Chemical Evaluation of Eumelanin Maturation by ToF-SIMS and Alkaline Peroxide Oxidation HPLC Analysis
Abstract
1. Introduction
2. Results
2.1. Maturation
2.2. AHPO Analysis
2.3. ToF-SIMS
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Maturation at High Temperatures and Pressures
4.3. ToF-SIMS
4.4. Alkaline Hydrogen Peroxide Oxidation (AHPO)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHPO | Alkaline hydrogen peroxide oxidation |
| ToF-SIMS | Time-of-flight secondary ion mass spectrometry |
| PCA | Principal component analysis |
| DHI | 5,6-Dihyroxyindole |
| DHICA | 5,6-Dihydroxyindole-2-carboxylic acid |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| HPLC | High performance liquid chromatography |
| PDCA | Pyrrole-2,3-dicarboxylic acid |
| PTCA | Pyrrole-2,3,5-tricarboxylic acid |
| PTeCA | Pyrrole-2,3,4,5-tetracarboxylic acid |
| Iso-PTCA | Pyrrole-2,3,4-tricarboxylic acid |
References
- Solano, F. Melanins: Skin pigments and much more—Types, structural models, biological functions, and formation routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef]
- Simon, J.D.; Peles, D.; Wakamatsu, K.; Ito, S. Current challenges in understanding melanogenesis: Bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res. 2009, 22, 563–579. [Google Scholar] [CrossRef] [PubMed]
- D’Ischia, M.; Wakamatsu, K.; Cicoira, F.; Di Mauro, E.; Garcia-Borron, J.C.; Commo, S.; Galván, I.; Ghanem, G.; Koike, K.; Meredith, P.; et al. Melanins and melanogenesis: From pigment cells to human health and technological applications. Pigment Cell Melanoma Res. 2015, 28, 520–544. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, J.; Sjövall, P.; Thiel, V.; Zheng, W.; Ito, S.; Wakamatsu, K.; Hauff, R.; Kear, B.P.; Engdahl, A.; Alwmark, C.; et al. Soft-tissue evidence for homeothermy and crypsis in a Jurassic ichthyosaur. Nature 2018, 564, 359–365. [Google Scholar] [CrossRef]
