Rhus coriaria L. (Sumac) Demonstrates Oncostatic Activity in the Therapeutic and Preventive Model of Breast Carcinoma
Abstract
1. Introduction
2. Results
2.1. Plant Secondary Metabolites in R. coriaria Methanolic Extract
2.2. 4T1 Therapeutic Model in Mice
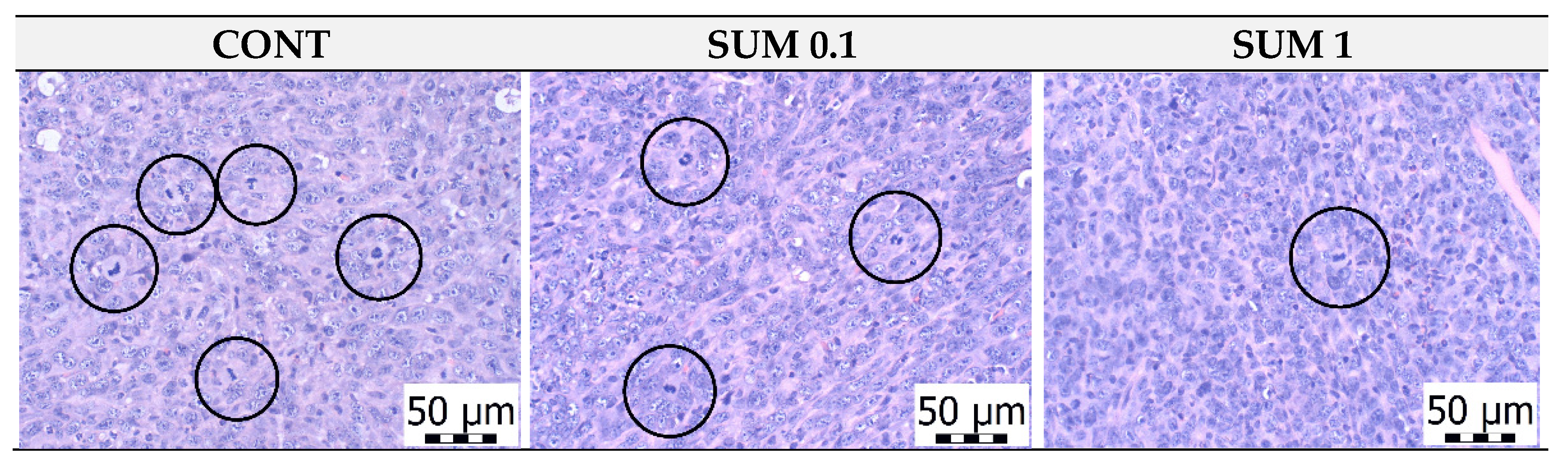
2.3. Chemoprevention Model-Parameters of Rat Mammary Carcinogenesis and Histopathology of Tumors
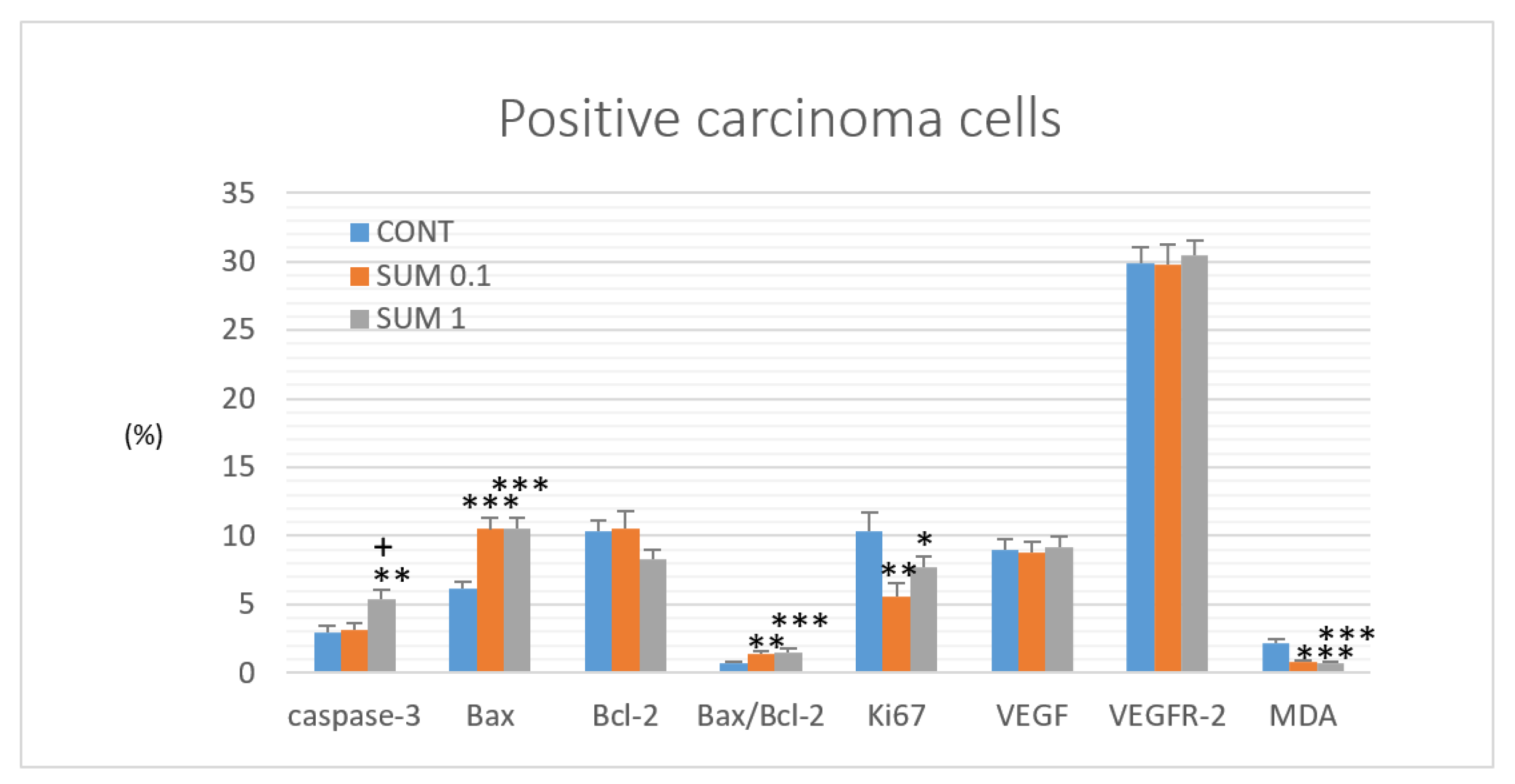
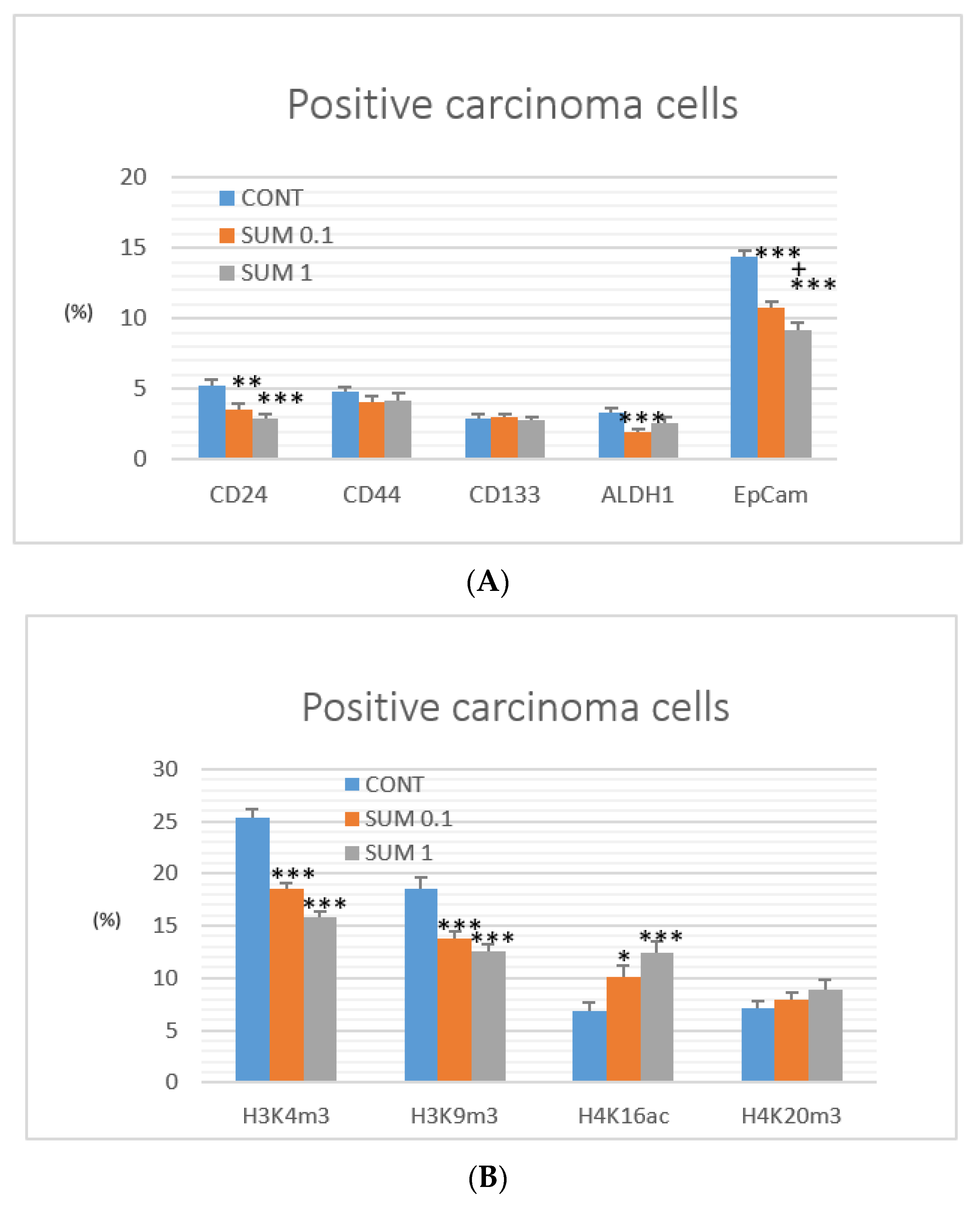
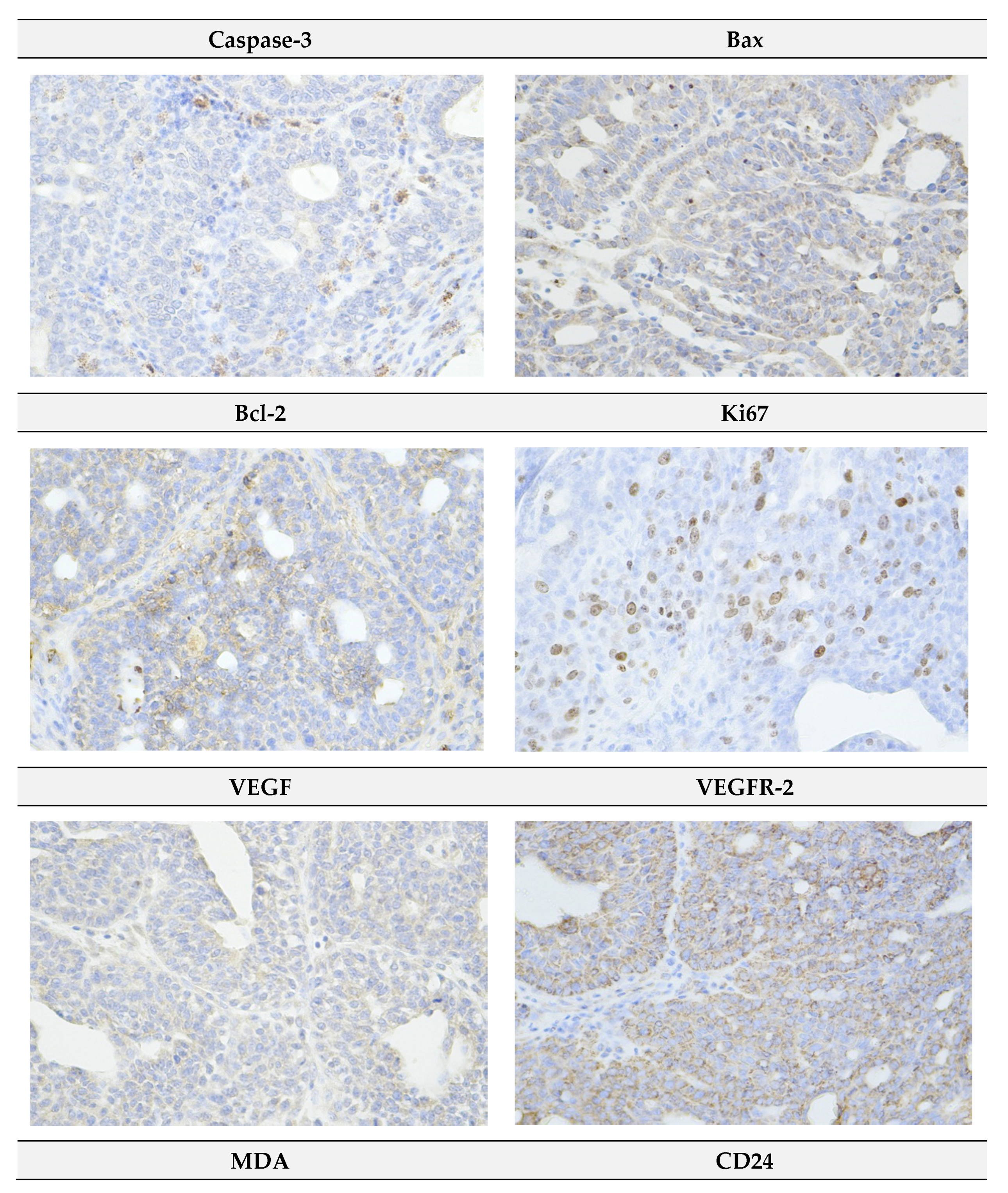
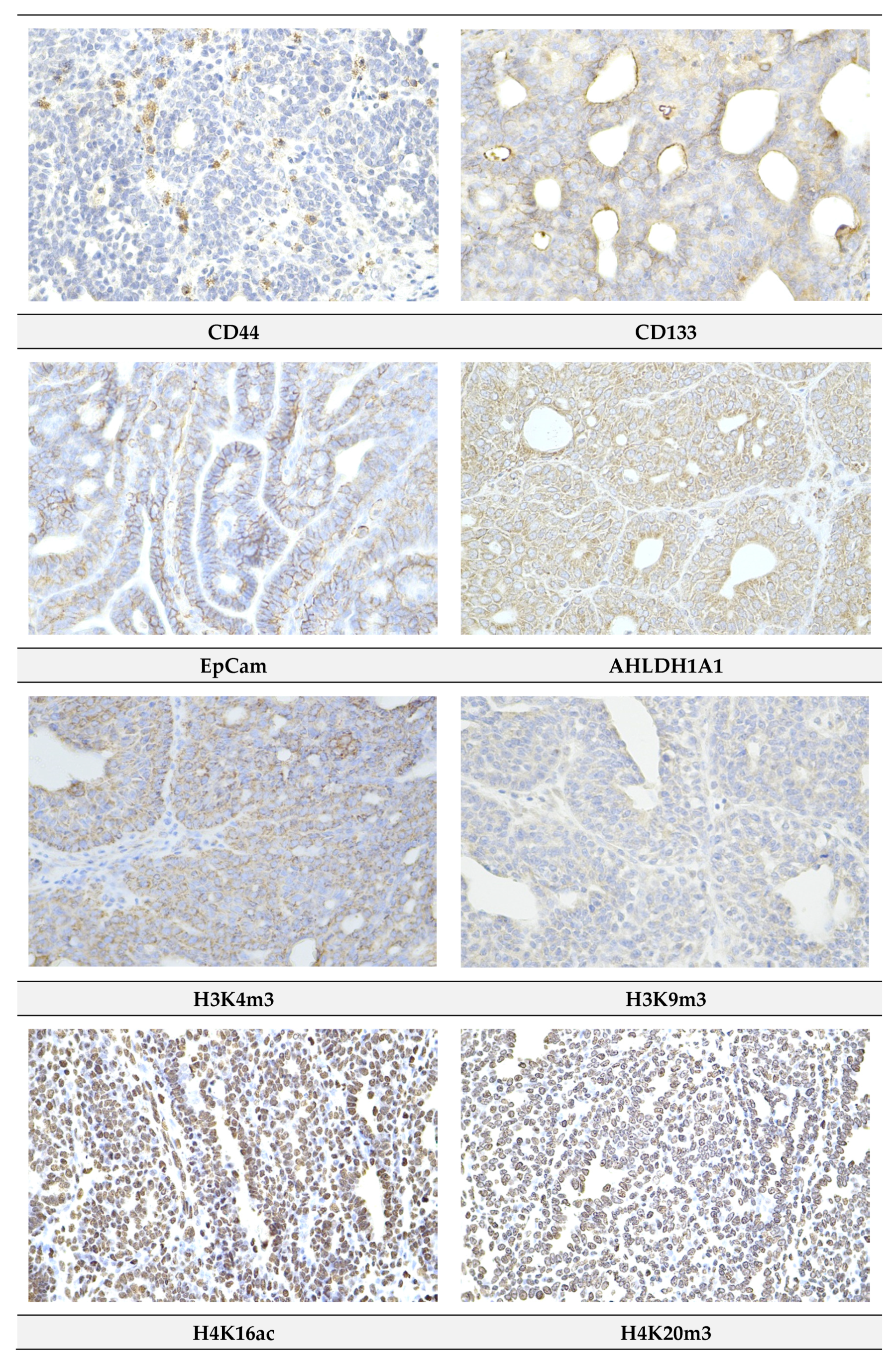
2.4. Immunohistochemistry of Rat Tumors
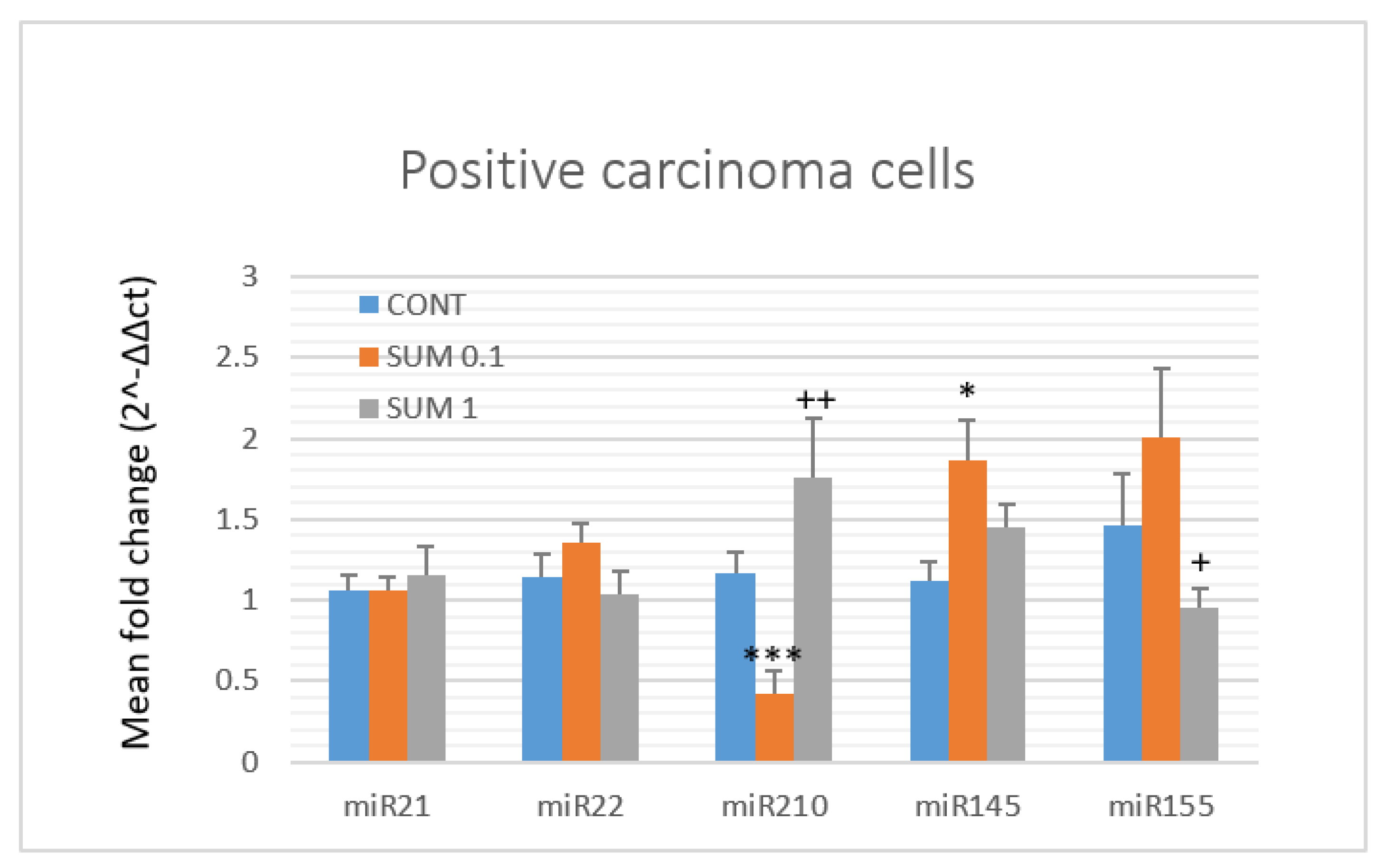
2.5. miRNA Expression
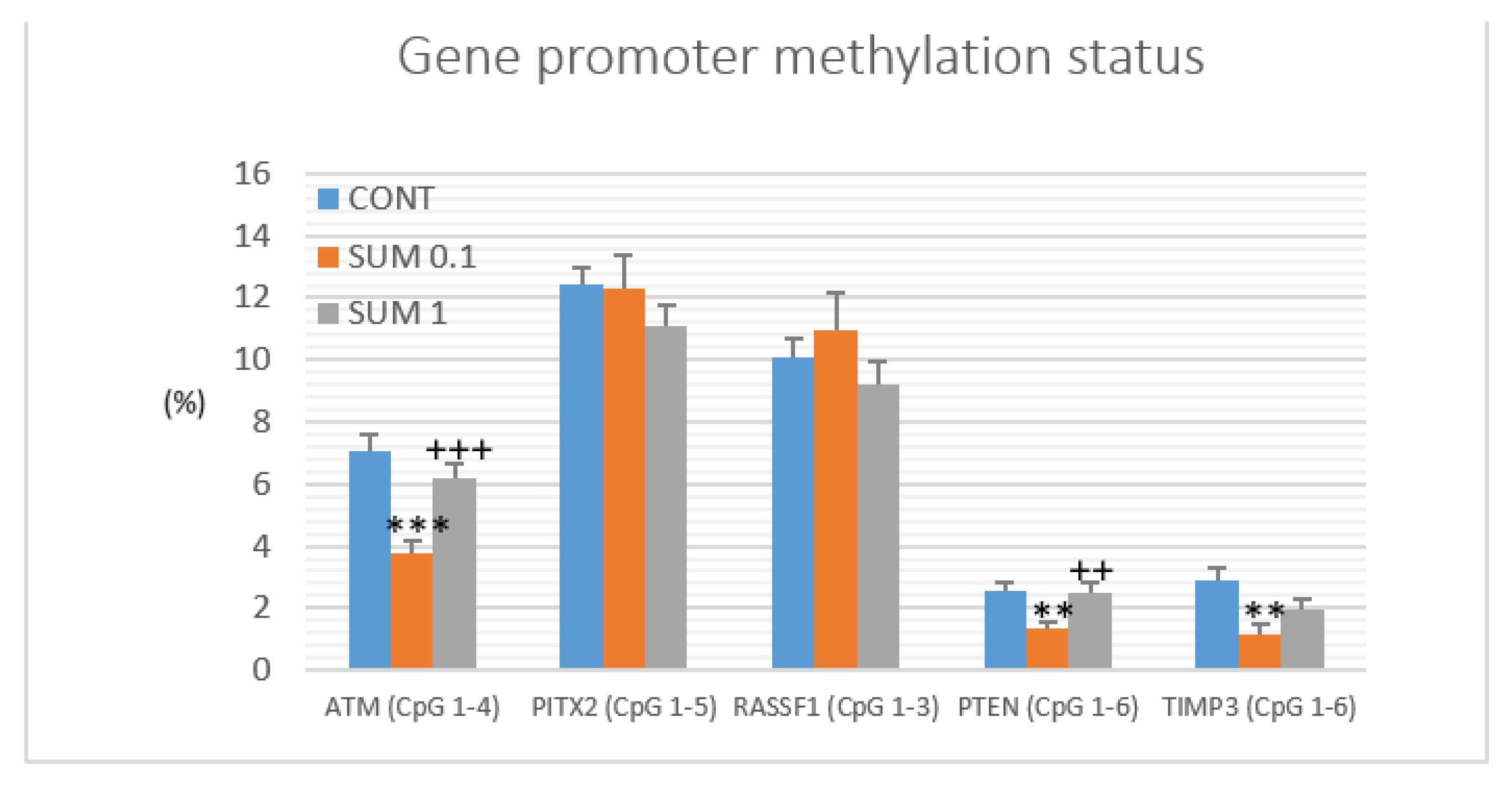
2.6. Gene Promoter Methylations Status
2.7. Physiological In Vivo Effects
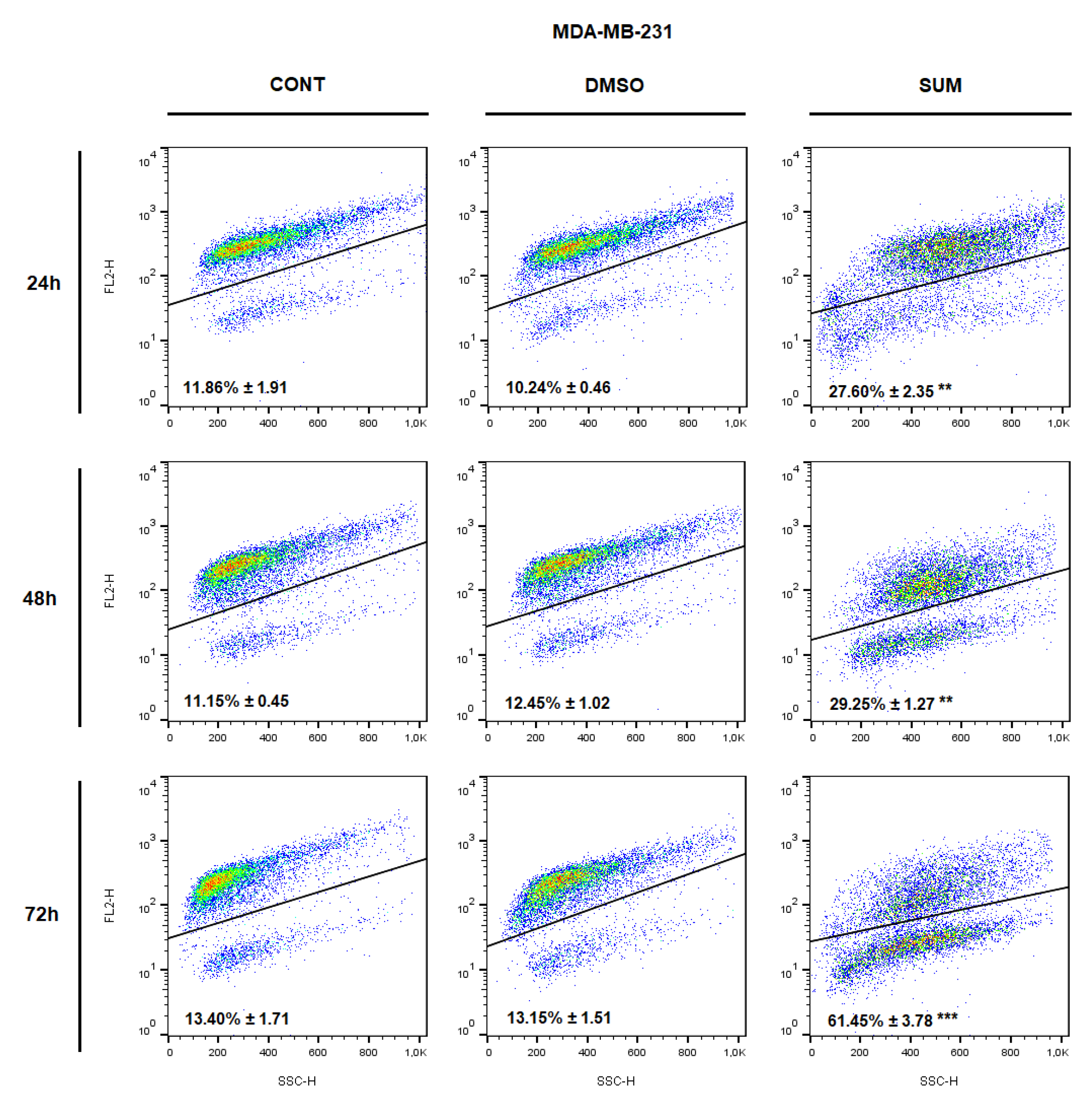
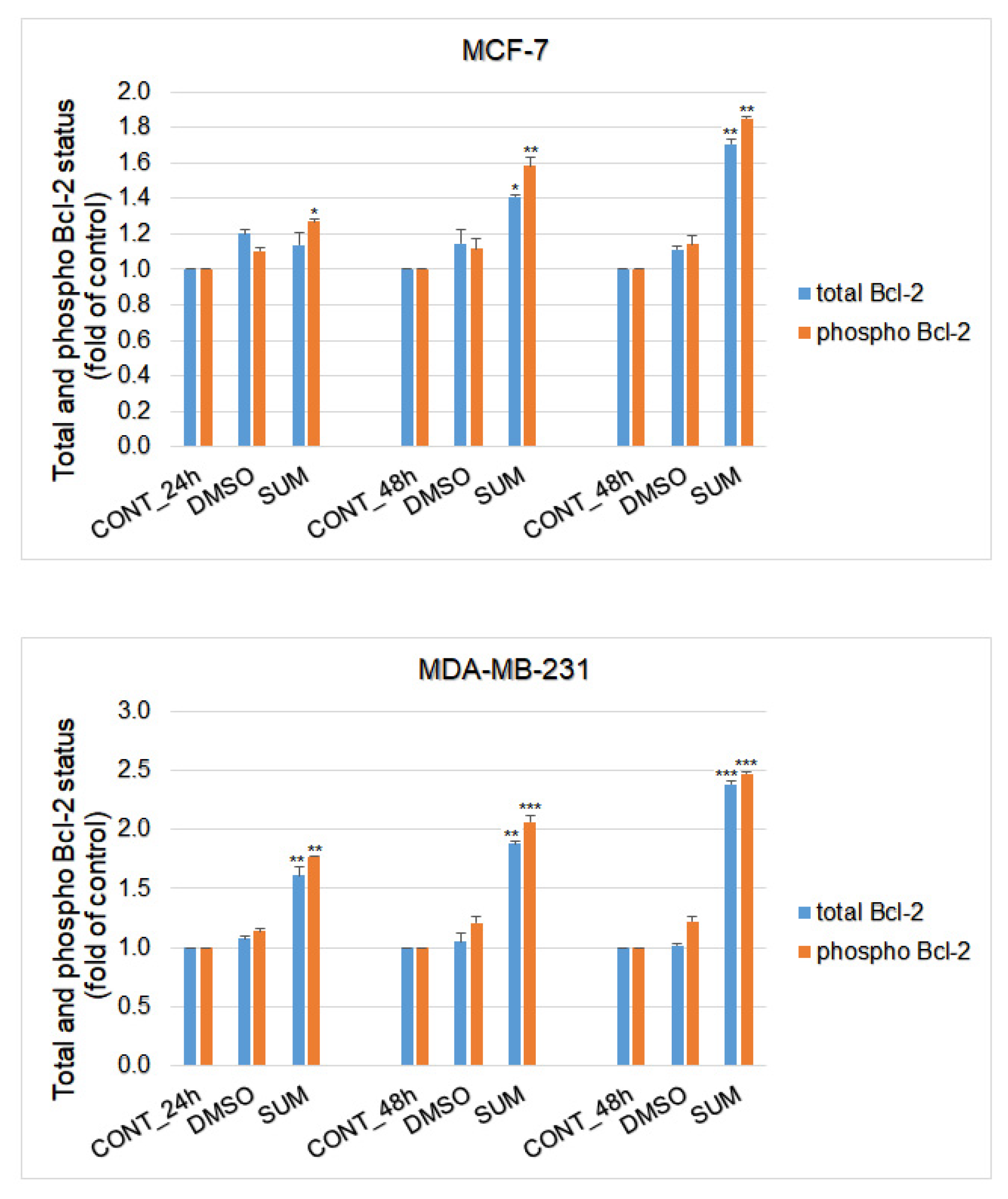
2.8. In Vitro Assessment on MCF-7 and MDA-MB-231 Cells
3. Discussion
4. Materials and Methods
4.1. Animal Models
4.2. Histopathological and Immunohistochemical Evaluations of Rat and Mouse Tumors
4.3. Analysis of miRNA Expression
4.4. DNA Isolation and Bisulfite Conversion
4.5. Determination of Methylated CpG Islands in the Promoter Regions (Pyrosequencing)
4.6. Cell Lines, Cell Cultures, and Experimental Design
4.7. Cytotoxicity Assay
4.8. Flow Cytometry Analyses Protocol
4.9. The Examinations of Plant Secondary Metabolites in Sumac Extract
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heer, E.; Harper, A.; Escandor, N.; Sung, H.; McCormack, V.; Fidler-Benaoudia, M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health 2020, 8, e1027–e1037. [Google Scholar] [CrossRef]
- Kapinova, A.; Kubatka, P.; Golubnitschaja, O.; Kello, M.; Zubor, P.; Solar, P.; Pec, M. Dietary phytochemicals in breast cancer research: Anticancer effects and potential utility for effective chemoprevention. Environ. Health Prev. Med. 2018, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- Uramova, S.; Kubatka, P.; Dankova, Z.; Kapinova, A.; Zolakova, B.; Samec, M.; Zubor, P.; Zulli, A.; Valentova, V.; Kwon, T.K.; et al. Plant natural modulators in breast cancer prevention: Status quo and future perspectives reinforced by predictive, preventive, and personalized medical approach. EPMA J. 2018, 9, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Liskova, A.; Koklesova, L.; Samec, M.; Varghese, E.; Abotaleb, M.; Samuel, S.M.; Smejkal, K.; Biringer, K.; Petras, M.; Blahutova, D.; et al. Implications of flavonoids as potential modulators of cancer neovascularity. J. Cancer Res. Clin. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Koklesova, L.; Liskova, A.; Samec, M.; Buhrmann, C.; Samuel, S.M.; Varghese, E.; Ashrafizadeh, M.; Najafi, M.; Shakibaei, M.; Büsselberg, D.; et al. Carotenoids in Cancer Apoptosis—The Road from Bench to Bedside and Back. Cancers (Basel) 2020, 12, 2425. [Google Scholar] [CrossRef] [PubMed]
- Samec, M.; Liskova, A.; Koklesova, L.; Samuel, S.M.; Zhai, K.; Buhrmann, C.; Varghese, E.; Abotaleb, M.; Qaradakhi, T.; Zulli, A.; et al. Flavonoids against the Warburg phenotype—Concepts of predictive, preventive and personalised medicine to cut the Gordian knot of cancer cell metabolism. EPMA J. 2020, 11, 377–398. [Google Scholar] [CrossRef] [PubMed]
- Koklesova, L.; Liskova, A.; Samec, M.; Qaradakhi, T.; Zulli, A.; Smejkal, K.; Kajo, K.; Jakubikova, J.; Behzadi, P.; Pec, M.; et al. Genoprotective activities of plant natural substances in cancer and chemopreventive strategies in the context of 3P medicine. EPMA J. 2020, 11, 261–287. [Google Scholar] [CrossRef]
- Liskova, A.; Koklesova, L.; Samec, M.; Smejkal, K.; Samuel, S.M.; Varghese, E.; Abotaleb, M.; Biringer, K.; Kudela, E.; Danko, J.; et al. Flavonoids in Cancer Metastasis. Cancers 2020, 12, 1498. [Google Scholar] [CrossRef]
