OsMre11 Is Required for Mitosis during Rice Growth and Development
Abstract
1. Introduction
2. Results
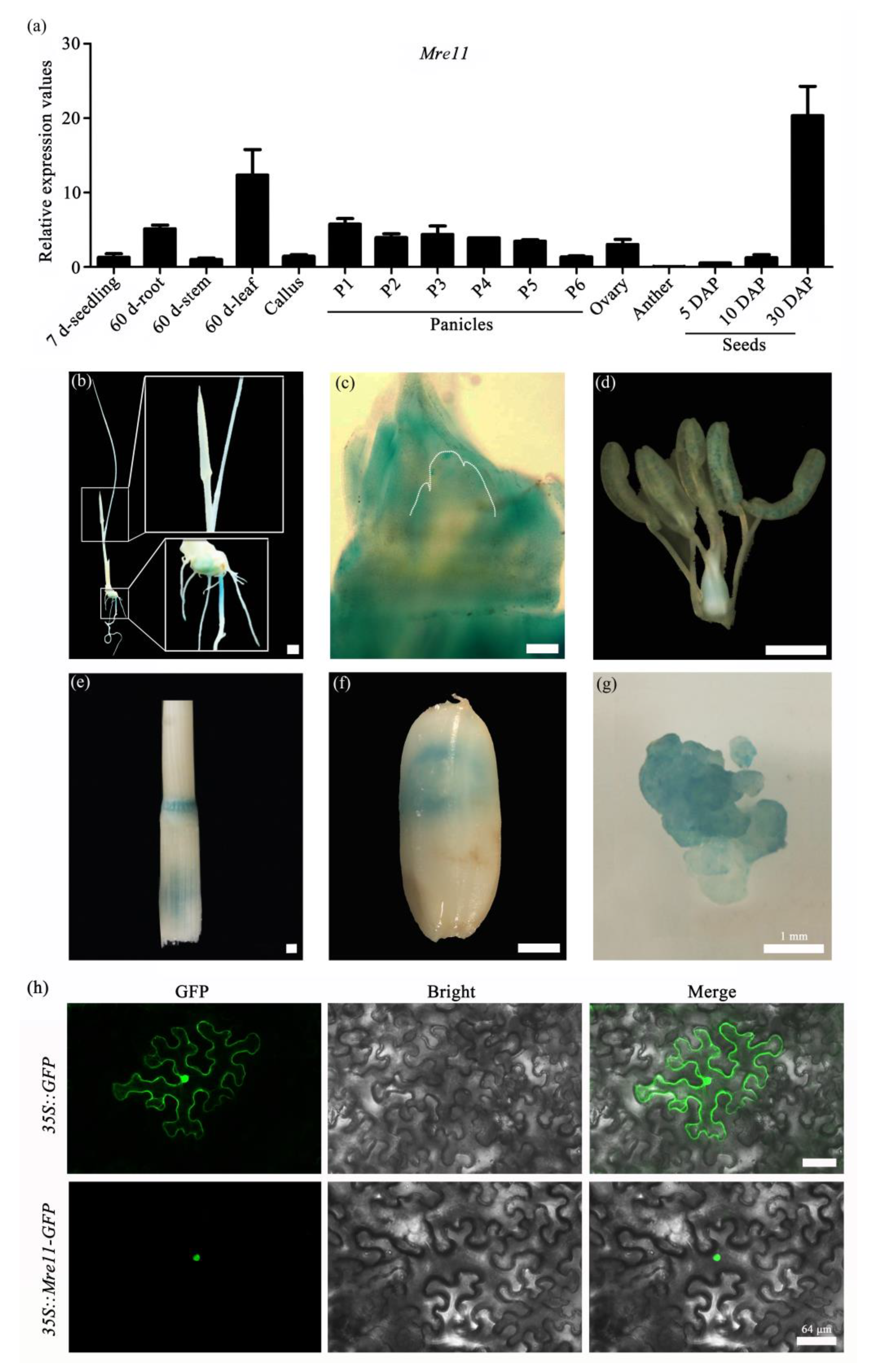
2.1. Mre11 Is Conserved in Various Species and Expressed Extensively during the Growth and Development of Rice
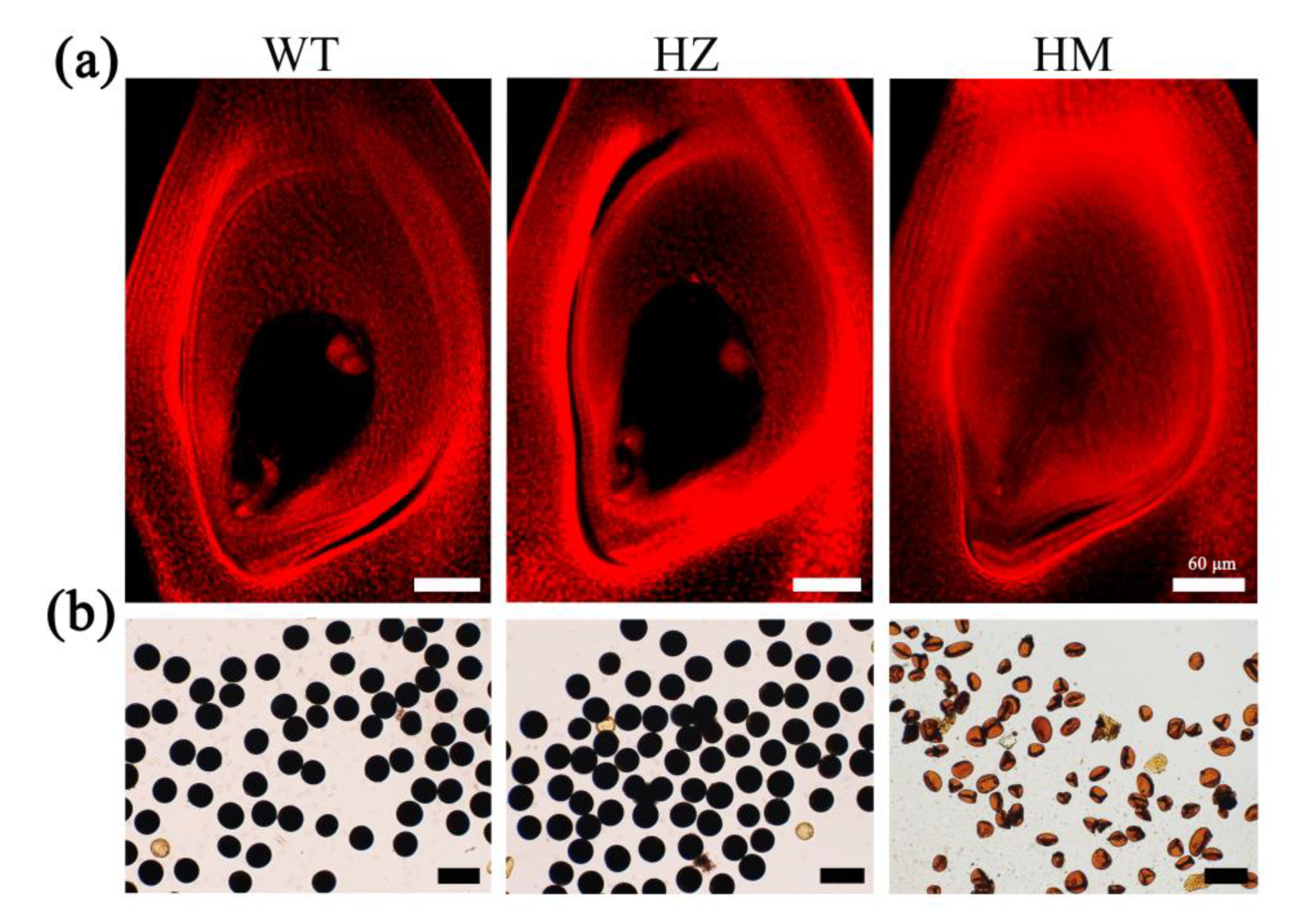
2.2. Knock-Out of OsMre11 Causes Dwarfism of the Rice Plant
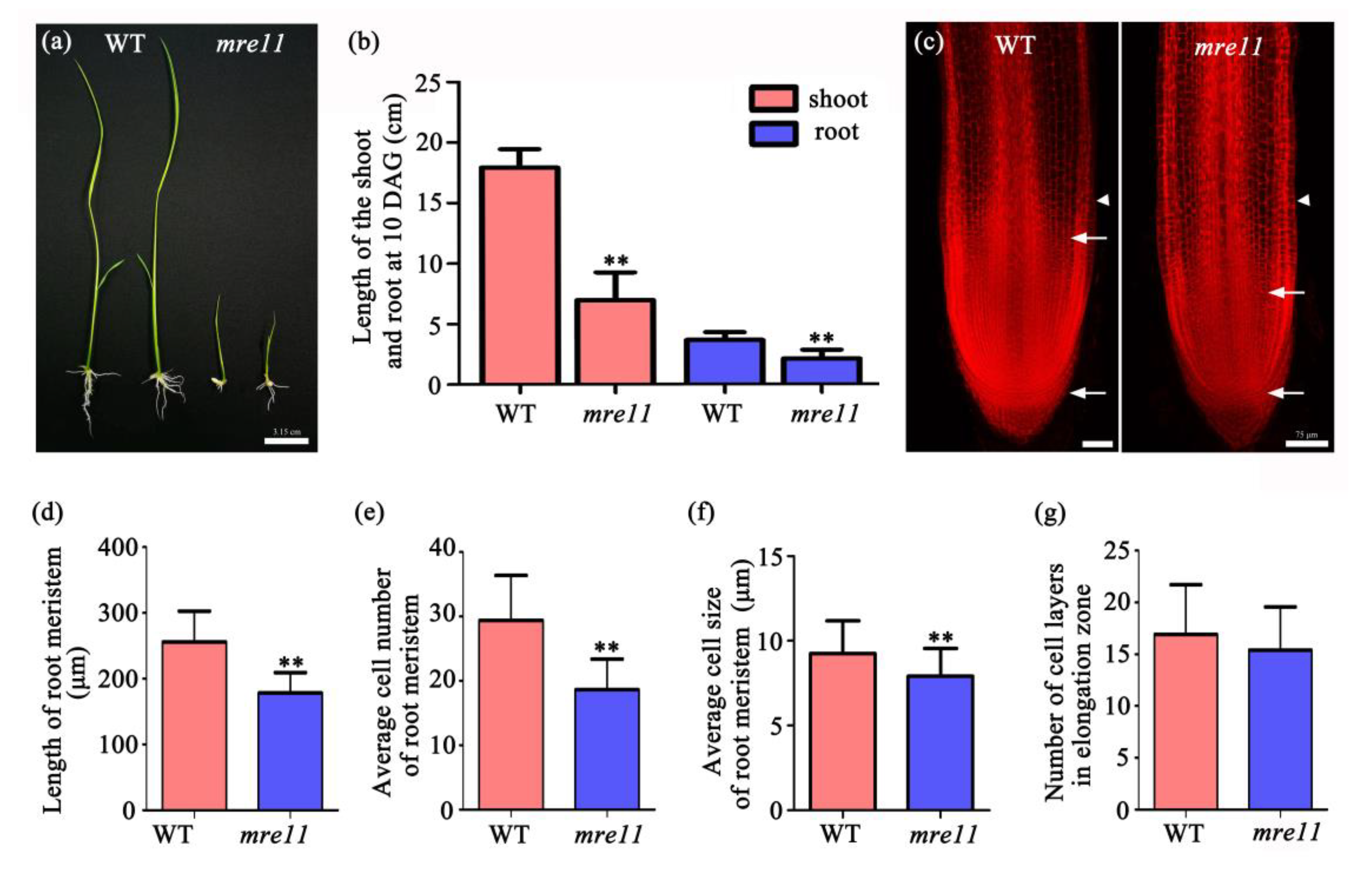
2.3. The Cell Division Activity in the Root Apical Meristem of the mre11 Mutant Was Inhibited
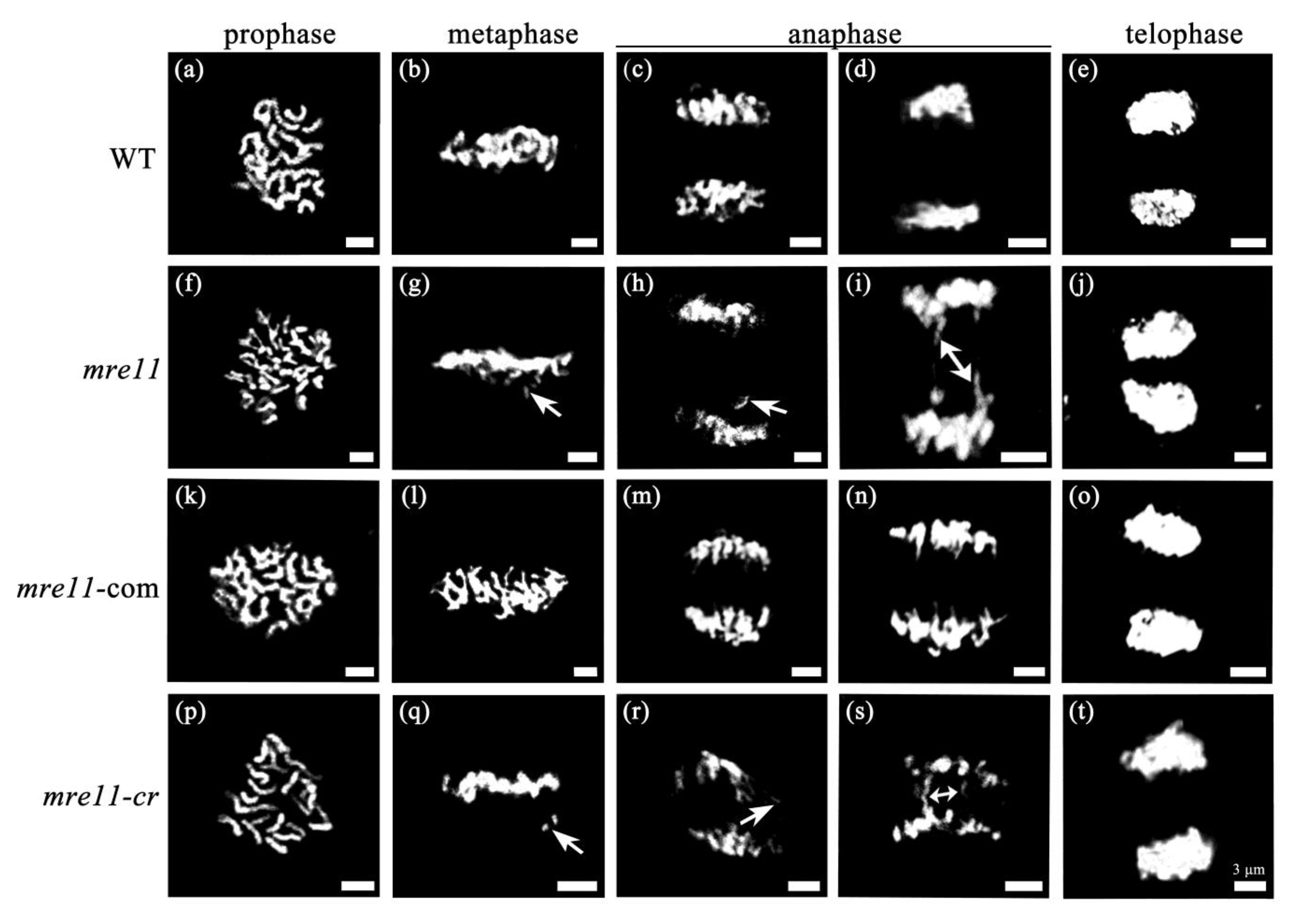
2.4. Mitotic Division Is Aberrant in the Root Apical Meristem of the mre11 Seedlings
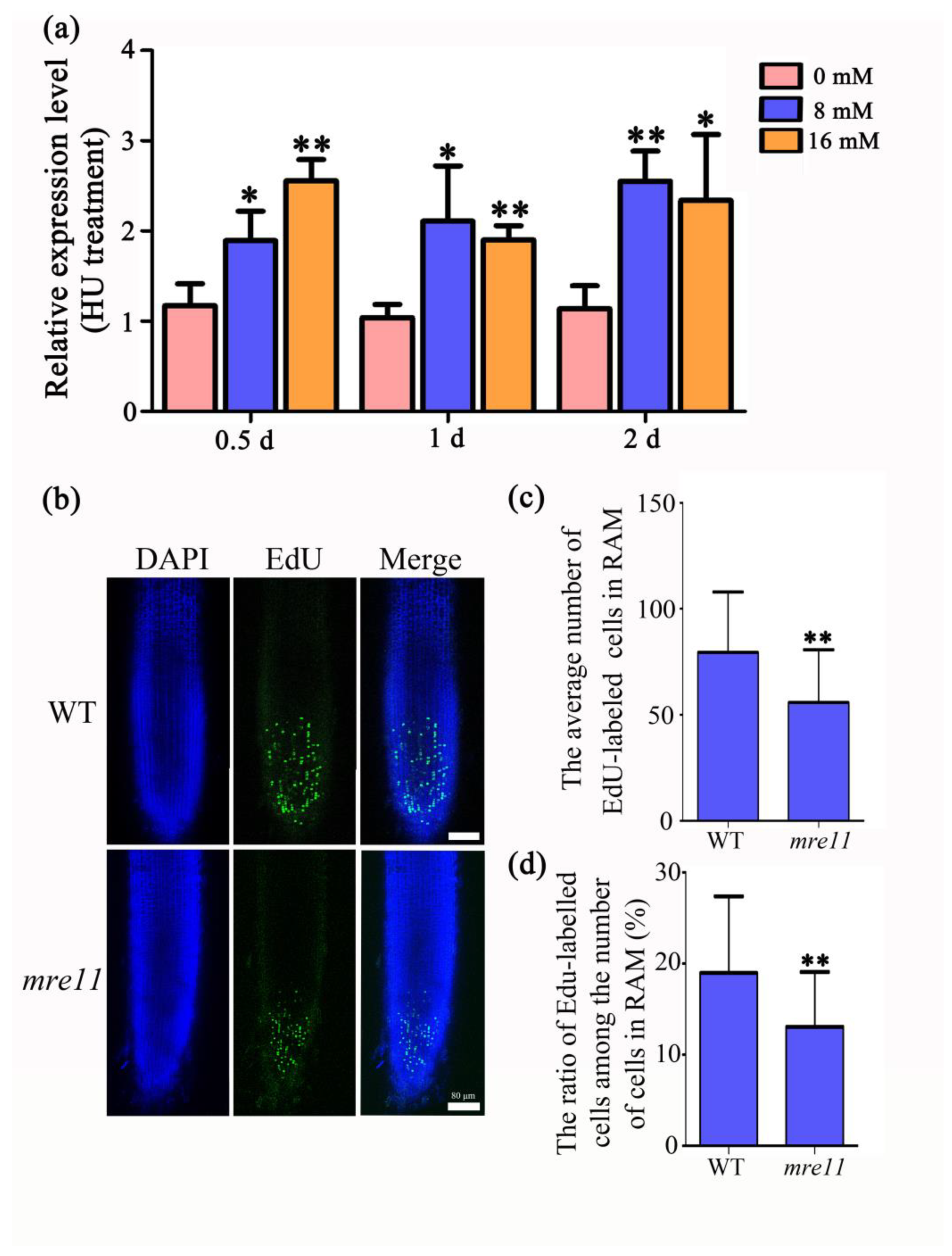
2.5. OsMre11 Functions in DNA Replication during Mitosis
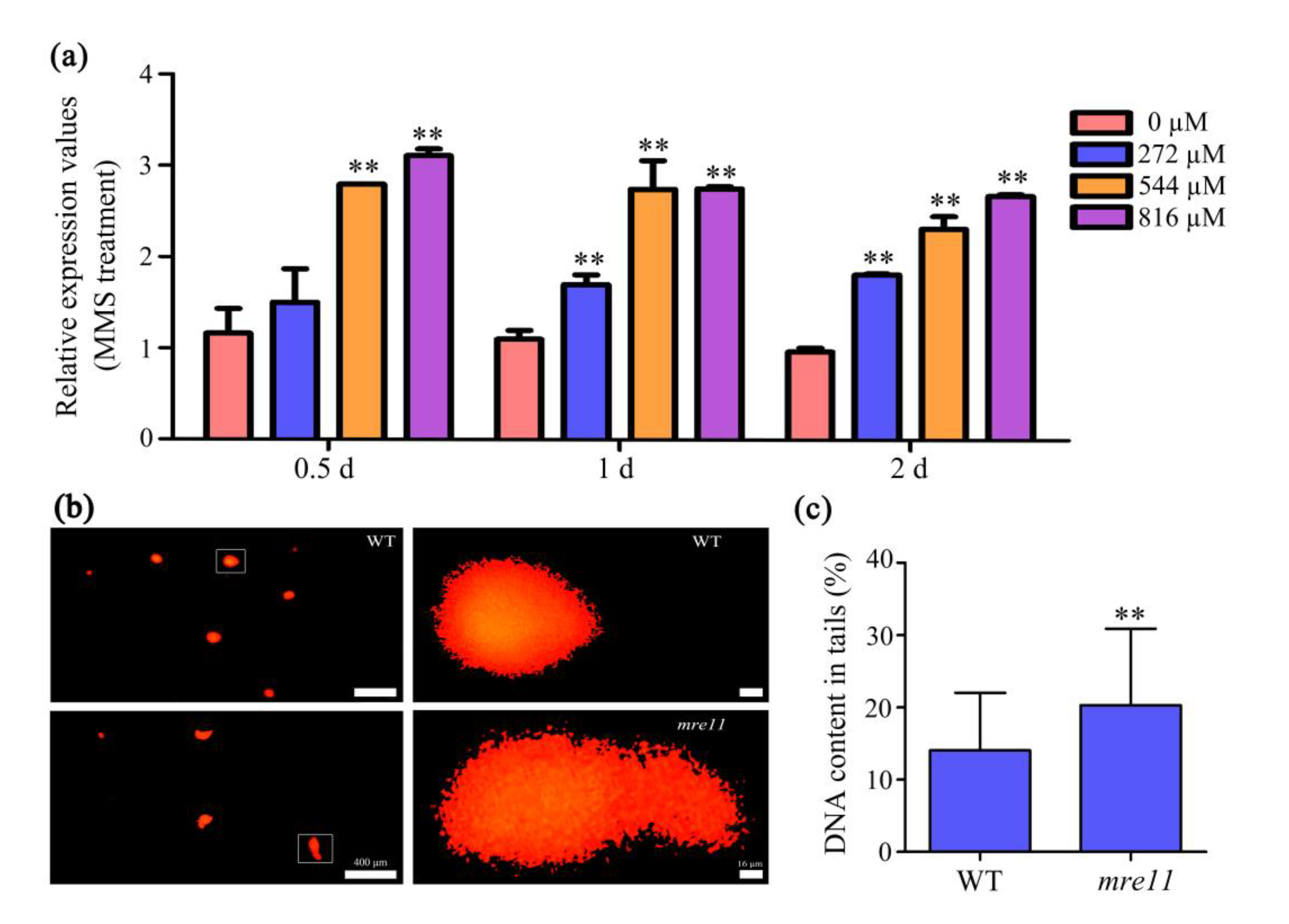
2.6. OsMre11 Is Essential for DNA Damage Repair in Somatic Cells
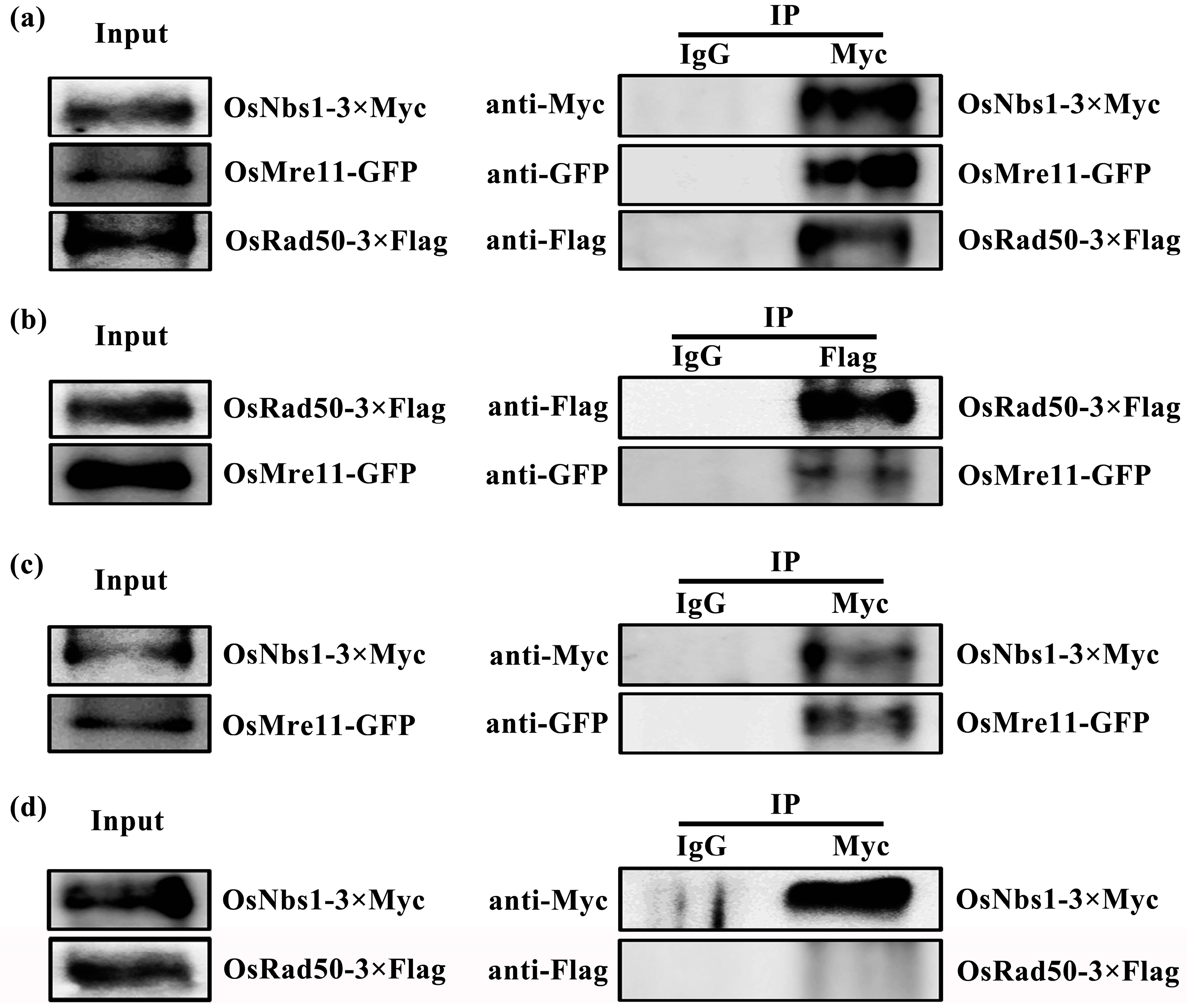
2.7. OsMre11 Plays Crucial Roles through Interacting with OsRad50 and OsNbs1
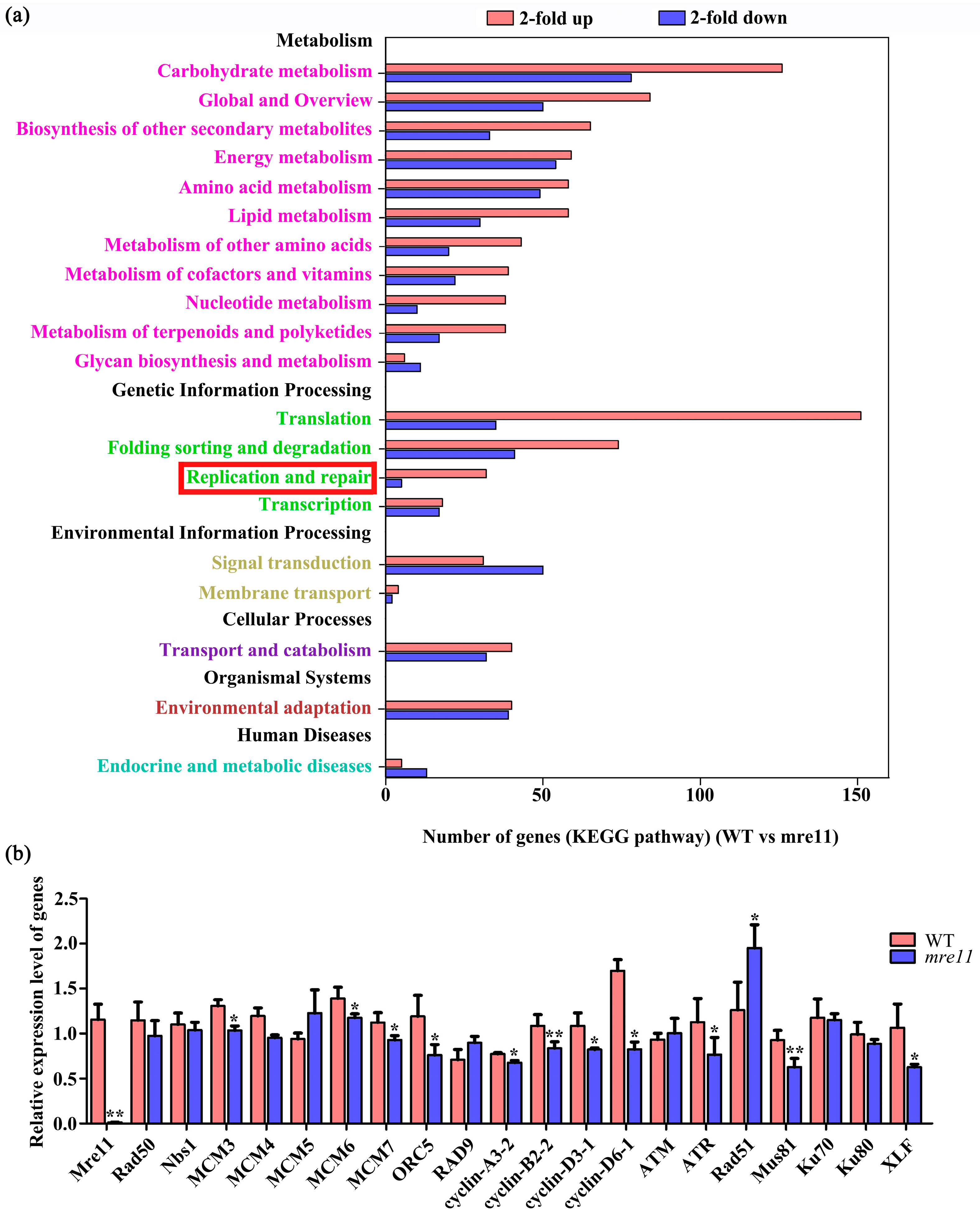
2.8. Transcriptome Comparative Analysis of Rice Wild Type and the mre11 Mutant
3. Discussion
3.1. Mre11 Is Indispensable for Growth and Development
3.2. OsMre11 Is Essential for Rice Mitosis
4. Conclusions
5. Materials and Methods
5.1. Plant Materials and Growth Conditions
5.2. Phylogenetic Analysis
5.3. qRT-PCR Assay
5.4. GUS Staining
5.5. Subcellular Localization of OsMre11
5.6. Plant Phenotype Analysis
5.7. Mitosis Assay
5.8. Treatment with DNA Replication Inhibitors and DNA Damage Reagents
5.9. EdU Staining Analysis
5.10. Comet Assay
5.11. Homology Modeling
5.12. BiFC Assay
5.13. Co-IP Analysis
5.14. RNA-Seq Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Con sent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waldminghaus, T.; Skarstad, K. The Escherichia coli SeqA protein. Plasmid 2009, 61, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.L.; Kleckner, N.E. coli oriC and the dnaA gene promoter are sequestered from dam methyltransferase following the passage of the chromosomal replication fork. Cell 1990, 62, 967–979. [Google Scholar] [CrossRef]
- Fragkos, M.; Ganier, O.; Coulombe, P.; Mechali, M. DNA replication origin activation in space and time. Nat. Rev. Mol. Cell Biol. 2015, 16, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, H.; Lygerou, Z. Control of DNA replication licensing in a cell cycle. Genes Cells 2002, 7, 523–534. [Google Scholar] [CrossRef]
