Antiproliferative Effects of St. John’s Wort, Its Derivatives, and Other Hypericum Species in Hematologic Malignancies
Abstract
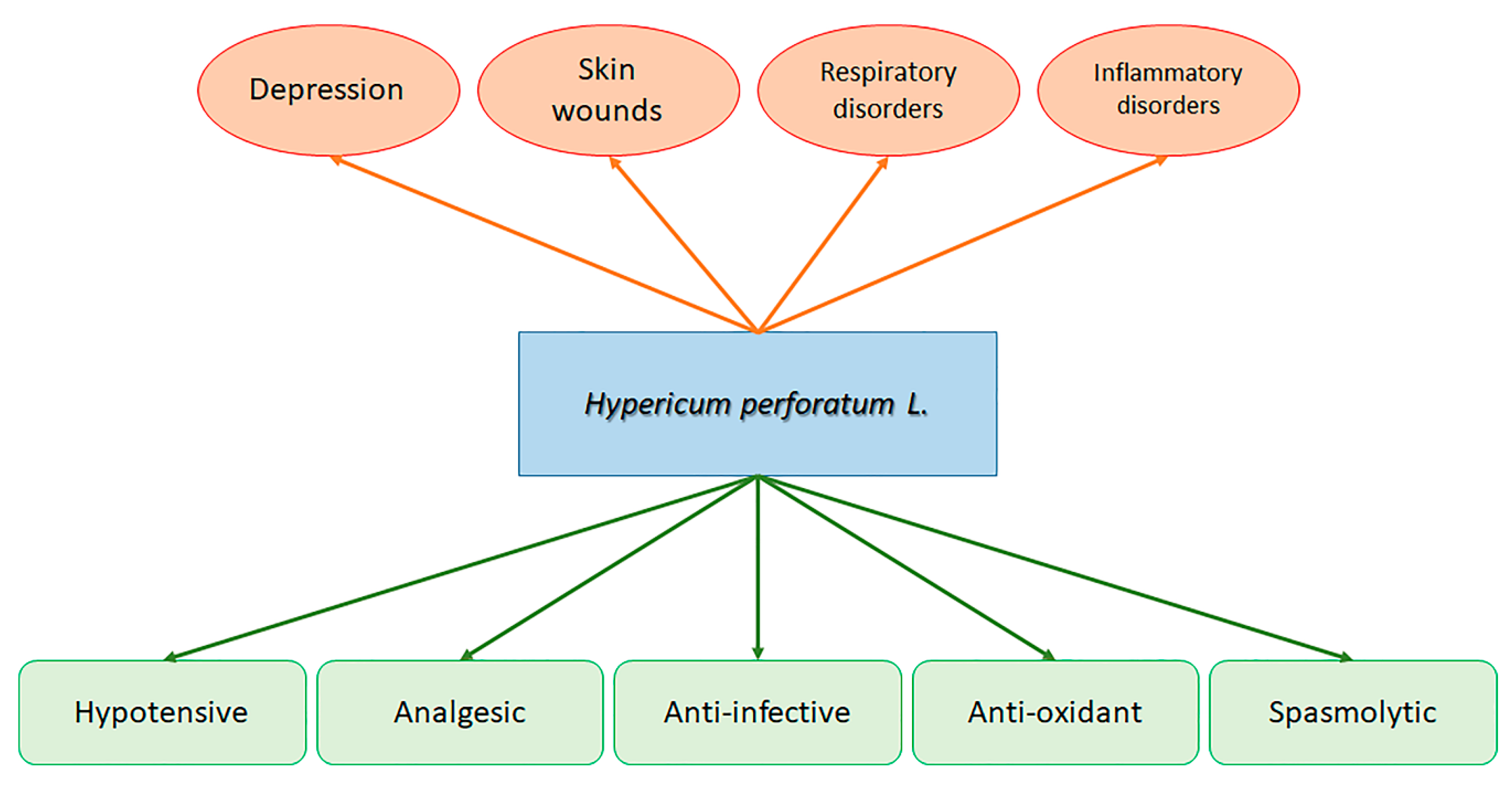
1. Introduction
2. Antiproliferative Activities of Hypericum Derivatives on Myeloid and Lymphoid Cells
2.1. Photochemical Features of Hypericin
2.2. Drug Interactions. Effects of Hypericum Derivatives on Mechanism of Multidrug Resistance
3. New By-Products and Hematologic Malignancies
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Marrelli, M.; Statti, G.; Conforti, F. Hypericum spp.: An Update on the Biological Activities and Metabolic Profiles. Mini-Rev. Med. Chem. 2020, 20, 66–87. [Google Scholar] [CrossRef] [PubMed]
- Clement, K.; Covertson, C.R.; Johnson, M.J.; Dearing, K. St. John’s wort and the treatment of mild to moderate depression: A systematic review. Holist. Nurs. Pract. 2006, 20, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.A.; Martinez-Poveda, B.; Amores-Sanchez, M.I.; Quesada, A.R. Hyperforin: More than an antidepressant bioactive compound? Life Sci. 2006, 79, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Linde, K.; Berner, M.M.; Kriston, L. St. John’s wort for major depression. Cochrane Database Syst. Rev. 2008, 4, CD000448. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. St. John’s wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2001, 53, 583–600. [Google Scholar] [CrossRef]
- Whiskey, E.; Werneke, U.; Taylor, D. A systematic review and meta-analysis of Hypericum perforatum in depression: A comprehensive clinical review. Int. Clin. Psychopharmacol. 2001, 16, 239–252. [Google Scholar] [CrossRef]
- Klemow, K.M.; Bartlow, A.; Crawford, J.; Kocher, N.; Shah, J.; Ritsick, M. Chapter 11 Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press; Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Assiri, K.; Alyami, Y.; Uyanik, J.M.; Romero-Reyes, M. Hypericum perforatum (St. John’s Wort) as a possible therapeutic alternative for the management of Trigeminal Neuralgia (TN)—A case report. Complement. Ther. Med. 2017, 30, 36–39. [Google Scholar] [CrossRef]
- Galeotti, N. Hypericum perforatum (St. John’s wort) beyond depression: A therapeutic perspective for pain conditions. J. Ethnopharmacol. 2017, 200, 136–146. [Google Scholar] [CrossRef]
- Deng, M.; Tao, L.; Qiao, Y.; Sun, W.; Xie, S.; Shi, Z.; Qi, C.; Zhang, Y. New cytotoxic secondary metabolites against human pancreatic cancer cells from the Hypericum perforatum endophytic fungus Aspergillus terreus. Fitoterapia 2020, 146, 104685. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Hosseini, F.; Ahmadi, A.; Mozafari, M.; Amjadi, I. Antiproliferative Activity of Hypericum perforatum, Achillea millefolium, and Aloevera in Interaction with the Prostatic Activity of CD82. Rep. Biochem. Mol. Biol. 2019, 8, 260–268. [Google Scholar]
- Ferraz, A.B.; Grivicich, I.; Von Poser, G.L.; Faria, D.H.; Kayser, G.B.; Schwartsmann, G.; Henriques, A.T.; da Rocha, A.B. Antitumor activity of three benzopyrans isolated from Hypericum polyanthemum. Fitoterapia 2005, 76, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Viveros-Valdez, E.; Rivas-Morales, C.; Oranday-Cárdenas, A.; Castro-Garza, J.; Carranza-Rosales, P. Antiproliferative effect from the Mexican poleo (Hedeoma drummondii). J. Med. Food 2010, 13, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Petelka, J.; Plagg, B.; Säumel, I.; Zerbe, S. Traditional medicinal plants in South Tyrol (northern Italy, southern Alps): Biodiversity and use. J. Ethnobiol. Ethnomed. 2020, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Madunić, J.; Matulić, M.; Friščić, M.; Pilepić, K.H. Evaluation of the cytotoxic activity of Hypericum spp. on human glioblastoma A1235 and breast cancer MDA MB-231 cells. J. Environ. Sci. Health A 2016, 51, 1157–1163. [Google Scholar] [CrossRef]