- Lindgren, J.; Kuriyama, T.; Madsen, H.; Sjövall, P.; Zheng, W.; Uvdal, P.; Engdahl, A.; Moyer, A.E.; Gren, J.A.; Kamezaki, N.; et al. Biochemistry and adaptive colouration of an exceptionally preserved juvenile fossil sea turtle. Sci. Rep. 2017, 7, 13324. [Google Scholar] [CrossRef]
- Glass, K.; Ito, S.; Wilby, P.R.; Sota, T.; Nakamura, A.; Bowers, C.R.; Vinther, J.; Dutta, S.; Summons, R.; Briggs, D.E.G.; et al. Direct chemical evidence for eumelanin pigment from the Jurassic period. Proc. Natl. Acad. Sci. USA 2012, 109, 10218–10223. [Google Scholar] [CrossRef]
- Lindgren, J.; Uvdal, P.; Sjövall, P.; Nilsson, D.E.; Engdahl, A.; Schultz, B.P.; Thiel, V. Molecular preservation of the pigment melanin in fossil melanosomes. Nat. Commun. 2012, 3, 824. [Google Scholar] [CrossRef]
- Carney, R.M.; Vinther, J.; Shawkey, M.D.; D’Alba, L.; Ackermann, J. New evidence on the colour and nature of the isolated Archaeopteryx feather. Nat. Commun. 2012, 3, 637. [Google Scholar] [CrossRef]
- Manning, P.L.; Edwards, N.P.; Bergmann, U.; Anné, J.; Sellers, W.I.; van Veelen, A.; Sokaras, D.; Egerton, V.M.; Alonso-Mori, R.; Ignatyev, K.; et al. Pheomelanin pigment remnants mapped in fossils of an extinct mammal. Nat. Commun. 2019, 10, 2250. [Google Scholar] [CrossRef]
- Colleary, C.; Dolocan, A.; Gardner, J.; Singh, S.; Wuttke, M.; Rabenstein, R.; Habersetzer, J.; Schaal, S.; Feseha, M.; Clemens, M.; et al. Chemical, experimental, and morphological evidence for diagenetically altered melanin in exceptionally preserved fossils. Proc. Natl. Acad. Sci. USA 2015, 112, 12592–12597. [Google Scholar] [CrossRef]