- Johnson, C.; Gali, V.K.; Takahashi, T.S.; Kubota, T. PCNA Retention on DNA into G2/M Phase Causes Genome Instability in Cells Lacking Elg1. Cell Rep. 2016, 16, 684–695. [Google Scholar] [CrossRef]
- Knoll, A.; Fauser, F.; Puchta, H. DNA recombination in somatic plant cells: Mechanisms and evolutionary consequences. Chromosome Res. 2014, 22, 191–201. [Google Scholar] [CrossRef]
- Rich, T.; Allen, R.L.; Wyllie, A.H. Defying death after DNA damage. Nature 2000, 407, 777–783. [Google Scholar] [CrossRef]
- Puchta, H. The repair of double-strand breaks in plants: Mechanisms and consequences for genome evolution. J. Exp. Bot. 2005, 56, 1–14. [Google Scholar] [CrossRef]
- Lukas, J.; Lukas, C. Molecular biology. Shielding broken DNA for a quick fix. Science 2013, 339, 652–653. [Google Scholar] [CrossRef]
- Hopfner, K.P. DNA Double-Strand Breaks Come into Focus. Cell 2009, 139, 25–27. [Google Scholar] [CrossRef]
- Czornak, K.; Chughtai, S.; Chrzanowska, K.H. Mystery of DNA repair: The role of the MRN complex and ATM kinase in DNA damage repair. J. Appl. Genet. 2008, 49, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Amiard, S.; Charbonnel, C.; Allain, E.; Depeiges, A.; White, C.I.; Gallego, M.E. Distinct Roles of the ATR Kinase and the Mre11-Rad50-Nbs1 Complex in the Maintenance of Chromosomal Stability in Arabidopsis. Plant Cell 2010, 22, 3020–3033. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.T.; Wang, W.B.; Li, S.Y.; Zhan, J.; Li, H.X.; Zhao, M.M.; Zhou, X.A.; Li, S.W.; Li, X.M.; Huo, Y.F.; et al. C1QBP Promotes Homologous Recombination by Stabilizing MRE11 and Controlling the Assembly and Activation of MRE11/RAD50/NBS1 Complex. Mol. Cell 2019, 75, 1299–1314.e6. [Google Scholar] [CrossRef] [PubMed]
- Aravind, L.; Walker, D.R.; Koonin, E.V. Conserved domains in DNA repair proteins and evolution of repair systems. Nucleic Acids Res. 1999, 27, 1223–1242. [Google Scholar] [CrossRef]
- Paull, T.T.; Gellert, M. Nbs1 potentiates ATP-driven DNA unwinding and endonuclease cleavage by the Mre11/Rad50 complex. Genes Dev. 1999, 13, 1276–1288. [Google Scholar] [CrossRef]
- Paull, T.T.; Gellert, M. The 3′ to 5′ exonuclease activity of Mre 11 facilitates repair of DNA double-strand breaks. Mol. Cell 1998, 1, 969–979. [Google Scholar] [CrossRef]
- Paull, T.T. 20 Years of Mre11 Biology: No End in Sight. Mol. Cell 2018, 71, 419–427. [Google Scholar] [CrossRef]
- Hopfner, K.P.; Craig, L.; Moncalian, G.; Zinkel, R.A.; Usui, T.; Owen BA, L.; Karcher, A.; Henderson, B.; Bodmer, J.L.; McMurray, C.T.; et al. The Rad50 zinc-hook is a structure joining Mre11 complexes in DNA recombination and repair. Nature 2002, 418, 562–566. [Google Scholar] [CrossRef]