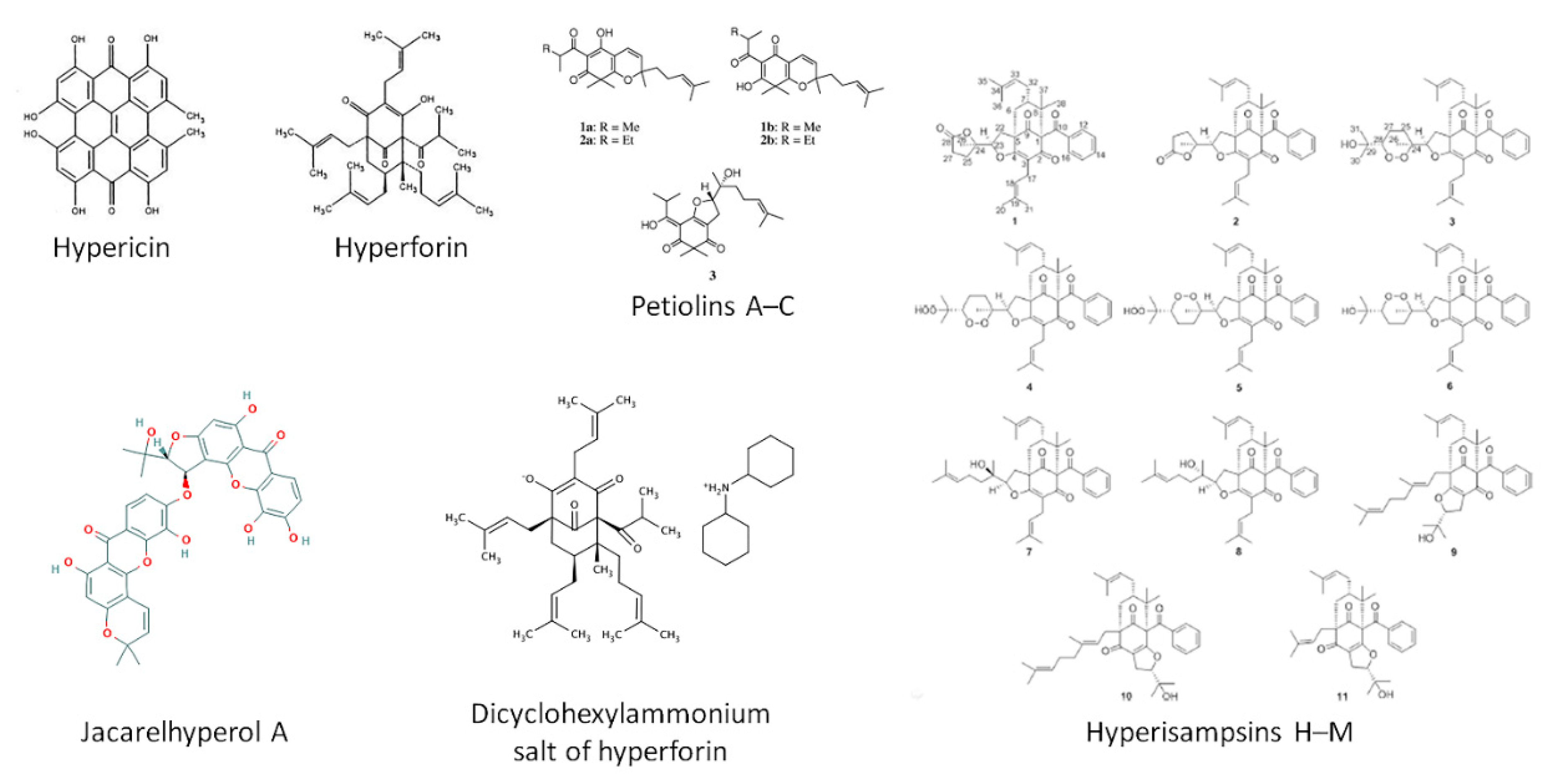
- Bridi, H.; de Carvalho Meirelles, G.; von Poser, G.L. Structural diversity and biological activities of phloroglucinol derivatives from Hypericum species. Phytochemistry 2018, 155, 203–232. [Google Scholar] [CrossRef]
- Smelcerovic, A.; Spiteller, M.; Zuehlke, S. Comparison of methods for the exhaustive extraction of hypericins, flavonoids, and hyperforin from Hypericum perforatum L. J. Agric. Food Chem. 2006, 54, 2750–2753. [Google Scholar] [CrossRef]
- Butterweck, V.; Schmidt, M. St. John’s wort: Role of active compounds for its mechanism of action and efficacy. Wien. Med. Wochenschr. 2007, 157, 356–361. [Google Scholar] [CrossRef]
- Tatsis, E.C.; Boeren, S.; Exarchou, V.; Troganis, A.N.; Vervoort, J.; Gerothanassis, I.P. Identification of the major constituents of Hypericumperforatum by LC/SPE/NMR and/or LC/MS. Phytochemistry 2007, 68, 383–393. [Google Scholar] [CrossRef]
- Saddiqe, Z.; Naeem, I.; Maimoona, A. A review of the antibacterial activity of Hypericum perforatum L. J. Ethnopharmacol. 2010, 131, 511–521. [Google Scholar] [CrossRef]
- Karioti, A.; Bilia, A.R. Hypericins as potential leads for new therapeutics. Int. J. Mol. Sci. 2010, 11, 562–594. [Google Scholar] [CrossRef]
- Murthy, H.N.; Kim, Y.S.; Park, S.Y.; Paek, K.Y. Hypericins: Biotechnological production from cell and organ cultures. Appl. Microbiol. Biotechnol. 2014, 98, 9187–9198. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Wang, S.J. Hypericin, the active component of St. John’s wort, inhibits glutamate release in the rat cerebrocortical synaptosomes via a mitogen-activated protein kinase-dependent pathway. Eur. J. Pharmacol. 2010, 634, 53–61. [Google Scholar] [CrossRef]
- Meruelo, D.; Lavie, G.; Lavie, D. Therapeutic agents with dramatic antiretroviral activity and little toxicity at effective doses: Aromatic polycyclic diones hypericin and pseudohypericin. Proc. Natl. Acad. Sci. USA 1988, 85, 5230–5234. [Google Scholar] [CrossRef] [PubMed]
- Joniova, J.; Misuth, M.; Sureau, F.; Miskovsky, P.; Nadova, Z. Effect of PKCalpha expression on Bcl-2 phosphorylation and cell death by hypericin. Apoptosis 2014, 19, 1779–1792. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Vandenbogaerde, A.; Donella-Deana, A.; Pinna, L.A.; Lee, K.T.; Goris, J.; Merlevede, W.; Vandenheede, J.R.; De Witte, P. Photosensitized inhibition of growth factor-regulated protein kinases by hypericin. Biochem. Pharmacol. 1995, 49, 1615–1622. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Heinrich, M.; Langosch, J.M.; Kammerer, N.; Lieb, K. Antibacterial activity of hyperforin from St. John’s wort. Lancet 1999, 354, 777. [Google Scholar] [CrossRef]
- Richard, A.J.; Amini, Z.J.; Ribnicky, D.M.; Stephens, J.M. St. John’s Wort inhibits insulin signaling in murine and human adipocytes. Biochim. Biophys. Acta 2012, 1822, 557–563. [Google Scholar] [CrossRef]
- Valipour, A.; Jäger, M.; Wu, P.; Schmitt, J.; Bunch, C.; Weberschock, T. Interventions for mycosis fungoides. Cochrane Database Syst. Rev. 2020, 7, CD008946. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X.; Cheng, W.; Wang, Y.; Yi, K.; Wang, Z.; Zhang, Y.; Shao, L.; Zhao, T. Hypericin-photodynamic therapy inhibits the growth of adult T-cell leukemia cells through induction of apoptosis and suppression of viral transcription. Retrovirology 2019, 16, 5. [Google Scholar] [CrossRef]
- Beerhues, L. Hyperforin. Phytochemistry 2006, 67, 2201–2207. [Google Scholar] [CrossRef]
