Activation of Kinin B1R Upregulates ADAM17 and Results in ACE2 Shedding in Neurons
Abstract
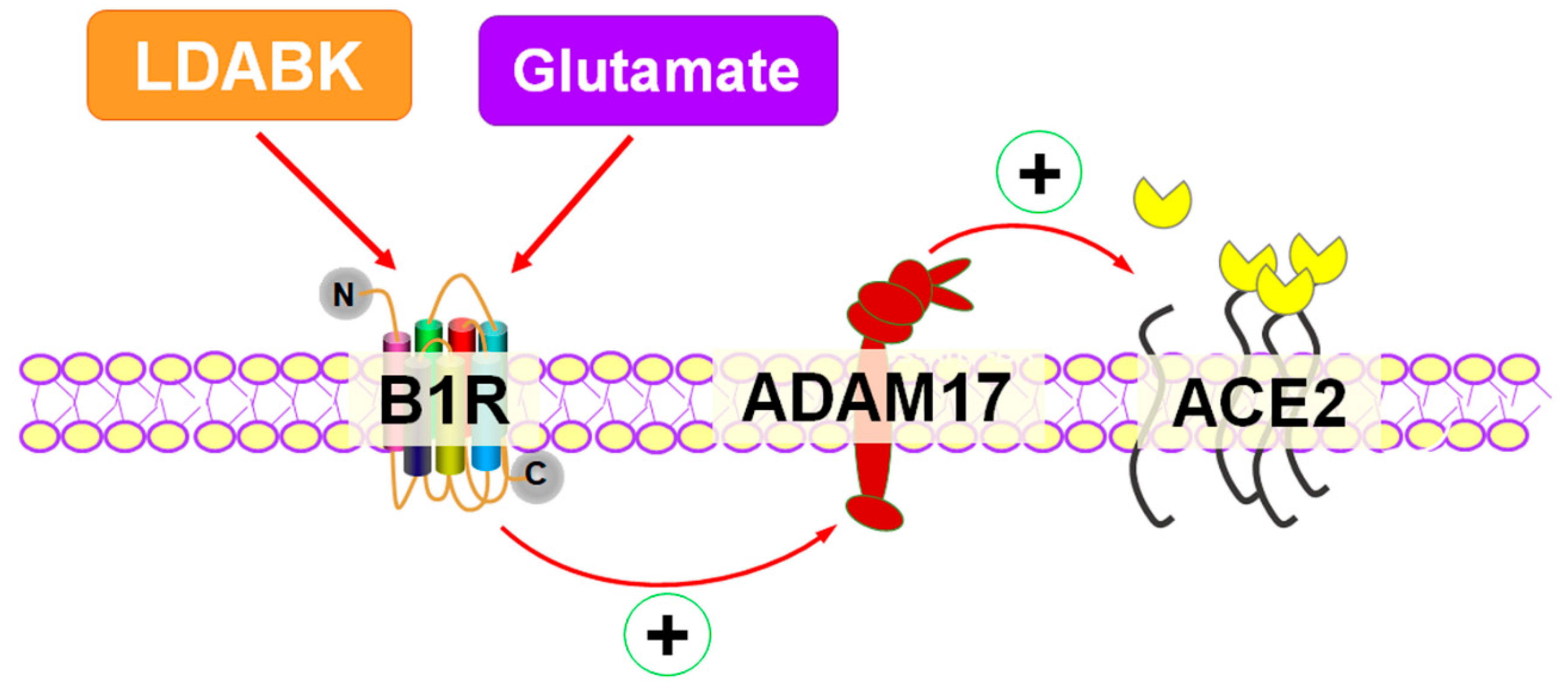
1. Introduction
2. Results
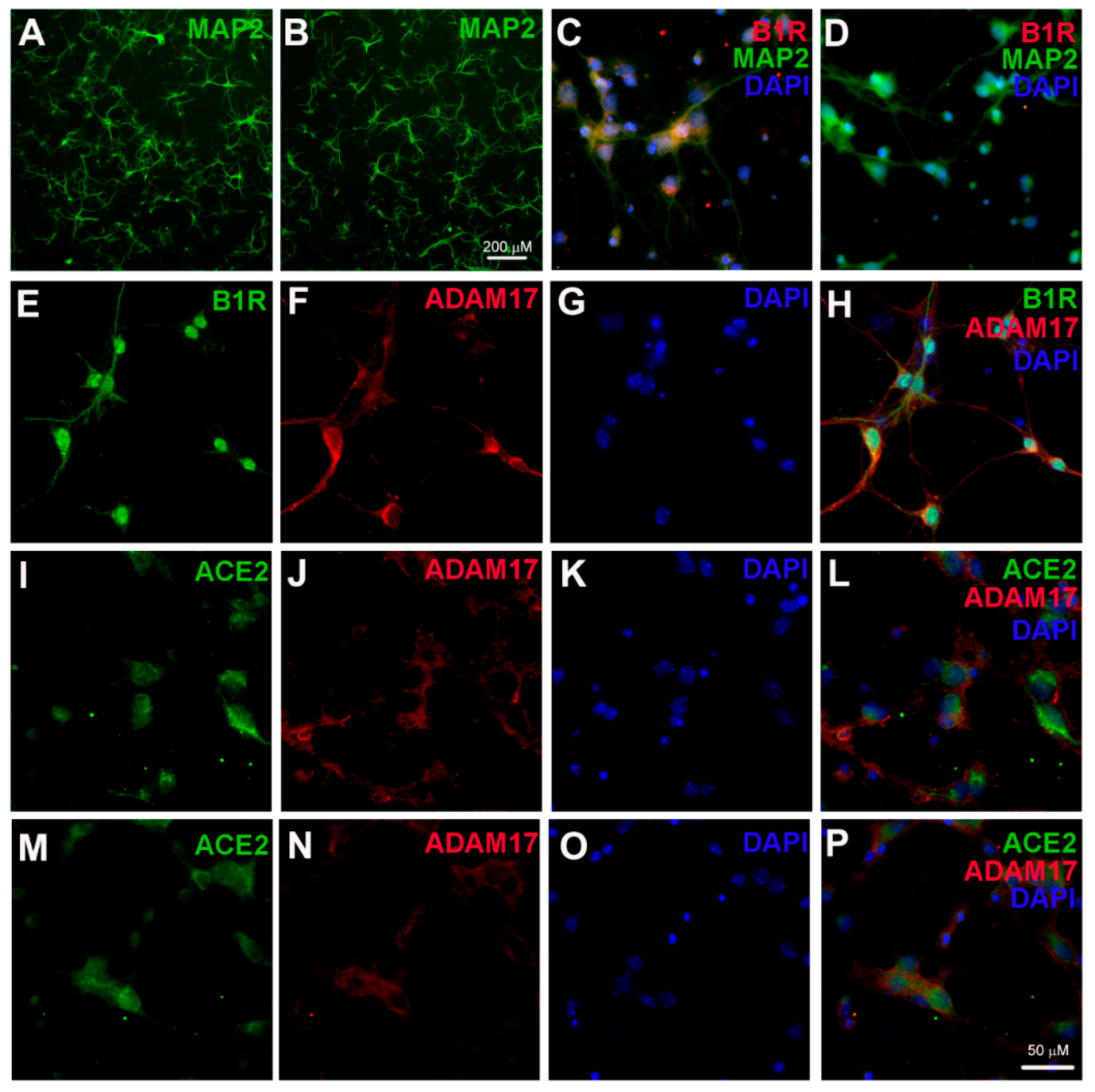
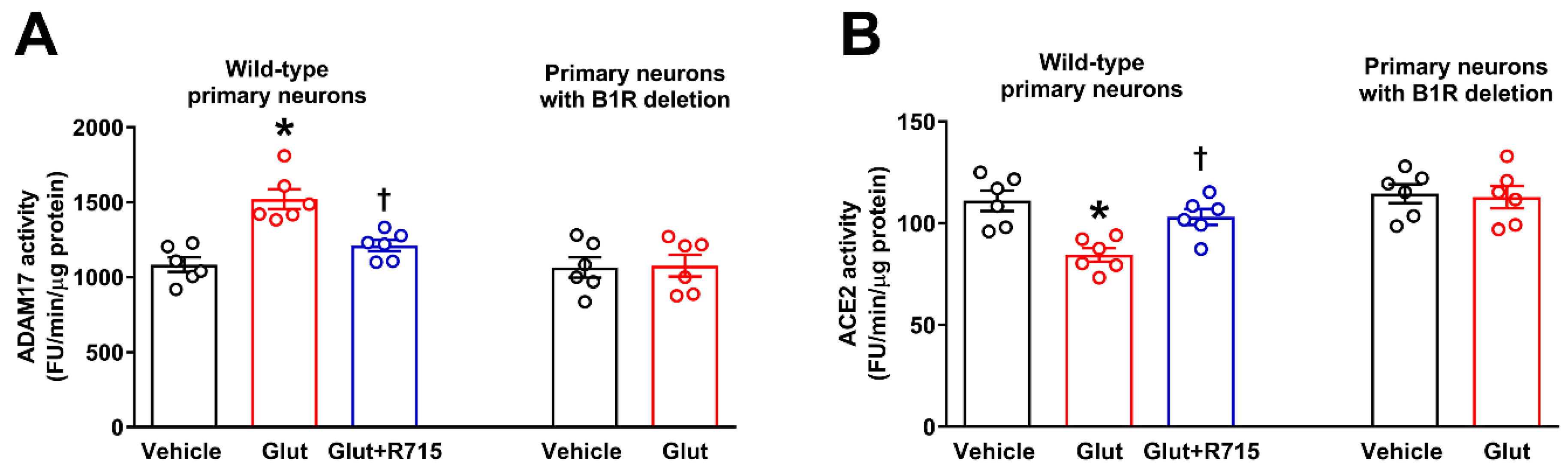
2.1. B1R Activation Increases ADAM17 Activity in Primary Hypothalamic Neurons
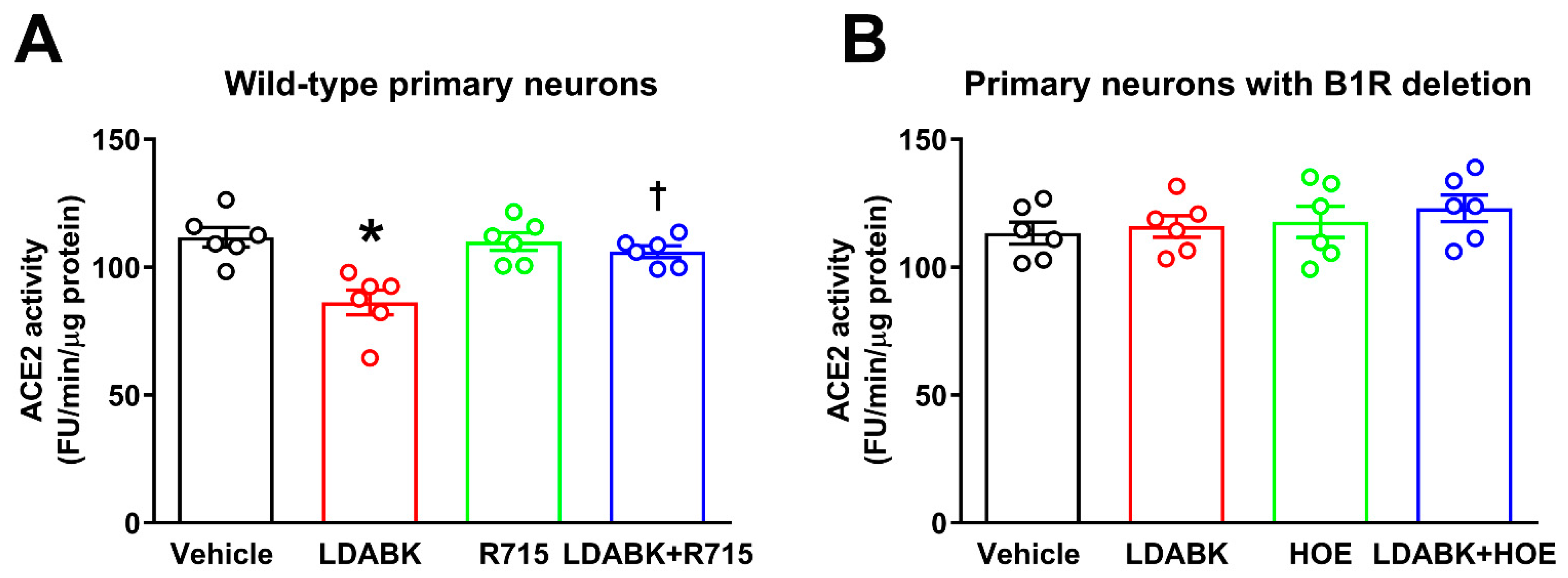
2.2. B1R Activation Results in Reduced ACE2 Activity in Primary Hypothalamic Neurons
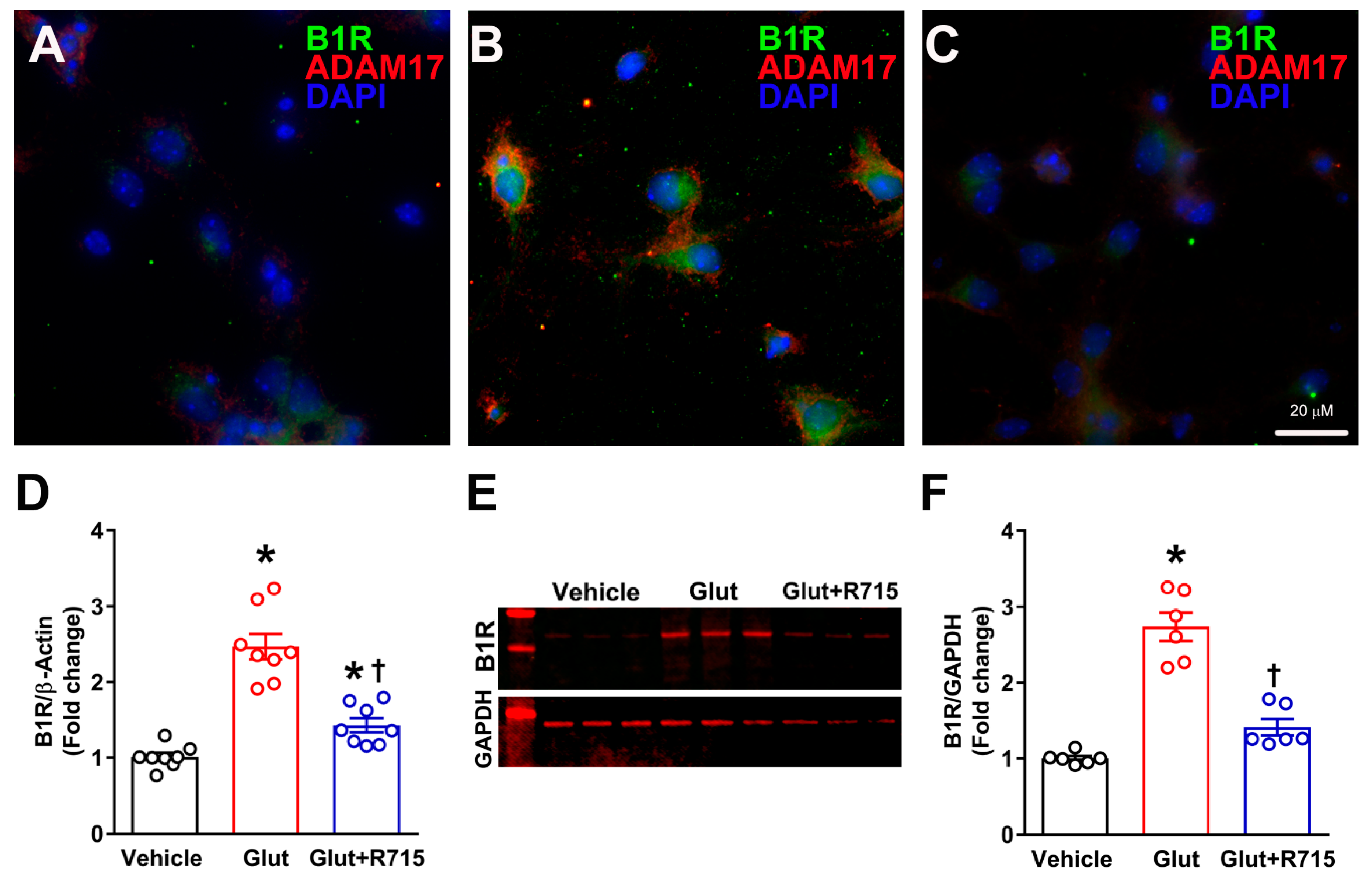
2.3. Stimulation with Glutamate Induces B1R Expression
2.4. Glutamate Induces ADAM17-Mediated ACE2 Shedding via B1R Activation
3. Discussion
4. Materials and Methods
4.1. Primary Neuronal Cell Culture
4.2. Immunofluorescence Staining
4.3. ACE2 Activity Assay
4.4. ADAM17 Activity Assay
4.5. Gene Expression Analysis by Real Time qRT-PCR
4.6. Protein Analysis by Western Blot
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE2 | Angiotensin converting enzyme 2 |
| ADAM17 | A Disintegrin And Metalloprotease 17 |
| Ara-C | Cytosine Arabinofuranoside |
| Ang II | Angiotensin II |
| AT1R | Angiotensin II type I receptor |
| B1R | Kinin B1 receptor |
| LDABK | Lys-Des-Arg9-Bradykinin |
References
- Xu, P.; Sriramula, S.; Lazartigues, E. ACE2/ANG-(1-7)/Mas pathway in the brain: The axis of good. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R804–R817. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Yue, X.; Lazartigues, E. ACE2 mouse models: A toolbox for cardiovascular and pulmonary research. Nat. Commun. 2020, 11, 5165. [Google Scholar] [CrossRef] [PubMed]