- Vergara, D.; Simeone, P.; Bettini, S.; Tinelli, A.; Valli, L.; Storelli, C.; Leo, S.; Santino, A.; Maffia, M. Antitumor activity of the dietary diterpene carnosol against a panel of human cancer cell lines. Food Funct. 2014, 5, 1261–1269. [Google Scholar] [CrossRef]
- Mao, Y.; Hao, J.; Jin, Z.-Q.; Niu, Y.-Y.; Yang, X.; Liu, D.; Cao, R.; Wu, X.-Z. Network pharmacology-based and clinically relevant prediction of the active ingredients and potential targets of Chinese herbs in metastatic breast cancer patients. Oncotarget 2017, 8, 27007–27021. [Google Scholar] [CrossRef]
- Pourzand, A.; Tajaddini, A.; Pirouzpanah, S.; Asghari-Jafarabadi, M.; Samadi, N.; Ostadrahimi, A.-R.; Sanaat, Z. Associations between Dietary Allium Vegetables and Risk of Breast Cancer: A Hospital-Based Matched Case-Control Study. J. Breast Cancer 2016, 19, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lv, K. Cruciferous vegetables intake is inversely associated with risk of breast cancer: A meta-analysis. Breast 2013, 22, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Chiuve, S.E.; Willett, W.C.; Hankinson, S.E.; Hu, F.B.; Holmes, M.D. Intake of specific fruits and vegetables in relation to risk of estrogen receptor-negative breast cancer among postmenopausal women. Breast Cancer Res. Treat. 2013, 138, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef]
- El Hasasna, H.; Saleh, A.; Samri, H.A.; Athamneh, K.; Attoub, S.; Arafat, K.; Benhalilou, N.; Alyan, S.; Viallet, J.; Dhaheri, Y.A.; et al. Rhus coriaria suppresses angiogenesis, metastasis and tumor growth of breast cancer through inhibition of STAT3, NFκB and nitric oxide pathways. Sci. Rep. 2016, 6, 21144. [Google Scholar] [CrossRef] [PubMed]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC-DAD-ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef]
- El Hasasna, H.; Athamneh, K.; Al Samri, H.; Karuvantevida, N.; Al Dhaheri, Y.; Hisaindee, S.; Ramadan, G.; Al Tamimi, N.; AbuQamar, S.; Eid, A.; et al. Rhus coriaria induces senescence and autophagic cell death in breast cancer cells through a mechanism involving p38 and ERK1/2 activation. Sci. Rep. 2015, 5, 13013. [Google Scholar] [CrossRef]
- Mirian, M.; Behrooeian, M.; Ghanadian, M.; Dana, N.; Sadeghi-Aliabadi, H. Cytotoxicity and antiangiogenic effects of Rhus coriaria, Pistacia vera and Pistacia khinjuk oleoresin methanol extracts. Res. Pharm. Sci. 2015, 10, 233–240. [Google Scholar]
- Ghorbani, P.; Namvar, F.; Homayouni-Tabrizi, M.; Soltani, M.; Karimi, E.; Yaghmaei, P. Apoptotic efficacy and antiproliferative potential of silver nanoparticles synthesised from aqueous extract of sumac (Rhus coriaria L.). IET Nanobiotechnol. 2018, 12, 600–603. [Google Scholar] [CrossRef]
- Athamneh, K.; Hasasna, H.E.; Samri, H.A.; Attoub, S.; Arafat, K.; Benhalilou, N.; Rashedi, A.A.; Dhaheri, Y.A.; AbuQamar, S.; Eid, A.; et al. Rhus coriaria increases protein ubiquitination, proteasomal degradation and triggers non-canonical Beclin-1-independent autophagy and apoptotic cell death in colon cancer cells. Sci. Rep. 2017, 7, 11633. [Google Scholar] [CrossRef]
- Kubatka, P.; Kello, M.; Kajo, K.; Samec, M.; Jasek, K.; Vybohova, D.; Uramova, S.; Liskova, A.; Sadlonova, V.; Koklesova, L.; et al. Chemopreventive and Therapeutic Efficacy of Cinnamomum zeylanicum L. Bark in Experimental Breast Carcinoma: Mechanistic In Vivo and In Vitro Analyses. Molecules 2020, 25, 1399. [Google Scholar] [CrossRef] [PubMed]
- Kubatka, P.; Uramova, S.; Kello, M.; Kajo, K.; Samec, M.; Jasek, K.; Vybohova, D.; Liskova, A.; Mojzis, J.; Adamkov, M.; et al. Anticancer Activities of Thymus vulgaris L. in Experimental Breast Carcinoma in Vivo and in Vitro. Int. J. Mol. Sci. 2019, 20, 1749. [Google Scholar] [CrossRef] [PubMed]