- Tauchi, H.; Kobayashi, J.; Morishima, K.; van Gent, D.C.; Shiraishi, T.; Verkaik, N.S.; vanHeems, D.; Ito, E.; Nakamura, A.; Sonodo, E.; et al. Nbs1 is essential for DNA repair by homologous recombination in higher vertebrate cells. Nature 2002, 420, 93–98. [Google Scholar] [CrossRef]
- Falck, J.; Coates, J.; Jackson, S.P. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature 2005, 434, 605–611. [Google Scholar] [CrossRef]
- Lee, J.H.; Paull, T.T. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science 2005, 308, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Higgins, J.D.; He, Y.; Lu, P.; Zhang, D.; Liang, W. Resolvase OsGEN1 Mediates DNA Repair by Homologous Recombination. Plant Physiol. 2017, 173, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.B.; Daley, J.M.; Kwon, Y.; Krasner, D.S.; Sung, P. Plasticity of the Mre11-Rad50-Xrs2-Sae2 nuclease ensemble in the processing of DNA-bound obstacles. Genes Dev. 2017, 31, 2331–2336. [Google Scholar] [CrossRef] [PubMed]
- Cannavo, E.; Cejka, P. Sae2 promotes dsDNA endonuclease activity within Mre11-Rad50-Xrs2 to resect DNA breaks. Nature 2014, 514, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Reginato, G.; Cannavo, E.; Cejka, P. Physiological protein blocks direct the Mre11-Rad50-Xrs2 and Sae2 nuclease complex to initiate DNA end resection. Genes Dev. 2017, 31, 2325–2330. [Google Scholar] [CrossRef] [PubMed]
- Lavin, M.F.; Birrell, G.; Chen, P.; Kozlov, S.; Scott, S.; Gueven, N. ATM signaling and genomic stability in response to DNA damage. Mutat. Res. 2005, 569, 123–132. [Google Scholar] [CrossRef]
- Mimitou, E.P.; Symington, L.S. DNA end resection: Many nucleases make light work. DNA Repair 2009, 8, 983–995. [Google Scholar] [CrossRef]
- Gallego, M.E.; Jeanneau, M.; Granier, F.; Bouchez, D.; Bechtold, N.; White, C.I. Disruption of the Arabidopsis RAD50 gene leads to plant sterility and MMS sensitivity. Plant J. 2001, 25, 31–41. [Google Scholar]
- Waterworth, W.M.; Altun, C.; Armstrong, S.J.; Roberts, N.; Dean, P.J.; Young, K.; Weil, C.F.; Bray, C.M.; West, C.E. NBS1 is involved in DNA repair and plays a synergistic role with ATM in mediating meiotic homologous recombination in plants. Plant J. 2007, 52, 41–52. [Google Scholar] [CrossRef]
- Ajimura, M.; Leem, S.H.; Ogawa, H. Identification of New Genes Required for Meiotic Recombination in Saccharomyces-Cerevisiae. Genetics 1993, 133, 51–66. [Google Scholar]