- Schempp, C.M.; Kirkin, V.; Simon-Haarhaus, B.; Kersten, A.; Kis, J.; Termeer, C.C.; Gilb, B.; Kaufmann, T.; Borner, C.; Sleeman, J.P.; et al. Inhibition of tumour cell growth by hyperforin, a novel anticancer drug from St. John’s Wort that acts by induction of apoptosis. Oncogene 2002, 21, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Dona, M.; Dell’Aica, I.; Pezzato, E.; Sartor, L.; Calabrese, F.; Barbera, M.D.; Donella-Deana, A.; Appendino, G.; Borsarini, A.; Caniato, R.; et al. Hyperforin inhibits cancer invasion and metastasis. Cancer Res. 2004, 64, 6225–6232. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Poveda, B.; Quesada, A.R.; Medina, M.A. Hyperforin, a bio-active compound of St. John’s Wort, is a new inhibitor of angiogenesis targeting several key steps of the process. Int. J. Cancer 2005, 117, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Quiney, C.; Billard, C.; Mirshahi, P.; Fourneron, J.D.; Kolb, J.P. Hyperforin inhibits MMP-9secretion by B-CLL cells and microtubule formation by endothelial cells. Leukemia 2006, 20, 583–589. [Google Scholar] [CrossRef]
- Hostanska, K.; Reichling, J.; Bommer, S.; Weber, M.; Saller, R. Hyperforin a constituent of St. John’s Wort (Hypericum perforatum L.) extract induces apoptosis by triggering activation of caspases and with hypericin synergistically exerts cytotoxicity towards human malignant cell lines. Eur. J. Pharm. Biopharm. 2003, 56, 121–132. [Google Scholar] [CrossRef]
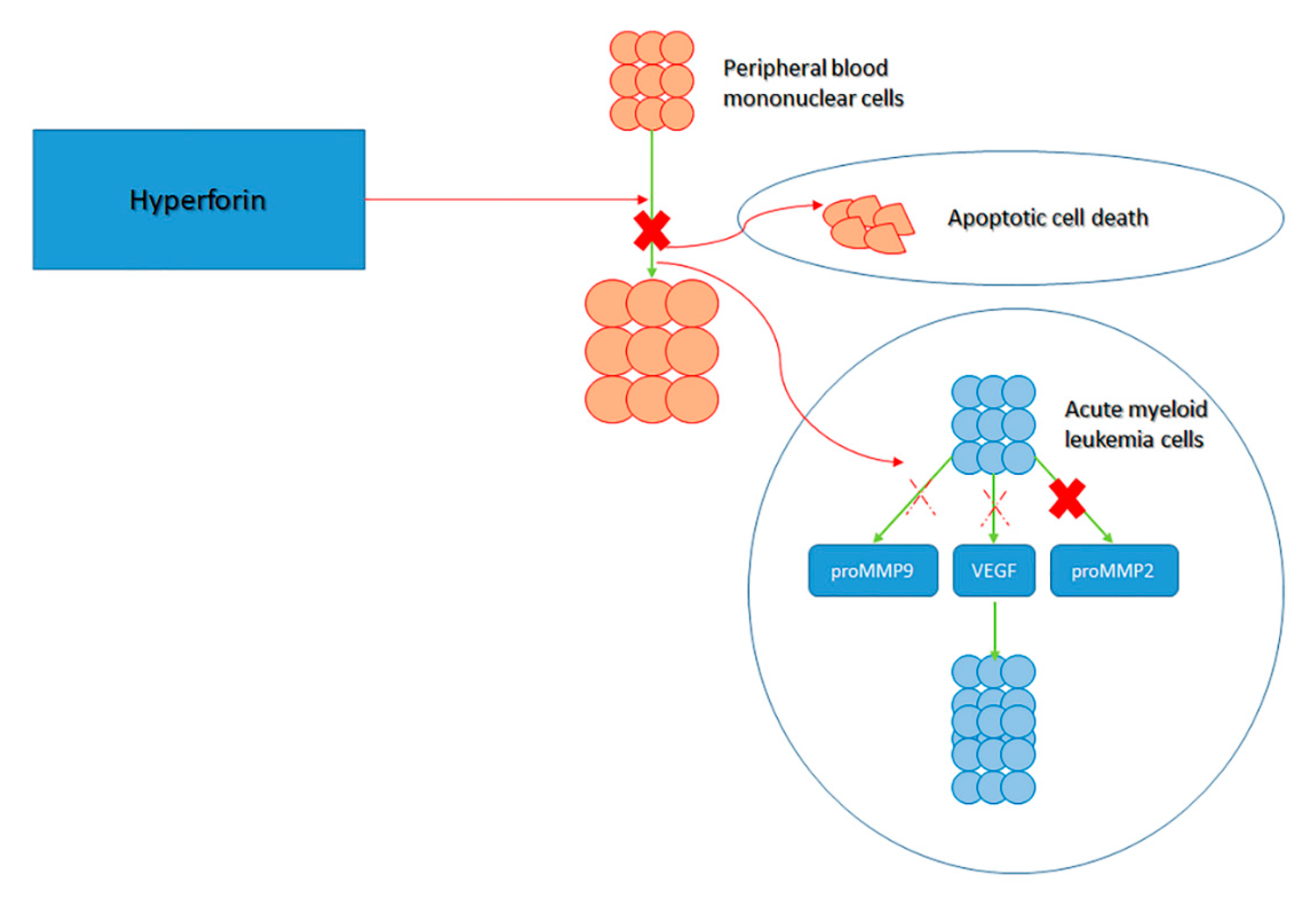
- Merhi, F.; Tang, R.; Piedfer, M.; Mathieu, J.; Bombarda, I.; Zaher, M.; Kolb, J.P.; Billard, C.; Bauvois, B. Hyperforin inhibits Akt1 kinase activity and promotes caspase-mediated apoptosis involving bad and noxa activation in human myeloid tumor cells. PLoS ONE 2011, 6, e25963. [Google Scholar] [CrossRef]
- Liu, J.Y.; Liu, Z.; Wang, D.M.; Li, M.M.; Wang, S.X.; Wang, R.; Chen, J.P.; Wang, Y.F.; Yang, D.P. Induction of apoptosis in K562 cells by dicyclohexylammonium salt of hyperforin through a mitochondrial-related pathway. Chem. Biol. Interact. 2011, 190, 91–101. [Google Scholar] [CrossRef]
- Martelli, A.M.; Nyakern, M.; Tabellini, G.; Bortul, R.; Tazzari, P.L.; Evangelisti, C.; Cocco, L. Phosphoinositide 3-kinase/Akt signaling pathway and its therapeutical implications for human acute myeloid leukemia. Leukemia 2006, 20, 911–928. [Google Scholar] [CrossRef]
- Datta, S.R.; Brunet, A.; Greenberg, M.E. Cellular survival: A play in three Akts. Genes Dev. 1999, 13, 2905–2927. [Google Scholar] [CrossRef]
- Dias, S.; Hattori, K.; Zhu, Z.; Heissig, B.; Choy, M.; Lane, W.; Wu, Y.; Chadburn, A.; Hyjek, E.; Gill, M.; et al. Autocrine stimulation of VEGFR-2 activates human leukemic cell growth and migration. J. Clin. Investig. 2000, 106, 511–521. [Google Scholar] [CrossRef]
- Billard, C.; Merhi, F.; Bauvois, B. Mechanistic insights into the antileukemic activity of hyperforin. Curr. Cancer Drug Targets 2013, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Quiney, C.; Billard, C.; Faussat, A.M.; Salanoubat, C.; Ensaf, A.; Naït-Si, Y.; Fourneron, J.D.; Kolb, J.P. Pro-apoptotic properties of hyperforin in leukemic cells from patients with B-cell chronic lymphocytic leukemia. Leukemia 2006, 20, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Quiney, C.; Billard, C.; Faussat, A.M.; Salanoubat, C.; Kolb, J.P. Hyperforin inhibits P-gp and BCRP activities in chronic lymphocytic leukaemia cells and myeloid cells. Leuk. Lymphoma 2007, 48, 1587–1599. [Google Scholar] [CrossRef] [PubMed]
- Burden, D.A.; Osheroff, N. Mechanism of action of eukaryotic topoisomerase II and drugs targeted to the enzyme. Biochim. Biophys. Acta 1998, 1400, 139–154. [Google Scholar] [CrossRef]