- Sriramula, S.; Xia, H.; Xu, P.; Lazartigues, E. Brain-targeted angiotensin-converting enzyme 2 overexpression attenuates neurogenic hypertension by inhibiting cyclooxygenase-mediated inflammation. Hypertension 2015, 65, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Sriramula, S.; Cardinale, J.P.; Lazartigues, E.; Francis, J. ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc. Res. 2011, 92, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Sriramula, S.; Chhabra, K.H.; Lazartigues, E. Brain angiotensin-converting enzyme type 2 shedding contributes to the development of neurogenic hypertension. Circ. Res. 2013, 113, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sriramula, S.; Xia, H.; Moreno-Walton, L.; Culicchia, F.; Domenig, O.; Poglitsch, M.; Lazartigues, E. Clinical Relevance and Role of Neuronal AT1 Receptors in ADAM17-Mediated ACE2 Shedding in Neurogenic Hypertension. Circ. Res. 2017, 121, 43–55. [Google Scholar] [CrossRef]
- Donoghue, M.; Hsieh, F.; Baronas, E.; Godbout, K.; Gosselin, M.; Stagliano, N.; Donovan, M.; Woolf, B.; Robison, K.; Jeyaseelan, R.; et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ. Res. 2000, 87, E1–E9. [Google Scholar] [CrossRef]
- Xu, J.; Sriramula, S.; Lazartigues, E. Excessive Glutamate Stimulation Impairs ACE2 Activity Through ADAM17-Mediated Shedding in Cultured Cortical Neurons. Cell. Mol. Neurobiol. 2018, 38, 1235–1243. [Google Scholar] [CrossRef]
- Deshotels, M.R.; Xia, H.; Sriramula, S.; Lazartigues, E.; Filipeanu, C.M. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension 2014, 64, 1368–1375. [Google Scholar] [CrossRef]