- Pralea, I.-E.; Moldovan, R.-C.; Petrache, A.-M.; Ilies, M.; Heghes, S.-C.; Ielciu, I.; Nicoară, R.; Moldovan, M.; Ene, M.; Radu, M.; et al. From extraction to advanced analytical methods: The challenges of melanin analysis. Int. J. Mol. Sci. 2019, 20, 3943. [Google Scholar] [CrossRef] [PubMed]
- Ito, S. A chemist’s view of melanogenesis. Pigment Cell Res. 2003, 16, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Pezzella, A.; Napolitano, A.; d’Ischia, M.; Prota, G. Oxidative polymerisation of 5,6-dihydroxyindole-2-carboxylic acid to melanin: A new insight. Tetrahedron 1996, 52, 7913–7920. [Google Scholar] [CrossRef]
- D’Ischia, M.; Napolitano, A.; Pezzella, A.; Land, E.J.; Ramsden, C.A.; Riley, P.A. 5,6-Dihydroxyindoles and Indole-5,6-diones. Adv. Heterocycl. Chem. 2005, 89, 1–63. [Google Scholar] [CrossRef]
- D’Ischia, M.; Napolitano, A.; Pezzella, A. 5,6-Dihydroxyindole chemistry: Unexplored opportunities beyond eumelanin. Eur. J. Org. Chem. 2011, 2011, 5501–5516. [Google Scholar] [CrossRef]
- Micillo, R.; Panzella, L.; Iacomino, M.; Prampolini, G.; Cacelli, I.; Ferretti, A.; Crescenzi, O.; Koike, K.; Napolitano, A.; d’Ischia, M. Eumelanin broadband absorption develops from aggregation-modulated chromophore interactions under structural and redox control. Sci. Rep. 2017, 7, 41532. [Google Scholar] [CrossRef]
- Micillo, R.; Panzella, L.; Koike, K.; Monfrecola, G.; Napolitano, A.; d’Ischia, M. “Fifty shades” of black and red or how carboxyl groups fine tune eumelanin and pheomelanin properties. Int. J. Mol. Sci. 2016, 17, 746. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K.; Glass, K.; Simon, J.D. High-performance liquid chromatography estimation of cross-linking of dihydroxyindole moiety in eumelanin. Anal. Biochem. 2013, 434, 221–225. [Google Scholar] [CrossRef]
