Novel Detection of Nasty Bugs, Prevention Is Better than Cure
Abstract
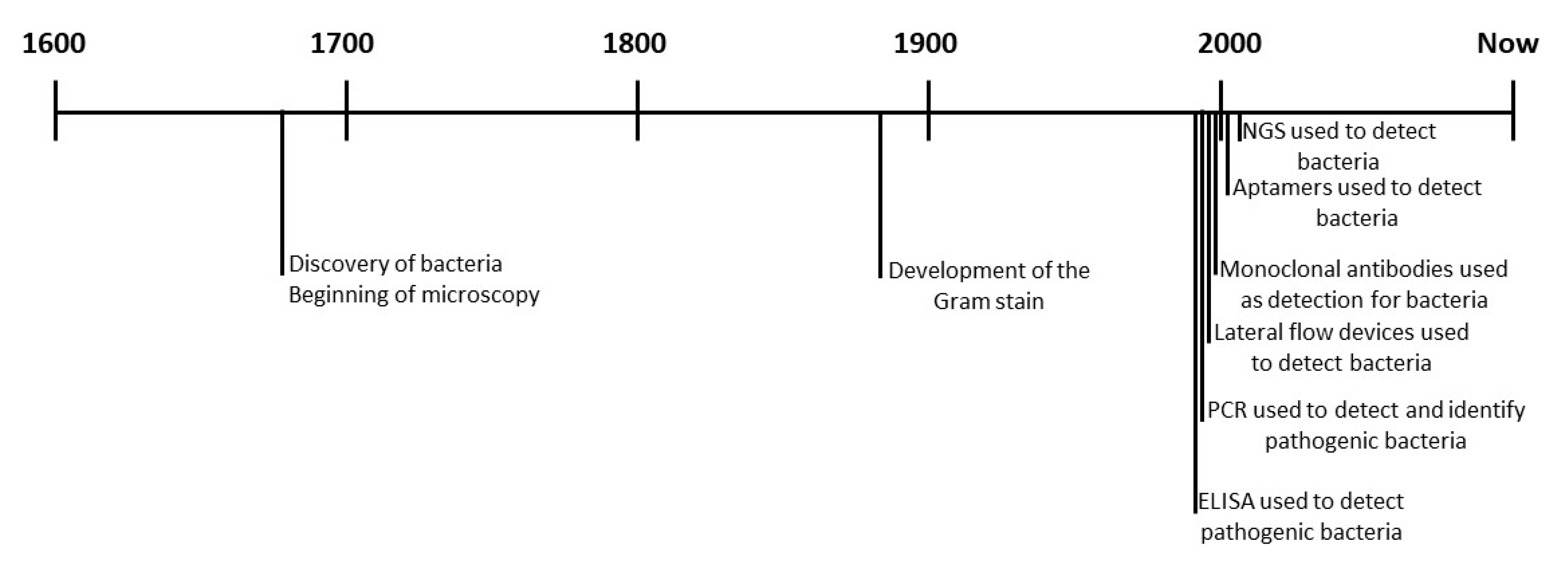
1. Introduction
2. Transmission, Resistance, and Persistence
3. Detection Systems
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AuNP | Gold nanoparticle |
| BSI | Bloodstream infection |
| CAUTI | Catheter-associated urinary tract infection |
| DNA | Deoxyribonucleic acid |
| ELISA | Enzyme-linked immune-sorbent assay |
| GI | Gastrointestinal |
| HAI | Hospital acquired infection |
| HCW | Health care worker |
| HIV | Human immunodeficiency virus |
| ICU | Intensive care unit |
| LFA | Lateral flow assay |
| MB | Methylene blue |
| MDR | Multi-drug resistant |
| MDRB | Multi-drug resistant bacteria |
| MRSA | Methicillin-resistance S. aureus |
| NGS | Next generation sequencing |
| PA | Protein A |
| PBP2a | Penicillin binding protein 2a |
| PCR | Polymerase chain reaction |
| POC | Point-of-care |
| RCA | Rolling circle amplification |
| SELEX | Systematic evolution of ligands via exponential enrichment |
| SSI | Surgical site infection |
| UTI | Urinary tract infection |
| VAP | Ventilator-associated pneumoniae |
| VRE | Vancomycin resistant enterococci |
References
- Cassir, N.; Thomas, G.; Hraiech, S.; Brunet, J.; Fournier, P.-E.; la Scola, B.; Papazian, L. Chlorhexidine daily bathing: Impact on health care–associated infections caused by gram-negative bacteria. Am. J. Infect. Control. 2015, 43, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Rello, J.; Marshall, J.K.; Silva, E.; Anzueto, A.; Martin-Loeches, I.; Moreno, R.; Lipman, J.; Gomersall, C.; Sakr, Y.; et al. International Study of the Prevalence and Outcomes of Infection in Intensive Care Units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef] [PubMed]
- Graves, N.; Weinhold, D.; Tong, E.N.; Birrell, F.; Doidge, S.; Ramritu, P.; Halton, K.; Lairson, D.; Whitby, M. Effect of Healthcare-Acquired Infection on Length of Hospital Stay and Cost. Infect. Control. Hosp. Epidemiol. 2007, 28, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lv, X.; Han, X.; Man, Y.; Saeed, Y.; Qing, H.; Deng, Y. Whole-cell based aptamer selection for selective capture of microorganisms using microfluidic devices. Anal. Methods 2015, 7, 6339–6345. [Google Scholar] [CrossRef]
- Jenkins, D.R. Nosocomial infections, and infection control. Medicine 2017, 45, 629–633. [Google Scholar] [CrossRef]
- Tan, R.; Wang, H.; Li, M.; Huang, J.; Sun, J.; Qu, H. Epidemiology and antimicrobial resistance among commonly encountered bacteria associated with infections and colonization in intensive care units in a university-affiliated hospital in Shanghai. J. Microbiol. Immunol. Infect. 2014, 47, 87–94. [Google Scholar] [CrossRef]
- Khan, H.A.; Baig, F.K.; Mehboob, R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pac. J. Trop. Biomed. 2017, 7, 478–482. [Google Scholar] [CrossRef]
- Khan, H.A.; Ahmad, A.; Mehboob, R. Nosocomial infections and their control strategies. Asian Pac. J. Trop. Biomed. 2015, 5, 509–514. [Google Scholar] [CrossRef]
- Cornejo-Juárez, P.; Vilar-Compte, D.; Pérez-Jiménez, C.; Ñamendys-Silva, S.; Sandoval-Hernández, S.; Volkow-Fernández, P. The impact of hospital-acquired infections with multidrug-resistant bacteria in an oncology intensive care unit. Int. J. Infect. Dis. 2015, 31, 31–34. [Google Scholar] [CrossRef]
- Le, N.K.; Hf, W.; Vu, P.D.; Khu, D.T.K.; Le, H.T.; Hoang, B.T.N.; Vo, V.T.; Lam, Y.M.; Vu, D.T.V.; Nguyen, T.H.; et al. High prevalence of hospital-acquired infections caused by gram-negative carbapenem resistant strains in Vietnamese pediatric ICUs: A multi-centre point prevalence survey. Medicine 2016, 95, e4099. [Google Scholar] [CrossRef]
- Arefian, H.; Hagel, S.; Heublein, S.; Rissner, F.; Scherag, A.; Brunkhorst, F.M.; Baldessarini, R.J.; Hartmann, M. Extra length of stay and costs because of health care–associated infections at a German university hospital. Am. J. Infect. Control. 2016, 44, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Schmier, J.K.; Hulme-Lowe, C.K.; Semenova, S.; Klenk, J.A.; DeLeo, P.C.; Sedlak, R.; Carlson, P.A. Estimated hospital costs associated with preventable health care-associated infections if health care antiseptic products were unavailable. Clin. Outcomes Res. 2016, 8, 197–205. [Google Scholar] [CrossRef]
- Kaye, K.S.; Pogue, J.M. Infections Caused by Resistant Gram-Negative Bacteria: Epidemiology and Management. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2015, 35, 949–962. [Google Scholar] [CrossRef]
- Royer, S.; Faria, A.L.S.; Seki, L.M.; Chagas, T.P.G.; de Campos, P.A.; Batistão, D.W.D.F.; Asensi, M.D.; Filho, P.P.G.; Ribas, R.M. Spread of multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa clones in patients with ventilator-associated pneumonia in an adult intensive care unit at a university hospital. Braz. J. Infect. Dis. 2015, 19, 350–357. [Google Scholar] [CrossRef] [PubMed]
