In Vitro Photodynamic Effects of the Inclusion Nanocomplexes of Glucan and Chlorin e6 on Atherogenic Foam Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of Inclusion Nanocomplexes of Glucan and Chlorin e6 (Glu/Ce6)
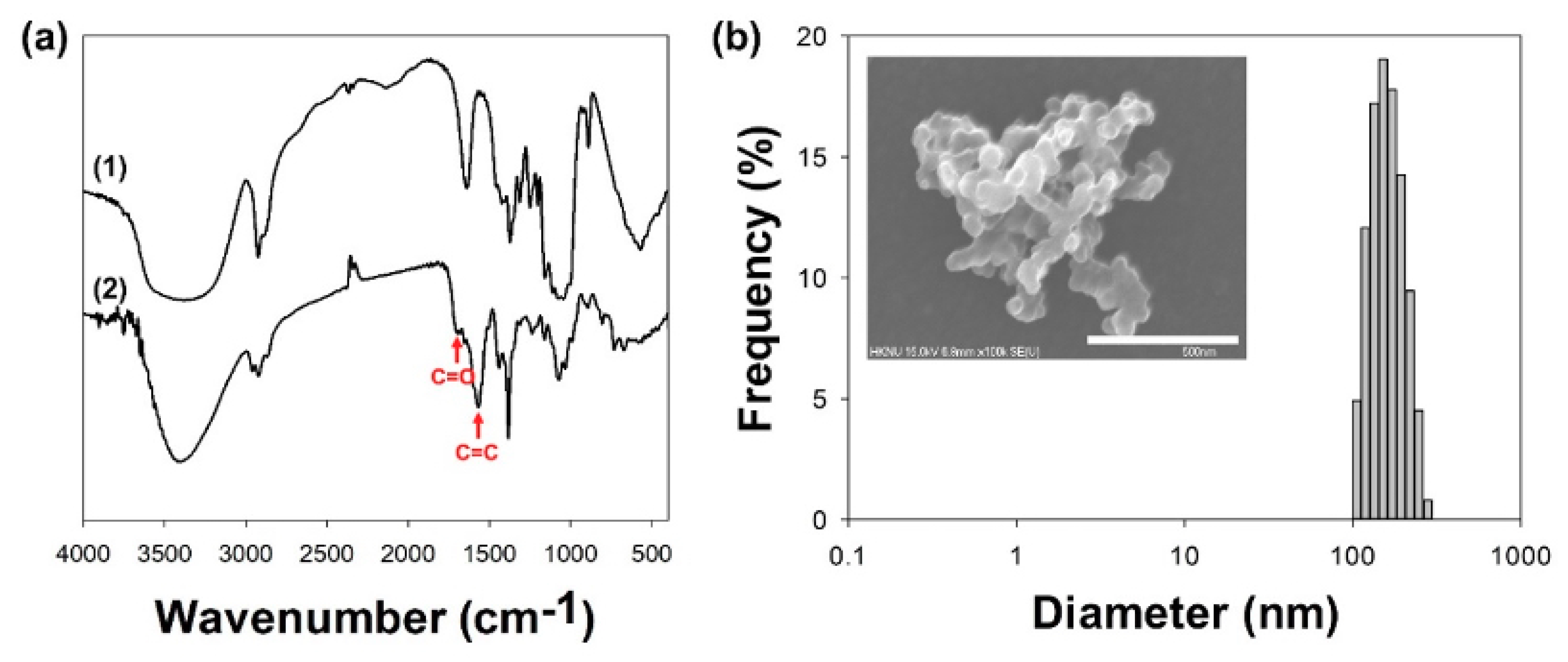
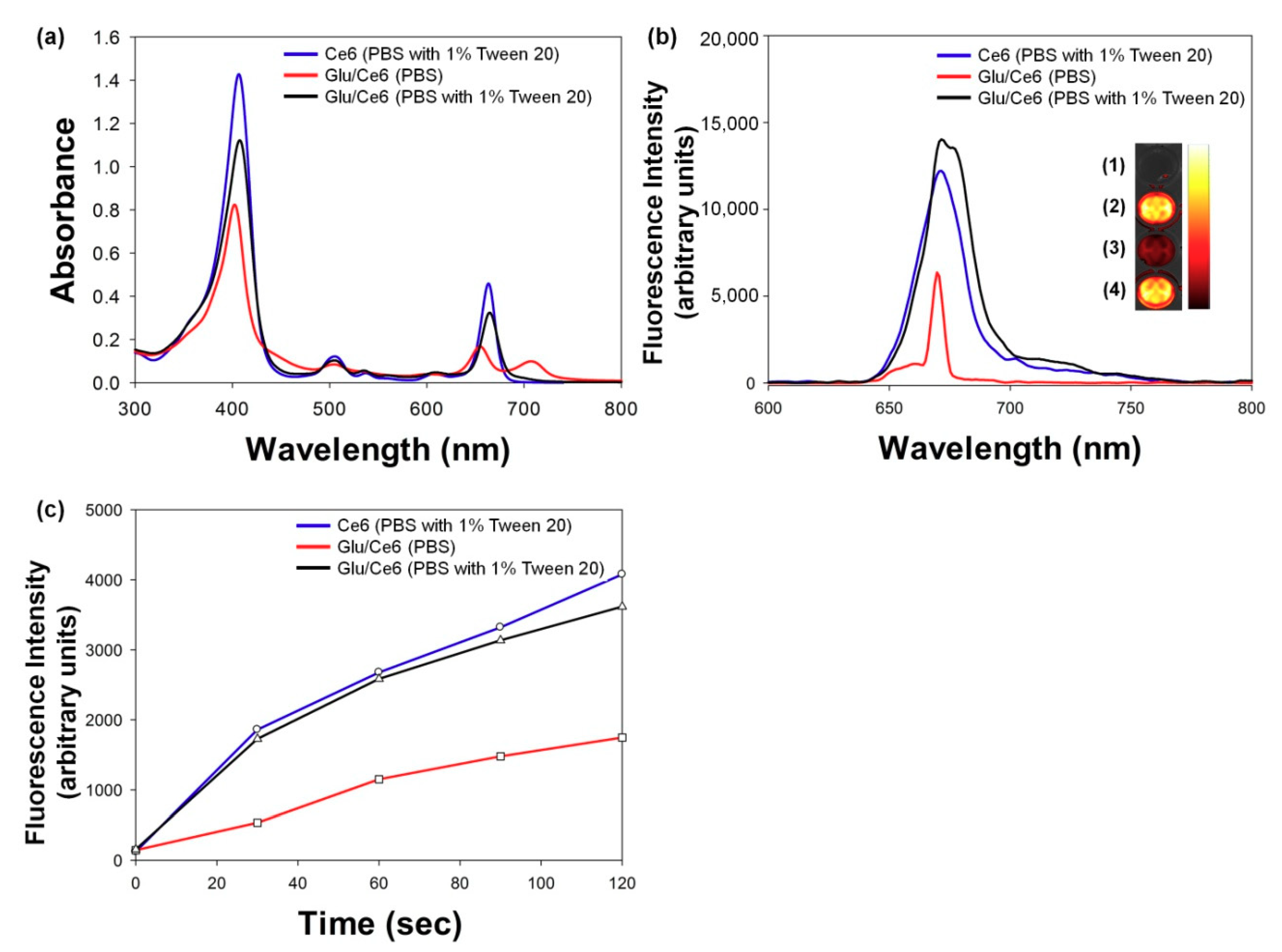
2.2. Characterizations of Glu/Ce6 Nanocomplexes
2.3. Singlet Oxygen (SO) Generation Study
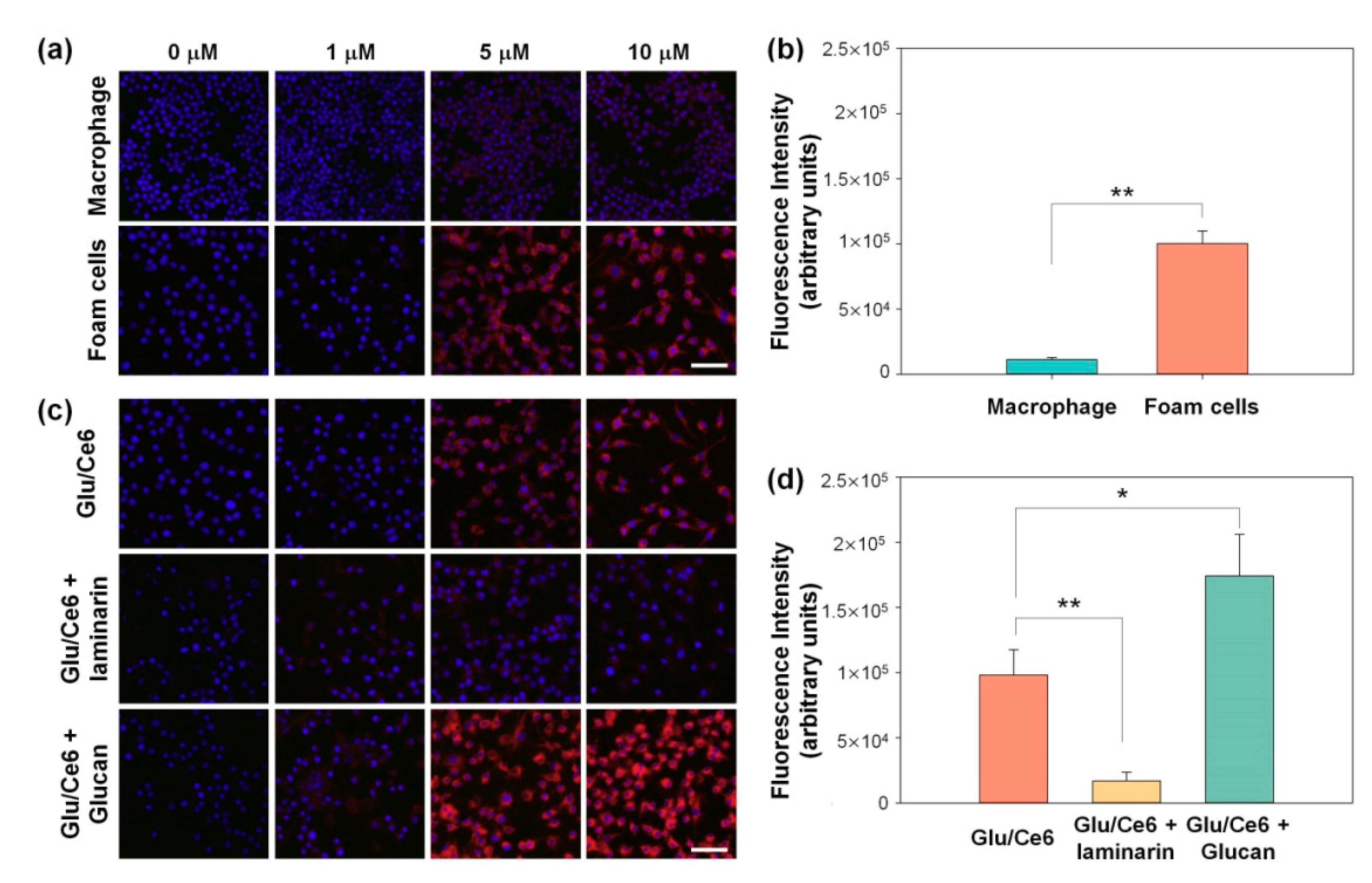
2.4. In Vitro Cytotoxic Study of Glu/Ce6 Nanocomplexes on Macrophages and Foam Cells
2.5. Intracellular Uptake of Glu/Ce6 Nanocomplexes by Foam Cells
2.6. In Vitro Phototoxic Effects of Glu/Ce6 Nanocomplexes
3. Materials and Methods
3.1. Materials
3.2. Fabrication of Glu and Ce6 (Glu/Ce6) Inclusion Nanocomplexes
3.3. Characterizations of Glu/Ce6 Nanocomplexes
3.4. Singlet-Oxygen-Generation Study
3.5. Cells and Culture Condition
3.6. Cytocompatibility Study
3.7. Intracellular Uptake of Glu/Ce6
3.8. In Vitro Phototoxic Effects of Glu/Ce6
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Garlanda, C.; Locati, M. Macrophage diversity and polarization in atherosclerosis: A question of balance. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Chinetti-Gbaguidi, G.; Colin, S.; Staels, B. Macrophage subsets in atherosclerosis. Nat. Rev. Cardiol. 2015, 12, 10–17. [Google Scholar] [CrossRef]
- Beckers, L.; Heeneman, S.; Wang, L.; Burkly, L.C.; Rousch, M.M.; Davidson, N.O.; Gijbels, M.J.; de Winther, M.P.; Daemen, M.J.; Lutgens, E. Disruption of hedgehog signalling in ApoE -/- mice reduces plasma lipid levels, but increases atherosclerosis due to enhanced lipid uptake by macrophages. J. Pathol. 2007, 212, 420–428. [Google Scholar] [CrossRef]
- Rader, D.J.; Pure, E. Lipoproteins, macrophage function, and atherosclerosis: Beyond the foam cell? Cell Metab. 2005, 1, 223–230. [Google Scholar] [CrossRef]
