Spatial and Pregnancy-Related Changes in the Protein, Amino Acid, and Carbohydrate Composition of Bovine Oviduct Fluid
Abstract
1. Introduction
2. Results
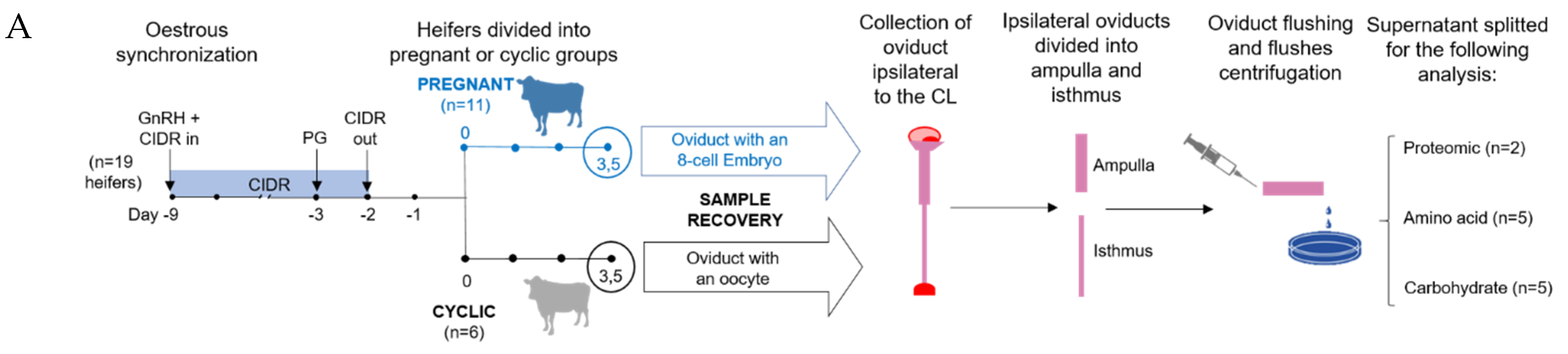
2.1. Sample Collection
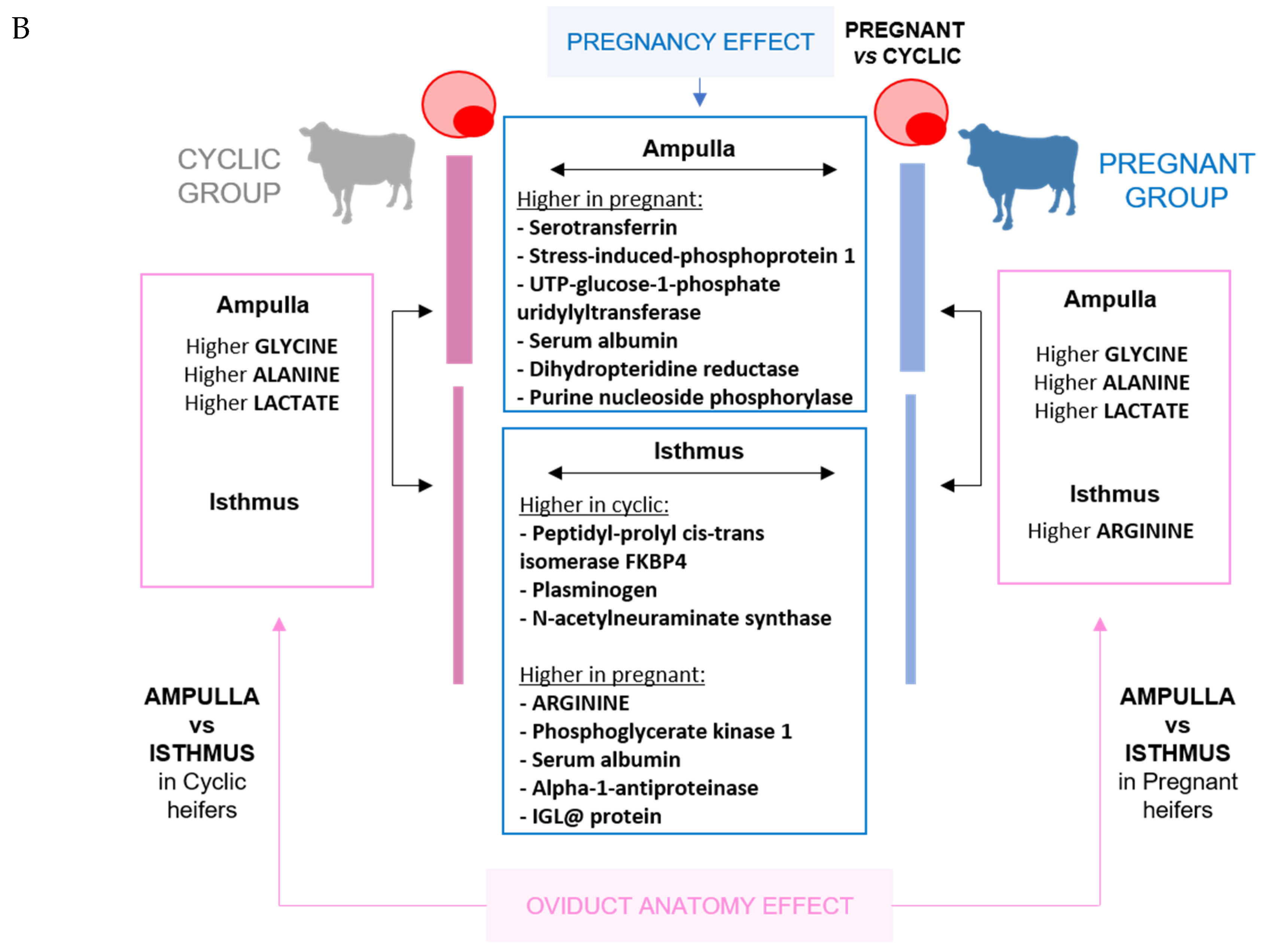
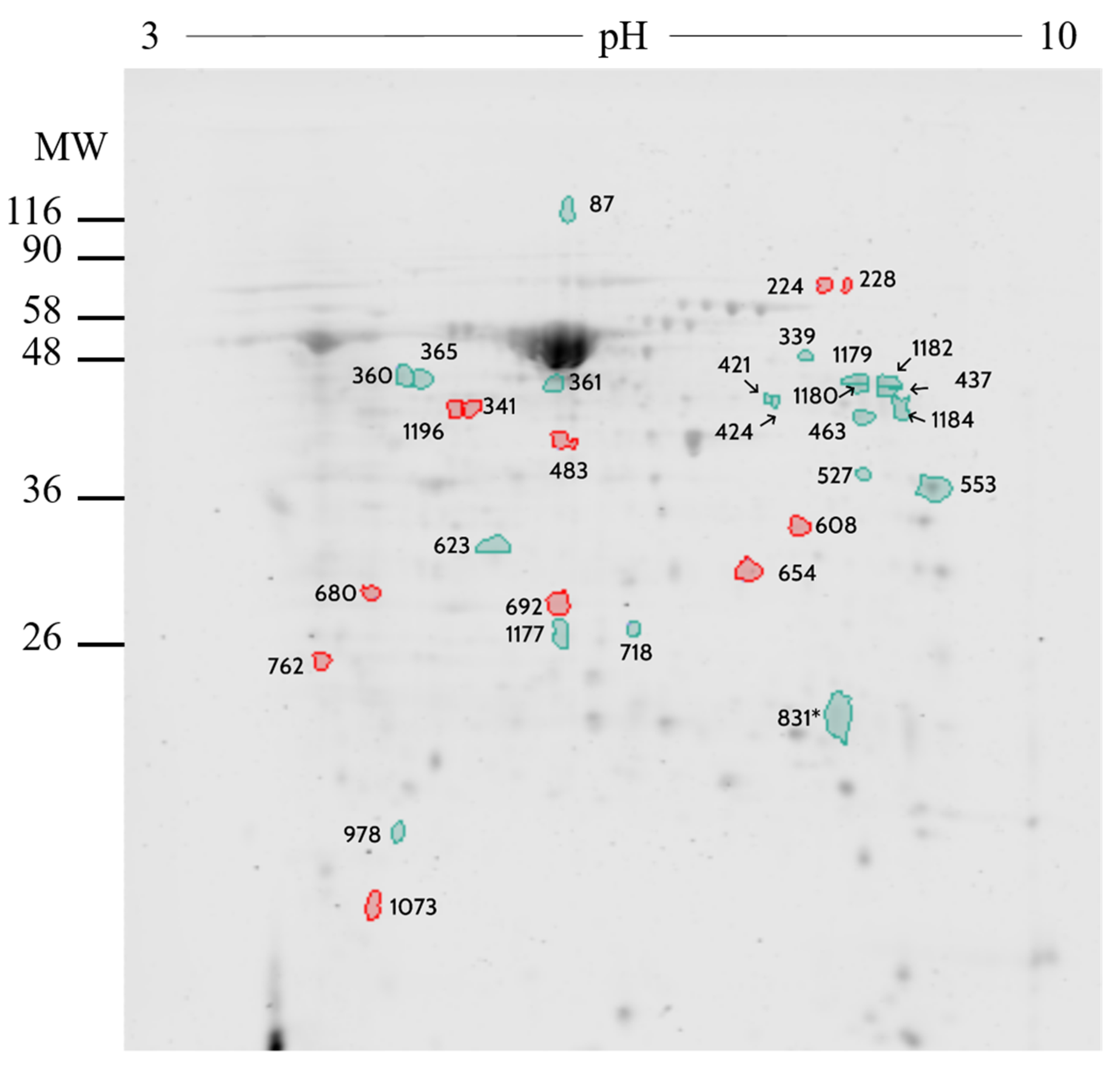
2.2. Proteomic Analysis
2.3. Amino Acid Analysis
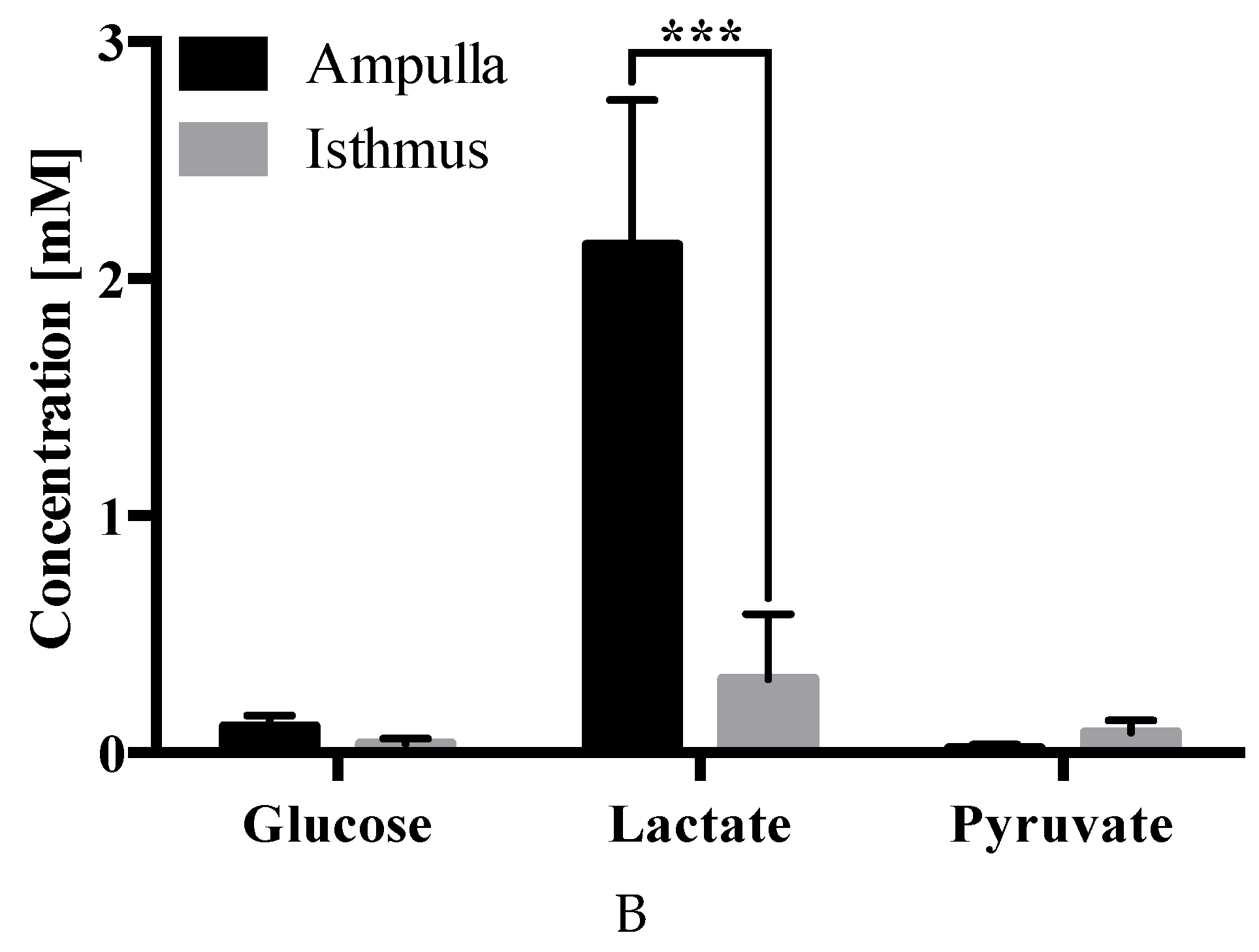
2.4. Carbohydrate Analysis
3. Discussion
4. Material and Methods
4.1. Animal Model
4.2. Sample Collection
4.3. Proteomic Analysis
4.4. Amino acid Analyses
4.5. Carbohydrate Analyses
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hackett, A.J.; Durnford, R.; Mapletoft, R.J.; Marcus, G.J. Location and status of embryos in the genital tract of superovulated cows 4 to 6 days after insemination. Theriogenology 1993, 40, 1147–1153. [Google Scholar] [CrossRef]
- Fernandez-Fuertes, B.; Rodríguez-Alonso, B.; Sánchez, J.M.; Simintiras, C.A.; Lonergan, P.; Rizos, D. Looking at the big picture: Understanding how the oviduct s dialogue with gametes and the embryo shapes reproductive success. Anim. Reprod. 2018, 15, 751–764. [Google Scholar] [CrossRef]
- Aguilar, J.; Reyley, M. The uterine tubal fluid: Secretion, composition and biological effects. Anim. Reprod. 2005, 2, 91–105. [Google Scholar]
- Leese, H.J. Studies on the movement of glucose, pyruvate and lactate into the ampulla and isthmus of the rabbit oviduct. Q. J. Exp. Physiol. 1983, 68, 89–96. [Google Scholar] [CrossRef]
- Grippo, A.A.; Henault, M.A.; Anderson, S.H.; Killian, G.J. Cation concentrations in fluid from the oviduct ampulla and isthmus of cows during the estrous cycle. J. Dairy Sci. 1992, 75, 58–65. [Google Scholar] [CrossRef]
- Grippo, A.A.; Anderson, S.H.; Chapman, D.A.; Henault, M.A.; Killian, G.J. Cholesterol, phospholipid and phospholipase activity of ampullary and isthmic fluid from the bovine oviduct. J. Reprod. Fertil. 1994, 102, 87–93. [Google Scholar] [CrossRef]
- Seytanoglu, A.; Georgiou, A.S.; Sostaric, E.; Watson, P.F.; Holt, W.V.; Fazeli, A. Oviductal Cell Proteome Alterations during the Reproductive Cycle in Pigs. J. Proteome Res. 2008, 7, 2825–2833. [Google Scholar] [CrossRef]
- Hugentobler, S.A.; Sreenan, J.M.; Humpherson, P.G.; Leese, H.J.; Diskin, M.G.; Morris, D.G. Effects of changes in the concentration of systemic progesterone on ions, amino acids and energy substrates in cattle oviduct and uterine fluid and blood. Reprod. Fertil. Dev. 2010, 22, 684–694. [Google Scholar] [CrossRef]
- Soleilhavoup, C.; Riou, C.; Tsikis, G.; Labas, V.; Harichaux, G.; Kohnke, P.; Reynaud, K.; de Graaf, S.P.; Gerard, N.; Druart, X. Proteomes of the Female Genital Tract During the Oestrous Cycle. Mol. Cell. Proteom. 2016, 15, 93–108. [Google Scholar] [CrossRef]
- Lamy, J.; Liere, P.; Pianos, A.; Aprahamian, F.; Mermillod, P.; Saint-Dizier, M. Steroid hormones in bovine oviductal fluid during the estrous cycle. Theriogenology 2016, 86, 1409–1420. [Google Scholar] [CrossRef]
- Lamy, J.; Gatien, J.; Dubuisson, F.; Nadal-Desbarats, L.; Salvetti, P.; Mermillod, P.; Saint-Dizier, M. Metabolomic profiling of bovine oviductal fluid across the oestrous cycle using proton nuclear magnetic resonance spectroscopy. Reprod. Fertil. Dev. 2018, 30, 1021–1028. [Google Scholar] [CrossRef]