- MacGowan, A.; Macnaughton, E. Antibiotic resistance. Medicine 2017, 45, 622–628. [Google Scholar] [CrossRef]
- Kong, L.Y.; Dendukuri, N.; Schiller, I.; Bourgault, A.-M.; Brassard, P.; Poirier, L.; Lamothe, F.; Béliveau, C.; Michaud, S.; Turgeon, N.; et al. Predictors of asymptomatic Clostridium difficile colonization on hospital admission. Am. J. Infect. Control 2015, 43, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Longtin, Y.; Paquet-Bolduc, B.; Gilca, R.; Garenc, C.; Fortin, E.; Longtin, J.; Trottier, S.; Gervais, P.; Roussy, J.-F.; Lévesque, S.; et al. Effect of Detecting and Isolating Clostridium difficile Carriers at Hospital Admission on the Incidence of C difficile Infections: A Quasi-Experimental Controlled Study. JAMA Intern. Med. 2016, 176, 796–804. [Google Scholar] [CrossRef]
- Abt, M.C.; McKenney, P.T.; Pamer, E.G. Clostridium difficile colitis: Pathogenesis and host defence. Nat. Rev. Genet. 2016, 14, 609–620. [Google Scholar] [CrossRef]
- Dapa, T.; Unnikrishnan, M. Biofilm formation byClostridium difficile. Gut Microbes 2013, 4, 397–402. [Google Scholar] [CrossRef]
- Thaden, J.T.; Li, Y.; Ruffin, F.; Maskarinec, S.A.; Hill-Rorie, J.M.; Wanda, L.C.; Reed, S.D.; Fowler, V.G. Increased Costs Associated with Bloodstream Infections Caused by Multidrug-Resistant Gram-Negative Bacteria Are Due Primarily to Patients with Hospital-Acquired Infections. Antimicrob. Agents Chemother. 2016, 61, e01709-16. [Google Scholar] [CrossRef]
- Fisher, R.A.; Gollan, B.; Helaine, S. Persistent bacterial infections and persister cells. Nat. Rev. Genet. 2017, 15, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Price, L.B.; Hungate, B.A.; Koch, B.J.; Davis, G.S.; Liu, C.M. Colonizing opportunistic pathogens (COPs): The beasts in all of us. PLoS Pathog. 2017, 13, e1006369. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, A.M.; Perez, K.K.; Musick, W.L.; Ikwuagwu, J.O.; Attia, E.; Fasoranti, O.O.; Cernoch, P.L.; Olsen, R.J.; Musser, J.M. Integrating Rapid Diagnostics and Antimicrobial Stewardship in Two Community Hospitals Improved Process Measures and Antibiotic Adjustment Time. Infect. Control. Hosp. Epidemiol. 2016, 37, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Templier, V.; Roux, A.; Roupioz, Y.; Livache, T. Ligands for label-free detection of whole bacteria on biosensors: A review. TrAC Trends Anal. Chem. 2016, 79, 71–79. [Google Scholar] [CrossRef]
- Vuotto, C.; Longo, F.; Balice, M.P.; Donelli, G.; Varaldo, P.E. Antibiotic Resistance Related to Biofilm Formation in Klebsiella pneumoniae. Pathogens 2014, 3, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Hu, B.; Gao, X.; Bao, R.; Chen, M.; Li, H. Sources of sporadic Pseudomonas aeruginosa colonizations/infections in surgical ICUs: Association with contaminated sink trap. J. Infect. Chemother. 2016, 22, 450–455. [Google Scholar] [CrossRef]
- Brown, S.P.; Cornforth, D.M.; Mideo, N. Evolution of virulence in opportunistic pathogens: Generalism, plasticity, and control. Trends Microbiol. 2012, 20, 336–342. [Google Scholar] [CrossRef]
- Lefebvre, A.; Bertrand, X.; Quantin, C.; Vanhems, P.; Lucet, J.C.; Nuemi, G.; Astruc, K.; Chavanet, P.; Aho-Glélé, L.S. Association between Pseudomonas aeruginosa positive water samples and healthcare-associated cases: Nine-year study at one university hospital. J. Hosp. Infect. 2017, 96, 238–243. [Google Scholar] [CrossRef]
- Saxena, S.; Banerjee, G.; Garg, R.; Singh, M. Comparative Study of Biofilm Formation in Pseudomonas aeruginosa Isolates from Patients of Lower Respiratory Tract Infection. J. Clin. Diagn. Res. 2014, 8, DC09–DC11. [Google Scholar]
- Wang, K.-Y.; Zeng, Y.-L.; Yang, X.-Y.; Li, W.-B.; Lan, X.-P. Utility of aptamer-fluorescence in situ hybridization for rapid detection of Pseudomonas aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 30, 273–278. [Google Scholar] [CrossRef]
- Cosgrove, S.E.; Qi, Y.; Kaye, K.S.; Harbarth, S.; Karchmer, A.W.; Carmeli, Y. The Impact of Methicillin Resistance in Staphylococcus aureus Bacteremia on Patient Outcomes: Mortality, Length of Stay, and Hospital Charges. Infect. Control. Hosp. Epidemiol. 2005, 26, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Ou, Q.; Lin, J.; Peng, Y.; Yao, Z. A meta-analysis of the rates of Staphylococcus aureus and methicillin-resistant S aureus contamination on the surfaces of environmental objects that health care workers frequently touch. Am. J. Infect. Control. 2017, 45, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Falugi, F.; Kim, H.K.; Missiakas, D.M.; Schneewind, O. Role of Protein A in the Evasion of Host Adaptive Immune Responses by Staphylococcus aureus. mBio 2013, 4, e00575-13. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, K.; Lucar, J.; Blackshear, C.; Hobbs, C.V. Methicillin-susceptible and Methicillin-resistant Staphylococcus aureus Bacteremia: Nationwide Estimates of 30-Day Readmission, In-hospital Mortality, Length of Stay, and Cost in the United States. Clin. Infect. Dis. 2019, 69, 2112–2118. [Google Scholar] [CrossRef] [PubMed]