- Chellat, F.; Merhi, Y.; Moreau, A.; Yahia, L. Therapeutic potential of nanoparticulate systems for macrophage targeting. Biomaterials 2005, 26, 7260–7275. [Google Scholar] [CrossRef]
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging macrophages with nanoparticles. Nat. Mater. 2014, 13, 125–138. [Google Scholar] [CrossRef]
- Amirbekian, V.; Lipinski, M.J.; Briley-Saebo, K.C.; Amirbekian, S.; Aguinaldo, J.G.; Weinreb, D.B.; Vucic, E.; Frias, J.C.; Hyafil, F.; Mani, V.; et al. Detecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRI. Proc. Natl. Acad. Sci. USA 2007, 104, 961–966. [Google Scholar] [CrossRef]
- Quillard, T.; Libby, P. Molecular imaging of atherosclerosis for improving diagnostic and therapeutic development. Circ. Res. 2012, 111, 231–244. [Google Scholar] [CrossRef]
- Jaffer, F.A.; Libby, P.; Weissleder, R. Molecular imaging of cardiovascular disease. Circulation 2007, 116, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.R. Nanomedicine and Cardiovascular Disease. Curr. Cardiovasc. Imaging Rep. 2010, 3, 42–49. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brown, G.D.; Taylor, P.R.; Reid, D.M.; Willment, J.A.; Williams, D.L.; Martinez-Pomares, L.; Wong, S.Y.; Gordon, S. Dectin-1 is a major beta-glucan receptor on macrophages. J. Exp. Med. 2002, 196, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, K.; Gijbels, M.J.; van der Velden, S.; Heinsbroek, S.E.; Kraal, G.; de Winther, M.P.; van den Berg, T.K. Dectin-1 deficiency does not affect atherosclerosis development in mice. Atherosclerosis 2015, 239, 318–321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharma, S.K.; Chiang, L.Y.; Hamblin, M.R. Photodynamic therapy with fullerenes in vivo: Reality or a dream? Nanomedicine (Lond.) 2011, 6, 1813–1825. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Huang, A.L.; Kyaw, T.S.; Bobik, A.; Peter, K. Atherosclerotic Plaque Rupture: Identifying the Straw That Breaks the Camel’s Back. Arterioscler. Thromb. Vasc. Biol. 2016, 36, e63–e72. [Google Scholar] [CrossRef]
- Houthoofd, S.; Vuylsteke, M.; Mordon, S.; Fourneau, I. Photodynamic therapy for atherosclerosis. The potential of indocyanine green. Photodiagnosis Photodyn. Ther. 2020, 29, 101568. [Google Scholar] [CrossRef]
- Kossodo, S.; LaMuraglia, G.M. Clinical potential of photodynamic therapy in cardiovascular disorders. Am. J. Cardiovasc. Drugs 2001, 1, 15–21. [Google Scholar] [CrossRef]
- Peng, C.; Li, Y.; Liang, H.; Cheng, J.; Li, Q.; Sun, X.; Li, Z.; Wang, F.; Guo, Y.; Tian, Z.; et al. Detection and photodynamic therapy of inflamed atherosclerotic plaques in the carotid artery of rabbits. J. Photochem. Photobiol. B 2011, 102, 26–31. [Google Scholar] [CrossRef]
- Yi, B.G.; Park, O.K.; Jeong, M.S.; Kwon, S.H.; Jung, J.I.; Lee, S.; Ryoo, S.; Kim, S.E.; Kim, J.W.; Moon, W.J.; et al. In vitro photodynamic effects of scavenger receptor targeted-photoactivatable nanoagents on activated macrophages. Int. J. Biol. Macromol. 2017, 97, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; McEwan, P.E.; Moore, T.I.; Pakala, R.; Kolodgie, F.D.; Hellinga, D.G.; Seabron, R.C.; Rychnovsky, S.J.; Vasek, J.; Scott, R.W.; et al. PhotoPoint photodynamic therapy promotes stabilization of atherosclerotic plaques and inhibits plaque progression. J. Am. Coll. Cardiol. 2008, 52, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, Y.; Kim, I.H.; Kim, K.; Choi, Y. ROS-responsive activatable photosensitizing agent for imaging and photodynamic therapy of activated macrophages. Theranostics 2013, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Juzeniene, A. Chlorin e6-based photosensitizers for photodynamic therapy and photodiagnosis. Photodiagnosis Photodyn. Ther. 2009, 6, 94–96. [Google Scholar] [CrossRef]