- Kubatka, P.; Uramova, S.; Kello, M.; Kajo, K.; Kruzliak, P.; Mojzis, J.; Vybohova, D.; Adamkov, M.; Jasek, K.; Lasabova, Z.; et al. Antineoplastic effects of clove buds (Syzygium aromaticum L.) in the model of breast carcinoma. J. Cell. Mol. Med. 2017, 21, 2837–2851. [Google Scholar] [CrossRef] [PubMed]
- Kubatka, P.; Kello, M.; Kajo, K.; Kruzliak, P.; Výbohová, D.; Mojžiš, J.; Adamkov, M.; Fialová, S.; Veizerová, L.; Zulli, A.; et al. Oregano demonstrates distinct tumour-suppressive effects in the breast carcinoma model. Eur. J. Nutr. 2017, 56, 1303–1316. [Google Scholar] [CrossRef]
- Kubatka, P.; Kapinová, A.; Kello, M.; Kruzliak, P.; Kajo, K.; Výbohová, D.; Mahmood, S.; Murin, R.; Viera, T.; Mojžiš, J.; et al. Fruit peel polyphenols demonstrate substantial anti-tumour effects in the model of breast cancer. Eur. J. Nutr. 2016, 55, 955–965. [Google Scholar] [CrossRef]
- Kubatka, P.; Kello, M.; Kajo, K.; Kruzliak, P.; Výbohová, D.; Šmejkal, K.; Maršík, P.; Zulli, A.; Gönciová, G.; Mojžiš, J.; et al. Young Barley Indicates Antitumor Effects in Experimental Breast Cancer In Vivo and In Vitro. Nutr. Cancer 2016, 68, 611–621. [Google Scholar] [CrossRef]
- Kubatka, P.; Kapinová, A.; Kružliak, P.; Kello, M.; Výbohová, D.; Kajo, K.; Novák, M.; Chripková, M.; Adamkov, M.; Péč, M.; et al. Antineoplastic effects of Chlorella pyrenoidosa in the breast cancer model. Nutrition 2015, 31, 560–569. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef]
- Kessler, E.R.; Su, L.-J.; Gao, D.; Torkko, K.C.; Wacker, M.; Anduha, M.; Chronister, N.; Maroni, P.; Crawford, E.D.; Flaig, T.W.; et al. Phase II Trial of Acai Juice Product in Biochemically Recurrent Prostate Cancer. Integr. Cancer Ther. 2018, 17, 1103–1108. [Google Scholar] [CrossRef]
- Lesinski, G.B.; Reville, P.K.; Mace, T.A.; Young, G.S.; Ahn-Jarvis, J.; Thomas-Ahner, J.; Vodovotz, Y.; Ameen, Z.; Grainger, E.; Riedl, K.; et al. Consumption of soy isoflavone enriched bread in men with prostate cancer is associated with reduced proinflammatory cytokines and immunosuppressive cells. Cancer Prev. Res. 2015, 8, 1036–1044. [Google Scholar] [CrossRef]
- Pantuck, A.J.; Leppert, J.T.; Zomorodian, N.; Aronson, W.; Hong, J.; Barnard, R.J.; Seeram, N.; Liker, H.; Wang, H.; Elashoff, R.; et al. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin. Cancer Res. 2006, 12, 4018–4026. [Google Scholar] [CrossRef] [PubMed]
- Grasselly, C.; Denis, M.; Bourguignon, A.; Talhi, N.; Mathe, D.; Tourette, A.; Serre, L.; Jordheim, L.P.; Matera, E.L.; Dumontet, C. The Antitumor Activity of Combinations of Cytotoxic Chemotherapy and Immune Checkpoint Inhibitors Is Model-Dependent. Front. Immunol 2018, 9, 2100. [Google Scholar] [CrossRef]
- Solár, P.; Sačková, V.; Hrčková, G.; Demečková, V.; Kassayová, M.; Bojková, B.; Mudroňová, D.; Gancarčíková, S.; Jendželovský, R.; Fedoročko, P. Antitumor effect of the combination of manumycin A and Immodin is associated with antiplatelet activity and increased granulocyte tumor infiltration in a 4T1 breast tumor model. Oncol. Rep. 2017, 37, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Demečková, V.; Solár, P.; Hrčková, G.; Mudroňová, D.; Bojková, B.; Kassayová, M.; Gancarčiková, S. Immodin and its immune system supportive role in paclitaxel therapy of 4T1 mouse breast cancer. Biomed. Pharmacother. 2017, 89, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Jeyabalan, J.; Aqil, F.; Munagala, R.; Annamalai, L.; Vadhanam, M.V.; Gupta, R.C. Chemopreventive and therapeutic activity of dietary blueberry against estrogen-mediated breast cancer. J. Agric. Food Chem. 2014, 62, 3963–3971. [Google Scholar] [CrossRef] [PubMed]
- Ravoori, S.; Vadhanam, M.V.; Aqil, F.; Gupta, R.C. Inhibition of estrogen-mediated mammary tumorigenesis by blueberry and black raspberry. J. Agric. Food Chem. 2012, 60, 5547–5555. [Google Scholar] [CrossRef]