- Tavassoli, M.; Shayeghi, M.; Nasim, A.; Watts, F.Z. Cloning and Characterization of the Schizosaccharomyces-Pombe Rad32 Gene—A Gene Required for Repair of Double-Strand Breaks and Recombination. Nucleic Acids Res. 1995, 23, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Chin, G.M.; Villeneuve, A.M. C-elegans mre-11 is required for meiotic recombination and DNA repair but is dispensable for the meiotic G(2) DNA damage checkpoint. Genes Dev. 2001, 15, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Buis, J.; Wu, Y.P.; Deng, Y.B.; Leddon, J.; Westfield, G.; Eckersdorff, M.; Sekiguchi, J.M.; Chang, S.; Ferguson, D.O. Mre11 nuclease activity has essential roles in DNA repair and genomic stability distinct from ATM activation. Cell 2008, 135, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Carney, J.P.; Maser, R.S.; Olivares, H.; Davis, E.M.; Le Beau, M.; Yates, J.R.; Hays, L.; Morgan, W.F.; Petrini, J.H. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: Linkage of double-strand break repair to the cellular DNA damage response. Cell 1998, 93, 477–486. [Google Scholar] [CrossRef]
- Ji, J.H.; Tang, D.; Wang, M.; Li, Y.F.; Zhang, L.; Wang, K.J.; Li, M.; Cheng, Z.K. MRE11 is required for homologous synapsis and DSB processing in rice meiosis. Chromosoma 2013, 122, 363–376. [Google Scholar] [CrossRef]
- Maser, R.S.; Zinkel, R.; Petrini JH, J. An alternative mode of translation permits production of a variant NBS1 protein from the common Nijmegen breakage syndrome allele. Nat. Genet. 2001, 27, 417–421. [Google Scholar] [CrossRef]
- Hayashi, K.; Hasegawa, J.; Matsunaga, S. The boundary of the meristematic and elongation zones in roots: Endoreduplication precedes rapid cell expansion. Sci. Rep. 2013, 3, 2723. [Google Scholar] [CrossRef]
- Ostling, O.; Johanson, K.J. Microelectrophoretic Study of Radiation-Induced DNA Damages in Individual Mammalian-Cells. Biochem. Biophys. Res. Commun. 1984, 123, 291–298. [Google Scholar] [CrossRef]
- Gobbini, E.; Cassani, C.; Villa, M.; Bonetti, D.; Longhese, M.P. Functions and regulation of the MRX complex at DNA double-strand breaks. Microb. Cell 2016, 3, 329–337. [Google Scholar] [CrossRef]
- Lu, H.M.; Shamanna, R.A.; Keijzers, G.; Anand, R.; Rasmussen, L.J.; Cejka, P.; Croteau, D.L.; Bohr, V.A. RECQL4 Promotes DNA End Resection in Repair of DNA Double-Strand Breaks. Cell Rep. 2016, 16, 161–173. [Google Scholar] [CrossRef]