- Lambert, D.W.; Clarke, N.E.; Hooper, N.M.; Turner, A.J. Calmodulin interacts with angiotensin-converting enzyme-2 (ACE2) and inhibits shedding of its ectodomain. FEBS Lett. 2008, 582, 385–390. [Google Scholar] [CrossRef]
- Lambert, D.W.; Yarski, M.; Warner, F.J.; Thornhill, P.; Parkin, E.T.; Smith, A.I.; Hooper, N.M.; Turner, A.J. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J. Biol. Chem. 2005, 280, 30113–30119. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.W.; Hanchapola, I.; Steer, D.L.; Smith, A.I. Angiotensin-converting enzyme 2 ectodomain shedding cleavage-site identification: Determinants and constraints. Biochemistry 2011, 50, 5182–5194. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.H.; Li, W.; Choe, H.; Farzan, M. Angiotensin-converting enzyme 2: A functional receptor for SARS coronavirus. Cell. Mol. Life Sci. 2004, 61, 2738–2743. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, H.; Pohlmann, S. Cellular entry of the SARS coronavirus. Trends Microbiol. 2004, 12, 466–472. [Google Scholar] [CrossRef]
- Zipeto, D.; Palmeira, J.D.F.; Arganaraz, G.A.; Arganaraz, E.R. ACE2/ADAM17/TMPRSS2 Interplay May Be the Main Risk Factor for COVID-19. Front. Immunol. 2020, 11, 576745. [Google Scholar] [CrossRef]
- Sriramula, S. Kinin B1 receptor: A target for neuroinflammation in hypertension. Pharmacol. Res. 2020, 155, 104715. [Google Scholar] [CrossRef]
- Leeb-Lundberg, L.M.; Marceau, F.; Muller-Esterl, W.; Pettibone, D.J.; Zuraw, B.L. International union of pharmacology. XLV. Classification of the kinin receptor family: From molecular mechanisms to pathophysiological consequences. Pharmacol. Rev. 2005, 57, 27–77. [Google Scholar] [CrossRef]