- Singletary, K.; MacDonald, C.; Wallig, M. Inhibition by rosemary and carnosol of 7,12-dimethylbenz[a]anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation. Cancer Lett. 1996, 104, 43–48. [Google Scholar] [CrossRef]
- Bishayee, A.; Mandal, A.; Bhattacharyya, P.; Bhatia, D. Pomegranate exerts chemoprevention of experimentally induced mammary tumorigenesis by suppression of cell proliferation and induction of apoptosis. Nutr. Cancer 2016, 68, 120–130. [Google Scholar] [CrossRef]
- Cao, Y.; Himmeldirk, K.B.; Qian, Y.; Ren, Y.; Malki, A.; Chen, X. Biological and biomedical functions of Penta-O-galloyl-D-glucose and its derivatives. J. Nat. Med. 2014, 68, 465–472. [Google Scholar] [CrossRef]
- Kiss, A.K.; Piwowarski, J.P. Ellagitannins, Gallotannins and their Metabolites—The Contribution to the Anti-Inflammatory Effect of Food Products and Medicinal Plants. Curr. Med. Chem. 2018, 25, 4946–4967. [Google Scholar] [CrossRef]
- Kapinova, A.; Stefanicka, P.; Kubatka, P.; Zubor, P.; Uramova, S.; Kello, M.; Mojzis, J.; Blahutova, D.; Qaradakhi, T.; Zulli, A.; et al. Are plant-based functional foods better choice against cancer than single phytochemicals? A critical review of current breast cancer research. Biomed. Pharmacother. 2017, 96, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Pi, C.; Feng, X.; Wang, Y.; Fu, S.; Zhang, X.; Zhao, L.; Wei, Y. Antitumor Activity In Vivo and Vitro of New Chiral Derivatives of Baicalin and Induced Apoptosis via the PI3K/Akt Signaling Pathway. Mol. Ther. Oncol. 2020, 19, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Samuel, S.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Hodroj, M.H.; Al Bast, N. al H.; Taleb, R.I.; Borjac, J.; Rizk, S. Nettle Tea Inhibits Growth of Acute Myeloid Leukemia Cells In Vitro by Promoting Apoptosis. Nutrients 2020, 12, 2629. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, F.; Fakhri, S.; Khan, H. Targeting apoptosis and autophagy following spinal cord injury: Therapeutic approaches to polyphenols and candidate phytochemicals. Pharmacol. Res. 2020, 160, 105069. [Google Scholar] [CrossRef] [PubMed]
- Gjerdrum, C.; Tiron, C.; Høiby, T.; Stefansson, I.; Haugen, H.; Sandal, T.; Collett, K.; Li, S.; McCormack, E.; Gjertsen, B.T.; et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc. Natl. Acad. Sci. USA 2010, 107, 1124–1129. [Google Scholar] [CrossRef]
- Chen, J.; Lu, L.; Feng, Y.; Wang, H.; Dai, L.; Li, Y.; Zhang, P. PKD2 mediates multi-drug resistance in breast cancer cells through modulation of P-glycoprotein expression. Cancer Lett. 2011, 300, 48–56. [Google Scholar] [CrossRef]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef]
- Rafi, M.M.; Vastano, B.C. Identification of a structure specific Bcl-2 phosphorylating homoisoflavone molecule from Vietnamese coriander (Polygonatum odoratum) that induces apoptosis and G2/M cell cycle arrest in breast cancer cell lines. Food Chem. 2007, 1, 332–340. [Google Scholar] [CrossRef]
- Hyun, H.-B.; Lee, W.S.; Go, S.-I.; Nagappan, A.; Park, C.; Han, M.H.; Hong, S.H.; Kim, G.; Kim, G.Y.; Cheong, J.; et al. The flavonoid morin from Moraceae induces apoptosis by modulation of Bcl-2 family members and Fas receptor in HCT 116 cells. Int. J. Oncol. 2015, 46, 2670–2678. [Google Scholar] [CrossRef]
- Domínguez-López, I.; Yago-Aragón, M.; Salas-Huetos, A.; Tresserra-Rimbau, A.; Hurtado-Barroso, S. Effects of Dietary Phytoestrogens on Hormones throughout a Human Lifespan: A Review. Nutrients 2020, 12, 24565. [Google Scholar] [CrossRef] [PubMed]
- Raju, S.R.; Balakrishnan, S.; Kollimada, S.; Chandrashekara, K.N.; Jampani, A. Anti-tumor effects of Artemisia nilagirica extract on MDA-MB-231 breast cancer cells: Deciphering the biochemical and biomechanical properties via TGF-β upregulation. Heliyon 2020, 6, e05088. [Google Scholar] [CrossRef] [PubMed]