- Wright, W.D.; Shah, S.S.; Heyer, W.D. Homologous recombination and the repair of DNA double-strand breaks. J. Biol. Chem. 2018, 293, 10524–10535. [Google Scholar] [CrossRef] [PubMed]
- Jasin, M.; Rothstein, R. Repair of strand breaks by homologous recombination. Cold Spring Harb. Perspect. Biol. 2013, 5, a012740. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 2017, 18, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Pannunzio, N.R.; Watanabe, G.; Lieber, M.R. Nonhomologous DNA end-joining for repair of DNA double-strand breaks. J. Biol. Chem. 2018, 293, 10512–10523. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Nagase, Y.; Tsubouchi, H.; Murakami-Murofushi, K.; Shibata, T.; Ohta, K. Distinct roles of two separable in vitro activities of yeast Mre11 in mitotic and meiotic recombination. EMBO J. 1998, 17, 6412–6425. [Google Scholar] [CrossRef] [PubMed]
- Aravind, L.; Koonin, E.V. Phosphoesterase domains associated with DNA polymerases of diverse origins. Nucleic Acids Res. 1998, 26, 3746–3752. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.S.; Maser, R.S.; Stankovic, T.; Bressan, D.A.; Kaplan, M.I.; Jaspers NG, J.; Raams, A.; Byrd, P.J.; Petrini JH, J.; Taylor AM, R. The DNA double-strand break repair gene hMRE11 is mutated in individuals with an ataxia-telangiectasia-like disorder. Cell 1999, 99, 577–587. [Google Scholar] [CrossRef]
- Schiller, C.B.; Lammens, K.; Guerini, I.; Coordes, B.; Feldmann, H.; Schlauderer, F.; Mockel, C.; Schele, A.; Strasser, K.; Jackson, S.P.; et al. Structure of Mre11-Nbs1 complex yields insights into ataxia-telangiectasia-like disease mutations and DNA damage signaling. Nat. Struct. Mol. Biol. 2012, 19, 693. [Google Scholar] [CrossRef]
- Bundock, P.; Hooykaas, P. Severe developmental defects, hypersensitivity to DNA-damaging agents, and lengthened telomeres in Arabidopsis MRE11 mutants. Plant Cell 2002, 14, 2451–2462. [Google Scholar] [CrossRef]
- Li, G.; Zou, W.X.; Jian, L.F.; Qian, J.; Deng, Y.T.; Zhao, J. Non-SMC elements 1 and 3 are required for early embryo and seedling development in Arabidopsis. J. Exp. Bot. 2017, 68, 1039–1054. [Google Scholar] [CrossRef]