- Hsiang, Y.; Stonefield, M.; Bower, R.D.; Fragoso, M.; Tsang, V.; Crespo, M.T.; Lundkvist, A. Assessing Photofrin uptake in atherosclerosis with a fluorescent probe: Comparison with photography and tissue measurements. Lasers Surg. Med. 1993, 13, 271–278. [Google Scholar] [CrossRef]
- Kereiakes, D.J.; Szyniszewski, A.M.; Wahr, D.; Herrmann, H.C.; Simon, D.I.; Rogers, C.; Kramer, P.; Shear, W.; Yeung, A.C.; Shunk, K.A.; et al. Phase I drug and light dose-escalation trial of motexafin lutetium and far red light activation (phototherapy) in subjects with coronary artery disease undergoing percutaneous coronary intervention and stent deployment: Procedural and long-term results. Circulation 2003, 108, 1310–1315. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. Immune recognition. A new receptor for beta-glucans. Nature 2001, 413, 36–37. [Google Scholar] [CrossRef]
- Drummond, R.A.; Brown, G.D. The role of Dectin-1 in the host defence against fungal infections. Curr. Opin. Microbiol. 2011, 14, 392–399. [Google Scholar] [CrossRef]
- Huang, H.; Ostroff, G.R.; Lee, C.K.; Wang, J.P.; Specht, C.A.; Levitz, S.M. Distinct patterns of dendritic cell cytokine release stimulated by fungal beta-glucans and toll-like receptor agonists. Infect. Immun. 2009, 77, 1774–1781. [Google Scholar] [CrossRef]
- Soto, E.R.; Ostroff, G.R. Characterization of multilayered nanoparticles encapsulated in yeast cell wall particles for DNA delivery. Bioconjug. Chem. 2008, 19, 840–848. [Google Scholar] [CrossRef]