- Mehraban, F.; Mostafazadeh, M.; Sadeghi, H.; Azizi, A.; Akbartabar Toori, M.; Gramizadeh, B.; Barati, V.; Sadeghi, H. Anticancer activity of Astragalus ovinus against 7, 12 dimethyl benz (a) anthracene (DMBA)-induced breast cancer in rats. Avicenna J. Phytomed. 2020, 10, 533–545. [Google Scholar] [PubMed]
- Wang, H.; Khor, T.O.; Shu, L.; Su, Z.-Y.; Fuentes, F.; Lee, J.-H.; Kong, A.-N.T. Plants vs. cancer: A review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med. Chem. 2012, 12, 1281–1305. [Google Scholar] [CrossRef]
- Niland, S.; Eble, J.A. Neuropilins in the Context of Tumor Vasculature. Int. J. Mol. Sci. 2019, 20, 639. [Google Scholar] [CrossRef]
- Hu, W.-H.; Dai, D.K.; Zheng, B.Z.-Y.; Duan, R.; Dong, T.T.-X.; Qin, Q.-W.; Tsim, K.W.-K. Piceatannol, a Natural Analog of Resveratrol, Exerts Anti-angiogenic Efficiencies by Blockage of Vascular Endothelial Growth Factor Binding to Its Receptor. Molecules 2020, 25, 3769. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Zhang, X.; Pan, L.; Jiang, L. The Protective Effects of Juglanin in Cerebral Ischemia Reduce Blood–Brain Barrier Permeability via Inhibition of VEGF/VEGFR2 Signaling. Drug Des. Dev. Ther. 2020, 14, 3165–3175. [Google Scholar] [CrossRef]
- Cho, H.-D.; Kim, J.-H.; Park, J.-K.; Hong, S.-M.; Kim, D.-H.; Seo, K.-I. Kochia scoparia seed extract suppresses VEGF-induced angiogenesis via modulating VEGF receptor 2 and PI3K/AKT/mTOR pathways. Pharm. Biol. 2019, 57, 684–693. [Google Scholar] [CrossRef]
- Sayır, F.; Şehitoğulları, A.; Demir, H.; Aslan, M.; Çobanoğlu, U.; Bilgin, C. Serum prolidase activity, total oxidant/antioxidant, and nitric oxide levels in patients with esophageal squamous cell carcinoma. Turk. Gogus Kalp. Damar. Cerrahisi Derg. 2019, 27, 206–211. [Google Scholar] [CrossRef]
- Günes, M.; Eryilmaz, R.; Aslan, R.; Taken, K.; Demir, H.; Demir, C. Oxidant-antioxidant levels in patients with bladder tumours. Aging Male 2020, 1–6. [Google Scholar] [CrossRef]
- Sevastre-Berghian, A.C.; Ielciu, I.; Mitre, A.O.; Filip, G.A.; Oniga, I.; Vlase, L.; Benedec, D.; Gheldiu, A.-M.; Toma, V.A.; Mihart, B.; et al. Targeting Oxidative Stress Reduction and Inhibition of HDAC1, MECP2, and NF-kB Pathways in Rats With Experimentally Induced Hyperglycemia by Administration of Thymus marshallianus Willd. Extracts. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Das, P.K.; Islam, F.; Lam, A.K. The Roles of Cancer Stem Cells and Therapy Resistance in Colorectal Carcinoma. Cells 2020, 9, 1392. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 1–35. [Google Scholar] [CrossRef]
- Shima, H.; Yamada, A.; Ishikawa, T.; Endo, I. Are breast cancer stem cells the key to resolving clinical issues in breast cancer therapy? Gland Surg. 2017, 6, 82–88. [Google Scholar] [CrossRef]
- Liu, T.T.; Li, X.F.; Wang, L.; Yang, J.L. CD133 expressionand clinicopathologic significance in benign and malignant breast lesions. Cancer Biomark. 2020, 28, 293–299. [Google Scholar] [CrossRef]
- Rennó, A.L.; Alves-Júnior, M.J.; Rocha, R.M.; De Souza, P.C.; de Souza, V.B.; Jampietro, J.; Vassallo, J.; Hyslop, S.; Anhê, G.F.; de Moraes Schenka, N.G.; et al. Decreased expression of stem cell markers by simvastatin in 7,12-dimethylbenz(a)anthracene (DMBA)-induced breast cancer. Toxicol. Pathol. 2015, 43, 400–410. [Google Scholar] [CrossRef]
- Levi, E.; Misra, S.; Du, J.; Patel, B.B.; Majumdar, A.P.N. Combination of aging and dimethylhydrazine treatment causes an increase in cancer-stem cell population of rat colonic crypts. Biochem. Biophys. Res. Commun. 2009, 385, 430–433. [Google Scholar] [CrossRef][Green Version]
- Liskova, A.; Kubatka, P.; Samec, M.; Zubor, P.; Mlyncek, M.; Bielik, T.; Samuel, S.M.; Zulli, A.; Kwon, T.K.; Büsselberg, D. Dietary Phytochemicals Targeting Cancer Stem Cells. Molecules 2019, 24, 899. [Google Scholar] [CrossRef]