- Maillo, V.; Sánchez-Calabuig, M.J.; Lopera-Vasquez, R.; Hamdi, M.; Gutierrez-Adan, A.; Lonergan, P.; Rizos, D. Oviductal response to gametes and early embryos in mammals. Reprod. Camb. Engl. 2016, 152, R127–R141. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, A.S.; Sostaric, E.; Wong, C.H.; Snijders, A.P.L.; Wright, P.C.; Moore, H.D.; Fazeli, A. Gametes Alter the Oviductal Secretory Proteome. Mol. Cell. Proteom. 2005, 4, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, A.S.; Snijders, A.P.L.; Sostaric, E.; Aflatoonian, R.; Vazquez, J.L.; Vazquez, J.M.; Roca, J.; Martinez, E.A.; Wright, P.C.; Fazeli, A. Modulation of the oviductal environment by gametes. J. Proteome Res. 2007, 6, 4656–4666. [Google Scholar] [CrossRef]
- Artemenko, K.; Horáková, J.; Steinberger, B.; Besenfelder, U.; Brem, G.; Bergquist, J.; Mayrhofer, C. A proteomic approach to monitor the dynamic response of the female oviductal epithelial cell surface to male gametes. J. Proteom. 2015, 113, 1–14. [Google Scholar] [CrossRef]
- Smits, K.; Nelis, H.; Van Steendam, K.; Govaere, J.; Roels, K.; Ververs, C.; Leemans, B.; Wydooghe, E.; Deforce, D.; Van Soom, A. Proteome of equine oviducal fluid: Effects of ovulation and pregnancy. Reprod. Fertil. Dev. 2017, 29, 1085. [Google Scholar] [CrossRef]
- Maillo, V.; de Frutos, C.; O’Gaora, P.; Forde, N.; Burns, G.W.; Spencer, T.E.; Gutierrez-Adan, A.; Lonergan, P.; Rizos, D. Spatial differences in gene expression in the bovine oviduct. Reproduction 2016, 152, 37–46. [Google Scholar] [CrossRef]
- Maillo, V.; Gaora, P.Ó.; Forde, N.; Besenfelder, U.; Havlicek, V.; Burns, G.W.; Spencer, T.E.; Gutierrez-Adan, A.; Lonergan, P.; Rizos, D. Oviduct-Embryo Interactions in Cattle: Two-Way Traffic or a One-Way Street? Biol. Reprod. 2015, 92, 144. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Gómez, E.; Caamaño, J.N.; Corrales, F.J.; Díez, C.; Correia-Álvarez, E.; Martín, D.; Trigal, B.; Carrocera, S.; Mora, M.I.; Pello-Palma, J.; et al. Embryonic Sex Induces Differential Expression of Proteins in Bovine Uterine Fluid. J. Proteome Res. 2013, 12, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Simintiras, C.; Sánchez, J.M.; McDonald, M.; Lonergan, P. The biochemistry surrounding bovine conceptus elongation. Biol. Reprod. 2019, 101, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Bäckryd, E.; Edström, S.; Gerdle, B.; Ghafouri, B. Do fragments and glycosylated isoforms of alpha-1-antitrypsin in CSF mirror spinal pathophysiological mechanisms in chronic peripheral neuropathic pain? An exploratory, discovery phase study. BMC Neurol. 2018, 18, 116. [Google Scholar] [CrossRef]