- Baylay, A.J.; Piddock, L.J.; Webber, M.A. Molecular Mechanisms of Antibiotic Resistance—Part I. Bact. Resist. Antibiot. Mol. Man 2019, 13, 1–26. [Google Scholar] [CrossRef]
- Murni, I.K.; Duke, T.; Kinney, S.; Daley, A.J.; Soenarto, Y. Reducing hospital-acquired infections and improving the rational use of antibiotics in a developing country: An effectiveness study. Arch. Dis. Child. 2015, 100, 454–459. [Google Scholar] [CrossRef]
- Zarpellon, M.N.; Gales, A.C.; Sasaki, A.L.; Selhorst, G.J.; Menegucci, T.C.; Cardoso, C.L.; Garcia, L.B.; Tognim, M.C.B. Survival of vancomycin-intermediate Staphylococcus aureus on hospital surfaces. J. Hosp. Infect. 2015, 90, 347–350. [Google Scholar] [CrossRef]
- Ling, M.L.; How, K.B. Pseudomonas aeruginosa outbreak linked to sink drainage design. Heal. Infect. 2013, 18, 143–146. [Google Scholar] [CrossRef]
- Durmaz, G.; Us, T.; Aydinli, A.; Kiremitci, A.; Kiraz, N.; Akgün, Y. Optimum Detection Times for Bacteria and Yeast Species with the BACTEC 9120 Aerobic Blood Culture System: Evaluation for a 5-Year Period in a Turkish University Hospital. J. Clin. Microbiol. 2003, 41, 819–821. [Google Scholar] [CrossRef]
- Kim, H.; Chung, D.-R.; Kang, M. A new point-of-care test for the diagnosis of infectious diseases based on multiplex lateral flow immunoassays. Analyst 2019, 144, 2460–2466. [Google Scholar] [CrossRef]
- Khanal, R.; Sah, P.; Lamichhane, P.; Lamsal, A.; Upadhaya, S.; Pahwa, V.K. Nasal carriage of methicillin resistant Staphylococcus aureus among health care workers at a tertiary care hospital in Western Nepal. Antimicrob. Resist. Infect. Control. 2015, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Starlander, G.; Melhus, Å. Minor outbreak of extended-spectrum β-lactamase-producing Klebsiella pneumoniae in an intensive care unit due to a contaminated sink. J. Hosp. Infect. 2012, 82, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Conly, J.; Johnston, B. Where are all the new antibiotics? The new antibiotic paradox. Can. J. Infect. Dis. Med. Microbiol. 2005, 16, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Moser, C.; Wang, H.-Z.; Høiby, N.; Song, Z.-J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- Seifi, K.; Kazemian, H.; Heidari, H.; Rezagholizadeh, F.; Saee, Y.; Shirvani, F.; Houri, H. Evaluation of Biofilm Formation Among Klebsiella pneumoniae Isolates and Molecular Characterization by ERIC-PCR. Jundishapur J. Microbiol. 2016, 9, e30682. [Google Scholar] [CrossRef] [PubMed]
- Anderl, J.N.; Franklin, M.J.; Stewart, P.S. Role of Antibiotic Penetration Limitation in Klebsiella pneumoniae Biofilm Resistance to Ampicillin and Ciprofloxacin. Antimicrob. Agents Chemother. 2000, 44, 1818–1824. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Harms, A.; Maisonneuve, E.; Gerdes, K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 2016, 354, aaf4268. [Google Scholar] [CrossRef]
- Harbarth, S.J.; Sax, H.; Gastmeier, P. The preventable proportion of nosocomial infections: An overview of published reports. J. Hosp. Infect. 2003, 54, 258–266. [Google Scholar] [CrossRef]
- Torres-Chavolla, E.; Alocilja, E.C. Aptasensors for detection of microbial and viral pathogens. Biosens. Bioelectron. 2009, 24, 3175–3182. [Google Scholar] [CrossRef]
- Byrne, B.; Stack, E.; Gilmartin, N.; O’Kennedy, R.J. Antibody-Based Sensors: Principles, Problems and Potential for Detection of Pathogens and Associated Toxins. Sensors 2009, 9, 4407–4445. [Google Scholar] [CrossRef] [PubMed]
- Zowawi, H.M.; Harris, P.N.A.; Roberts, M.J.; Tambyah, P.A.; Schembri, M.A.; Pezzani, M.D.; Williamson, D.A.; Paterson, D.L. The emerging threat of multidrug-resistant Gram-negative bacteria in urology. Nat. Rev. Urol. 2015, 12, 570–584. [Google Scholar] [CrossRef]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Höök, M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Genet. 2014, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Yuling, Z.; Zhao, Y.; Liu, C.; Chen, Z.; Zhou, D. Molecular pathogenesis ofKlebsiella pneumoniae. Futur. Microbiol. 2014, 9, 1071–1081. [Google Scholar] [CrossRef]
- Stentzel, S.; Sundaramoorthy, N.; Michalik, S.; Nordengrün, M.; Schulz, S.; Kolata, J.; Kloppot, P.; Engelmann, S.; Steil, L.; Hecker, M.; et al. Specific serum IgG at diagnosis of Staphylococcus aureus bloodstream invasion is correlated with disease progression. J. Proteom. 2015, 128, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.; Chang, P.; Benton, D.J.; McCauley, J.W.; Iqbal, M.; Cass, A. Dual Recognition Element Lateral Flow Assay Toward Multiplex Strain Specific Influenza Virus Detection. Anal. Chem. 2017, 89, 6781–6786. [Google Scholar] [CrossRef]