- Naujokat, C.; McKee, D.L. The “Big Five” Phytochemicals Targeting Cancer Stem Cells: Curcumin, EGCG, Sulforaphane, Resveratrol and Genistein. Curr. Med. Chem. 2020. [Google Scholar] [CrossRef]
- Hudlikar, R.; Wang, L.; Wu, R.; Li, S.; Peter, R.; Shannar, A.; Chou, P.J.; Liu, X.; Liu, Z.; Kuo, H.-C.D.; et al. Epigenetics/epigenomics and prevention of early stages of cancer by isothiocyanates. Cancer Prev. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Kuo, H.-C.D.; Yin, R.; Wu, R.; Liu, X.; Wang, L.; Hudlikar, R.; Peter, R.M.; Kong, A.-N. Epigenetics/epigenomics of triterpenoids in cancer prevention and in health. Biochem. Pharmacol. 2020, 175, 113890. [Google Scholar] [CrossRef]
- Samec, M.; Liskova, A.; Kubatka, P.; Uramova, S.; Zubor, P.; Samuel, S.M.; Zulli, A.; Pec, M.; Bielik, T.; Biringer, K.; et al. The role of dietary phytochemicals in the carcinogenesis via the modulation of miRNA expression. J. Cancer Res. Clin. Oncol. 2019, 145, 1665–1679. [Google Scholar] [CrossRef] [PubMed]
- Jasek, K.; Kubatka, P.; Samec, M.; Liskova, A.; Smejkal, K.; Vybohova, D.; Bugos, O.; Biskupska-Bodova, K.; Bielik, T.; Zubor, P.; et al. DNA Methylation Status in Cancer Disease: Modulations by Plant-Derived Natural Compounds and Dietary Interventions. Biomolecules 2019, 9, 289. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.-X.; Yuan, Y.; Li, L.-L.; Wang, Z.-H.; Dan, L.-Y.; Chen, Y.; Ren, G.-S.; Tao, Q. Aberrant promoter CpG methylation and its translational applications in breast cancer. Chin. J. Cancer 2013, 32, 12–20. [Google Scholar] [CrossRef]
- Wang, L.-S.; Kuo, C.-T.; Huang, T.H.-M.; Yearsley, M.; Oshima, K.; Stoner, G.D.; Yu, J.; Lechner, J.F.; Huang, Y.-W. Black raspberries protectively regulate methylation of Wnt pathway genes in precancerous colon tissue. Cancer Prev. Res. 2013, 6, 1317–1327. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Gu, F.; Dombkowski, A.; Wang, L.-S.; Stoner, G.D. Black raspberries demethylate Sfrp4, a WNT pathway antagonist, in rat esophageal squamous cell papilloma. Mol. Carcinog. 2016, 55, 1867–1875. [Google Scholar] [CrossRef]
- Romanowska, J.; Joshi, A. From Genotype to Phenotype: Through Chromatin. Genes 2019, 10, 76. [Google Scholar] [CrossRef]
- Chatterjee, B.; Ghosh, K.; Kanade, S.R. Resveratrol modulates epigenetic regulators of promoter histone methylation and acetylation that restores BRCA1, p53, p21CIP1 in human breast cancer cell lines. Biofactors 2019, 45, 818–829. [Google Scholar] [CrossRef]
- Royston, K.; Udayakumar, N.; Lewis, K.; Tollefsbol, T. A Novel Combination of Withaferin A and Sulforaphane Inhibits Epigenetic Machinery, Cellular Viability and Induces Apoptosis of Breast Cancer Cells. Int. J. Mol. Sci. 2017, 18, 1092. [Google Scholar] [CrossRef]
- Samec, M.; Liskova, A.; Koklesova, L.; Mestanova, V.; Franekova, M.; Kassayova, M.; Bojkova, B.; Uramova, S.; Zubor, P.; Janikova, K.; et al. Fluctuations of Histone Chemical Modifications in Breast, Prostate, and Colorectal Cancer: An Implication of Phytochemicals as Defenders of Chromatin Equilibrium. Biomolecules 2019, 9, 829. [Google Scholar] [CrossRef] [PubMed]
- Son, S.W.; Lee, H.Y.; Moeng, S.; Kuh, H.J.; Choi, S.Y.; Park, J.K. Participation of MicroRNAs in the Treatment of Cancer with Phytochemicals. Molecules 2020, 25, 4701. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Li, Y.; Liu, X.; Wu, Z.; Li, J.; Ma, Z. Roles of plant-derived bioactive compounds and related microRNAs in cancer therapy. Phytother. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Varghese, E.; Liskova, A.; Kubatka, P.; Samuel, S.M.; Büsselberg, D. Anti-Angiogenic Effects of Phytochemicals on miRNA Regulating Breast Cancer Progression. Biomolecules 2020, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Ding, M.; Zhang, H.; Xu, X.; Tang, J. Molecular mechanisms and clinical applications of miR-22 in regulating malignant progression in human cancer (Review). Int. J. Oncol. 2016, 50, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Imani, S.; Zhang, X.; Hosseinifard, H.; Fu, S.; Fu, J. The diagnostic role of microRNA-34a in breast cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 23177–23187. [Google Scholar] [CrossRef]