- Peebles, K.A.; Baker, R.K.; Kurz, E.U.; Schneider, B.J.; Kroll, D.J. Catalytic inhibition of human DNA topoisomerase IIalpha by hypericin, a naphthodianthrone from St. John’s wort (Hypericum perforatum). Biochem. Pharmacol. 2001, 62, 1059–1070. [Google Scholar] [CrossRef]
- Valletta, E.; Rinaldi, A.; Marini, M.; Franzese, O.; Roscetti, G. Distinct Hypericum perforatum L. total extracts exert different antitumour activity on erythroleukemic K562 cells. Phytother. Res. 2018, 32, 1803–1811. [Google Scholar] [CrossRef]
- Masuda, T.; Oyama, Y.; Yamamoto, N.; Umebayashi, C.; Nakao, H.; Toi, Y.; Takeda, Y.; Nakamoto, K.; Kuninaga, H.; Nishizato, Y.; et al. Cytotoxic screening of medicinal and edible plants in Okinawa, Japan, and identification of the main toxic constituent of Rhodeajaponica (Omoto). Biosci. Biotechnol. Biochem. 2003, 67, 1401–1404. [Google Scholar] [CrossRef]
- Zhang, R.; Ji, Y.; Zhang, X.; Kennelly, E.J.; Long, C. Ethnopharmacology of Hypericum species in China: A comprehensive review on ethnobotany, phytochemistry and pharmacology. J. Ethnopharmacol. 2020, 254, 112686. [Google Scholar] [CrossRef]
- Chen, X.Q.; Li, Y.; Cheng, X.; Wang, K.; He, J.; Pan, Z.H.; Li, M.M.; Peng, L.Y.; Xu, G.; Zhao, Q.S. Polycyclic polyprenylated acylphloroglucinols and chromone O-glucosides from Hypericumhenryi subsp. uraloides. Chem. Biodivers. 2010, 7, 196–204. [Google Scholar] [CrossRef]
- Zhang, S.; Yin, J.; Li, X.; Zhang, J.; Yue, R.; Diao, Y.; Li, H.; Wang, H.; Shan, L.; Zhang, W. Jacarelhyperol A induced apoptosis in leukaemia cancer cell through inhibition the activity of Bcl-2 proteins. BMC Cancer 2014, 14, 689. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, C.; Tong, Q.; Chen, X.; Yang, J.; Liu, J.; Sun, B.; Wang, J.; Yao, G.; Luo, Z.; et al. Hyperisampsins H-M, Cytotoxic Polycyclic Polyprenylated Acylphloroglucinols from Hypericum sampsonii. Sci. Rep. 2015, 5, 14772. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Kubota, T.; Ishiyama, H.; Araki, A.; Kashiwada, Y.; Takaishi, Y.; Mikami, Y.; Kobayashi, J. Petiolins A-C, phloroglucinol derivatives from Hypericum pseudopetiolatum var. kiusianum. Bioorg. Med. Chem. 2008, 16, 5619–5623. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, P.; Vinod Kumar, S.; Dhanaraj, S.A.; Mukherjee, P.K.; Suresh, B. In vitro cytotoxicity and antitumour properties of Hypericum mysorense and Hypericum patulum. Phytother. Res. 2003, 17, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Dongre, S.H.; Badami, S.; Godavarthi, A. Antitumor activity of Hypericum hookerianum against DLA induced tumor in mice and its possible mechanism of action. Phytother. Res. 2008, 22, 23–29. [Google Scholar] [CrossRef]
- Puthur, S.; Anoopkumar, A.N.; Rebello, S.; Aneesh, E.M. Hypericum japonicum: A Double-Headed Sword to Combat Vector Control and Cancer. Appl. Biochem. Biotechnol. 2018, 186, 1–11. [Google Scholar] [CrossRef]
- Momekov, G.; Ferdinandov, D.; Zheleva-Dimitrova, D.; Nedialkov, P.; Girreser, U.; Kitanov, G. Cytotoxic effects of hyperatomarin, a prenylated phloroglucinol from Hypericum annulatum Moris subsp. annulatum, in a panel of malignant cell lines. Phytomedicine 2008, 15, 1010–1015. [Google Scholar] [CrossRef]
- Imbesi, S.; Musolino, C.; Allegra, A.; Saija, A.; Morabito, F.; Calapai, G.; Gangemi, S. Oxidative stress in oncohematologic diseases: An update. Expert Rev. Hematol. 2013, 6, 317–325. [Google Scholar] [CrossRef]
- Gangemi, S.; Allegra, A.; Aguennouz, M.; Alonci, A.; Speciale, A.; Cannavò, A.; Cristani, M.; Russo, S.; Spatari, G.; Alibrandi, A.; et al. Relationship between advanced oxidation protein products, advanced glycation end products, and S-nitrosylated proteins with biological risk and MDR-1 polymorphisms in patients affected by B-chronic lymphocytic leukemia. Cancer Investig. 2012, 30, 20–26. [Google Scholar] [CrossRef]
