, 53–63. [Google Scholar] [CrossRef][Green Version]
- Woo, J.; Shin, K.O.; Park, S.Y.; Jang, K.S.; Kang, S. Effects of exercise and diet change on cognition function and synaptic plasticity in high fat diet induced obese rats. Lipids Health Dis. 2013, 12, 144. [Google Scholar] [CrossRef]
- Cheng, J.; Chen, L.; Han, S.; Qin, L.; Chen, N.; Wan, Z. Treadmill Running and Rutin Reverse High Fat Diet Induced Cognitive Impairment in Diet Induced Obese Mice. J. Nutr. Health Aging 2016, 20, 503–508. [Google Scholar] [CrossRef]
- Spencer, S.J.; D’Angelo, H.; Soch, A.; Watkins, L.R.; Maier, S.F.; Barrientos, R.M. High-fat diet and aging interact to produce neuroinflammation and impair hippocampal- and amygdalar-dependent memory. Neurobiol. Aging 2017, 58, 88–101. [Google Scholar] [CrossRef]
- De Senna, P.N.; Ilha, J.; Baptista, P.P.; do Nascimento, P.S.; Leite, M.C.; Paim, M.F.; Gonccalves, C.A.; Achaval, M.; Xavier, L.L. Effects of physical exercise on spatial memory and astroglial alterations in the hippocampus of diabetic rats. Metab. Brain Dis. 2011, 26, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Jung, S.Y.; Kim, T.W.; Lee, K.S.; Kim, K. Treadmill exercise decreases incidence of Alzheimer’s disease by suppressing glycogen synthase kinase-3beta expression in streptozotocin-induced diabetic rats. J. Exerc. Rehabil. 2015, 11, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Masri, B.; Daviaud, D.; Gesta, S.; Guigne, C.; Mazzucotelli, A.; Castan-Laurell, I.; Tack, I.; Knibiehler, B.; Carpene, C.; et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 2005, 146, 1764–1771. [Google Scholar] [CrossRef]
- Vinel, C.; Lukjanenko, L.; Batut, A.; Deleruyelle, S.; Pradere, J.P.; Le Gonidec, S.; Dortignac, A.; Geoffre, N.; Pereira, O.; Karaz, S.; et al. The exerkine apelin reverses age-associated sarcopenia. Nat. Med. 2018, 24, 1360–1371. [Google Scholar] [CrossRef]