- Venturutti, L.; Romero, L.V.; Urtreger, A.J.; Chervo, M.F.; Cordo Russo, R.I.; Mercogliano, M.F.; Inurrigarro, G.; Pereyra, M.G.; Proietti, C.J.; Izzo, F.; et al. Stat3 regulates ErbB-2 expression and co-opts ErbB-2 nuclear function to induce miR-21 expression, PDCD4 downregulation and breast cancer metastasis. Oncogene 2016, 35, 2208–2222. [Google Scholar] [CrossRef]
- Jung, D.E.; Park, S.B.; Kim, K.; Kim, C.; Song, S.Y. CG200745, an HDAC inhibitor, induces anti-tumour effects in cholangiocarcinoma cell lines via miRNAs targeting the Hippo pathway. Sci. Rep. 2017, 7, 10921. [Google Scholar] [CrossRef]
- Kang, H. MicroRNA-Mediated Health-Promoting Effects of Phytochemicals. Int. J. Mol. Sci. 2019, 20, 2535. [Google Scholar] [CrossRef]
- Kubatka, P.; Kajo, K.; Zihlavnikova, K.; Adamicova, K.; Vybohova, D.; Pec, M.; Nosal, V.; Stollarova, N.; Bojkova, B.; Kassayova, M.; et al. Immunohistochemical and histomorphological analysis of rat mammary tumors after simvastatin treatment. Neoplasma 2012, 59, 516–523. [Google Scholar] [CrossRef][Green Version]


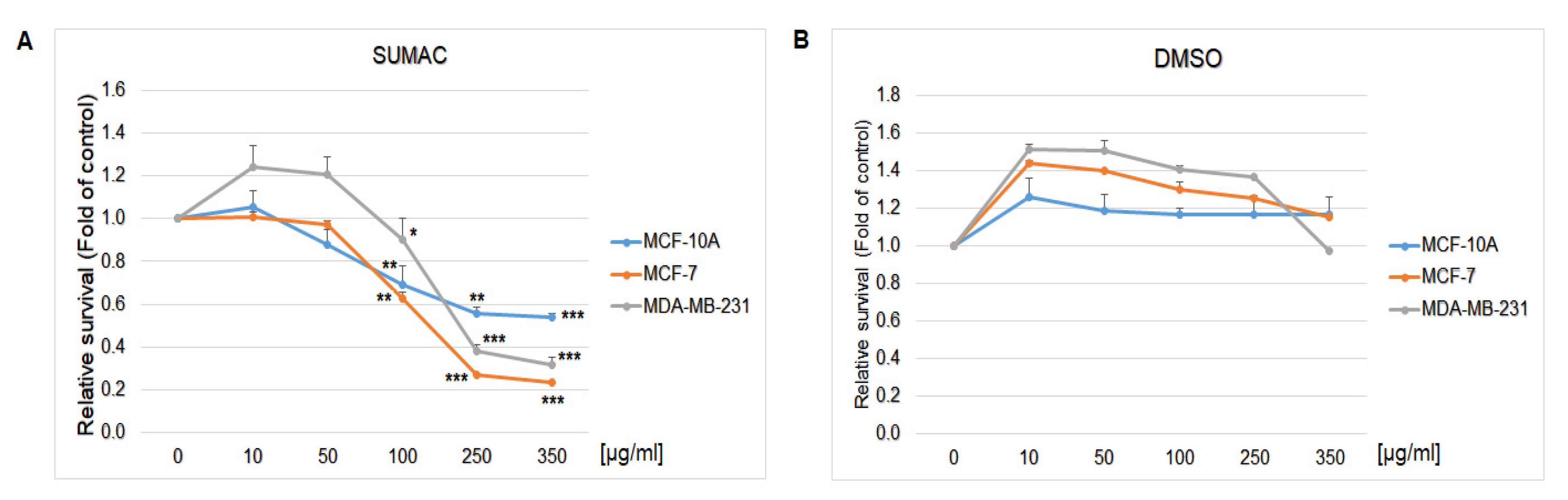
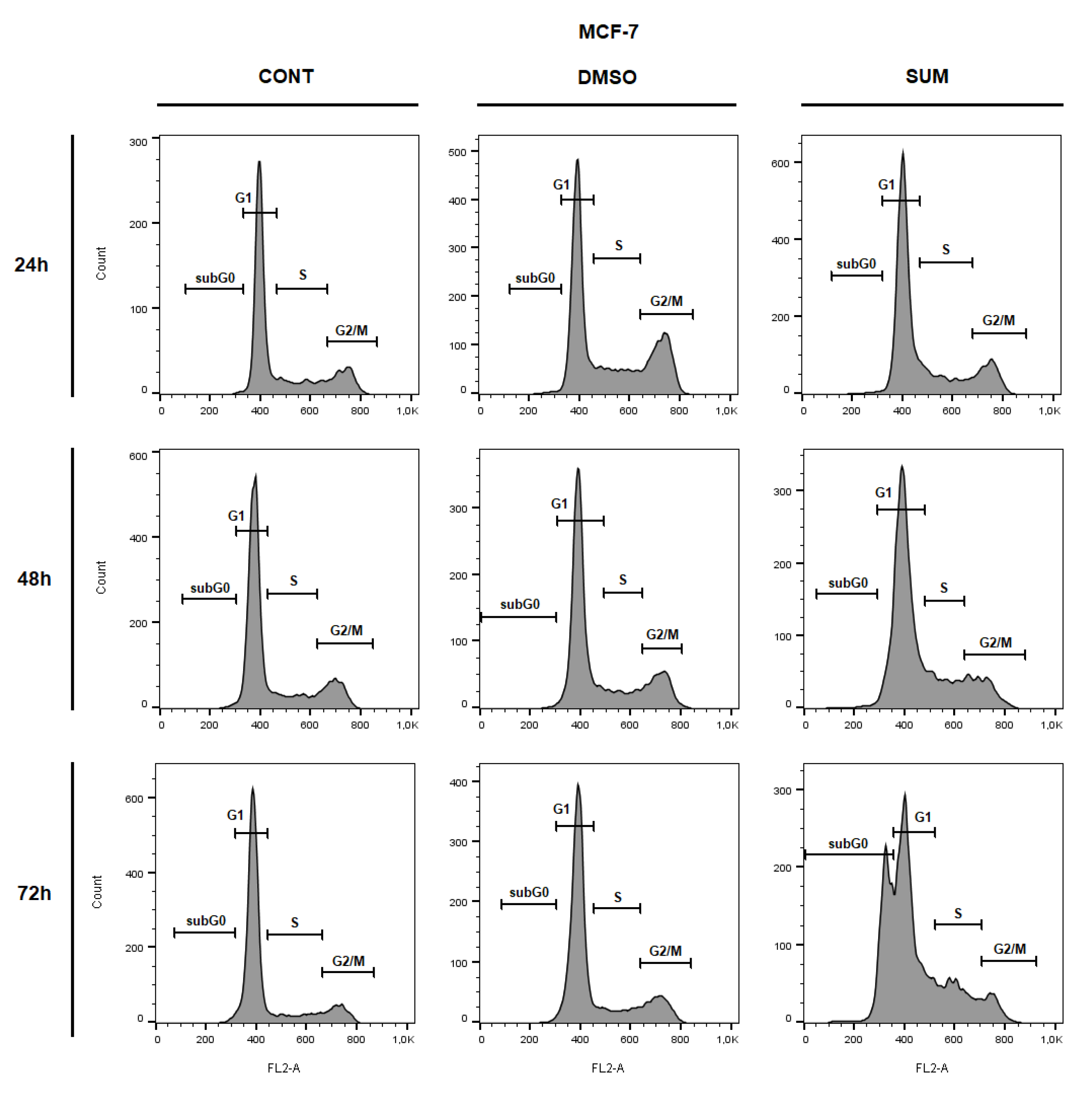
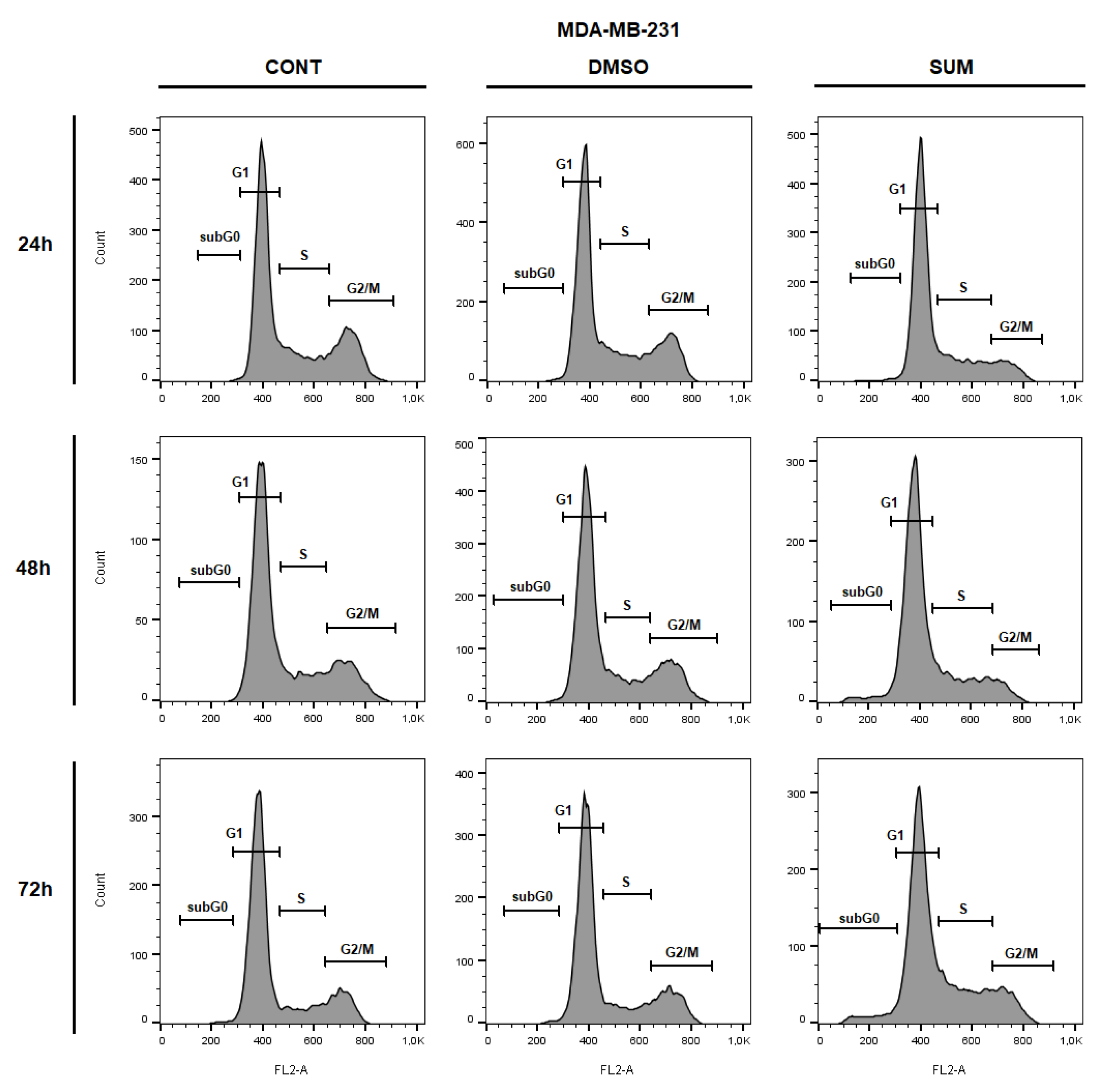
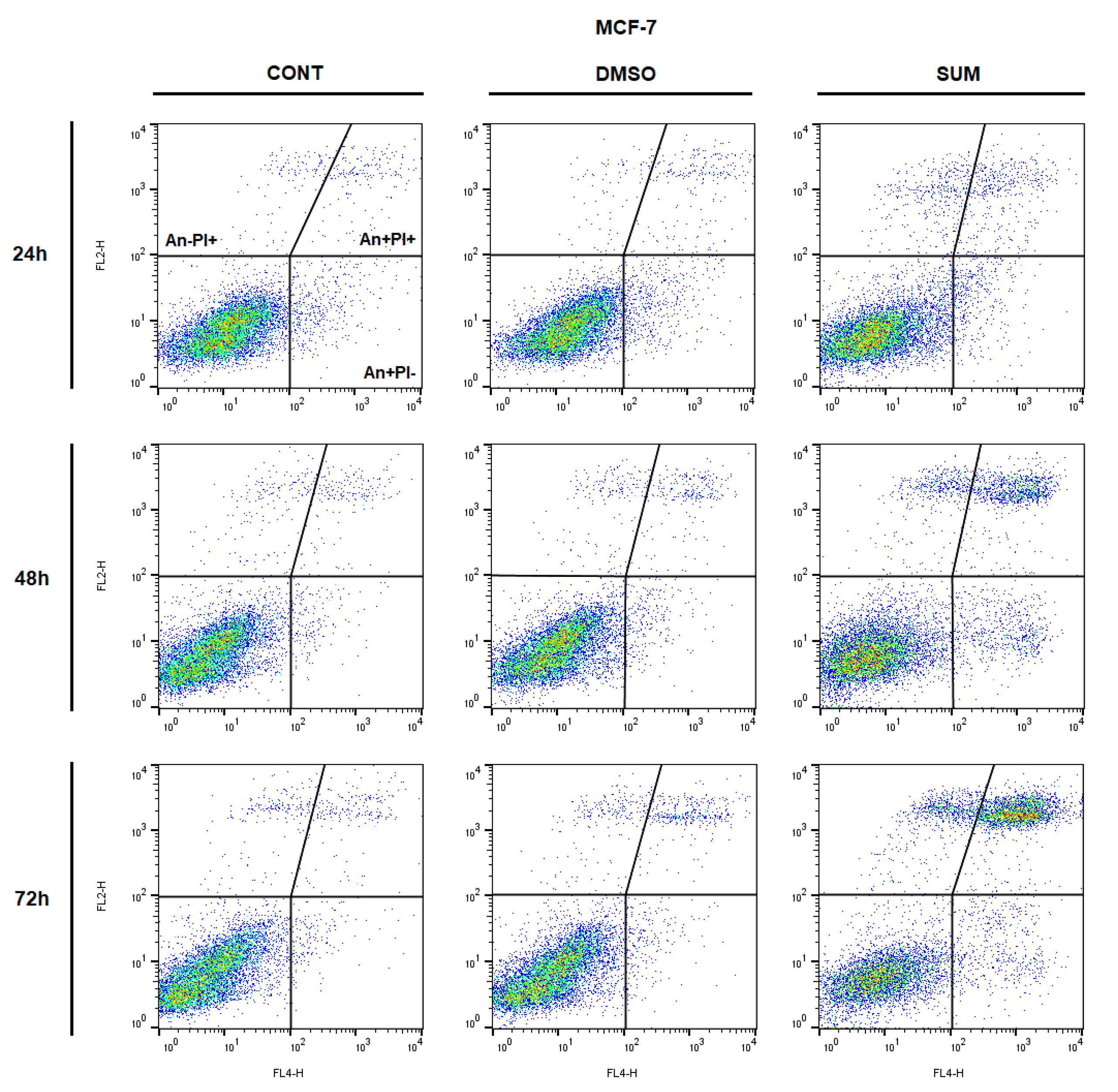



| Parameter | CONT | SUM 0.1 | SUM 1 |
|---|---|---|---|
| Necrosis/whole tumor area | 7.65 ± 2.47 | 18.19 ± 5.32 | 7.97 ± 2.50 |
| Mitotic activity index | 38.60 ± 2.56 | 24.53 ± 2.86 *** | 18.86 ± 1.95 *** |
| Group | CONT | SUM 0.1 | SUM 1 |
|---|---|---|---|
| tumor bearing animals/all animals | 25/25 | 22/25 | 20/25 |
| tumor frequency per group # | 4.00 ± 0.70 | 2.84 ± 0.59 | 4.16 ± 0.81 |
| tumor latency #(days) | 71.12 ± 2.79 | 73.36 ± 3.43 | 73.36 ± 3.77 |
| tumor incidence (%) | 100 | 88 | 80 * |
| average tumor volume # (cm3) | 0.48 ± 0.08 | 0.75 ± 0.15 | 0.69 ± 0.13 |
| high/low grade carcinomas ratio | 42/58 (=0.724) | 14/57 (0.246) ** | 17/87 (0.195) *** |
| Time (h) | 24 | 48 | 72 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | CONT | DMSO | SUM | CONT | DMSO | SUM | CONT | DMSO | SUM |
| Sub-G0/G1 | 0.77 ± 0.03 | 1.14 ± 0.38 | 1.30 ± 0.20 | 1.23 ± 0.01 | 1.09 ± 0.02 | 1.95 ± 0.11 | 1.54 ± 0.13 | 1.42 ± 0.41 | 23.30 ± 3.18 ** |
| G1 | 60.00 ± 3.43 | 53.35 ± 1.59 * | 66.80 ± 1.96 * | 68.50 ± 1.47 | 64.75 ± 1.84 | 66.55 ± 0.20 | 75.15 ± 0.78 | 70.45 ± 0.45 | 49.55 ± 0.12 ** |
| S | 19.45 ± 1.51 | 22.20 ± 1.80 | 18.35 ± 0.04 | 15.40 ± 0.16 | 14.25 ± 1.10 | 15.40 ± 1.06 | 11.65 ± 0.69 | 12.40 ± 0.73 | 17.65 ± 0.53 |
| G2/M | 19.80 ± 1.88 | 23.30 ± 3.02 | 13.55 ± 1.84 * | 14.90 ± 1.63 | 19.90 ± 0.73 | 16.10 ± 1.14 | 11.66 ± 0.04 | 15.75 ± 0.12 | 9.50 ± 2.54 |
| Time (h) | 24 | 48 | 72 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | CONT | DMSO | SUM | CONT | DMSO | SUM | CONT | DMSO | SUM |
| Sub-G0/G1 | 0.58 ± 0.09 | 0.74 ± 0.09 | 2.02 ± 0.16 | 0.69 ± 0.02 | 0.91 ± 0.05 | 4.35 ± 0.35 | 0.98 ± 0.02 | 0.99 ± 0.06 | 4.79 ± 0.31 * |
| G1 | 56.30 ± 0.49 | 58.20 ± 0.73 | 66.55 ± 1.96 * | 62.30 ± 1.06 | 61.65 ± 1.18 | 64.20 ± 0.08 | 68.60 ± 2.04 | 66.75 ± 0.20 | 62.55 ± 0.35 * |
| S | 19.75 ± 0.45 | 18.95 ± 1.51 | 21.65 ± 0.37 | 17.20 ± 0.57 | 16.50 ± 0.98 | 25.30 ± 0.01 * | 11.90 ± 0.33 | 14.55 ± 0.53 | 20.45 ± 2.00 * |
| G2/M | 23.35 ± 0.04 | 22.10 ± 0.90 | 9.80 ± 0.08 * | 19.80 ± 0.49 | 20.90 ± 0.16 | 6.16 ± 0.13 * | 18.50 ± 2.37 | 17.70 ± 0.41 | 12.20 ± 0.16 * |