- Regoli, D.; Barabe, J. Pharmacology of bradykinin and related kinins. Pharmacol. Rev. 1980, 32, 1–46. [Google Scholar]
- Raidoo, D.M.; Bhoola, K.D. Kinin receptors on human neurones. J. Neuroimmunol. 1997, 77, 39–44. [Google Scholar] [CrossRef]
- Sriramula, S.; Lazartigues, E. Kinin B1 Receptor Promotes Neurogenic Hypertension Through Activation of Centrally Mediated Mechanisms. Hypertension 2017, 70, 1122–1131. [Google Scholar] [CrossRef]
- Meeker, R.B.; Greenwood, R.S.; Hayward, J.N. Glutamate receptors in the rat hypothalamus and pituitary. Endocrinology 1994, 134, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Maher, P.; Van Leyen, K.; Dey, P.N.; Honrath, B.; Dolga, A.; Methner, A. The role of Ca(2+) in cell death caused by oxidative glutamate toxicity and ferroptosis. Cell Calcium 2018, 70, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Rueda, C.B.; Llorente-Folch, I.; Traba, J.; Amigo, I.; Gonzalez-Sanchez, P.; Contreras, L.; Juaristi, I.; Martinez-Valero, P.; Pardo, B.; Del Arco, A.; et al. Glutamate excitotoxicity and Ca2+-regulation of respiration: Role of the Ca2+ activated mitochondrial transporters (CaMCs). Biochim. Biophys. Acta 2016, 1857, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Chaparro-Huerta, V.; Rivera-Cervantes, M.C.; Flores-Soto, M.E.; Gomez-Pinedo, U.; Beas-Zarate, C. Proinflammatory cytokines and apoptosis following glutamate-induced excitotoxicity mediated by p38 MAPK in the hippocampus of neonatal rats. J. Neuroimmunol. 2005, 165, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.B.; Chodavarapu, H.; Porretta, C.; Robinson, L.K.; Lazartigues, E. Dynamics of ADAM17-Mediated Shedding of ACE2 Applied to Pancreatic Islets of Male db/db Mice. Endocrinology 2015, 156, 4411–4425. [Google Scholar] [CrossRef]