- Yamamoto, T.; Habata, Y.; Matsumoto, Y.; Yasuhara, Y.; Hashimoto, T.; Hamajyo, H.; Anayama, H.; Fujii, R.; Fuse, H.; Shintani, Y.; et al. Apelin-transgenic mice exhibit a resistance against diet-induced obesity by increasing vascular mass and mitochondrial biogenesis in skeletal muscle. Biochim. Biophys. Acta 2011, 1810, 853–862. [Google Scholar] [CrossRef]
- Xu, J.; Jackson, C.W.; Khoury, N.; Escobar, I.; Perez-Pinzon, M.A. Brain SIRT1 Mediates Metabolic Homeostasis and Neuroprotection. Front. Endocrinol. 2018, 9, 702. [Google Scholar] [CrossRef]
- Fan, J.; Guang, H.; Zhang, H.; Chen, D.; Ding, L.; Fan, X.; Gan, Z.; Wang, Y.; Mao, S. SIRT1 Mediates Apelin-13 in Ameliorating Chronic Normobaric Hypoxia-induced Anxiety-like Behavior by Suppressing NF-kappaB Pathway in Mice Hippocampus. Neuroscience 2018, 381, 22–34. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Ying, Z. Differential effects of exercise and dietary docosahexaenoic acid on molecular systems associated with control of allostasis in the hypothalamus and hippocampus. Neuroscience 2010, 168, 130–137. [Google Scholar] [CrossRef]
- Lin, J.Y.; Kuo, W.W.; Baskaran, R.; Kuo, C.H.; Chen, Y.A.; Chen, W.S.; Ho, T.; Day, C.H.; Mahalakshmi, B.; Huang, C. Swimming exercise stimulates IGF1/ PI3K/Akt and AMPK/SIRT1/PGC1alpha survival signaling to suppress apoptosis and inflammation in aging hippocampus. Aging 2020, 12, 6852–6864. [Google Scholar] [CrossRef]
- De Mota, N.; Lenkei, Z.; Llorens-Cortes, C. Cloning, pharmacological characterization and brain distribution of the rat apelin receptor. Neuroendocrinology 2000, 72, 400–407. [Google Scholar] [CrossRef]
- Zhou, S.; Guo, X.; Chen, S.; Xu, Z.; Duan, W.; Zeng, B. Apelin-13 regulates LPS-induced N9 microglia polarization involving STAT3 signaling pathway. Neuropeptides 2019, 76, 101938. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Yan, Z.Y.; Lan, F.J.; Dong, Y.Q.; Xiong, Y. Suppression of NLRP3 inflammasome attenuates stress-induced depression-like behavior in NLGN3-deficient mice. Biochem. Biophys. Res. Commun. 2018, 501, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Wei, M.; Yuan, X.; Liu, Z.; Wang, X.; Zhang, D.; Luo, L.; Wu, J.; Guo, W.; Qin, Z. TIGAR regulates mitochondrial functions through SIRT1-PGC1alpha pathway and translocation of TIGAR into mitochondria in skeletal muscle. FASEB J. 2019, 33, 6082–6098. [Google Scholar] [CrossRef] [PubMed]