- Jayol, A.; Nordmann, P.; Desroches, M.; Decousser, J.-W.; Poirel, L. Acquisition of Broad-Spectrum Cephalosporin Resistance Leading to Colistin Resistance in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2016, 60, 3199–3201. [Google Scholar] [CrossRef]
- Verdoodt, N.; Basso, C.R.; Rossi, B.F.; Pedrosa, V.A. Development of a rapid and sensitive immunosensor for the detection of bacteria. Food Chem. 2017, 221, 1792–1796. [Google Scholar] [CrossRef]
- Gosiewski, T.; Ludwig-Galezowska, A.H.; Huminska, K.; Sroka-Oleksiak, A.; Radkowski, P.; Salamon, D.; Wojciechowicz, J.; Kus-Slowinska, M.; Bulanda, M.; Wołkow, P.P. Comprehensive detection and identification of bacterial DNA in the blood of patients with sepsis and healthy volunteers using next-generation sequencing method - the observation of DNAemia. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 329–336. [Google Scholar] [CrossRef]
- Ivnitski, D.; Abdel-Hamid, I.; Atanasov, P.; Wilkins, E. Biosensors for detection of pathogenic bacteria. Biosens. Bioelectron. 1999, 14, 599–624. [Google Scholar] [CrossRef]
- Sanvicens, N.; Pastells, C.; Pascual, N.; Marco, M.-P. Nanoparticle-based biosensors for detection of pathogenic bacteria. TrAC Trends Anal. Chem. 2009, 28, 1243–1252. [Google Scholar] [CrossRef]
- Ahmed, A.; Rushworth, J.V.; Hirst, N.A.; Millner, P.A. Biosensors for Whole-Cell Bacterial Detection. Clin. Microbiol. Rev. 2014, 27, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, H.; Zhang, P.; Sun, C.; Wang, X.; Wang, X.; Yang, R.; Wang, C.; Zhou, L. Rapid multiplex detection of 10 foodborne pathogens with an up-converting phosphor technology-based 10-channel lateral flow assay. Sci. Rep. 2016, 6, 21342. [Google Scholar] [CrossRef] [PubMed]
- Brosel-Oliu, S.; Ferreira, R.; Uria, N.; Abramova, N.; Gargallo, R.; Muñoz-Pascual, F.-X.; Bratov, A. Novel impedimetric aptasensor for label-free detection of Escherichia coli O157:H7. Sens. Actuators B Chem. 2018, 255, 2988–2995. [Google Scholar] [CrossRef]
- Paniel, N.; Baudart, J.; Hayat, A.; Barthelmebs, L. Aptasensor and genosensor methods for detection of microbes in real world samples. Methods 2013, 64, 229–240. [Google Scholar] [CrossRef]
- Majdinasab, M.; Hayat, A.; Marty, J. Aptamer-based assays and aptasensors for detection of pathogenic bacteria in food samples. TrAC Trends Anal. Chem. 2018, 107, 60–77. [Google Scholar] [CrossRef]
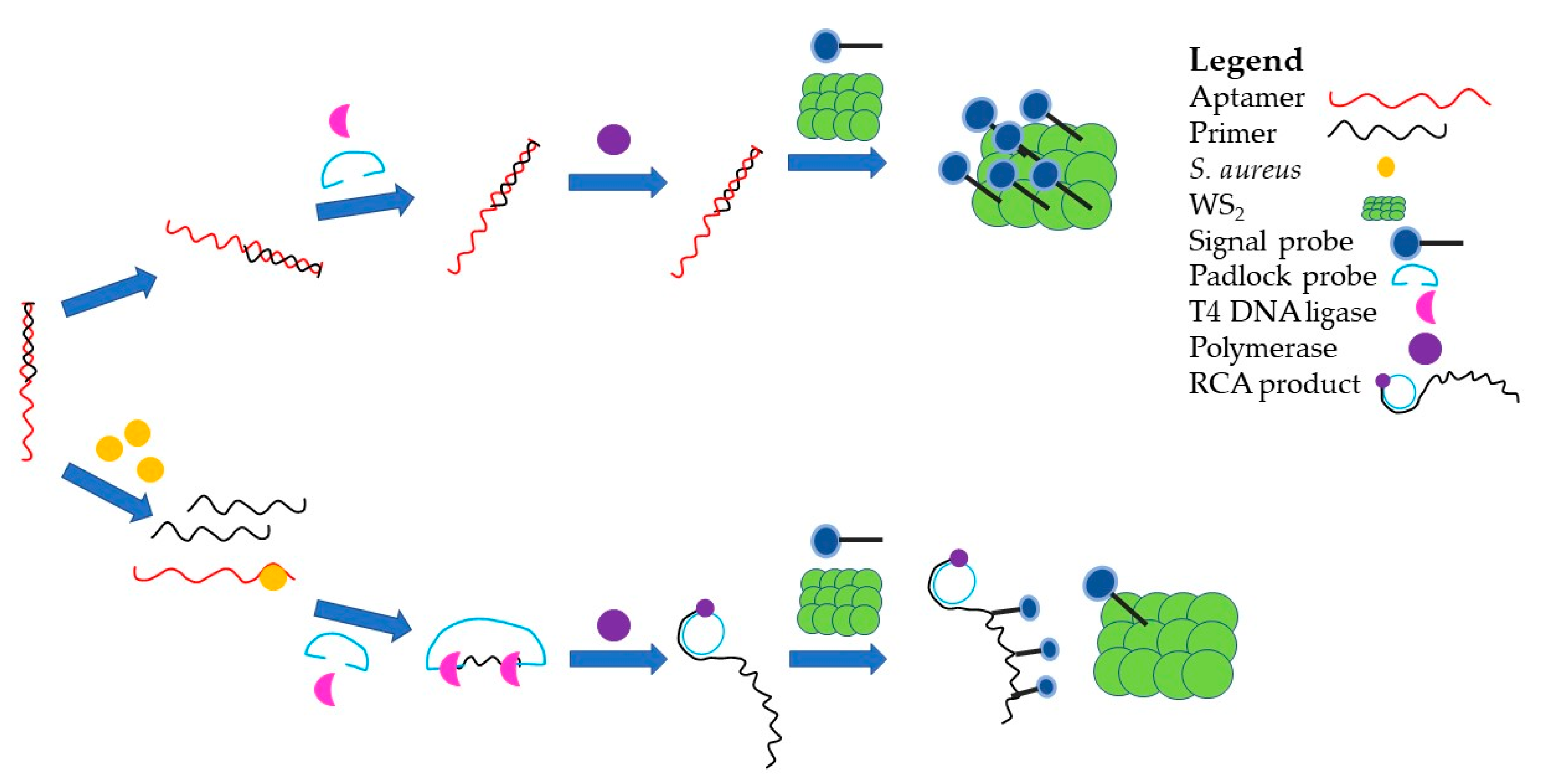
- Hao, L.; Gu, H.; Duan, N.; Wu, S.; Ma, X.; Xia, Y.; Tao, Z.; Wang, Z. An enhanced chemiluminescence resonance energy transfer aptasensor based on rolling circle amplification and WS2 nanosheet for Staphylococcus aureus detection. Anal. Chim. Acta 2017, 959, 83–90. [Google Scholar] [CrossRef]
- Bartholomew, J.W.; Mittwer, T. The Gram stain. Bacteriol. Rev. 1952, 16, 1–29. [Google Scholar] [CrossRef]
- Cother, E.J.; Vruggink, H. Detection of viable and non-viable cells ofErwinia carotovora var.atroseptica in inoculated tubers of var. Bintje with enzyme-linked immunosorbent assay (ELISA). Potato Res. 1980, 23, 133–135. [Google Scholar] [CrossRef]
- Steffan, R.J.; Atlas, R.M. DNA amplification to enhance detection of genetically engineered bacteria in environmental samples. Appl. Environ. Microb. 1988, 54, 2185. [Google Scholar] [CrossRef]
- Fong, W.K.; Modrusan, Z.; McNevin, J.P.; Marostenmaki, J.; Zin, B.; Bekkaoui, F. Rapid Solid-Phase Immunoassay for Detection of Methicillin-Resistant Staphylococcus aureus Using Cycling Probe Technology. J. Clin. Microbiol. 2000, 38, 2525. [Google Scholar] [CrossRef] [PubMed]
- Fratamico, P.; Strobaugh, T.; Medina, M.; Gehring, A. Detection of Escherichia coli 0157:H7 using a surface plasmon resonance biosensor. Biotechnol. Tech. 1998, 12, 571–576. [Google Scholar] [CrossRef]
- Bruno, J.G.; Kiel, J.L. In vitro selection of DNA aptamers to anthrax spores with electrochemiluminescence detection. Biosens. Bioelectron. 1999, 14, 457–464. [Google Scholar] [CrossRef]
- Wurtzel, O.; Dori-Bachash, M.; Pietrokovski, S.; Jurkevitch, E.; Sorek, R. Mutation Detection with Next-Generation Resequencing through a Mediator Genome. PLoS ONE 2010, 5, e15628. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Dobrindt, U.; Hacker, J.; Hasnain, S.E. Genomic fluidity and pathogenic bacteria: Applications in diagnostics, epidemiology and intervention. Nat. Rev. Genet. 2008, 6, 387–394. [Google Scholar] [CrossRef]
- Felföldi, T.; Heéger, Z.; Vargha, M.; Márialigeti, K. Detection of potentially pathogenic bacteria in the drinking water distribution system of a hospital in Hungary. Clin. Microbiol. Infect. 2010, 16, 89–92. [Google Scholar] [CrossRef]