- Panzella, L.; Gentile, G.; D’Errico, G.; Della Vecchia, N.F.; Errico, M.E.; Napolitano, A.; Carfagna, C.; d’Ischia, M. Atypical structural and π-electron features of a melanin polymer that lead to superior free-radical-scavenging properties. Angew. Chem. Int. Ed. 2013, 52, 12684–12687. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Ito, S. Advanced chemical methods in melanin determination. Pigment Cell Res. 2002, 15, 174–183. [Google Scholar] [CrossRef]
- Chen, C.-T.; Chuang, C.; Cao, J.; Ball, V.; Ruch, D.; Buehler, M.J. Excitonic effects from geometric order and disorder explain broadband optical absorption in eumelanin. Nat. Commun. 2014, 5, 3859. [Google Scholar] [CrossRef] [PubMed]
- Pezzella, A.; Panzella, L.; Natangelo, A.; Arzillo, M.; Napolitano, A.; d’Ischia, M. 5,6-Dihydroxyindole tetramers with “Anomalous” interunit bonding patterns by oxidative coupling of 5,5′,6,6′-tetrahydroxy-2,7′-biindolyl: Emerging complexities on the way toward an improved model of eumelanin buildup. J. Org. Chem. 2007, 72, 9225–9230. [Google Scholar] [CrossRef] [PubMed]
- Glass, K.; Ito, S.; Wilby, P.R.; Sota, T.; Nakamura, A.; Russell Bowers, C.; Miller, K.E.; Dutta, S.; Summons, R.E.; Briggs, D.E.G.; et al. Impact of diagenesis and maturation on the survival of eumelanin in the fossil record. Org. Geochem. 2013, 64, 29–37. [Google Scholar] [CrossRef]
- Ito, S.; Nakanishi, Y.; Valenzuela, R.K.; Brilliant, M.H.; Kolbe, L.; Wakamatsu, K. Usefulness of alkaline hydrogen peroxide oxidation to analyze eumelanin and pheomelanin in various tissue samples: Application to chemical analysis of human hair melanins. Pigment Cell Melanoma Res. 2011, 24, 605–613. [Google Scholar] [CrossRef]