- Qian, J.; Chen, Y.Y.; Hu, Y.; Deng, Y.T.; Liu, Y.; Li, G.; Zou, W.X.; Zhao, J. Arabidopsis replication factor C4 is critical for DNA replication during the mitotic cell cycle. Plant J. 2018, 94, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Šamanić, I.; Cvitanić, R.; Simunić, J.; Puizina, J. Arabidopsis thaliana MRE11 is essential for activation of cell cycle arrest, transcriptional regulation and DNA repair upon the induction of double-stranded DNA breaks. Plant Biol. 2016, 18, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Wang, Y.H.; Wang, C.M.; Lyu, J.; Wang, Y.L.; Dong, H.; Long, W.H.; Wang, D.; Kong, W.Y.; Wang, L.W.; et al. ALR encoding dCMP deaminase is critical for DNA damage repair, cell cycle progression and plant development in rice. J. Exp. Bot. 2017, 68, 5773–5786. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kalhorzadeh, P.; Hu, Z.B.; Cools, T.; Amiard, S.; Willing, E.M.; De Winne, N.; Gevaert, K.; De Jaeger, G.; Schneeberger, K.; White, C.I.; et al. Arabidopsis thaliana RNase H2 Deficiency Counteracts the Needs for the WEE1 Checkpoint Kinase but Triggers Genome Instability. Plant Cell 2014, 26, 3680–3692. [Google Scholar] [CrossRef]
- Vesela, E.; Chroma, K.; Turi, Z.; Mistrik, M. Common Chemical Inductors of Replication Stress: Focus on Cell-Based Studies. Biomolecules 2017, 7, 19. [Google Scholar] [CrossRef]
- Bleuyard, J.Y.; Gallego, M.E.; White, C.I. Meiotic defects in the Arabidopsis rad50 mutant point to conservation of the MRX complex function in early stages of meiotic recombination. Chromosoma 2004, 113, 197–203. [Google Scholar] [CrossRef]
- Puizina, J.; Siroky, J.; Mokros, P.; Schweizer, D.; Riha, K. Mre11 deficiency in Arabidopsis is associated with chromosomal instability in somatic cells and Spo11-dependent genome fragmentation during meiosis. Plant Cell 2004, 6, 1968–1978. [Google Scholar] [CrossRef]
- Lobachev, K.; Vitriol, E.; Stemple, J.; Resnick, M.A.; Bloom, K. Chromosome fragmentation after induction of a double-strand break is an active process prevented by the RMX repair complex. Curr. Biol. 2004, 14, 2107–2112. [Google Scholar] [CrossRef]
- Kaye, J.A.; Melo, J.A.; Cheung, S.K.; Vaze, M.B.; Haber, J.E.; Toczyski, D.P. DNA breaks promote genomic instability by impeding proper chromosome segregation. Curr. Biol. 2004, 14, 2096–2106. [Google Scholar] [CrossRef]
- Wiltzius, J.J.; Hohl, M.; Fleming, J.C.; Petrini, J.H. The Rad50 hook domain is a critical determinant of Mre11 complex functions. Nat. Struct. Mol. Biol. 2005, 12, 403–407. [Google Scholar] [CrossRef]