- Zou, Y.; Liang, J.; She, Z.; Kraatz, H. Gold nanoparticles-based multifunctional nanoconjugates for highly sensitive and enzyme-free detection of E. coli K12. Talanta 2019, 193, 15–22. [Google Scholar] [CrossRef]
- Zelada-Guillén, G.A.; Sebastián-Avila, J.L.; Blondeau, P.; Riu, J.; Rius, F.X. Label-free detection of Staphylococcus aureus in skin using real-time potentiometric biosensors based on carbon nanotubes and aptamers. Biosens. Bioelectron. 2012, 31, 226–232. [Google Scholar] [CrossRef]
- Ferguson, C.; Booth, N.; Allan, E. An ELISA for the detection of Bacillus subtilis L-form bacteria confirms their symbiosis in strawberry. Lett. Appl. Microbiol. 2000, 31, 390–394. [Google Scholar] [CrossRef]
- Králík, P.; Ricchi, M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef]
- Kinghorn, A.B.; Dirkzwager, R.M.; Liang, S.; Cheung, Y.-W.; Fraser, L.A.; Shiu, S.C.-C.; Tang, M.S.L.; Tanner, J.A. Aptamer Affinity Maturation by Resampling and Microarray Selection. Anal. Chem. 2016, 88, 6981–6985. [Google Scholar] [CrossRef] [PubMed]
- Brody, E.N.; Gold, L. Aptamers as therapeutic and diagnostic agents. Rev. Mol. Biotechnol. 2000, 74, 5–13. [Google Scholar] [CrossRef]
- Toh, S.Y.; Citartan, M.; Gopinath, S.C.; Tang, T.-H. Aptamers as a replacement for antibodies in enzyme-linked immunosorbent assay. Biosens. Bioelectron. 2015, 64, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Tombelli, S.; Minunni, M.; Mascini, M. Analytical applications of aptamers. Internat. Congr. Opt. Optoelectron. 2007, 20. [Google Scholar] [CrossRef] [PubMed]
- Cerchia, L.; de Franciscis, V. Targeting cancer cells with nucleic acid aptamers. Trends Biotechnol. 2010, 28, 517–525. [Google Scholar] [CrossRef]
- Shigdar, S.; Qian, C.; Lv, L.; Pu, C.; Li, Y.; Li, L.; Marappan, M.; Lin, J.; Wang, L.; Duan, W. The Use of Sensitive Chemical Antibodies for Diagnosis: Detection of Low Levels of Epcam in Breast Cancer. PLoS ONE 2013, 8, e57613. [Google Scholar] [CrossRef]
- Song, M.Y.; Nguyen, D.; Hong, S.W.; Kim, B.C. Broadly reactive aptamers targeting bacteria belonging to different genera using a sequential toggle cell-SELEX. Sci. Rep. 2017, 7, srep43641. [Google Scholar] [CrossRef]
- Hanif, A.; Farooq, R.; Rehman, M.U.; Khan, R.; Majid, S.; Ganaie, M.A. Aptamer based nanobiosensors: Promising healthcare devices. Saudi Pharm. J. 2018, 27, 312–319. [Google Scholar] [CrossRef]
- Griffiths, A.D.; Duncan, A.R. Strategies for selection of antibodies by phage display. Curr. Opin. Biotechnol. 1998, 9, 102–108. [Google Scholar] [CrossRef]
- Bu, T.; Yao, X.; Huang, L.; Dou, L.; Zhao, B.; Yang, B.; Li, T.; Wang, J.; Zhang, D. Dual recognition strategy and magnetic enrichment based lateral flow assay toward Salmonella enteritidis detection. Talanta 2020, 206, 120204. [Google Scholar] [CrossRef]
- Xu, L.; Dai, Q.; Shi, Z.; Liu, X.; Gao, L.; Wang, Z.; Zhu, X.; Li, Z. Accurate MRSA identification through dual-functional aptamer and CRISPR-Cas12a assisted rolling circle amplification. J. Microbiol. Methods 2020, 173, 105917. [Google Scholar] [CrossRef] [PubMed]
- Gürtler, V. Predicting genome variations between passages of Clostridium difficle by ribotypes. Microbiol. Aust. 2015, 36, 109–110. [Google Scholar] [CrossRef][Green Version]
- Bourgeois, I.; Camiade, E.; Biswas, R.; Courtin, P.; Gibert, L.; Götz, F.; Chapot-Chartier, M.-P.; Pons, J.-L.; Pestel-Caron, M. Characterization of AtlL, a bifunctional autolysin of Staphylococcus lugdunensis with N-acetylglucosaminidase and N-acetylmuramoyl-l-alanine amidase activities. FEMS Microbiol. Lett. 2009, 290, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, T. Rapid detection of Klebsiella pneumoniae, Klebsiella oxytoca, Raoultella ornithinolytica and other related bacteria in food by lateral-flow test strip immunoassays. J. Microbiol. Methods 2018, 147, 43–49. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Scharinger, E.J.; Dietrich, R.; Wittwer, T.; Märtlbauer, E.; Schauer, K. Multiplexed Lateral Flow Test for Detection and Differentiation of Cronobacter sakazakii Serotypes O1 and O2. Front. Microbiol. 2017, 8, 1826. [Google Scholar] [CrossRef]
- Wang, R.; Kim, K.; Choi, N.; Wang, X.; Lee, J.; Jeon, J.H.; Rhie, G.-E.; Choo, J. Highly sensitive detection of high-risk bacterial pathogens using SERS-based lateral flow assay strips. Sens. Actuators B Chem. 2018, 270, 72–79. [Google Scholar] [CrossRef]
- Su, L.; Jia, W.; Hou, C.; Lei, Y. Microbial biosensors: A review. Biosens. Bioelectron. 2011, 26, 1788–1799. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Q. Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosens. Bioelectron. 2016, 75, 273–284. [Google Scholar] [CrossRef]
- Bang, G.S.; Cho, S.; Kim, B.-G. A novel electrochemical detection method for aptamer biosensors. Biosens. Bioelectron. 2005, 21, 863–870. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wang, L.; Li, J.; Fan, C.; Zhao, J. Aptamer-based biosensors. TrAC Trends Anal. Chem. 2008, 27, 108–117. [Google Scholar] [CrossRef]