- Lindgren, J.; Nilsson, D.-E.; Sjövall, P.; Jarenmark, M.; Ito, S.; Wakamatsu, K.; Kear, B.P.; Schultz, B.P.; Sylvestersen, R.L.; Madsen, H.; et al. Fossil insect eyes shed light on trilobite optics and the arthropod pigment screen. Nature 2019, 573, 122–125. [Google Scholar] [CrossRef]
- Tanaka, G.; Parker, A.R.; Hasegawa, Y.; Siveter, D.J.; Yamamoto, R.; Miyashita, K.; Takahashi, Y.; Ito, S.; Wakamatsu, K.; Mukuda, T.; et al. Mineralized rods and cones suggest colour vision in a 300 Myr-old fossil fish. Nat. Commun. 2014, 5, 5920. [Google Scholar] [CrossRef]
- Sanyova, J.; Cersoy, S.; Richardin, P.; Laprévote, O.; Walter, P.; Brunelle, A. Unexpected materials in a Rembrandt painting characterized by high spatial resolution cluster-TOF-SIMS imaging. Anal. Chem. 2011, 83, 753–760. [Google Scholar] [CrossRef]
- Stephan, T.; Jessberger, E.K.; Heiss, C.H.; Rost, D. TOF-SIMS analysis of polycyclic aromatic hydrocarbons in Allan Hills 84001. Meteorit. Planet. Sci. 2003, 38, 109–116. [Google Scholar] [CrossRef]
- Lindgren, J.; Sjövall, P.; Carney, R.M.; Uvdal, P.; Gren, J.A.; Dyke, G.; Schultz, B.P.; Shawkey, M.D.; Barnes, K.R.; Polcyn, M.J. Skin pigmentation provides evidence of convergent melanism in extinct marine reptiles. Nature 2014, 506, 484–488. [Google Scholar] [CrossRef]
- McNamara, M.E.; van Dongen, B.E.; Lockyer, N.P.; Bull, I.D.; Orr, P.J. Fossilization of melanosomes via sulfurization. Palaeontology 2016, 59, 337–350. [Google Scholar] [CrossRef]