- Musolino, C.; Allegra, A.; Alonci, A.; Saija, A.; Russo, S.; Cannavò, A.; Cristani, M.; Centorrino, R.; Saitta, S.; Alibrandi, A.; et al. Carbonyl group serum levels are associated with CD38 expression in patients with B chronic lymphocytic leukemia. Clin. Biochem. 2011, 44, 1487–1490. [Google Scholar] [CrossRef]
- Gangemi, S.; Allegra, A.; Alonci, A.; Cristani, M.; Russo, S.; Speciale, A.; Penna, G.; Spatari, G.; Cannavò, A.; Bellomo, G.; et al. Increase of novel biomarkers for oxidative stress in patients with plasma cell disorders and in multiple myeloma patients with bone lesions. Inflamm. Res. 2012, 61, 1063–1067. [Google Scholar] [CrossRef]
- Allegra, A.; Pioggia, G.; Tonacci, A.; Musolino, C.; Gangemi, S. Oxidative Stress and Photodynamic Therapy of Skin Cancers: Mechanisms, Challenges and Promising Developments. Antioxidants 2020, 9, 448. [Google Scholar] [CrossRef]
- Lavie, G.; Mazur, Y.; Lavie, D.; Meruelo, D. The chemical and biological properties of hypericin—A compound with a broad spectrum of biological activities. Med. Res. Rev. 1995, 15, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.C.; Prusti, R.K.; Walker, E.B.; Song, P.S.; Watanabe, M.; Furuya, M. Photodynamic action in Stentor coeruleus sensitized by endogenous pigment stentorin. Photochem. Photobiol. 1986, 43, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Duran, N.; Song, P.S. Hypericin and its photodynamic action. Photochem. Photobiol. 1986, 43, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Zeisser-Labouebe, M.; Lange, N.; Gurny, R.; Delie, F. Hypericin-loaded nanoparticles for the photodynamic treatment of ovarian cancer. Int. J. Pharm. 2006, 326, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Bublik, M.; Head, C.; Benharash, P.; Paiva, M.; Eshraghi, A.; Kim, T.; Saxton, R. Hypericin and pulsed laser therapy of squamous cell cancer in vitro. Photomed. Laser Surg. 2006, 24, 341–347. [Google Scholar] [CrossRef]
- Hadjur, C.; Richard, M.J.; Parat, M.O.; Jardon, P.; Favier, A. Photodynamic effects of hypericin on lipid peroxidation and antioxidant status in melanoma cells. Photochem. Photobiol. 1996, 64, 375–381. [Google Scholar] [CrossRef]
- Kocisova, E.; Chinsky, L.; Miskovsky, P. Sequence specific interaction of the antiretrovirally active drug hypericin with 5′ATGGCAGGATAT3′ oligonucleotide: A resonance Raman spectroscopy study. J. Biomol. Struct. Dyn. 1998, 15, 1147–1154. [Google Scholar] [CrossRef]
- Johnson, S.A.; Dalton, A.E.; Pardini, R.S. Time-course of hypericin phototoxicity and effect on mitochondrial energies in EMT6 mouse mammary carcinoma cells. Free Radic. Biol. Med. 1998, 25, 144–152. [Google Scholar] [CrossRef]
- Hamilton, H.B.; Hinton, D.R.; Law, R.E.; Gopalakrishna, R.; Su, Y.Z.; Chen, Z.H.; Weiss, M.H.; Couldwell, W.T. Inhibition of cellular growth and induction of apoptosis in pituitary adenoma cell lines by the protein kinase C inhibitor hypericin: Potential therapeutic application. J. Neurosurg. 1996, 85, 329–334. [Google Scholar] [CrossRef]
- Zhang, W.; Law, R.E.; Hinton, D.R.; Couldwell, W.T. Inhibition of human malignant glioma cell motility and invasion in vitro by hypericin, a potent protein kinase C inhibitor. Cancer Lett. 1997, 120, 31–38. [Google Scholar] [CrossRef]
- Vandenbogaerde, A.L.; Cuveele, J.F.; Proot, P.; Himpens, B.E.; Merlevede, W.J.; de Witte, P.A. Differential cytotoxic effects induced after photosensitization by hypericin. J. Photochem. Photobiol. B 1997, 38, 136–142. [Google Scholar] [CrossRef]