- Talamo, F.; D’Ambrosio, C.; Arena, S.; Del Vecchio, P.; Ledda, L.; Zehender, G.; Ferrara, L.; Scaloni, A. Proteins from bovine tissues and biological fluids: Defining a reference electrophoresis map for liver, kidney, muscle, plasma and red blood cells. Proteomics 2003, 3, 440–460. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, I.; Das, S.K.; Dey, S.K. Differential expression of vascular endothelial growth factor and its receptor mRNAs in the mouse uterus around the time of implantation. J. Endocrinol. 1995, 147, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, G.; Bowling, A.; Eng, L.A.; Keen, S.; Randall, P.A. The permeability of rabbit oviduct to proteins present in the serum. Biol. Reprod. 1978, 18, 516–520. [Google Scholar] [CrossRef]
- Schmaltz-Panneau, B.; Cordova, A.; Dhorne-Pollet, S.; Hennequet-Antier, C.; Uzbekova, S.; Martinot, E.; Doret, S.; Martin, P.; Mermillod, P.; Locatelli, Y. Early bovine embryos regulate oviduct epithelial cell gene expression during in vitro co-culture. Anim. Reprod. Sci. 2014, 149, 103–116. [Google Scholar] [CrossRef]
- Nang, C.F.; Osman, K.; Budin, S.B.; Ismail, M.I.; Jaffar, F.H.F.; Mohamad, S.F.S.; Ibrahim, S.F. Bovine serum albumin: Survival and osmolarity effect in bovine spermatozoa stored above freezing point. Andrologia 2012, 44, 447–453. [Google Scholar] [CrossRef]
- Herrick, J.R.; Lyons, S.M.; Greene, A.F.; Broeckling, C.D.; Schoolcraft, W.B.; Krisher, R.L. Direct and Osmolarity-Dependent Effects of Glycine on Preimplantation Bovine Embryos. PLoS ONE 2016, 11, e0159581. [Google Scholar] [CrossRef]
- Rizos, D.; Gutiérrez-Adán, A.; Pérez-Garnelo, S.; de la Fuente, J.; Boland, M.P.; Lonergan, P. Bovine Embryo Culture in the Presence or Absence of Serum: Implications for Blastocyst Development, Cryotolerance, and Messenger RNA Expression1. Biol. Reprod. 2003, 68, 236–243. [Google Scholar] [CrossRef]
- Lazzari, G.; Wrenzycki, C.; Herrmann, D.; Duchi, R.; Kruip, T.; Niemann, H.; Galli, C. Cellular and molecular deviations in bovine in vitro-produced embryos are related to the large offspring syndrome. Biol. Reprod. 2002, 67, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.G.; Gardner, D.K.; Anne Pugh, P.; McMillan, W.H.; Robin Tervit, H. Lamb Birth Weight is Affected by Culture System Utilized during in Vitro Pre-Elongation Development of Ovine Embryos. Biol. Reprod. 1995, 53, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- van Wagtendonk-de Leeuw, A.M.; Mullaart, E.; de Roos, A.P.W.; Merton, J.S.; den Daas, J.H.G.; Kemp, B.; de Ruigh, L. Effects of different reproduction techniques: AI, moet or IVP, on health and welfare of bovine offspring. Theriogenology 2000, 53, 575–597. [Google Scholar] [CrossRef]
- Lamy, J.; Labas, V.; Harichaux, G.; Tsikis, G.; Mermillod, P.; Saint-Dizier, M. Regulation of the bovine oviductal fluid proteome. Reproduction 2016, 152, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Sun, J.; Dong, H.; Luo, O.; Zheng, X.; Obergfell, C.; Tang, Y.; Bi, J.; O’Neill, R.; Ruan, Y.; et al. Transcriptional profiles of bovine in vivo pre-implantation development. BMC Genom. 2014, 15, 756. [Google Scholar] [CrossRef] [PubMed]
- Wydooghe, E.; Heras, S.; Dewulf, J.; Piepers, S.; Van den Abbeel, E.; De Sutter, P.; Vandaele, L.; Van Soom, A. Replacing serum in culture medium with albumin and insulin, transferrin and selenium is the key to successful bovine embryo development in individual culture. Reprod. Fertil. Dev. 2014, 26, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Nasr-Esfahani, M.H.; Johnson, M.H. How does transferrin overcome the in vitro block to development of the mouse preimplantation embryo? Reproduction 1992, 96, 41–48. [Google Scholar] [CrossRef][Green Version]