- Iwata, M.; Silva Enciso, J.E.; Greenberg, B.H. Selective and specific regulation of ectodomain shedding of angiotensin-converting enzyme 2 by tumor necrosis factor alpha-converting enzyme. Am. J. Physiol. Cell Physiol. 2009, 297, C1318–C1329. [Google Scholar] [CrossRef]
- Patel, V.B.; Clarke, N.; Wang, Z.; Fan, D.; Parajuli, N.; Basu, R.; Putko, B.; Kassiri, Z.; Turner, A.J.; Oudit, G.Y. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: A positive feedback mechanism in the RAS. J. Mol. Cell Cardiol. 2014, 66, 167–176. [Google Scholar] [CrossRef]
- Jia, H.P.; Look, D.C.; Tan, P.; Shi, L.; Hickey, M.; Gakhar, L.; Chappell, M.C.; Wohlford-Lenane, C.; McCray, P.B., Jr. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 297, L84–L96. [Google Scholar] [CrossRef]
- Guy, J.L.; Lambert, D.W.; Turner, A.J.; Porter, K.E. Functional angiotensin-converting enzyme 2 is expressed in human cardiac myofibroblasts. Exp. Physiol. 2008, 93, 579–588. [Google Scholar] [CrossRef]
- Lew, R.A.; Warner, F.J.; Hanchapola, I.; Yarski, M.A.; Ramchand, J.; Burrell, L.M.; Smith, A.I. Angiotensin-converting enzyme 2 catalytic activity in human plasma is masked by an endogenous inhibitor. Exp. Physiol. 2008, 93, 685–693. [Google Scholar] [CrossRef]
- Xiao, F.; Hiremath, S.; Knoll, G.; Zimpelmann, J.; Srivaratharajah, K.; Jadhav, D.; Fergusson, D.; Kennedy, C.R.; Burns, K.D. Increased urinary angiotensin-converting enzyme 2 in renal transplant patients with diabetes. PLoS ONE 2012, 7, e37649. [Google Scholar] [CrossRef] [PubMed]