- Hamula, C.L.A.; Zhang, H.; Guan, L.L.; Li, X.-F.; Le, X.C. Selection of Aptamers against Live Bacterial Cells. Anal. Chem. 2008, 80, 7812–7819. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, S.C.B.; Lakshmipriya, T.; Chen, Y.; Phang, W.-M.; Hashim, U. Aptamer-based ‘point-of-care testing’. Biotechnol. Adv. 2016, 34, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Duan, N.; Wu, S.; Shen, M.; Wang, Z. Selection, Identification, and Binding Mechanism Studies of an ssDNA Aptamer Targeted to Different Stages of E. coli O157:H7. J. Agric. Food Chem. 2018, 66, 5677–5682. [Google Scholar] [CrossRef]
- White, R.; Rusconi, C.P.; Scardino, E.; Wolberg, A.S.; Lawson, J.H.; Hoffman, M.; A Sullenger, B. Generation of Species Cross-reactive Aptamers Using “Toggle” SELEX. Mol. Ther. 2001, 4, 567–573. [Google Scholar] [CrossRef]
- la Housse, M.; Park, H.-C.; Lee, S.-C.; Ha, N.-R.; Jung, I.-P.; Schlesinger, S.R.; Shackelford, K.; Yoon, M.-Y.; Kim, S.J. Inhibition of anthrax lethal factor by ssDNA aptamers. Arch. Biochem. Biophys. 2018, 646, 16–23. [Google Scholar] [CrossRef]
- Biondi, E.; Lane, J.D.; Das, D.; Dasgupta, S.; Piccirilli, J.A.; Hoshika, S.; Bradley, K.M.; Krantz, B.A.; Benner, S.A. Laboratory evolution of artificially expanded DNA gives redesignable aptamers that target the toxic form of anthrax protective antigen. Nucleic Acids Res. 2016, 44, 9565–9577. [Google Scholar] [CrossRef]
- Dwivedi, H.P.; Smiley, R.D.; Jaykus, L.-A. Selection and characterization of DNA aptamers with binding selectivity to Campylobacter jejuni using whole-cell SELEX. Appl. Microbiol. Biotechnol. 2010, 87, 2323–2334. [Google Scholar] [CrossRef]
- Marton, S.; Cleto, F.; Krieger, M.A.; Cardoso, J. Isolation of an Aptamer that Binds Specifically to E. coli. PLoS ONE 2016, 11, e0153637. [Google Scholar] [CrossRef]
- Renders, M.; Miller, E.; Lam, C.H.; Perrin, D. Whole cell-SELEX of aptamers with a tyrosine-like side chain against live bacteria. Org. Biomol. Chem. 2017, 15, 1980–1989. [Google Scholar] [CrossRef] [PubMed]
- Amraee, M.; Oloomi, M.; Yavari, A.; Bouzari, S. DNA aptamer identification and characterization for E. coli O157 detection using cell-based SELEX method. Anal. Biochem. 2017, 536, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Gu, L.; Ren, W.; Ma, X.; Qin, M.; Lyu, M.; Wang, S. Recognition of Helicobacter pylori by protein-targeting aptamers. Helicobacter 2019, 24, e12577. [Google Scholar] [CrossRef] [PubMed]
- Graziani, A.C.; Stets, M.I.; Lopes, A.L.K.; Schluga, P.H.C.; Marton, S.; Mendes, I.F.; de Andrade, A.S.R.; Krieger, M.A.; Cardoso, J. High Efficiency Binding Aptamers for a Wide Range of Bacterial Sepsis Agents. J. Microbiol. Biotechnol. 2017, 27, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Mozioglu, E.; Gokmen, O.; Tamerler, C.; Kocagoz, Z.T.; Akgoz, M. Selection of Nucleic Acid Aptamers Specific for Mycobacterium tuberculosis. Appl. Biochem. Biotechnol. 2015, 178, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Zimbres, F.M.; Tárnok, A.; Ulrich, H.D.; Wrenger, C. Aptamers: Novel Molecules as Diagnostic Markers in Bacterial and Viral Infections? BioMed Res. Int. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Soundy, J.; Day, D.J. Selection of DNA aptamers specific for live Pseudomonas aeruginosa. PLoS ONE 2017, 12, e0185385. [Google Scholar] [CrossRef]
- Duan, N.; Wu, S.; Chen, X.; Huang, Y.; Xia, Y.; Ma, X.; Wang, Z. Selection and Characterization of Aptamers against Salmonella typhimurium Using Whole-Bacterium Systemic Evolution of Ligands by Exponential Enrichment (SELEX). J. Agric. Food Chem. 2013, 61, 3229–3234. [Google Scholar] [CrossRef]
- Sedighian, H.; Halabian, R.; Amani, J.; Heiat, M.; Amin, M.; Fooladi, A.A.I. Staggered Target SELEX, a novel approach to isolate non-cross-reactive aptamer for detection of SEA by apta-qPCR. J. Biotechnol. 2018, 286, 45–55. [Google Scholar] [CrossRef]
- Wang, K.; Gan, L.; Jiang, L.; Zhang, X.; Yang, X.; Chen, M.; Lan, X. Neutralization of Staphylococcal Enterotoxin B by an Aptamer Antagonist. Antimicrob. Agents Chemother. 2015, 59, 2072–2077. [Google Scholar] [CrossRef]
- Stoltenburg, R.; Krafčiková, P.; Víglaský, V.; Strehlitz, B. G-quadruplex aptamer targeting Protein A and its capability to detect Staphylococcus aureus demonstrated by ELONA. Sci. Rep. 2016, 6, 33812. [Google Scholar] [CrossRef] [PubMed]
- Stoltenburg, R.; Strehlitz, B. Refining the Results of a Classical SELEX Experiment by Expanding the Sequence Data Set of an Aptamer Pool Selected for Protein A. Int. J. Mol. Sci. 2018, 19, 642. [Google Scholar] [CrossRef] [PubMed]