- Scheufler, C.; Brinker, A.; Bourenkov, G.; Pegoraro, S.; Moroder, L.; Bartunik, H.; Hartl, F.U.; Moarefi, I. Structure of TPR domain-peptide complexes: Critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell 2000, 101, 199–210. [Google Scholar] [CrossRef]
- Schmid, A.B.; Lagleder, S.; Gräwert, M.A.; Röhl, A.; Hagn, F.; Wandinger, S.K.; Cox, M.B.; Demmer, O.; Richter, K.; Groll, M.; et al. The architecture of functional modules in the Hsp90 co-chaperone Sti1/Hop. EMBO J. 2012, 31, 1506–1517. [Google Scholar] [CrossRef]
- Almiñana, C.; Tsikis, G.; Labas, V.; Uzbekov, R.; da Silveira, J.C.; Bauersachs, S.; Mermillod, P. Deciphering the oviductal extracellular vesicles content across the estrous cycle: Implications for the gametes-oviduct interactions and the environment of the potential embryo. BMC Genom. 2018, 19, 622. [Google Scholar] [CrossRef]
- Demant, M.; Deutsch, D.R.; Fröhlich, T.; Wolf, E.; Arnold, G.J. Proteome analysis of early lineage specification in bovine embryos. Proteomics 2015, 15, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Beraldo, F.H.; Soares, I.N.; Goncalves, D.F.; Fan, J.; Thomas, A.A.; Santos, T.G.; Mohammad, A.H.; Roffé, M.; Calder, M.D.; Nikolova, S.; et al. Stress-inducible phosphoprotein 1 has unique cochaperone activity during development and regulates cellular response to ischemia via the prion protein. FASEB J. 2013, 27, 3594–3607. [Google Scholar] [CrossRef] [PubMed]
- Winuthayanon, W.; Bernhardt, M.L.; Padilla-Banks, E.; Myers, P.H.; Edin, M.L.; Lih, F.B.; Hewitt, S.C.; Korach, K.S.; Williams, C.J. Oviductal estrogen receptor a signaling prevents protease-mediated embryo death. Elife 2015, 4, e10453. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.V.; Weber, D.M.; Phinney, B.S.; Selvaraj, V. Profiling of proteins secreted in the bovine oviduct reveals diverse functions of this luminal microenvironment. PLoS ONE 2017, 12, e0188105. [Google Scholar] [CrossRef]
- Fernandes, C.C.L.; Rodriguez-Villamil, P.; Vasconcelos, F.R.; Nagano, C.S.; Rossetto, R.; de Alencar Araripe Noronha Moura, A.; Rondina, D. Proteome of the periovulatory oviduct and uterus of goats as related to nutritional balance. Reprod. Domest. Anim. Zuchthyg. 2018, 53, 1085–1095. [Google Scholar] [CrossRef]
- Acuña, O.S.; Avilés, M.; López-Úbeda, R.; Guillén-Martínez, A.; Soriano-Úbeda, C.; Torrecillas, A.; Coy, P.; Izquierdo-Rico, M.J. Differential gene expression in porcine oviduct during the oestrous cycle. Reprod. Fertil. Dev. 2017, 29, 2387. [Google Scholar] [CrossRef]
- Chirico, W.J. Protein release through nonlethal oncotic pores as an alternative nonclassical secretory pathway. BMC Cell Biol. 2011, 12, 46. [Google Scholar] [CrossRef]
- Zieker, D.; Königsrainer, I.; Tritschler, I.; Löffler, M.; Beckert, S.; Traub, F.; Nieselt, K.; Bühler, S.; Weller, M.; Gaedcke, J.; et al. Phosphoglycerate kinase 1 a promoting enzyme for peritoneal dissemination in gastric cancer. Int. J. Cancer 2010, 126, 1513–1520. [Google Scholar] [CrossRef]