- Vandenbogaerde, A.L.; Delaey, E.M.; Vantieghem, A.M.; Himpens, B.E.; Merlevede, W.J.; de Witte, P.A. Cytotoxicity and antiproliferative effect of hypericin and derivatives after photosensitization. Photochem. Photobiol. 1998, 67, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Trepel, M.; Grimmel, C.; Schabet, M.; Bremen, D.; Krajewski, S.; Reed, J.C. Hypericin-induced apoptosis of human malignant glioma cells is light-dependent, independent of bcl-2 expression, and does not require wild-type p53. Neurol. Res. 1997, 19, 459. [Google Scholar] [CrossRef]
- Fehr, M.I.; Carpenter, S.L.; Petrich, J.W. The role of oxygen in the photoinduced antiviral activity of hypericin. Bioorg. Med. Chem. Lett. 1994, 4, 1339–1344. [Google Scholar] [CrossRef]
- Mirossay, A.; Mirossay, L.; Sarisský, M.; Papp, P.; Mojzis, J. Modulation of the phototoxic effect of hypericin in human leukemia CEM cell line by N-ethylmaleimide, amiloride and omeprazole. Physiol. Res. 2002, 51, 641–644. [Google Scholar]
- Chen, B.; Zupkó, I.; de Witte, P.A. Photodynamic therapy with hypericin in a mouse P388 tumor model: Vascular effects determine the efficacy. Int. J. Oncol. 2001, 18, 737–742. [Google Scholar] [CrossRef]
- Schempp, C.M.; Simon-Haarhaus, B.; Simon, J.C. Phototoxic and apoptosis-inducing capacity of pseudohypericin. Planta Med. 2002, 68, 171–173. [Google Scholar] [CrossRef]
- Borrelli, F.; Izzo, A.A. Herb–drug interactions with St. John’s wort (Hypericum perforatum): An update on clinical observations. AAPS J. 2009, 11, 710–727. [Google Scholar] [CrossRef]
- Mathijssen, R.H.; Verweij, J.; de Bruijn, P.; Loos, W.J.; Sparreboom, A. Effects of St. John’s wort on irinotecan metabolism. J. Natl. Cancer Inst. 2002, 94, 1247–1249. [Google Scholar] [CrossRef]
- Amjadi, I.; Mohajeri, M.; Borisov, A.; Hosseini, M.S. Antiproliferative Effects of Free and Encapsulated Hypericum Perforatum L. Extract and Its Potential Interaction with Doxorubicin for Esophageal Squamous Cell Carcinoma. J. Pharmacopunct. 2019, 22, 102–108. [Google Scholar] [CrossRef]
- Meruelo, D. The potential use of hypericin as inactivator of retroviruses and other viruses in blood products. Blood 1993, 82, 205A. [Google Scholar]
- Degar, S.; Prince, A.M.; Pascual, D.; Lavie, G.; Levin, B.; Mazur, Y.; Lavie, D.; Ehrlich, L.S.; Carter, C.; Meruelo, D. Inactivation of the human immunodeficiency virus by hypericin: Evidence for photochemical alterations of p24 and a block in uncoating. AIDS Res. Hum. Retrovir. 1992, 8, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Mitra, A.K. MDR- and CYP3A4-mediated drug-herbal interactions. Life Sci. 2006, 78, 2131–2145. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Buddha, B.; Dey, S.; Pal, D.; Mitra, A.K. In vitro interaction of the HIV protease inhibitor ritonavir with herbal constituents: Changes in P-gp and CYP3A4 activity. Am. J. Ther. 2004, 11, 262–277. [Google Scholar] [CrossRef]
- Mannel, M. Drug interactions with St. John’s wort: Mechanisms and clinical implications. Drug Saf. 2004, 27, 773–797. [Google Scholar] [CrossRef] [PubMed]
- Waka, A.; Sakaeda, T.; Takara, K.; Hirai, M.; Kinura, T.; Ohmoto, N.; Zhou, J.; Nakamura, T.; Kobayashi, H.; Okamura, N.; et al. Effect of St. John’s Wort and hypericin on cytotoxicity of anticancer drugs. Drug Metab. Pharmacokinet. 2002, 17, 467–474. [Google Scholar]
- Weber, C.C.; Kressmann, S.; Fricker, G.; Müller, W.E. Modulation of P-glycoprotein function by St. John’s wort extract and its major constituents. Pharmacopsychiatry 2004, 37, 292–298. [Google Scholar] [CrossRef]