- Vila, L.; Roca, C.; Elias, I.; Casellas, A.; Lage, R.; Franckhauser, S.; Bosch, F. AAV-mediated Sirt1 overexpression in skeletal muscle activates oxidative capacity but does not prevent insulin resistance. Mol. Ther. Methods Clin. Dev. 2016, 5, 16072. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.J.; Yu, S.P.; Zhang, L.; Wei, L. Neuroprotective effect of the endogenous neural peptide apelin in cultured mouse cortical neurons. Exp. Cell Res. 2010, 316, 1773–1783. [Google Scholar] [CrossRef]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef]
- Xiong, X.Q.; Chen, D.; Sun, H.J.; Ding, L.; Wang, J.J.; Chen, Q.; Li, Y.; Zhou, Y.; Han, Y.; Zhang, F.; et al. FNDC5 overexpression and irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis in obesity. Biochim. Biophys. Acta. 2015, 1852, 1867–1875. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.; Greenberg, M.; Spiegelman, B. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef]
- Mohammadi, S.; Oryan, S.; Komaki, A.; Eidi, A.; Zarei, M. Effects of Hippocampal Microinjection of Irisin, an Exercise-Induced Myokine, on Spatial and Passive Avoidance Learning and Memory in Male Rats. Int. J. Pept. Res. Ther. 2020, 26, 357–367. [Google Scholar] [CrossRef]
- Mohammadi, S.; Oryan, S.; Komaki, A.; Eidi, A.; Zarei, M. Effects of intra-dentate gyrus microinjection of myokine irisin on long-term potentiation in male rats. Arq. Neuropsiquiatr. 2019, 77, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yan, S.; Luo, L.; Yang, L. Irisin regulates the expression of BDNF and glycometabolism in diabetic rats. Mol. Med. Rep. 2019, 19, 1074–1082. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonccalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef]