- Tranguch, S.; Cheung-Flynn, J.; Daikoku, T.; Prapapanich, V.; Cox, M.B.; Xie, H.; Wang, H.; Das, S.K.; Smith, D.F.; Dey, S.K. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc. Natl. Acad. Sci. USA 2005, 102, 14326–14331. [Google Scholar] [CrossRef]
- Tranguch, S.; Wang, H.; Daikoku, T.; Xie, H.; Smith, D.F.; Dey, S.K. FKBP52 deficiency-conferred uterine progesterone resistance is genetic background and pregnancy stage specific. J. Clin. Investig. 2007, 117, 1824–1834. [Google Scholar] [CrossRef]
- Hirota, Y.; Acar, N.; Tranguch, S.; Burnum, K.E.; Xie, H.; Kodama, A.; Osuga, Y.; Ustunel, I.; Friedman, D.B.; Caprioli, R.M.; et al. Uterine FK506-binding protein 52 (FKBP52)-peroxiredoxin-6 (PRDX6) signaling protects pregnancy from overt oxidative stress. Proc. Natl. Acad. Sci. USA 2010, 107, 15577–15582. [Google Scholar] [CrossRef] [PubMed]
- Demetriou, C.; Chanudet, E.; GOSgene; Joseph, A.; Topf, M.; Thomas, A.C.; Bitner-Glindzicz, M.; Regan, L.; Stanier, P.; Moore, G.E. Exome sequencing identifies variants in FKBP4 that are associated with recurrent fetal loss in humans. Hum. Mol. Genet. 2019, 28, 3466–3474. [Google Scholar] [CrossRef] [PubMed]
- Swegen, A.; Grupen, C.G.; Gibb, Z.; Baker, M.A.; de Ruijter-Villani, M.; Smith, N.D.; Stout, T.A.E.; Aitken, R.J. From Peptide Masses to Pregnancy Maintenance: A Comprehensive Proteomic Analysis of The Early Equine Embryo Secretome, Blastocoel Fluid, and Capsule. Proteomics 2017, 17, 1600433. [Google Scholar] [CrossRef] [PubMed]
- Plow, E.F.; Herren, T.; Redlitz, A.; Miles, L.A.; Hoover-Plow, J.L. The cell biology of the plasminogen system. FASEB J. 1995, 9, 939–945. [Google Scholar] [CrossRef]
- Mondéjar, I.; Grullón, L.A.; García-Vázquez, F.A.; Romar, R.; Coy, P. Fertilization outcome could be regulated by binding of oviductal plasminogen to oocytes and by releasing of plasminogen activators during interplay between gametes. Fertil. Steril. 2012, 97, 453–461. [Google Scholar] [CrossRef]
- Roldán-Olarte, M.; Jiménez-Díaz, M.; Miceli, D.C. Plasminogen detection in oocytes and plasminogen activator activities in the porcine oviduct during the estrous cycle. Zygote Camb. Engl. 2005, 13, 115–123. [Google Scholar] [CrossRef]
- Coy, P.; Jiménez-Movilla, M.; García-Vázquez, F.A.; Mondéjar, I.; Grullón, L.; Romar, R. Oocytes use the plasminogen-plasmin system to remove supernumerary spermatozoa. Hum. Reprod. Oxf. Engl. 2012, 27, 1985–1993. [Google Scholar] [CrossRef]
- Roldán-Olarte, M.; Maillo, V.; Sánchez-Calabuig, M.J.; Beltrán-Breña, P.; Rizos, D.; Gutiérrez-Adán, A. Effect of urokinase type plasminogen activator on in vitro bovine oocyte maturation. Reprod. Camb. Engl. 2017, 154, 231–240. [Google Scholar]
- Hugentobler, S.A.; Morris, D.G.; Sreenan, J.M.; Diskin, M.G. Ion concentrations in oviduct and uterine fluid and blood serum during the estrous cycle in the bovine. Theriogenology 2007, 68, 538–548. [Google Scholar] [CrossRef]