- McNamara, M.E.; Briggs, D.E.G.; Orr, P.J.; Field, D.J.; Wang, Z. Experimental maturation of feathers: Implications for reconstructions of fossil feather colour. Biol. Lett. 2013, 9, 20130184. [Google Scholar] [CrossRef] [PubMed]
- Saitta, E.T.; Kaye, T.G.; Vinther, J. Sediment-encased maturation: A novel method for simulating diagenesis in organic fossil preservation. Palaeontology 2019, 62, 135–150. [Google Scholar] [CrossRef]
- Graham, D.J.; Wagner, M.S.; Castner, D.G. Information from complexity: Challenges of TOF-SIMS data interpretation. Appl. Surf. Sci. 2006, 252, 6860–6868. [Google Scholar] [CrossRef]
- Pinheiro, F.L.; Prado, G.; Ito, S.; Simon, J.D.; Wakamatsu, K.; Anelli, L.E.; Andrade, J.A.F.; Glass, K. Chemical characterization of pterosaur melanin challenges color inferences in extinct animals. Sci. Rep. 2019, 9, 15947. [Google Scholar] [CrossRef]
- Wiemann, J.; Fabbri, M.; Yang, T.-R.; Stein, K.; Sander, P.M.; Norell, M.A.; Briggs, D.E.G. Fossilization transforms vertebrate hard tissue proteins into N-heterocyclic polymers. Nat. Commun. 2018, 9, 4741. [Google Scholar] [CrossRef]
- Simonovic, B.R.; Wilczok, T. Direct evidence of melanin decomposition by simultaneous DTA, TGA and MS analysis. J. Serb. Chem. Soc. 1995, 60, 681–686. [Google Scholar]
- D’Ischia, M.; Wakamatsu, K.; Napolitano, A.; Briganti, S.; Garcia-Borron, J.-C.; Kovacs, D.; Meredith, P.; Pezzella, A.; Picardo, M.; Sarna, T.; et al. Melanins and melanogenesis: Methods, standards, protocols. Pigment Cell Melanoma Res. 2013, 26, 616–633. [Google Scholar] [CrossRef]
- Landais, P.; Michels, R.; Elie, M. Are time and temperature the only constraints to the simulation of organic matter maturation? Org. Geochem. 1994, 22, 617–630. [Google Scholar] [CrossRef]