- Siteneski, A.; Olescowicz, G.; Pazini, F.L.; Camargo, A.; Fraga, D.B.; Brocardo, P.S.; Gil-Mohapel, J.; Cunha, M.P.; Rodrigues, A.L.S. Antidepressant-like and pro-neurogenic effects of physical exercise: The putative role of FNDC5/irisin pathway. J. Neural Transm. 2020, 127, 355–370. [Google Scholar] [CrossRef]
- Lev-Vachnish, Y.; Cadury, S.; Rotter-Maskowitz, A.; Feldman, N.; Roichman, A.; Illouz, T.; Varvak, A.; Nicola, R.; Madar, R.; Okun, E. L-Lactate Promotes Adult Hippocampal Neurogenesis. Front. Neurosci. 2019, 13, 403. [Google Scholar] [CrossRef]
- Pierre, K.; Pellerin, L. Monocarboxylate transporters in the central nervous system: Distribution, regulation and function. J Neurochem. 2005, 94, 1–14. [Google Scholar] [CrossRef]
- EL, L.J.; Selfridge, J.E.; Burns, J.M.; Swerdlow, R.H. Lactate administration reproduces specific brain and liver exercise-related changes. J. Neurochem. 2013, 127, 91–100. [Google Scholar]
- Hadzic, A.; Nguyen, T.D.; Hosoyamada, M.; Tomioka, N.H.; Bergersen, L.H.; Storm-Mathisen, J.; Morland, C. The Lactate Receptor HCA1 Is Present in the Choroid Plexus, the Tela Choroidea, and the Neuroepithelial Lining of the Dorsal Part of the Third Ventricle. Int. J. Mol. Sci. 2020, 21, 6457. [Google Scholar] [CrossRef]
- Bozzo, L.; Puyal, J.; Chatton, J.Y. Lactate modulates the activity of primary cortical neurons through a receptor-mediated pathway. PLoS ONE 2013, 8, e71721. [Google Scholar] [CrossRef] [PubMed]
- Morland, C.; Andersson, K.A.; Haugen, O.P.; Hadzic, A.; Kleppa, L.; Gille, A.; Rinholm, J.E.; Palibrk, V.; Diget, E.H.; Kennedy, L.H.; et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat. Commun. 2017, 8, 15557. [Google Scholar] [CrossRef] [PubMed]
- Carrard, A.; Elsayed, M.; Margineanu, M.; Boury-Jamot, B.; Fragniere, L.; Meylan, E.M.; Petit, J.M.; Fiumelli, H.; Magistretti Pierre, J.; Martin, J.L. Peripheral administration of lactate produces antidepressant-like effects. Mol. Psychiatry 2018, 23, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, Z.; Castano, O.; Castells, A.A.; Mateos-Timoneda, M.A.; Planell, J.A.; Engel, E.; Alcantara, S. Neurogenesis and vascularization of the damaged brain using a lactate-releasing biomimetic scaffold. Biomaterials 2014, 35, 4769–4781. [Google Scholar] [CrossRef]