- Hugentobler, S.A.; Diskin, M.G.; Leese, H.J.; Humpherson, P.G.; Watson, T.; Sreenan, J.M.; Morris, D.G. Amino acids in oviduct and uterine fluid and blood plasma during the estrous cycle in the bovine. Mol. Reprod. Dev. 2007, 74, 445–454. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Leese, H.J. Metabolism of the preimplantation embryo: 40 years on. Reproduction 2012, 143, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.L.; Wade, M.G.; Nancarrow, C.D.; Kelleher, D.L.; Boland, M.P. Influence of ovine oviducal amino acid concentrations and an ovine oestrus-associated glycoprotein on development and viability of bovine embryos. Mol. Reprod. Dev. 1997, 47, 164–169. [Google Scholar] [CrossRef]
- Dawson, K.M.; Collins, J.L.; Baltz, J.M. Osmolarity-dependent glycine accumulation indicates a role for glycine as an organic osmolyte in early preimplantation mouse embryos. Biol. Reprod. 1998, 59, 225–232. [Google Scholar] [CrossRef]
- Steeves, C.L.; Baltz, J.M. Regulation of intracellular glycine as an organic osmolyte in early preimplantation mouse embryos. J. Cell. Physiol. 2005, 204, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.K.; Lane, M. Amino acids and ammonium regulate mouse embryo development in culture. Biol. Reprod. 1993, 48, 377–385. [Google Scholar] [CrossRef]
- Van Winkle, L.J.; Dickinson, H.R. Differences in amino acid content of preimplantation mouse embryos that develop in vitro versus in vivo: In vitro effects of five amino acids that are abundant in oviductal secretions. Biol. Reprod. 1995, 52, 96–104. [Google Scholar] [CrossRef]
- Sinclair, K.D.; Rooke, J.A.; McEvoy, T.G. Regulation of nutrient uptake and metabolism in pre-elongation ruminant embryos. Reprod. Camb. Engl. Suppl. 2003, 61, 371–385. [Google Scholar] [CrossRef]
- França, M.R.; da Silva, M.I.S.; Pugliesi, G.; Van Hoeck, V.; Binelli, M. Evidence of endometrial amino acid metabolism and transport modulation by peri-ovulatory endocrine profiles driving uterine receptivity. J. Anim. Sci. Biotechnol. 2017, 8, 54. [Google Scholar] [CrossRef]
- Moore, K.; Bondioli, K.R. Glycine and alanine supplementation of culture medium enhances development of in vitro matured and fertilized cattle embryos. Biol. Reprod. 1993, 48, 833–840. [Google Scholar] [CrossRef]
- Lee, E.S.; Fukui, Y. Synergistic effect of alanine and glycine on bovine embryos cultured in a chemically defined medium and amino acid uptake by vitro-produced bovine morulae and blastocysts. Biol. Reprod. 1996, 55, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wen, L.; Wang, S.; Bou, S. Development, freezability and amino acid consumption of bovine embryos cultured in synthetic oviductal fluid (SOF) medium containing amino acids at oviductal or uterine-fluid concentrations. Theriogenology 2006, 66, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Fukui, Y.; Lee, B.C.; Lim, J.M.; Hwang, W.S. Promoting effect of amino acids added to a chemically defined medium on blastocyst formation and blastomere proliferation of bovine embryos cultured in vitro. Anim. Reprod. Sci. 2004, 84, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Mateo, R.D.; Wu, G.; Bazer, F.W.; Park, J.C.; Shinzato, I.; Kim, S.W. Dietary l-Arginine Supplementation Enhances the Reproductive Performance of Gilts. J. Nutr. 2007, 137, 652–656. [Google Scholar] [CrossRef]