- Monthioux, M.; Landais, P.; Monin, J.-C. Comparison between natural and artificial maturation series of humic coals from the Mahakam delta, Indonesia. Org. Geochem. 1985, 8, 275–292. [Google Scholar] [CrossRef]
- Ito, S.; Del Bino, S.; Hirobe, T.; Wakamatsu, K. Improved HPLC conditions to determine eumelanin and pheomelanin contents in biological samples using an ion pair reagent. Int. J. Mol. Sci. 2020, 21, 5134. [Google Scholar] [CrossRef]
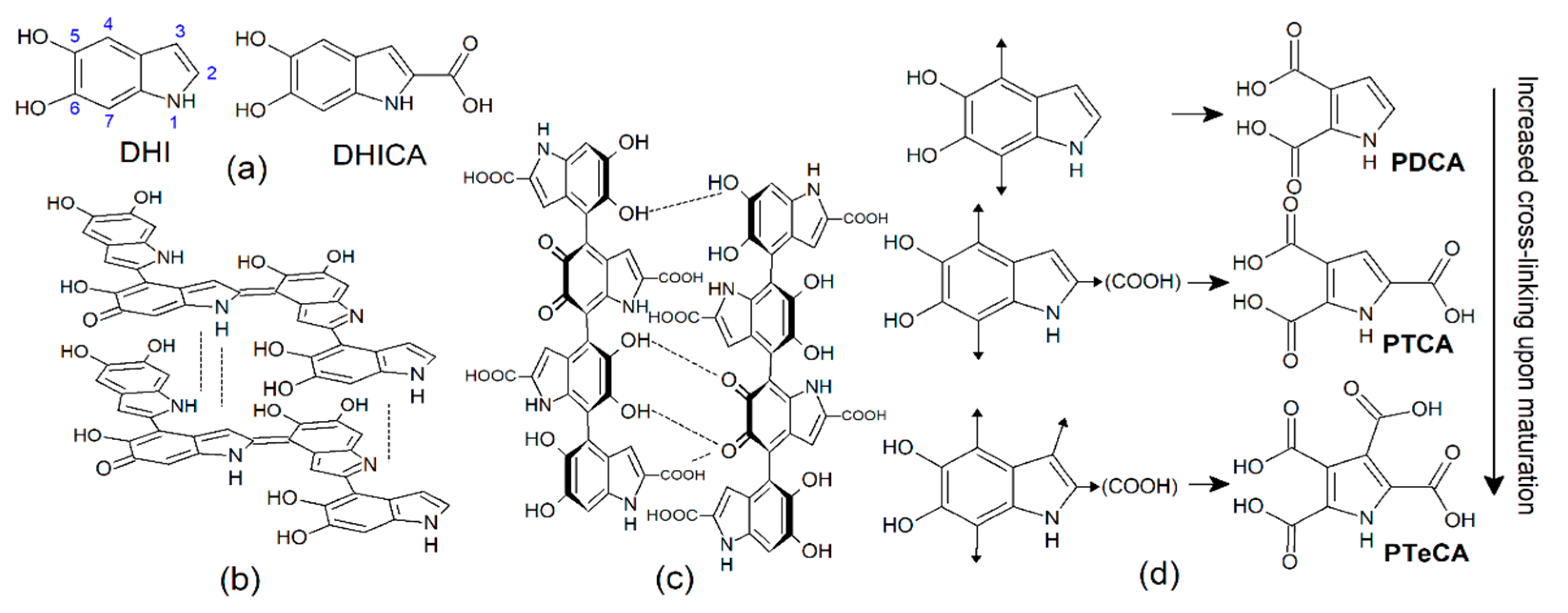
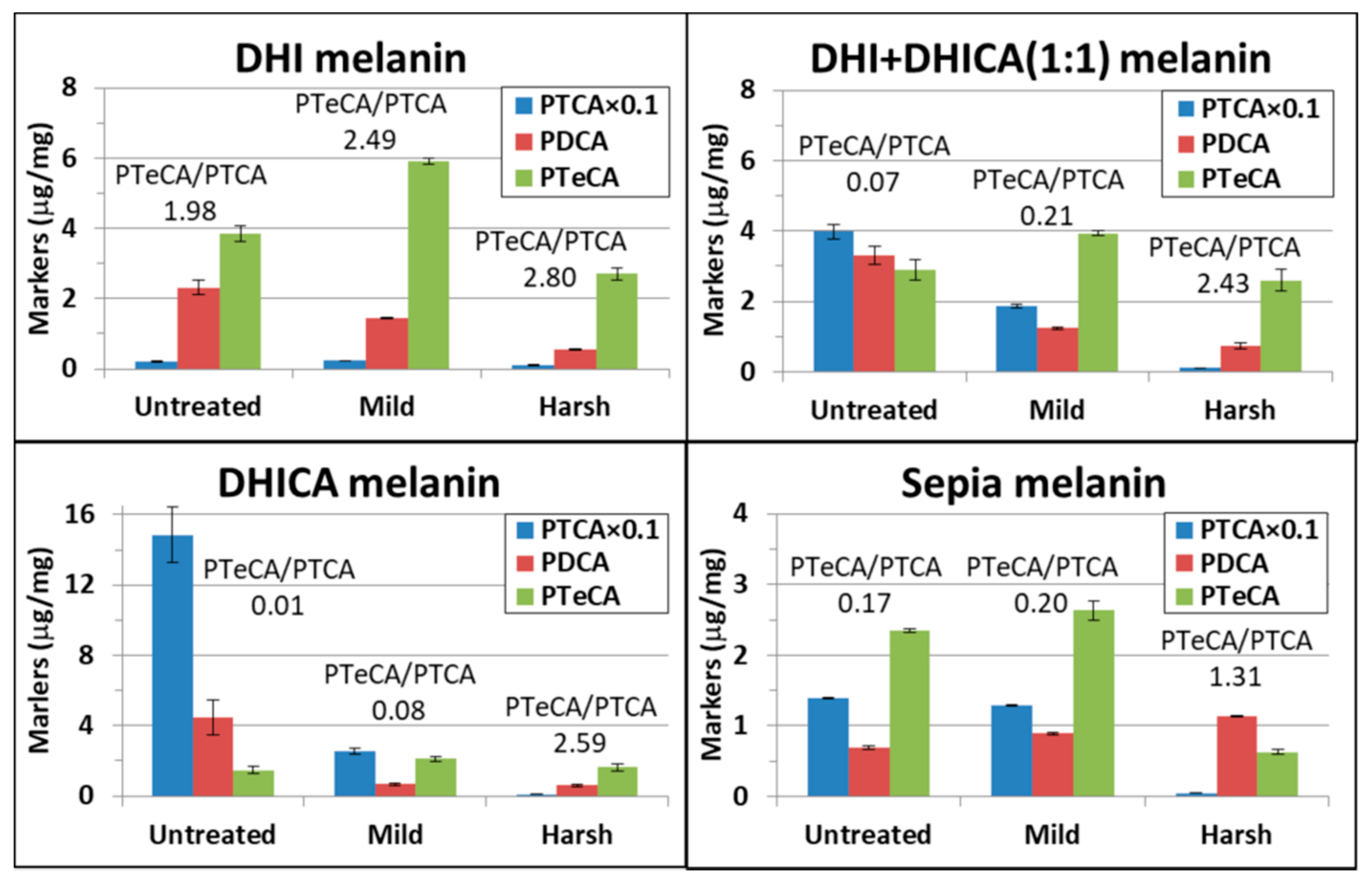
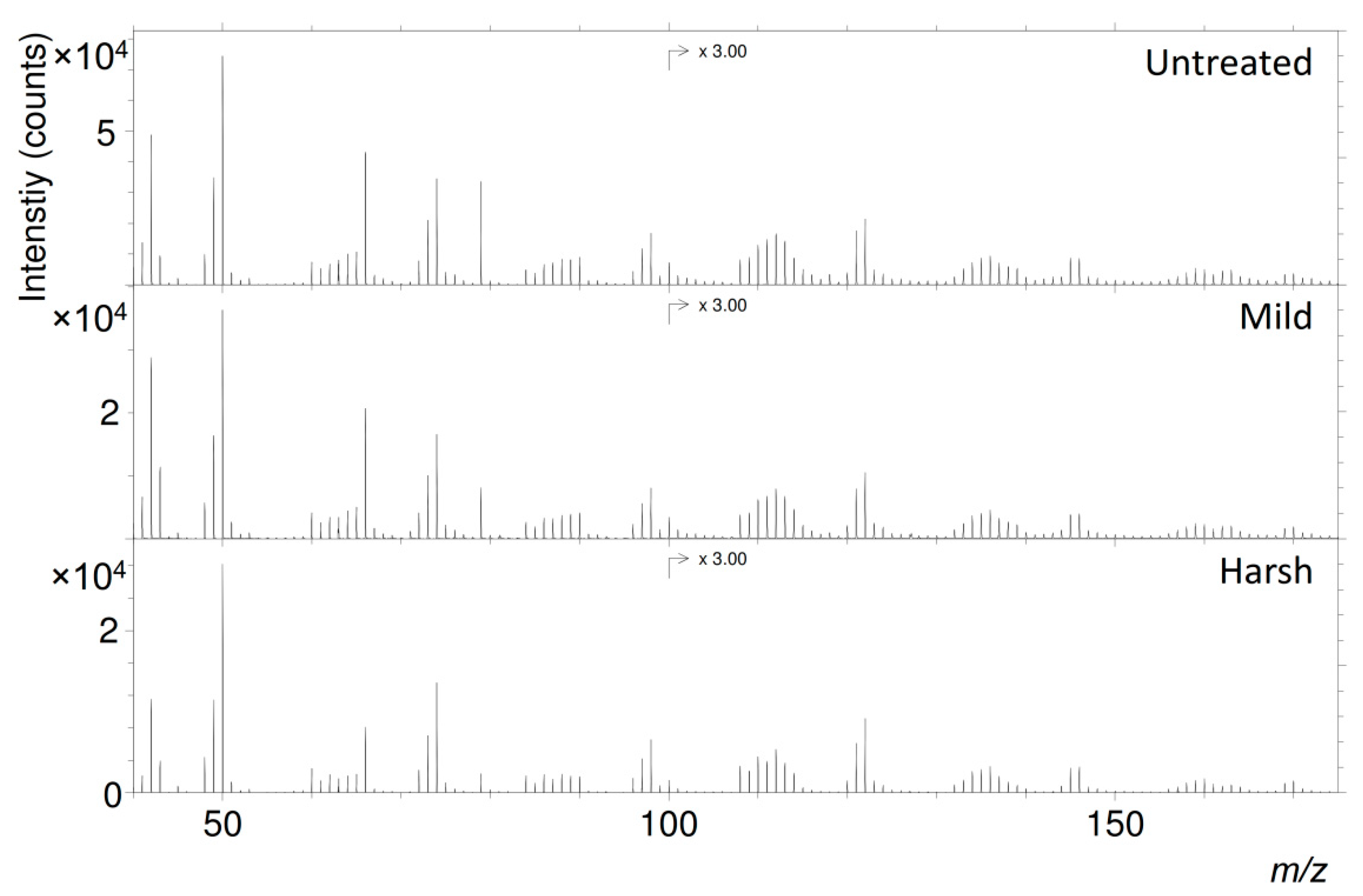
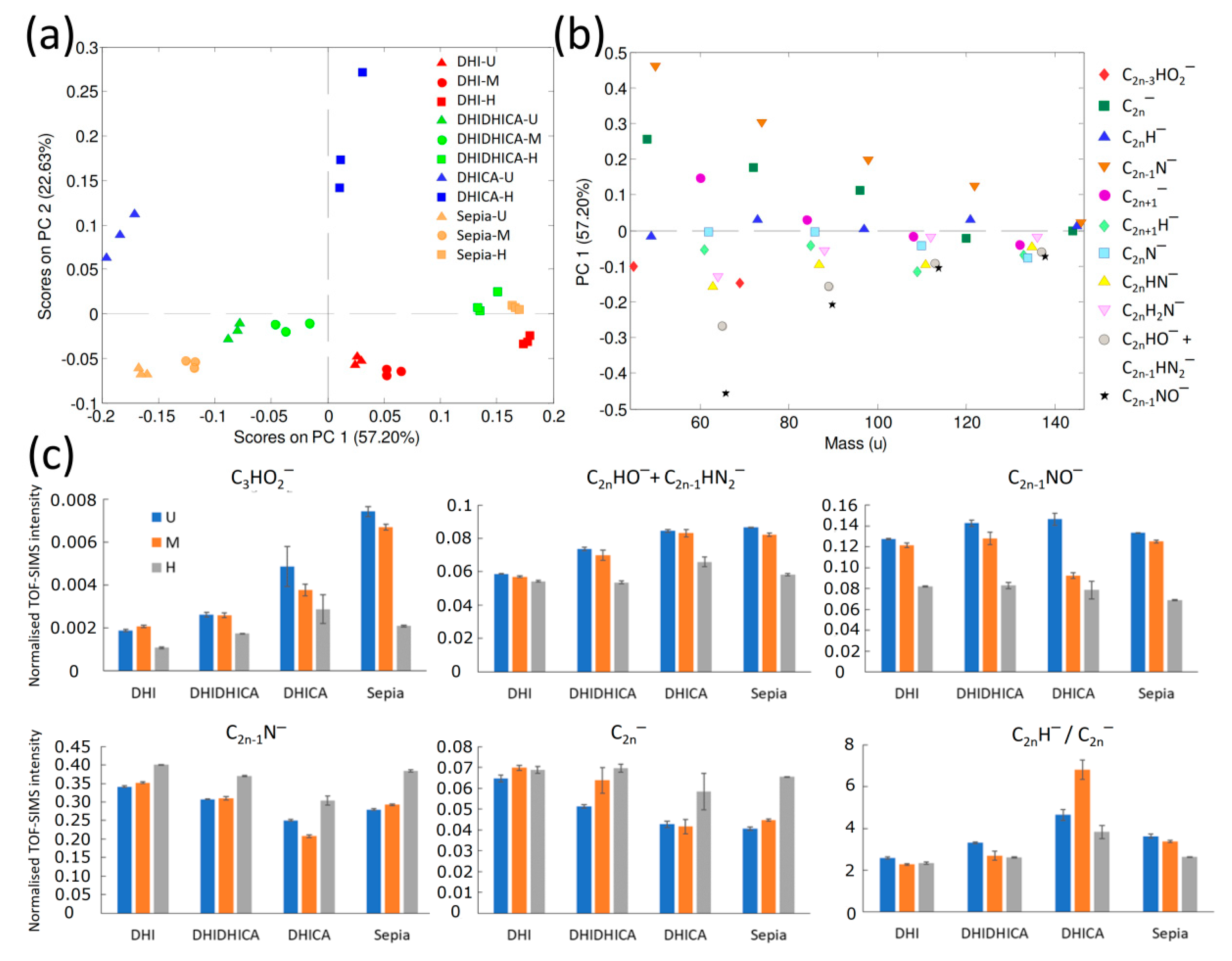
| DHI Melanin | DHI + DHICA (1:1) Melanin | DHICA Melanin | Sepia Melanin | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-treatment | U | M | H | U | M | H | U | M | H | U | M | H |
| Weight loss (%) | 0 | 6 | 14 | 0 | 3 | 18 | 0 | 9 | 20 | 0 | 5 | 47 |
| Observed Mass (m/z) | Ion Assignment | Theoretical Mass (m/z) |
|---|---|---|
| 44.999 | CHO2− | 44.998 |
| 48.001 | C4− | 48.000 |
| 49.010 | C4H− | 49.008 |
| 50.006 | C3N− | 50.003 |
| 60.001 | C5− | 60.000 |
| 61.008 | C5H− | 61.008 |
| 62.006 | C4N− | 62.003 |
| 63.013 | C4HN− | 63.011 |
| 64.011 | C4H2N− + C3N2− | 64.019/64.006 |
| 65.013 | C4HO− + C3HN2− | 65.003/65.014 |
| 66.000 | C3NO− | 65.998 |
| 68.999 | C3HO2− | 68.998 |
| 72.000 | C6− | 72.000 |
| 73.009 | C6H− | 73.008 |
| 74.006 | C5N− | 74.003 |
| 84.000 | C7− | 84.000 |
| 85.007 | C7H− | 85.008 |
| 86.006 | C6N− | 86.003 |
| 87.012 | C6HN− | 87.011 |
| 88.011 | C6H2N− + C5N2− | 88.019/88.006 |
| 89.014 | C6HO− + C5HN2− | 89.003/89.014 |
| 90.002 | C5NO− | 89.998 |
| 95.998 | C8− | 96.000 |
| 97.008 | C8H− | 97.008 |
| 98.004 | C7N− | 98.003 |
| 107.997 | C9− | 108.000 |
| 109.005 | C9H− | 109.008 |
| 110.005 | C8N− | 110.003 |