- Komoroski, B.J.; Zhang, S.; Cai, H.; Hutzler, J.M.; Frye, R.; Tracy, T.S.; Strom, S.C.; Lehmann, T.; Ang, C.Y.; Cui, Y.Y.; et al. Induction and inhibition of cytochromes P450 by the St. John’s wort constituent hyperforin in human hepatocyte cultures. Drug Metab. Dispos. 2004, 32, 512–518. [Google Scholar] [CrossRef]
- Haslam, I.S.; Jones, K.; Coleman, T.; Simmons, N.L. Induction of P-glycoprotein expression and function in human intestinal epithelial cells (T84). Biochem. Pharmacol. 2008, 76, 850–861. [Google Scholar] [CrossRef]
- Jendželovská, Z.; Jendželovský, R.; Hiľovská, L.; Kovaľ, J.; Mikeš, J.; Fedoročko, P. Single pre-treatment with hypericin, a St. John’s wort secondary metabolite, attenuates cisplatin- and mitoxantrone-induced cell death in A2780, A2780cis and HL-60 cells. Toxicol. In Vitro 2014, 28, 1259–1273. [Google Scholar] [CrossRef] [PubMed]
- Rothley, M.; Schmid, A.; Thiele, W.; Schacht, V.; Plaumann, D.; Gartner, M.; Yektaoglu, A.; Bruyère, F.; Noël, A.; Giannis, A.; et al. Hyperforin and aristoforin inhibit lymphatic endothelial cell proliferation in vitro and in vivo and suppress tumor-induced lymphangiogenesis in vivo. Int. J. Cancer 2009, 125, 34–42. [Google Scholar] [CrossRef]
- Gartner, M.; Muller, T.; Simon, J.C.; Giannis, A.; Sleeman, J.P. Aristoforin, a novel stable derivative of hyperforin, is a potent anticancer agent. ChemBioChem 2005, 6, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Pleyer, L.; Egle, A.; Hartmann, T.N.; Greil, R. Molecular and cellular mechanisms of CLL: Novel therapeutic approaches. Nat. Rev. Clin. Oncol. 2009, 6, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Dighiero, G.; Hamblin, D.J. Chronic lymphocytic leukemia. Lancet 2008, 371, 1017–1029. [Google Scholar] [CrossRef]
- Dell’Aica, I.; Niero, R.; Piazza, F.; Cabrelle, A.; Sartor, L.; Colalto, C.; Brunetta, E.; Lorusso, G.; Benelli, R.; Albini, A.; et al. Hyperforin blocks neutrophil activation of matrix metalloproteinase-9, motility and recruitment, and restrains inflammation-triggered angiogenesis and lung fibrosis. J. Pharmacol. Exp. Ther. 2007, 321, 492–500. [Google Scholar]
- Ang, C.Y.; Hu, L.; Heinze, T.M.; Cui, Y.; Freeman, J.P.; Kozak, K.; Luo, W.; Liu, F.F.; Mattia, A.; DiNovi, M. Instability of St. John’s wort (Hypericum perforatum L.) and degradation of hyperforin in aqueous solutions and functional beverage. J. Agric. Food Chem. 2004, 52, 6156–6164. [Google Scholar] [CrossRef]
- Verotta, L.; Appendino, G.; Belloro, E.; Jakupovic, J.; Bombardelli, E. Furohyperforin, a prenylated phloroglucinol from st. John’s wort (Hypericum perforatum). J. Nat. Prod. 1999, 62, 770–772. [Google Scholar] [CrossRef]
- Verotta, L.; Appendino, G.; Belloro, E.; Bianchi, F.; Sterner, O.; Lovati, M.; Bombardelli, E. Synthesis and biological evaluation of hyperforin analogues. Part I. Modification of the enolized cyclohexanedione moiety. J. Nat. Prod. 2002, 65, 433–438. [Google Scholar] [CrossRef]
- Martinez-Poveda, B.; Verotta, L.; Bombardelli, E.; Quesada, A.R.; Medina, M.A. Tetrahydrohyperforin and octahydrohyperforin are two new potent inhibitors of angiogenesis. PLoS ONE 2010, 5, e9558. [Google Scholar] [CrossRef]
- Chen, X.; Feng, W.; Chen, Q.; Yang, X.; Yang, D.; Wang, D.; Zhong, L. Effects of acetylate hyperforin on the processing of amyloid precursor protein. Int. J. Physiol. Pathophysiol. Pharmacol. 2009, 1, 76–82. [Google Scholar] [PubMed]