- Aouadi, M.; Tesz, G.J.; Nicoloro, S.M.; Wang, M.; Chouinard, M.; Soto, E.; Ostroff, G.R.; Czech, M.P. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature 2009, 458, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Soto, E.; Kim, Y.S.; Lee, J.; Kornfeld, H.; Ostroff, G. Glucan Particle Encapsulated Rifampicin for Targeted Delivery to Macrophages. Polymers 2010, 2, 681–689. [Google Scholar] [CrossRef]
- Nasrollahi, Z.; Mohammadi, S.R.; Mollarazi, E.; Yadegari, M.H.; Hassan, Z.M.; Talaei, F.; Dinarvand, R.; Akbari, H.; Atyabi, F. Functionalized nanoscale beta-1,3-glucan to improve Her2+ breast cancer therapy: In vitro and in vivo study. J. Control. Release 2015, 202, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, X.; Cai, F.; Zhang, L. Renaturation of triple helical polysaccharide lentinan in water-diluted dimethylsulfoxide solution. Carbohydr. Res. 2010, 345, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Sletmoen, M.; Stokke, B.T. Higher order structure of (1,3)-beta-D-glucans and its influence on their biological activities and complexation abilities. Biopolymers 2008, 89, 310–321. [Google Scholar] [CrossRef]
- Rota, C.; Chignell, C.F.; Mason, R.P. Evidence for free radical formation during the oxidation of 2’-7’-dichlorofluorescin to the fluorescent dye 2’-7’-dichlorofluorescein by horseradish peroxidase: Possible implications for oxidative stress measurements. Free Radic. Biol. Med. 1999, 27, 873–881. [Google Scholar] [CrossRef]
- Goodridge, H.S.; Reyes, C.N.; Becker, C.A.; Katsumoto, T.R.; Ma, J.; Wolf, A.J.; Bose, N.; Chan, A.S.; Magee, A.S.; Danielson, M.E.; et al. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature 2011, 472, 471–475. [Google Scholar] [CrossRef]
- Rothfuchs, A.G.; Bafica, A.; Feng, C.G.; Egen, J.G.; Williams, D.L.; Brown, G.D.; Sher, A. Dectin-1 interaction with Mycobacterium tuberculosis leads to enhanced IL-12p40 production by splenic dendritic cells. J. Immunol. 2007, 179, 3463–3471. [Google Scholar] [CrossRef]