- Reuven, N.; Adler, J.; Broennimann, K.; Myers, N.; Shaul, Y. Recruitment of DNA Repair MRN Complex by Intrinsically Disordered Protein Domain Fused to Cas9 Improves Efficiency of CRISPR-Mediated Genome Editing. Biomolecules 2019, 9, 584. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.L.; Yuan, D.K.; Liu, M.; Li, C.X.; Liu, Y.Y.; Zhang, S.C.; Yao, N.; Yang, C.W. AtMMS21, an SMC5/6 Complex Subunit, Is Involved in Stem Cell Niche Maintenance and DNA Damage Responses in Arabidopsis Roots. Plant Physiol. 2013, 161, 1755–1768. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Xiao, R.; Wang, H.F.; Cheng, Z.H.; Li, W.X.; Zhu, G.F.; Wang, Y.; Ma, H. The Arabidopsis RAD51 paralogs RAD51B, RAD51D and XRCC2 play partially redundant roles in somatic DNA repair and gene regulation. New Phytol. 2014, 201, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Da Ines, O.; Degroote, F.; Amiard, S.; Goubely, C.; Gallego, M.E.; White, C.I. Effects of XRCC2 and RAD51B mutations on somatic and meiotic recombination in Arabidopsis thaliana. Plant J. 2013, 74, 959–970. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, J.; Xu, M.; Ji, W.; Yu, M.; Tao, Y.; Gong, Z.; Gu, M.; Yu, H. Rice RAD51 paralogs play essential roles in somatic homologous recombination for DNA repair. Plant J. 2018, 95, 282–295. [Google Scholar] [CrossRef]
- Peng, X.J.; Liu, S.J.; Bao, C.M.; Liu, Y.Z.; Xie, H.W.; Cai, Y.H.; Li, B.M.; Hang, H.Y.; Ding, X. Regulation of ATRIP protein abundance by RAD9 in the DNA damage repair pathway. Cell Mol. Biol. 2015, 61, 31–36. [Google Scholar]
- Zhao, B.; Watanabe, G.; Morten, M.J.; Reid, D.A.; Rothenberg, E.; Lieber, M.R. The essential elements for the noncovalent association of two DNA ends during NHEJ synapsis. Nat. Commun. 2019, 10, 3588. [Google Scholar] [CrossRef]
- Samanić, I.; Simunić, J.; Riha, K.; Puizina, J. Evidence for distinct functions of MRE11 in Arabidopsis meiosis. PLoS ONE 2013, 8, e78760. [Google Scholar]
- Miao, J.; Guo, D.S.; Zhang, J.Z.; Huang, Q.P.; Qin, G.J.; Zhang, X.; Wan, J.M.; Gu, H.Y.; Qu, L.J. Targeted mutagenesis in rice using CRISPR-Cas system. Cell Res. 2013, 23, 1233–1236. [Google Scholar] [CrossRef]
- Zhong, H.; Simons, J.W. Direct comparison of GAPDH, beta-actin, cyclophilin, and 28S rRNA as internal standards for quantifying RNA levels under hypoxia. Biochem. Biophys. Res. Commun. 1999, 259, 523–526. [Google Scholar] [CrossRef]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. Gus Fusions—Beta-Glucuronidase as a Sensitive and Versatile Gene Fusion Marker in Higher-Plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.L.; Jiang, W.; Long, F.; Cheng, S.F.; Yang, W.J.; Zhao, Y.; Zhou, D.X. Rice Homeodomain Protein WOX11 Recruits a Histone Acetyltransferase Complex to Establish Programs of Cell Proliferation of Crown Root Meristem. Plant Cell 2017, 29, 1088–1104. [Google Scholar] [CrossRef]