- Zanoli, P.; Rivasi, M.; Baraldi, C.; Baraldi, M. Pharmacological activity of hyperforin acetate in rats. Behav. Pharmacol. 2002, 13, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Speciale, A.; Molonia, M.S.; Guglielmo, L.; Musolino, C.; Ferlazzo, G.; Costa, G.; Saija, A.; Cimino, F. Curcumin ameliorates the in vitro efficacy of carfilzomib in human multiple myeloma U266 cells targeting p53 and NF-κB pathways. Toxicol. In Vitro 2018, 47, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Innao, V.; Russo, S.; Gerace, D.; Alonci, A.; Musolino, C. Anticancer Activity of Curcumin and Its Analogues: Preclinical and Clinical Studies. Cancer Investig. 2017, 35, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Tonacci, A.; Pioggia, G.; Musolino, C.; Gangemi, S. Anticancer Activity of Rosmarinus officinalis L.: Mechanisms of Action and Therapeutic Potentials. Nutrients 2020, 12, 1739. [Google Scholar] [CrossRef]
- Frye, R.F.; Fitzgerald, S.M.; Lagattuta, T.F.; Hruska, M.W.; Egorin, M.J. Effect of St. John’s wort on imatinib mesylate pharmacokinetics. Clin. Pharmacol. Ther. 2004, 76, 323–329. [Google Scholar] [CrossRef]
- Allegra, A.; Alonci, A.; Campo, S.; Penna, G.; Petrungaro, A.; Gerace, D.; Musolino, C. Circulating microRNAs: New biomarkers in diagnosis, prognosis and treatment of cancer (review). Int. J. Oncol. 2012, 41, 1897–1912. [Google Scholar] [CrossRef]
- Campo, S.; Allegra, A.; D’Ascola, A.; Alonci, A.; Scuruchi, M.; Russo, S.; Avenoso, A.; Gerace, D.; Campo, G.M.; Musolino, C. MiRNome expression is deregulated in the peripheral lymphoid compartment of multiple myeloma. Br. J. Haematol. 2014, 165, 801–813. [Google Scholar] [CrossRef]
- Innao, V.; Allegra, A.; Pulvirenti, N.; Allegra, A.G.; Musolino, C. Therapeutic potential of antagomiRs in haematological and oncological neoplasms. Eur. J. Cancer Care 2020, 29, e13208. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, S.; Fu, Z.; Wang, Y.; Wang, N.; Liu, Y.; Zhao, C.; Wu, J.; Hu, Y.; Zhang, J.; et al. Effective detection and quantification of dietetically absorbed plant microRNAs in human plasma. J. Nutr. Biochem. 2015, 26, 505–512. [Google Scholar] [CrossRef]
- Galla, G.; Volpato, M.; Sharbel, T.F.; Barcaccia, G. Computational identification of conserved microRNAs and their putative targets in the Hypericum perforatum L. flower transcriptome. Plant Reprod. 2013, 26, 209–229. [Google Scholar] [CrossRef] [PubMed]
- Ergün, S. Cross-Kingdom Gene regulation via miRNAs of Hypericum perforatum (St. John’s wort) flower dietetically absorbed: An in silico approach to define potential biomarkers for prostate cancer. Comput. Biol. Chem. 2019, 80, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Nahrstedt, A.; Butterweck, V. Lessons learned from herbal medicinal products: The example of St. John’s Wort (perpendicular). J. Nat. Prod. 2010, 73, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allegra, A.; Tonacci, A.; Spagnolo, E.V.; Musolino, C.; Gangemi, S. Antiproliferative Effects of St. John’s Wort, Its Derivatives, and Other Hypericum Species in Hematologic Malignancies. Int. J. Mol. Sci. 2021, 22, 146. https://doi.org/10.3390/ijms22010146
Allegra A, Tonacci A, Spagnolo EV, Musolino C, Gangemi S. Antiproliferative Effects of St. John’s Wort, Its Derivatives, and Other Hypericum Species in Hematologic Malignancies. International Journal of Molecular Sciences. 2021; 22(1):146. https://doi.org/10.3390/ijms22010146
Chicago/Turabian StyleAllegra, Alessandro, Alessandro Tonacci, Elvira Ventura Spagnolo, Caterina Musolino, and Sebastiano Gangemi. 2021. "Antiproliferative Effects of St. John’s Wort, Its Derivatives, and Other Hypericum Species in Hematologic Malignancies" International Journal of Molecular Sciences 22, no. 1: 146. https://doi.org/10.3390/ijms22010146
APA StyleAllegra, A., Tonacci, A., Spagnolo, E. V., Musolino, C., & Gangemi, S. (2021). Antiproliferative Effects of St. John’s Wort, Its Derivatives, and Other Hypericum Species in Hematologic Malignancies. International Journal of Molecular Sciences, 22(1), 146. https://doi.org/10.3390/ijms22010146