- Herrera-Lopez, G.; Griego, E.; Galvan, E.J. Lactate induces synapse-specific potentiation on CA3 pyramidal cells of rat hippocampus. PLoS ONE 2020, 15, e0242309. [Google Scholar] [CrossRef]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef]
- Cali, C.; Tauffenberger, A.; Magistretti, P. The Strategic Location of Glycogen and Lactate: From Body Energy Reserve to Brain Plasticity. Front. Cell. Neurosci. 2019, 13, 82. [Google Scholar] [CrossRef]
- Mason, S. Lactate Shuttles in Neuroenergetics-Homeostasis, Allostasis and Beyond. Front. Neurosci. 2017, 11, 43. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Allaman, I. Lactate in the brain: From metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef]
- Barros, L.F.; Weber, B. CrossTalk proposal: An important astrocyte-to-neuron lactate shuttle couples neuronal activity to glucose utilisation in the brain. J. Physiol. 2018, 596, 347–350. [Google Scholar] [CrossRef]
- Powell, C.L.; Davidson, A.R.; Brown, A.M. Universal Glia to Neurone Lactate Transfer in the Nervous System: Physiological Functions and Pathological Consequences. Biosensors 2020, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Pierre, K.; Magistretti, P.J.; Pellerin, L. MCT2 is a major neuronal monocarboxylate transporter in the adult mouse brain. J. Cereb. Blood Flow Metab. 2002, 22, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.J.; Michel, C.B.; Marra, V.; Smalley, J.L.; Hennig, M.H.; Graham, B.P.; Forsythe, I.D. Glucose and lactate as metabolic constraints on presynaptic transmission at an excitatory synapse. J. Physiol. 2018, 596, 1699–1721. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8, 184. [Google Scholar] [CrossRef]