- Parekh, R.U.; Robidoux, J.; Sriramula, S. Kinin B1 Receptor Blockade Prevents Angiotensin II-induced Neuroinflammation and Oxidative Stress in Primary Hypothalamic Neurons. Cell Mol. Neurobiol. 2020, 40, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, R.T.; Keum, J.S.; El-Shewy, H.M.; Lee, M.H.; Wang, B.; Gooz, M.; Luttrell, D.K.; Luttrell, L.M.; Jaffa, A.A. Plasma kallikrein promotes epidermal growth factor receptor transactivation and signaling in vascular smooth muscle through direct activation of protease-activated receptors. J. Biol. Chem. 2010, 285, 35206–35215. [Google Scholar] [CrossRef] [PubMed]
- Singewald, N.; Philippu, A. Involvement of biogenic amines and amino acids in the central regulation of cardiovascular homeostasis. Trends Pharmacol. Sci. 1996, 17, 356–363. [Google Scholar] [CrossRef]
- Basting, T.; Xu, J.; Mukerjee, S.; Epling, J.; Fuchs, R.; Sriramula, S.; Lazartigues, E. Glutamatergic neurons of the paraventricular nucleus are critical contributors to the development of neurogenic hypertension. J. Physiol. 2018, 596, 6235–6248. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Molinas, A.J.R.; Mukerjee, S.; Morgan, D.A.; Rahmouni, K.; Zsombok, A.; Lazartigues, E. Activation of ADAM17 (A Disintegrin and Metalloprotease 17) on Glutamatergic Neurons Selectively Promotes Sympathoexcitation. Hypertension 2019, 73, 1266–1274. [Google Scholar] [CrossRef]
- Mukerjee, S.; Gao, H.; Xu, J.; Sato, R.; Zsombok, A.; Lazartigues, E. ACE2 and ADAM17 Interaction Regulates the Activity of Presympathetic Neurons. Hypertension 2019, 74, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Sriramula, S.; Pedersen, K.B.; Xia, H.; Lazartigues, E. Determining the Enzymatic Activity of Angiotensin-Converting Enzyme 2 (ACE2) in Brain Tissue and Cerebrospinal Fluid Using a Quenched Fluorescent Substrate. Methods Mol. Biol. 2017, 1527, 117–126. [Google Scholar]