| Time (h) | 24 | 48 | 72 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | CONT | DMSO | SUM | CONT | DMSO | SUM | CONT | DMSO | SUM |
| An−/PI− | 92.05 ± 0.53 | 91.15 ± 0.04 | 88.75 ± 1.18 | 94.80 ± 0.24 | 93.60 ± 0.57 | 77.15 ± 0.45 ** | 94.75 ± 0.53 | 92.85 ± 0.94 | 56.75 ± 0.29 ** |
| An+/PI− | 3.41 ± 0.62 | 4.32 ± 0.88 | 5.10 ± 0.68 | 1.40 ± 0.33 | 2.46 ± 0.23 | 6.43 ± 0.35 | 1.96 ± 0.20 | 2.03 ± 0.28 | 4.61 ± 0.76 |
| An+/PI+ | 2.41 ± 0.50 | 2.56 ± 0.02 | 3.70 ± 0.69 | 2.04 ± 0.14 | 2.39 ± 0.07 | 11.00 ± 0.24 * | 1.97 ± 0.19 | 2.88 ± 0.67 | 29.80 ± 0.01 ** |
| An−/PI+ | 2.12 ± 0.68 | 1.96 ± 0.89 | 2.47 ± 1.20 | 1.76 ± 0.06 | 1.56 ± 0.25 | 5.45 ± 0.54 | 1.35 ± 0.55 | 2.27 ± 0.01 | 8.83 ± 0.50 * |
| Time (h) | 24 | 48 | 72 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | CONT | DMSO | SUM | CONT | DMSO | SUM | CONT | DMSO | SUM |
| An−/PI− | 92.20 ± 1.22 | 92.40 ± 0.41 | 83.65 ± 1.21 * | 90.90 ± 0.41 | 89.95 ± 0.86 | 75.30 ± 2.61 ** | 89.95 ± 1.67 | 91.65 ± 1.27 | 50.00 ± 3.69 ** |
| An+/PI− | 2.87 ± 0.59 | 2.82 ± 0.18 | 4.25 ± 0.53 | 2.51 ± 0.18 | 3.08 ± 0.33 | 6.92 ± 3.09 | 1.85 ± 0.62 | 2.20 ± 0.98 | 6.22 ± 1.82 |
| An+/PI+ | 3.25 ± 0.36 | 3.01 ± 0.27 | 8.38 ± 1.12 * | 4.06 ± 0.10 | 4.08 ± 0.05 | 12.30 ± 0.16 * | 4.78 ± 0.60 | 3.71 ± 0.53 | 34.00 ± 2.10 ** |
| An−/PI+ | 1.69 ± 0.31 | 1.78 ± 0.05 | 3.75 ± 0.58 | 2.56 ± 0.34 | 2.86 ± 0.48 | 5.46 ± 0.32 | 3.46 ± 0.44 | 2.42 ± 0.22 | 9.78 ± 0.92 * |
| Analyses | Staining Solution | Company |
|---|---|---|
| Cell cycle * | 10% Triton X-100 0.5 mg/mL ribonuclease A 0.025 mg/mL propidium iodide–PI In 500 µL PBS | Sigma-Aldrich, Steinheim, Germany |
| Apoptosis | Annexin V-Alexa Fluor 647 1:100 (catalogue no. A23204) | Thermo Scientific, Rockford, IL, USA |
| PI (5 mg/mL) 1:500 | Sigma-Aldrich | |
| Mitochondrial membrane potential | TMRE (tetramethylrhodamine ethyl ester per chlorate) final conc. 0.1 µM | Molecular Probes, Eugene, OR, USA |
| Caspase activation | Cleaved caspase-3 rabbit mAb PE conjugate 1:100 (#9978) | Cell Signaling, Danvers, MA, USA |
| Cleaved caspase-7 rabbit mAb PE conjugate 1:100 (#42542) | ||
| Proteins analysis | Cleaved PARP rabbit mAb PE conjugate 1:100 (#8978) | |
| Bcl-2 mouse mAb PE conjugated 1:100 (#26295) | ||
| Phospho-Bcl-2 (Ser 70) rabbit mAb Alexa Fluor 488 conjugate 1:200 (#2834) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubatka, P.; Kello, M.; Kajo, K.; Samec, M.; Liskova, A.; Jasek, K.; Koklesova, L.; Kuruc, T.; Adamkov, M.; Smejkal, K.; et al. Rhus coriaria L. (Sumac) Demonstrates Oncostatic Activity in the Therapeutic and Preventive Model of Breast Carcinoma. Int. J. Mol. Sci. 2021, 22, 183. https://doi.org/10.3390/ijms22010183
Kubatka P, Kello M, Kajo K, Samec M, Liskova A, Jasek K, Koklesova L, Kuruc T, Adamkov M, Smejkal K, et al. Rhus coriaria L. (Sumac) Demonstrates Oncostatic Activity in the Therapeutic and Preventive Model of Breast Carcinoma. International Journal of Molecular Sciences. 2021; 22(1):183. https://doi.org/10.3390/ijms22010183
Chicago/Turabian StyleKubatka, Peter, Martin Kello, Karol Kajo, Marek Samec, Alena Liskova, Karin Jasek, Lenka Koklesova, Tomas Kuruc, Marian Adamkov, Karel Smejkal, and et al. 2021. "Rhus coriaria L. (Sumac) Demonstrates Oncostatic Activity in the Therapeutic and Preventive Model of Breast Carcinoma" International Journal of Molecular Sciences 22, no. 1: 183. https://doi.org/10.3390/ijms22010183
APA StyleKubatka, P., Kello, M., Kajo, K., Samec, M., Liskova, A., Jasek, K., Koklesova, L., Kuruc, T., Adamkov, M., Smejkal, K., Svajdlenka, E., Solar, P., Pec, M., Büsselberg, D., Sadlonova, V., & Mojzis, J. (2021). Rhus coriaria L. (Sumac) Demonstrates Oncostatic Activity in the Therapeutic and Preventive Model of Breast Carcinoma. International Journal of Molecular Sciences, 22(1), 183. https://doi.org/10.3390/ijms22010183