- Allen, A.; Messier, C. Plastic changes in the astrocyte GLUT1 glucose transporter and beta-tubulin microtubule protein following voluntary exercise in mice. Behav. Brain Res. 2013, 240, 95–102. [Google Scholar] [CrossRef]
- Swanson, R.A.; Benington, J.H. Astrocyte glucose metabolism under normal and pathological conditions in vitro. Dev. Neurosci. 1996, 18, 515–521. [Google Scholar] [CrossRef]
- Diaz-Garcia, C.M.; Yellen, G. Neurons rely on glucose rather than astrocytic lactate during stimulation. J. Neurosci. Res. 2019, 97, 883–889. [Google Scholar] [CrossRef]
- Netzahualcoyotzi, C.; Pellerin, L. Neuronal and astroglial monocarboxylate transporters play key but distinct roles in hippocampus-dependent learning and memory formation. Prog. Neurobiol. 2020, 194, 101888. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. beta-Hydroxybutyrate: A Signaling Metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef]
- Laffel, L. Ketone bodies: A review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab. Res. Rev. 1999, 15, 412–426. [Google Scholar] [CrossRef]
- Marosi, K.; Kim, S.W.; Moehl, K.; Scheibye-Knudsen, M.; Cheng, A.; Cutler, R.; Camandola, S.; Mattson, M.P. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J. Neurochem. 2016, 139, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.P.; Li, S.N.; Wang, J.F.; Li, Y.; Xie, S.S.; Xue, W.J.; Liu, H.; Huang, B.; Lv, Q.; Lei, L.; et al. BHBA suppresses LPS-induced inflammation in BV-2 cells by inhibiting NF-kappaB activation. Mediat. Inflamm. 2014, 2014, 983401. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Zhu, W.; Lu, M.; Ni, B.; Yang, J. D-beta-hydroxybutyrate promotes functional recovery and relieves pain hypersensitivity in mice with spinal cord injury. Br. J. Pharmacol. 2017, 174, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Yamanashi, T.; Iwata, M.; Kamiya, N.; Tsunetomi, K.; Kajitani, N.; Wada, N.; Iitsuka, T.; Yamauchi, T.; Miura, A.; Pu, S.; et al. Beta-hydroxybutyrate, an endogenic NLRP3 inflammasome inhibitor, attenuates stress-induced behavioral and inflammatory responses. Sci. Rep. 2017, 7, 7677. [Google Scholar] [CrossRef]