- Kim, J.; Song, G.; Wu, G.; Gao, H.; Johnson, G.A.; Bazer, F.W. Arginine, leucine, and glutamine stimulate proliferation of porcine trophectoderm cells through the MTOR-RPS6K-RPS6-EIF4EBP1 signal transduction pathway. Biol. Reprod. 2013, 88, 113. [Google Scholar] [CrossRef]
- Bazer, F.W.; Wang, X.; Johnson, G.A.; Wu, G. Select nutrients and their effects on conceptus development in mammals. Anim. Nutr. 2015, 1, 85–95. [Google Scholar] [CrossRef]
- Wang, X.; Burghardt, R.C.; Romero, J.J.; Hansen, T.R.; Wu, G.; Bazer, F.W. Functional roles of arginine during the peri-implantation period of pregnancy. III. Arginine stimulates proliferation and interferon tau production by ovine trophectoderm cells via nitric oxide and polyamine-TSC2-MTOR signaling pathways. Biol. Reprod. 2015, 92, 75. [Google Scholar] [CrossRef]
- Simintiras, C.A.; Sanchez, J.M.; McDonald, M.; Martins, T.; Binelli, M.; Lonergan, P. Biochemical characterization of progesterone-induced alterations in bovine uterine fluid amino acid and carbohydrate composition during the conceptus elongation window. Biol. Reprod. 2019, 100, 672–685. [Google Scholar] [CrossRef]
- Hugentobler, S.A.; Humpherson, P.G.; Leese, H.J.; Sreenan, J.M.; Morris, D.G. Energy substrates in bovine oviduct and uterine fluid and blood plasma during the oestrous cycle. Mol. Reprod. Dev. 2008, 75, 496–503. [Google Scholar] [CrossRef]
- Leese, H.J.; Gray, S.M. Vascular perfusion: A novel means of studying oviduct function. Am. J. Physiol. Endocrinol. Metab. 1985, 248, E624–E632. [Google Scholar] [CrossRef]
- Gardner, D.K. Lactate production by the mammalian blastocyst: Manipulating the microenvironment for uterine implantation and invasion? Bioessays 2015, 37, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Ireland, J.J.; Murphee, R.L.; Coulson, P.B. Accuracy of Predicting Stages of Bovine Estrous Cycle by Gross Appearance of the Corpus Luteum. J. Dairy Sci. 1980, 63, 155–160. [Google Scholar] [CrossRef]
- Carrasco, L.C.; Coy, P.; Avilés, M.; Gadea, J.; Romar, R. Glycosidase determination in bovine oviducal fluid at the follicular and luteal phases of the oestrous cycle. Reprod. Fertil. Dev. 2008, 20, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Stetson, I.; Izquierdo-Rico, M.J.; Moros, C.; Chevret, P.; Lorenzo, P.L.; Ballesta, J.; Rebollar, P.G.; Gutierrez-Gallego, R.; Aviles, M. Rabbit zona pellucida composition: A molecular, proteomic and phylogenetic approach. J. Proteom. 2012, 75, 5920–5935. [Google Scholar] [CrossRef]
- Simintiras, C.A.; Sturmey, R.G. Genistein crosses the bioartificial oviduct and alters secretion composition. Reprod. Toxicol. 2017, 71, 63–70. [Google Scholar] [CrossRef]
- Bauersachs, S.; Simintiras, C.A.; Sturmey, R.G.; Krebs, S.; Bick, J.; Blum, H.; Wolf, E.; Lonergan, P.; Forde, N. Effect of metabolic status on conceptus–maternal interactions on day 19 in dairy cattle: II. Effects on the endometrial transcriptome†. Biol. Reprod. 2017, 97, 413–425. [Google Scholar] [CrossRef]