- Pedersen, K.B.; Sriramula, S.; Chhabra, K.H.; Xia, H.; Lazartigues, E. Species-specific inhibitor sensitivity of angiotensin-converting enzyme 2 (ACE2) and its implication for ACE2 activity assays. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1293–R1299. [Google Scholar] [CrossRef]
- Ogunlade, B.; Guidry, J.J.; Mukerjee, S.; Sriramula, S.; Lazartigues, E.; Filipeanu, C.M. The Actin Bundling Protein Fascin-1 as an ACE2-Accessory Protein. Cell Mol. Neurobiol. 2020, 1–9. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parekh, R.U.; Sriramula, S. Activation of Kinin B1R Upregulates ADAM17 and Results in ACE2 Shedding in Neurons. Int. J. Mol. Sci. 2021, 22, 145. https://doi.org/10.3390/ijms22010145
Parekh RU, Sriramula S. Activation of Kinin B1R Upregulates ADAM17 and Results in ACE2 Shedding in Neurons. International Journal of Molecular Sciences. 2021; 22(1):145. https://doi.org/10.3390/ijms22010145
Chicago/Turabian StyleParekh, Rohan Umesh, and Srinivas Sriramula. 2021. "Activation of Kinin B1R Upregulates ADAM17 and Results in ACE2 Shedding in Neurons" International Journal of Molecular Sciences 22, no. 1: 145. https://doi.org/10.3390/ijms22010145
APA StyleParekh, R. U., & Sriramula, S. (2021). Activation of Kinin B1R Upregulates ADAM17 and Results in ACE2 Shedding in Neurons. International Journal of Molecular Sciences, 22(1), 145. https://doi.org/10.3390/ijms22010145