- Guex, N.; Peitsch, M.C.; Schwede, T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: A historical perspective. Electrophoresis 2009, 30, S162–S173. [Google Scholar] [CrossRef] [PubMed]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef] [PubMed]
- Seifert, F.U.; Lammens, K.; Hopfner, K.P. Structure of the catalytic domain of Mre11 from Chaetomium thermophilum. Acta Crystallogr. Sect. F-Struct. Biol. Commun. 2015, 71, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Sparkes, I.A.; Runions, J.; Kearns, A.; Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 2006, 1, 2019–2025. [Google Scholar] [CrossRef]
- Deng, Y.T.; Zou, W.X.; Li, G.; Zhao, J. TRANSLOCASE OF THE INNER MEMBRANE9 and 10 Are Essential for Maintaining Mitochondrial Function during Early Embryo Cell and Endosperm Free Nucleus Divisions in Arabidopsis. Plant Physiol. 2014, 166, 853–868. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef]
- Symington, L.S. Role of RAD52 epistasis group genes in homologous recombination and double-strand break repair. Microbiol. Mol. Biol. Rev. 2002, 66, 630–670. [Google Scholar] [CrossRef]
- Roy, S.; Choudhury, S.R.; Sengupta, D.N.; Das, K.P. Involvement of AtPollambda in the repair of high salt- and DNA cross-linking agent-induced double strand breaks in Arabidopsis. Plant Physiol. 2013, 162, 1195–1210. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, M.; Nie, Y.; Chen, Y.; Zhang, X.; Zhao, J. OsMre11 Is Required for Mitosis during Rice Growth and Development. Int. J. Mol. Sci. 2021, 22, 169. https://doi.org/10.3390/ijms22010169
Shen M, Nie Y, Chen Y, Zhang X, Zhao J. OsMre11 Is Required for Mitosis during Rice Growth and Development. International Journal of Molecular Sciences. 2021; 22(1):169. https://doi.org/10.3390/ijms22010169
Chicago/Turabian StyleShen, Miaomiao, Yanshen Nie, Yueyue Chen, Xiufeng Zhang, and Jie Zhao. 2021. "OsMre11 Is Required for Mitosis during Rice Growth and Development" International Journal of Molecular Sciences 22, no. 1: 169. https://doi.org/10.3390/ijms22010169
APA StyleShen, M., Nie, Y., Chen, Y., Zhang, X., & Zhao, J. (2021). OsMre11 Is Required for Mitosis during Rice Growth and Development. International Journal of Molecular Sciences, 22(1), 169. https://doi.org/10.3390/ijms22010169