| 111.011 | C8HN− | 111.011 |
| 112.010 | C8H2N− + C7N2− | 112.019/112.006 |
| 113.014 | C8HO− + C7HN2− | 113.003/113.014 |
| 114.009 | C7NO− | 113.998 |
| 119.994 | C10− | 120.000 |
| 121.007 | C10H− | 121.008 |
| 122.002 | C9N− | 122.003 |
| 131.996 | C11− | 132.000 |
| 133.002 | C11H− | 133.008 |
| 134.003 | C10N− | 134.003 |
| 135.009 | C10HN− | 135.011 |
| 136.008 | C10H2N− + C9N2− | 136.019/136.006 |
| 137.013 | C10HO− + C9HN2− | 137.003/137.014 |
| 138.013 | C9NO− | 137.998 |
| 143.992 | C12− | 144.000 |
| 145.004 | C12H− | 145.008 |
| 146.001 | C11N− | 146.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarenmark, M.; Sjövall, P.; Ito, S.; Wakamatsu, K.; Lindgren, J. Chemical Evaluation of Eumelanin Maturation by ToF-SIMS and Alkaline Peroxide Oxidation HPLC Analysis. Int. J. Mol. Sci. 2021, 22, 161. https://doi.org/10.3390/ijms22010161
Jarenmark M, Sjövall P, Ito S, Wakamatsu K, Lindgren J. Chemical Evaluation of Eumelanin Maturation by ToF-SIMS and Alkaline Peroxide Oxidation HPLC Analysis. International Journal of Molecular Sciences. 2021; 22(1):161. https://doi.org/10.3390/ijms22010161
Chicago/Turabian StyleJarenmark, Martin, Peter Sjövall, Shosuke Ito, Kazumasa Wakamatsu, and Johan Lindgren. 2021. "Chemical Evaluation of Eumelanin Maturation by ToF-SIMS and Alkaline Peroxide Oxidation HPLC Analysis" International Journal of Molecular Sciences 22, no. 1: 161. https://doi.org/10.3390/ijms22010161
APA StyleJarenmark, M., Sjövall, P., Ito, S., Wakamatsu, K., & Lindgren, J. (2021). Chemical Evaluation of Eumelanin Maturation by ToF-SIMS and Alkaline Peroxide Oxidation HPLC Analysis. International Journal of Molecular Sciences, 22(1), 161. https://doi.org/10.3390/ijms22010161