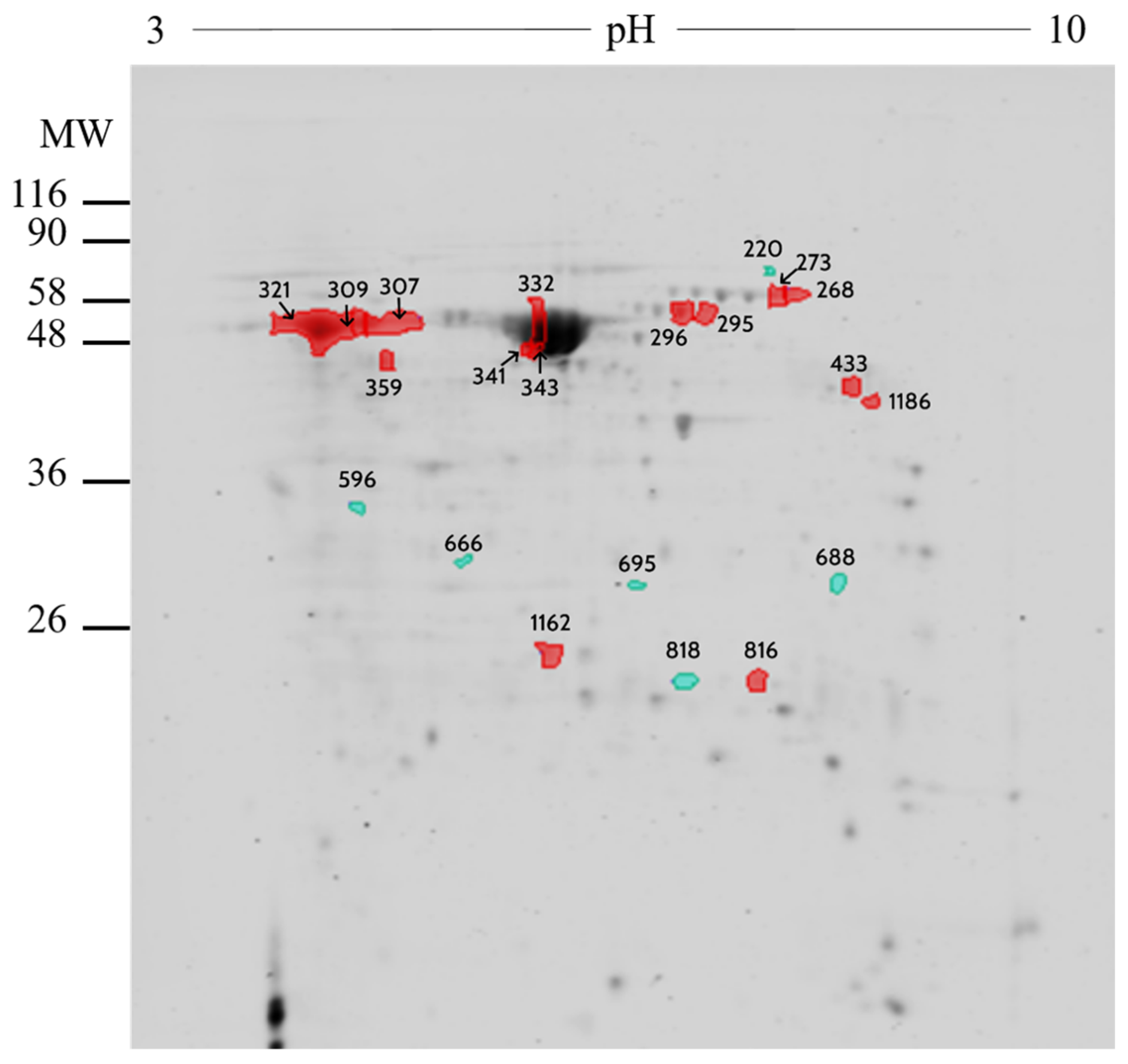
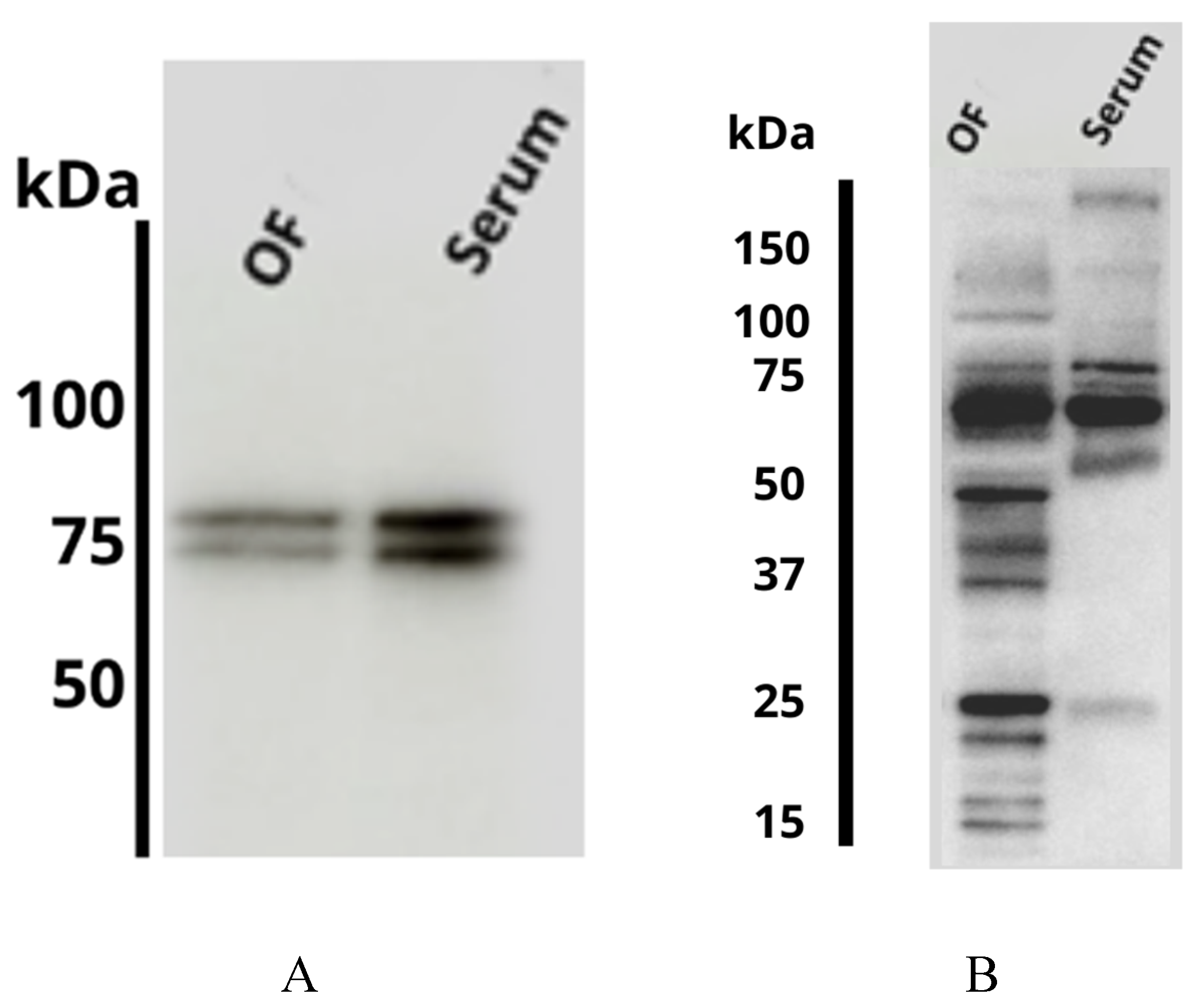
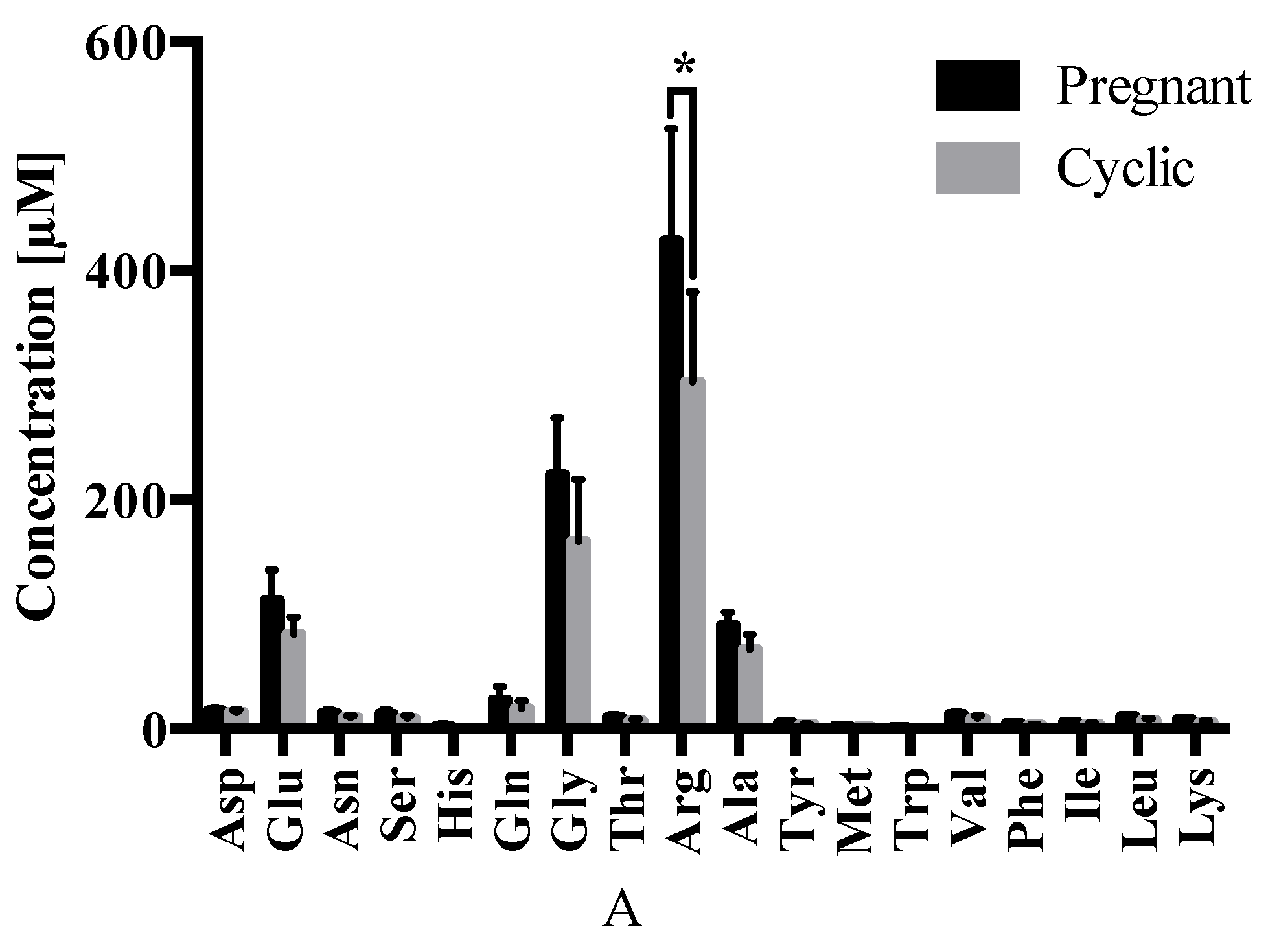
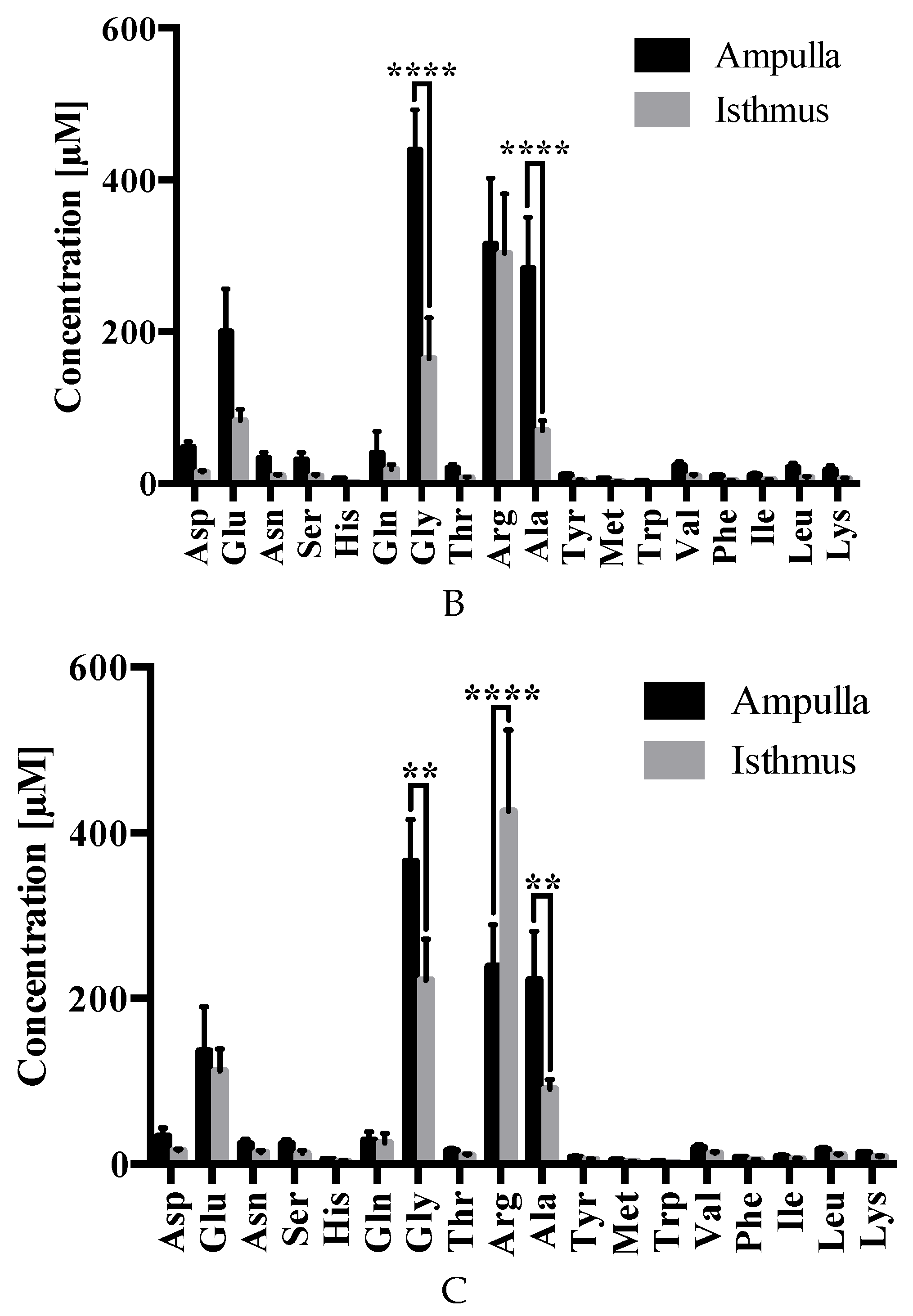
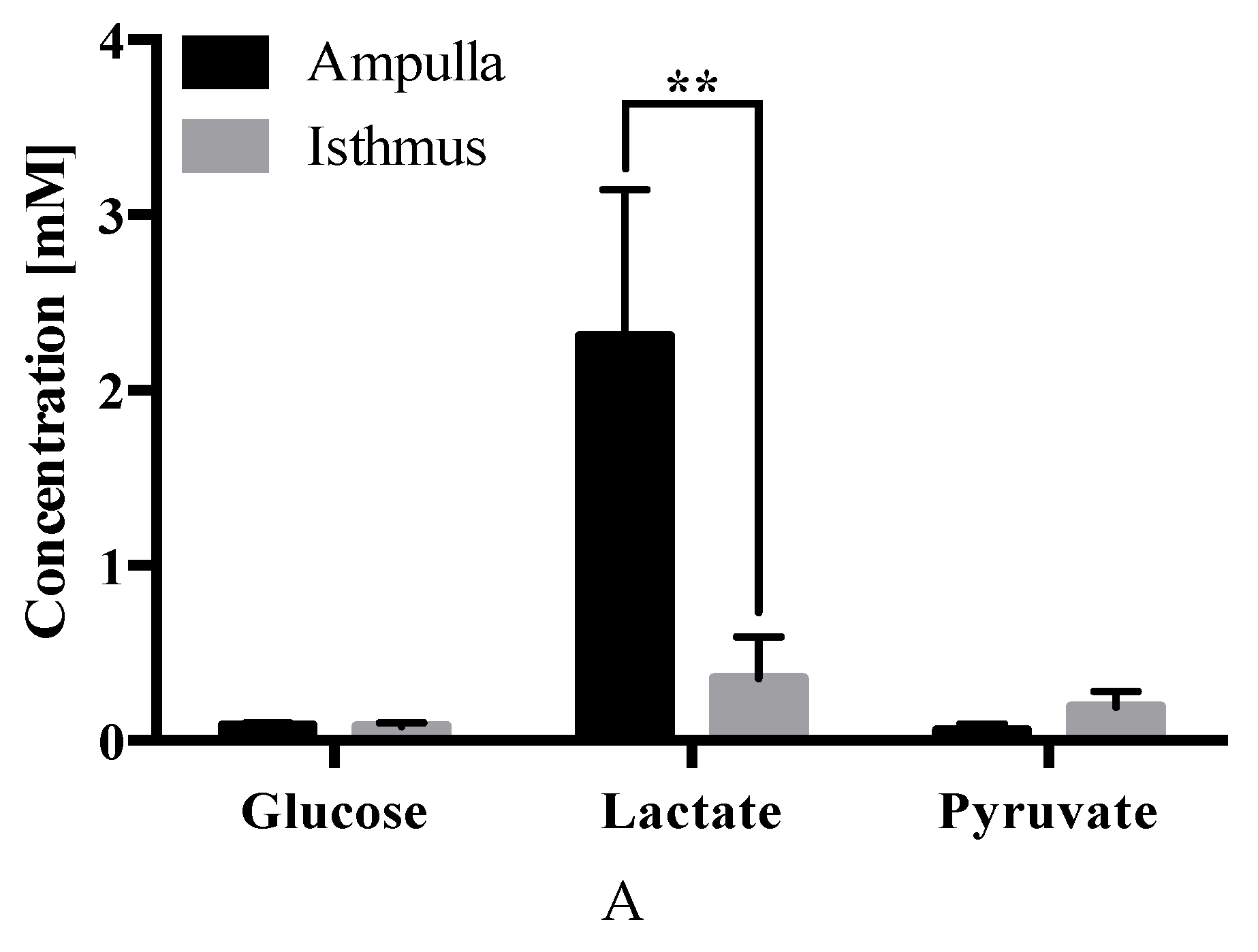
| Number | UniprotKB | Protein Name | Symbol | Fold |
|---|---|---|---|---|
| 309 | P02769 | Serum albumin | ALB | 1.19 |
| 332 | P02769 | Serum albumin | ALB | 1.27 |
| 341 | P02769 | Serum albumin | ALB | 1.41 |
| 343 | P02769 | Serum albumin | ALB | 1.21 |
| 1162 | A0A452DJA8 | Purine nucleoside phosphorylase | PNP | 1.03 |
| 816 | Q3T0Z7 | Dihydropteridine reductase | QDPR | 1.11 |
| 296 | A0A3Q1LW78 | Stress-induced-phosphoprotein 1 | STIP1 | 1.98 |
| 268 | Q29443 | Serotransferrin | TF | 2.27 |
| 273 | Q29443 | Serotransferrin | TF | 2.07 |
| 295 | Q29443 | Serotransferrin | TF | 1.42 |
| 433 | A0A3Q1M010 | UTP--glucose-1-phosphate uridylyltransferase | UGP2 | 1.54 |
| Number | UniprotKB | Protein Name | Symbol | Fold |
|---|---|---|---|---|
| 437 | P02769 | Serum albumin | ALB | 1.08 |
| 831 | Q9TRY0 | Peptidyl-prolyl cis-trans isomerase FKBP4 | FKBP4 | 1.24 |
| 1179 | - | - | IGHG | 1.49 |
| 463 | - | - | IGHG | 1.28 |
| 339 | - | - | IGHG | 1.27 |
| 978 | Q3T101 | IGL@ protein | IGL | 1.59 |
| 527 | Q1RMX7 | N-acetylneuraminate synthase | NANS | 1.12 |
| 718 | Q3T0P6 | Phosphoglycerate kinase 1 | PGK1 | 1.00 |
| 360 | P06868 | Plasminogen | PLG | 1.16 |
| 1182 | P06868 | Plasminogen | PLG | 1.08 |
| 1180 | P34955 | α-1-antiproteinase | SERPINA1 | 1.33 |
| 1184 | P34955 | α-1-antiproteinase | SERPINA1 | 1.23 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Alonso, B.; Maillo, V.; Acuña, O.S.; López-Úbeda, R.; Torrecillas, A.; Simintiras, C.A.; Sturmey, R.; Avilés, M.; Lonergan, P.; Rizos, D. Spatial and Pregnancy-Related Changes in the Protein, Amino Acid, and Carbohydrate Composition of Bovine Oviduct Fluid. Int. J. Mol. Sci. 2020, 21, 1681. https://doi.org/10.3390/ijms21051681
Rodríguez-Alonso B, Maillo V, Acuña OS, López-Úbeda R, Torrecillas A, Simintiras CA, Sturmey R, Avilés M, Lonergan P, Rizos D. Spatial and Pregnancy-Related Changes in the Protein, Amino Acid, and Carbohydrate Composition of Bovine Oviduct Fluid. International Journal of Molecular Sciences. 2020; 21(5):1681. https://doi.org/10.3390/ijms21051681
Chicago/Turabian StyleRodríguez-Alonso, Beatriz, Veronica Maillo, Omar Salvador Acuña, Rebeca López-Úbeda, Alejandro Torrecillas, Constantine A. Simintiras, Roger Sturmey, Manuel Avilés, Patrick Lonergan, and Dimitrios Rizos. 2020. "Spatial and Pregnancy-Related Changes in the Protein, Amino Acid, and Carbohydrate Composition of Bovine Oviduct Fluid" International Journal of Molecular Sciences 21, no. 5: 1681. https://doi.org/10.3390/ijms21051681
APA StyleRodríguez-Alonso, B., Maillo, V., Acuña, O. S., López-Úbeda, R., Torrecillas, A., Simintiras, C. A., Sturmey, R., Avilés, M., Lonergan, P., & Rizos, D. (2020). Spatial and Pregnancy-Related Changes in the Protein, Amino Acid, and Carbohydrate Composition of Bovine Oviduct Fluid. International Journal of Molecular Sciences, 21(5), 1681. https://doi.org/10.3390/ijms21051681