- Ramlal, S.; Mondal, B.; Lavu, P.S.; Kingston, J. Capture and detection of Staphylococcus aureus with dual labeled aptamers to cell surface components. Int. J. Food Microbiol. 2018, 265, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Liu, J.; Su, D.; Hu, D.; Hou, S.; Hu, T.; Yang, J.; Luo, Y.; Xi, Q.; Chu, B.; et al. Identification of ssDNA aptamers specific to clinical isolates of Streptococcus mutans strains with different cariogenicity. Acta Biochim. Biophys. Sin. 2016, 48, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Hamula, C.L.; Le, X.C.; Li, X.-F. DNA Aptamers Binding to Multiple Prevalent M-Types ofStreptococcus pyogenes. Anal. Chem. 2011, 83, 3640–3647. [Google Scholar] [CrossRef]
- Hamula, C.L.; Peng, H.; Wang, Z.; Tyrrell, G.J.; Li, X.-F.; Le, X.C. An improved SELEX technique for selection of DNA aptamers binding to M-type 11 of Streptococcus pyogenes. Methods 2016, 97, 51–57. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, M.; Su, H.; Xiao, H.; Wu, S.; Qin, X.; Li, S.; Mi, H.; Lu, Z.; Shi, D.; et al. Selection and characterization of ssDNA aptamers specifically recognizing pathogenic Vibrio alginolyticus. J. Fish. Dis. 2019, 42, 851–858. [Google Scholar] [CrossRef]
- Song, S.; Wang, X.; Xu, K.; Li, Q.; Ning, L.; Yang, X. Selection of highly specific aptamers to Vibrio parahaemolyticus using cell-SELEX powered by functionalized graphene oxide and rolling circle amplification. Anal. Chim. Acta 2019, 1052, 153–162. [Google Scholar] [CrossRef]
- Yan, W.; Gu, L.; Liu, S.; Ren, W.; Lyu, M.; Wang, S. Identification of a highly specific DNA aptamer for Vibrio vulnificus using systematic evolution of ligands by exponential enrichment coupled with asymmetric PCR. J. Fish. Dis. 2018, 41, 1821–1829. [Google Scholar] [CrossRef]
- Yan, A.C.; Levy, M. Aptamers and aptamer targeted delivery. RNA Biol. 2009, 6, 316–320. [Google Scholar] [CrossRef]
- Becker, S.; Theile, S.; Heppeler, N.; Michalczyk, A.; Wentzel, A.; Wilhelm, S.; Jaeger, K.-E.; Kolmar, H. A generic system for the Escherichia coli cell-surface display of lipolytic enzymes. FEBS Lett. 2005, 579, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Anis, E.; Hawkins, I.K.; Ilha, M.R.S.; Woldemeskel, M.W.; Saliki, J.T.; Wilkes, R.P. Evaluation of Targeted Next-Generation Sequencing for Detection of Bovine Pathogens in Clinical Samples. J. Clin. Microbiol. 2018, 56, e00399-18. [Google Scholar] [CrossRef] [PubMed]
- Motro, Y.; Moran-Gilad, J. Next-generation sequencing applications in clinical bacteriology. Biomol. Detect. Quantif. 2017, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Udugama, B.; Kadhiresan, P.; Kozlowski, H.N.; Malekjahani, A.; Osborne, M.; Li, V.Y.C.; Chen, H.; Mubareka, S.; Gubbay, J.B.; Chan, W.C.W. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano 2020, 14, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- Villalonga, A.; Pérez-Calabuig, A.M.; Villalonga, R. Electrochemical biosensors based on nucleic acid aptamers. Anal. Bioanal. Chem. 2020, 412, 55–72. [Google Scholar] [CrossRef] [PubMed]

| Infection | Overall Percentage (%) | Most Common Organisms (%) |
|---|---|---|
| Surgical Site Infections | (19.6%) | Staphylococcus aureus (17.9%) |
| Pneumonia | (19.4%) | Pseudomonas aeruginosa (17.4%) |
| Urinary tract infections | (19%) | Escherichia coli (36.2%) |
| Bloodstream infections | (10.6%) | Coagulase-negative Staphylococci (18.5%) |
| Gastrointestinal system infections | (7.6%) | Clostridium difficile (48%) |
| Other Lower Respiratory Tract Infections | (4.1%) | Staphylococcus aureus (12.6%) |
| Other infections | (19.7%) | Unspecified |
| Detection and Diagnostic System | Aptamer or Antibody Applicable | Advantages | Disadvantages | Location and Limit of Detection |
|---|---|---|---|---|
| Culturing and microscopy | Neither applicable | Detects presence of bacteria Easy technique Does not require specialist equipment Relatively cheap | Some bacteria are un-culturable Prone to false negatives Lack specificity—only detects presence or absence not species, which is not desired for a diagnostic Time-consuming [24,39,58,60,61,62,76,77,78] | Pathology laboratory Limit of detection: N/A, time is the factor rather than concentration, the bacteria will grow but will take longer with a lower cfu/mL |
| ELISA | Both applicable | Specific Little chemical preparation required Cheaper | Expensive equipment Requires specialist equipment Time-consuming Requires culturing [58,60,61,62,77,79] | Pathology laboratory Limit of detection: 104–106 cfu/mL |
| PCR | Neither applicable | Requires small amount of bacteria Specific—can identify species Easy technique Does not require specialist equipment | Requires specific probes Point mutations in bacterial genes can lead to false negatives and false positives Time-consuming Expensive [62,80] | Onsite or pathology laboratory Limit of detection: 103 cfu/mL |