- Zou, X.H.; Li, H.M.; Wang, S.; Leski, M.; Yao, Y.C.; Yang, X.D.; Huang, Q.; Chen, G. The effect of 3-hydroxybutyrate methyl ester on learning and memory in mice. Biomaterials 2009, 30, 1532–1541. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Q.; Li, S.; Lu, X.; Zhao, Y.; Guan, J.S.; Chen, J.; Wu, Q.; Chen, G. 3-Hydroxybutyrate methyl ester as a potential drug against Alzheimer’s disease via mitochondria protection mechanism. Biomaterials 2013, 34, 7552–7562. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body beta-hydroxybutyrate. Elife 2016, 5, e15092. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.J.; Knight, N.S.; Cole, M.A.; Cochlin, L.E.; Carter, E.; Tchabanenko, K.; Pichulik, T.; Gulston, M.K.; Atherton, H.J.; Schroeder, M.A.; et al. Novel ketone diet enhances physical and cognitive performance. FASEB J. 2016, 30, 4021–4032. [Google Scholar] [CrossRef] [PubMed]
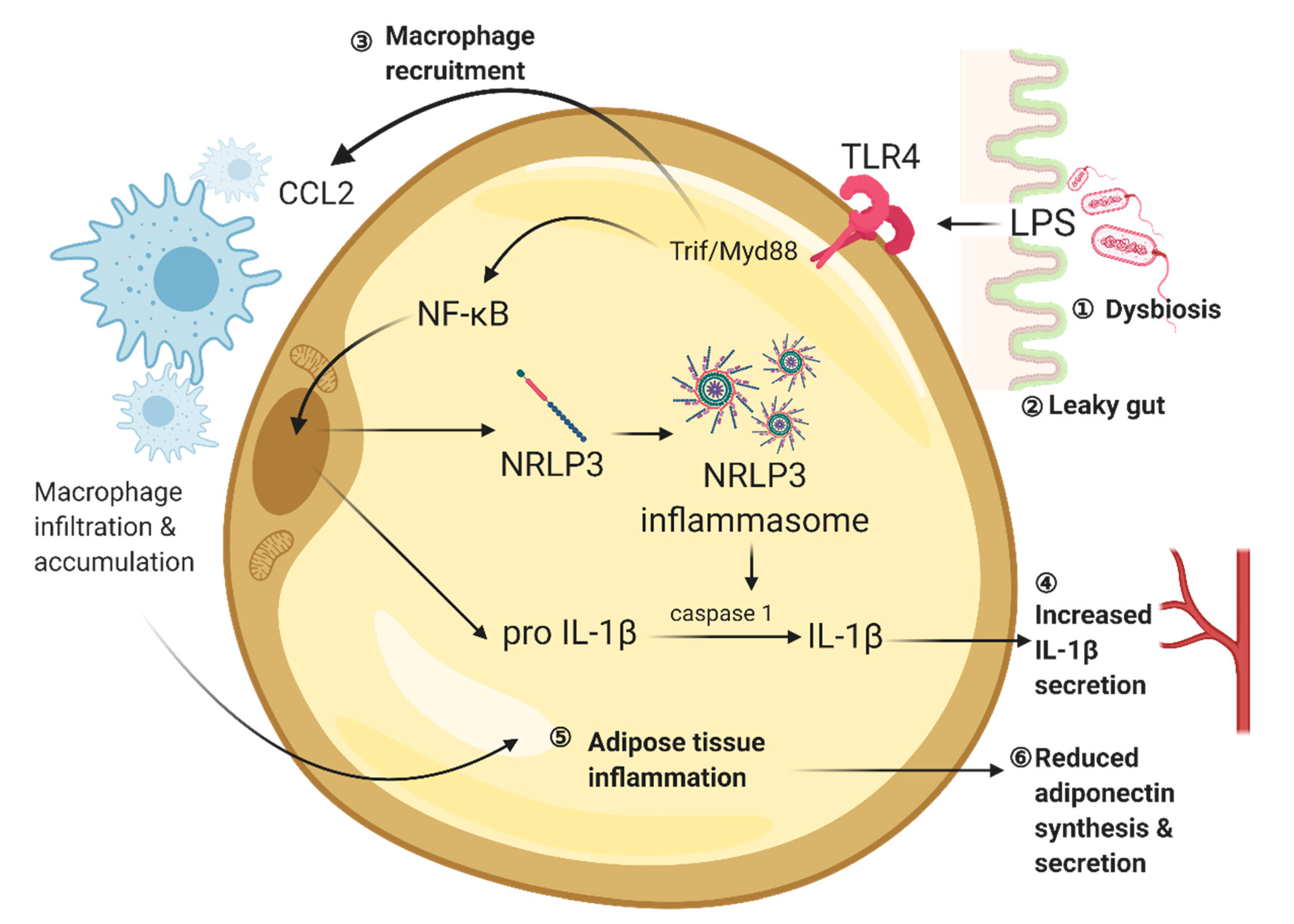
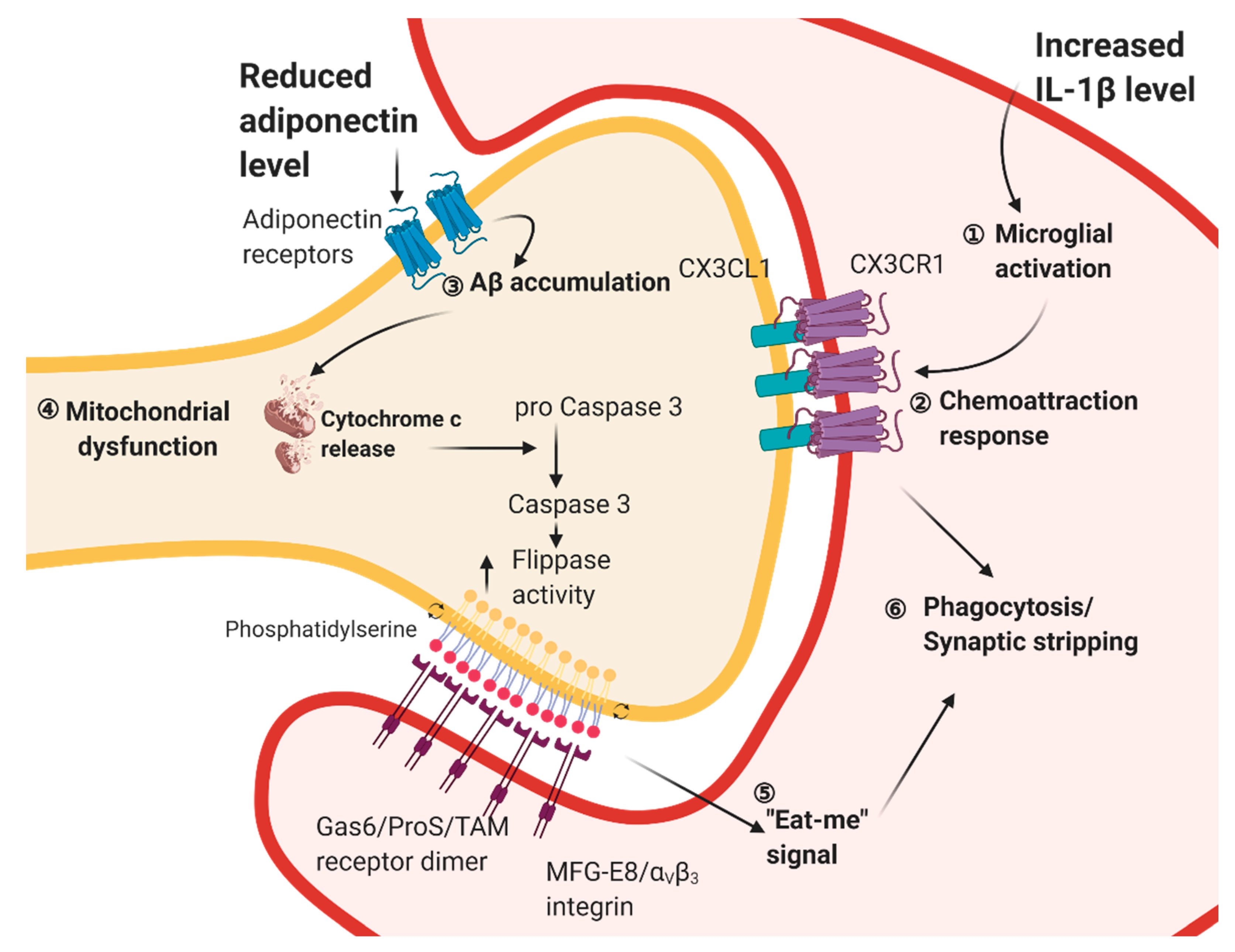
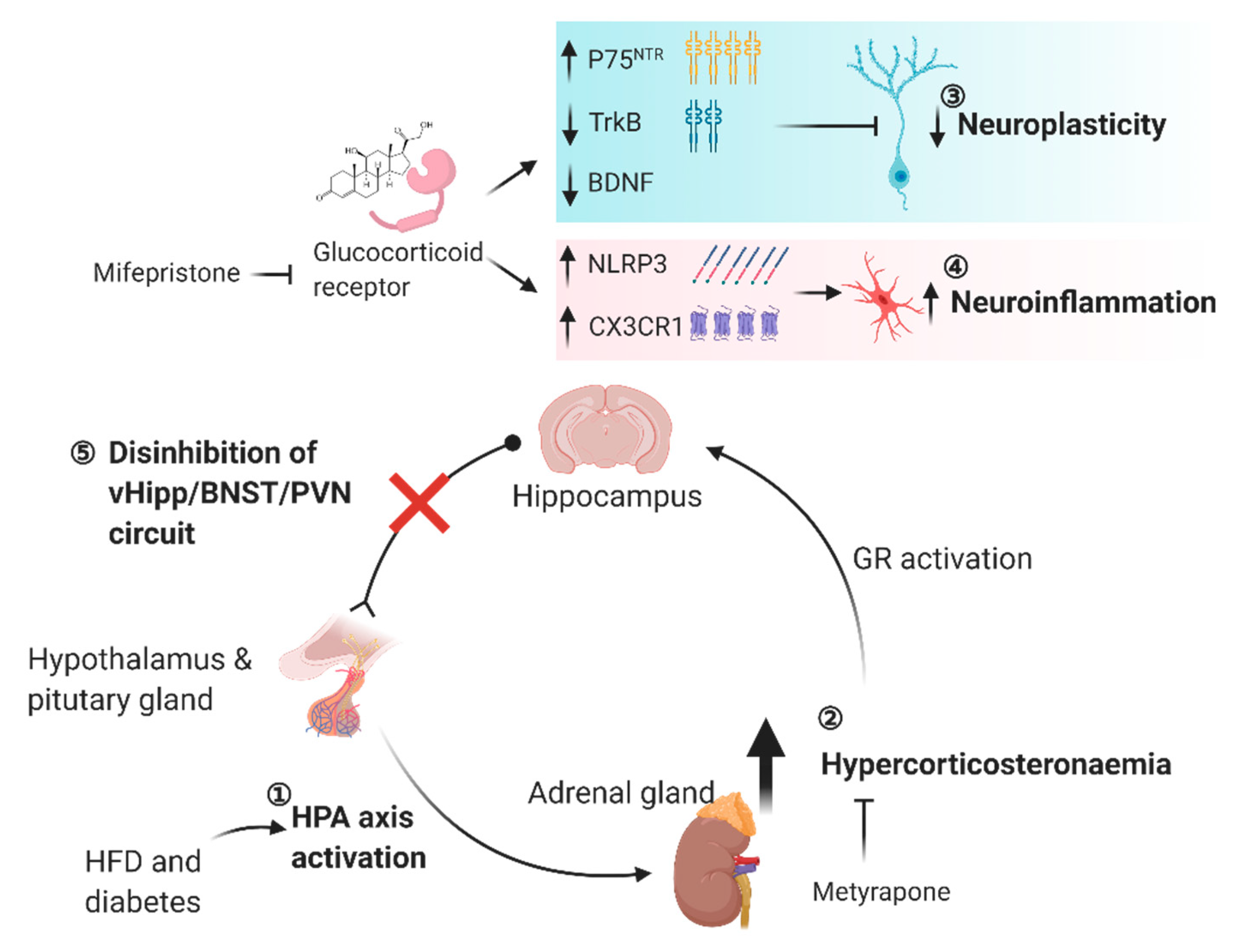

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.H.-y.; Yau, S.-y. From Obesity to Hippocampal Neurodegeneration: Pathogenesis and Non-Pharmacological Interventions. Int. J. Mol. Sci. 2021, 22, 201. https://doi.org/10.3390/ijms22010201
Lee TH-y, Yau S-y. From Obesity to Hippocampal Neurodegeneration: Pathogenesis and Non-Pharmacological Interventions. International Journal of Molecular Sciences. 2021; 22(1):201. https://doi.org/10.3390/ijms22010201
Chicago/Turabian StyleLee, Thomas Ho-yin, and Suk-yu Yau. 2021. "From Obesity to Hippocampal Neurodegeneration: Pathogenesis and Non-Pharmacological Interventions" International Journal of Molecular Sciences 22, no. 1: 201. https://doi.org/10.3390/ijms22010201
APA StyleLee, T. H.-y., & Yau, S.-y. (2021). From Obesity to Hippocampal Neurodegeneration: Pathogenesis and Non-Pharmacological Interventions. International Journal of Molecular Sciences, 22(1), 201. https://doi.org/10.3390/ijms22010201