- Li, X.; Luo, H.; Ye, Y.; Chen, X.; Zou, Y.; Duan, J.; Xiang, D. betaglucan, a dectin1 ligand, promotes macrophage M1 polarization via NFkappaB/autophagy pathway. Int. J. Oncol. 2019, 54, 271–282. [Google Scholar] [CrossRef]
- Crescenzi, E.; Varriale, L.; Iovino, M.; Chiaviello, A.; Veneziani, B.M.; Palumbo, G. Photodynamic therapy with indocyanine green complements and enhances low-dose cisplatin cytotoxicity in MCF-7 breast cancer cells. Mol. Cancer Ther. 2004, 3, 537–544. [Google Scholar]
- Wlodkowic, D.; Telford, W.; Skommer, J.; Darzynkiewicz, Z. Apoptosis and beyond: Cytometry in studies of programmed cell death. Methods Cell Biol. 2011, 103, 55–98. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, J.W.; Kim, J.H.; Park, K. In Vitro Photodynamic Effects of the Inclusion Nanocomplexes of Glucan and Chlorin e6 on Atherogenic Foam Cells. Int. J. Mol. Sci. 2021, 22, 177. https://doi.org/10.3390/ijms22010177
Ahn JW, Kim JH, Park K. In Vitro Photodynamic Effects of the Inclusion Nanocomplexes of Glucan and Chlorin e6 on Atherogenic Foam Cells. International Journal of Molecular Sciences. 2021; 22(1):177. https://doi.org/10.3390/ijms22010177
Chicago/Turabian StyleAhn, Jae Won, Jin Hyuk Kim, and Kyeongsoon Park. 2021. "In Vitro Photodynamic Effects of the Inclusion Nanocomplexes of Glucan and Chlorin e6 on Atherogenic Foam Cells" International Journal of Molecular Sciences 22, no. 1: 177. https://doi.org/10.3390/ijms22010177
APA StyleAhn, J. W., Kim, J. H., & Park, K. (2021). In Vitro Photodynamic Effects of the Inclusion Nanocomplexes of Glucan and Chlorin e6 on Atherogenic Foam Cells. International Journal of Molecular Sciences, 22(1), 177. https://doi.org/10.3390/ijms22010177