| Real time PCR | Neither applicable | Time-efficient Requires small amount of bacteria Specific—can identify species | Requires specific probes Point mutations in bacterial genes can lead to false negatives and false positives [62,80] | Pathology laboratory Limit of detection: 103 cfu/mL |
| Next generation sequencing | Neither applicable | Time-efficient Requires small amount of bacteria Specific | Requires specialist equipment Requires bioinformatics knowledge [59] | Sequencing company Limit of detection: 10–100 cfu/mL |
| Biosensors (Antibodies) | Antibody | Highly specific (nanomolar) Time-efficient | Batch-batch variation Expensive Prone to steric hindrance Degrades in heat and pH changes Can cause immune response [24,60,61] | Onsite or pathology laboratory Limit of detection: 103 cfu/mL |
| Biosensors (Aptamers) | Aptamer | Highly specific (nanomolar to femtomolar) Time-efficient High signal density Low steric hindrance Easily modifiable Cheaper Does not use animals Does not degrade in high heat or changing pH Reusable No immune response | Nuclease degradation Can be too small [24,81,82,83,84,85,86,87,88] | Onsite Limit of detection: 102 cfu/mL |
| Lateral flow devices | Both applicable | Time-efficient Cheap Simple | Can be prone to false binding Can be complex to use Can require complex equipment [63] | Onsite or pathology laboratory Limit of detection: 43 cfu/mL to 109 cfu/mL |
| Aptamers | Type of Aptamer | Organism | Target |
|---|---|---|---|
| ML6, ML7 and ML12 | DNA | Bacillus anthracis [107] | Lethal factor |
| PA1 | DNA | Bacillus anthracis [108] | Protective antigen |
| ONS-23 | DNA | Campylobacter jejuni (strain A9a) [109] | Whole bacteria |
| P12-31 | DNA | Escherichia coli (ATCC 25922) [110] | Whole bacteria |
| EA1 and EA7 | DNA | Escherichia coli (strain 11775) [4] | Whole bacteria |
| 8.10A, 8.14B, 8.18B and 8.28A | DNA | Escherichia coli DH5α [111] | Whole bacteria |
| AM1, AM2, AM3, AM4, AM5 and AM6 | DNA | Escherichia coli O157:H7 [112] | Whole bacteria |
| Apt-5 | DNA | Escherichia coli O157:H7 [105] | Whole bacteria |
| Hp4 | DNA | Helicobacter pylori [113] | Whole bacteria |
| hemag1, mag1 and hemag3 | DNA | Lactobacillus acidophilus (strain 4355, 4356, 4357) [103] | Whole bacteria |
| Antibac1 and Antibac2 | DNA | Multiple species [114] | Peptidoglycan |
| Mtb36 | DNA | Mycobacterium tuberculosis (strain H37Ra) [115] | Whole cell |
| NK2 | DNA | Mycobacterium tuberculosis (strain H37Rv) [116] | Membrane proteins |
| JN17, JN21, JN08 and JN27 | DNA | Pseudomonas aeruginosa [117] | Whole bacteria |
| 33 | DNA | Salmonella enterica serovar Typhimurium [116] | Outer membrane proteins (OMPs) |
| S-PS8.4 | RNA | Salmonella enterica serovar Typhimurium [116] | Type IVB pili |
| ST2, ST3, ST7 and ST9 | DNA | Salmonella typhimurium (strain ATCC 50761) [118] | Whole bacteria |
| C5, C7, C10, C13 and C16 | DNA | Staphylococcal Enterotoxin A [119] | Staphylococcal Enterotoxin A |
| A11 | DNA | Staphylococcal Enterotoxin B [120] | Staphylococcal Enterotoxin B |
| SA20, SA23, SA32, SA34 and SA43 | DNA | Staphylococcus aureus (strain MRSA) [116] | Whole bacteria |
| PA#2/8, PA#2/8[S1-58], PA#2/8[S1-50], PA#2/8[S1-43] and PA#2/8[S28-50] | DNA | Staphylococcus aureus [121] | Protein A |
| Pa-C10 and PA-C8 | DNA | Staphylococcus aureus [122] | Protein A |
| RAB10, RAB20, RAB28 and RAB35 | DNA | Staphylococcus aureus [123] | Whole bacteria |
| H1, H16, H4, L1, L10 and H19 | DNA | Streptococcus mutans [124] | Whole bacteria |
| 20A9, 20A24P, 20A9P, 20A12P, 20A14P and 15A3P | DNA | Streptococcus pyogenes [125] | M-Type bacteria |
| E-Cells 1, E-Cells 1P, E-CA 20, E-CA20P, D-Cells 9 and D-Cells9P | DNA | Streptococcus pyogenes [126] | Whole bacteria |
| VA2 and VA8 | DNA | Vibrio aliginolyticus [127] | Whole bacteria |
| Ap1, Ap2, Apt3 and Apt4 | DNA | Vibrio parahaemolyticus (ATCC 17802) [128] | Whole bacteria |
| Vapt2 | DNA | Vibrio vulnificus [129] | Whole bacteria |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strom, M.; Crowley, T.; Shigdar, S. Novel Detection of Nasty Bugs, Prevention Is Better than Cure. Int. J. Mol. Sci. 2021, 22, 149. https://doi.org/10.3390/ijms22010149
Strom M, Crowley T, Shigdar S. Novel Detection of Nasty Bugs, Prevention Is Better than Cure. International Journal of Molecular Sciences. 2021; 22(1):149. https://doi.org/10.3390/ijms22010149
Chicago/Turabian StyleStrom, Mia, Tamsyn Crowley, and Sarah Shigdar. 2021. "Novel Detection of Nasty Bugs, Prevention Is Better than Cure" International Journal of Molecular Sciences 22, no. 1: 149. https://doi.org/10.3390/ijms22010149
APA StyleStrom, M., Crowley, T., & Shigdar, S. (2021). Novel Detection of Nasty Bugs, Prevention Is Better than Cure. International Journal of Molecular Sciences, 22(1), 149. https://doi.org/10.3390/ijms22010149




