Renal Sympathetic Nerve-Derived Signaling in Acute and Chronic Kidney Diseases
Abstract
1. Introduction
2. Adrenergic Receptors and Norepinephrine in the Kidney
3. Renal Sympathetic Nervous System in AKI and CKD
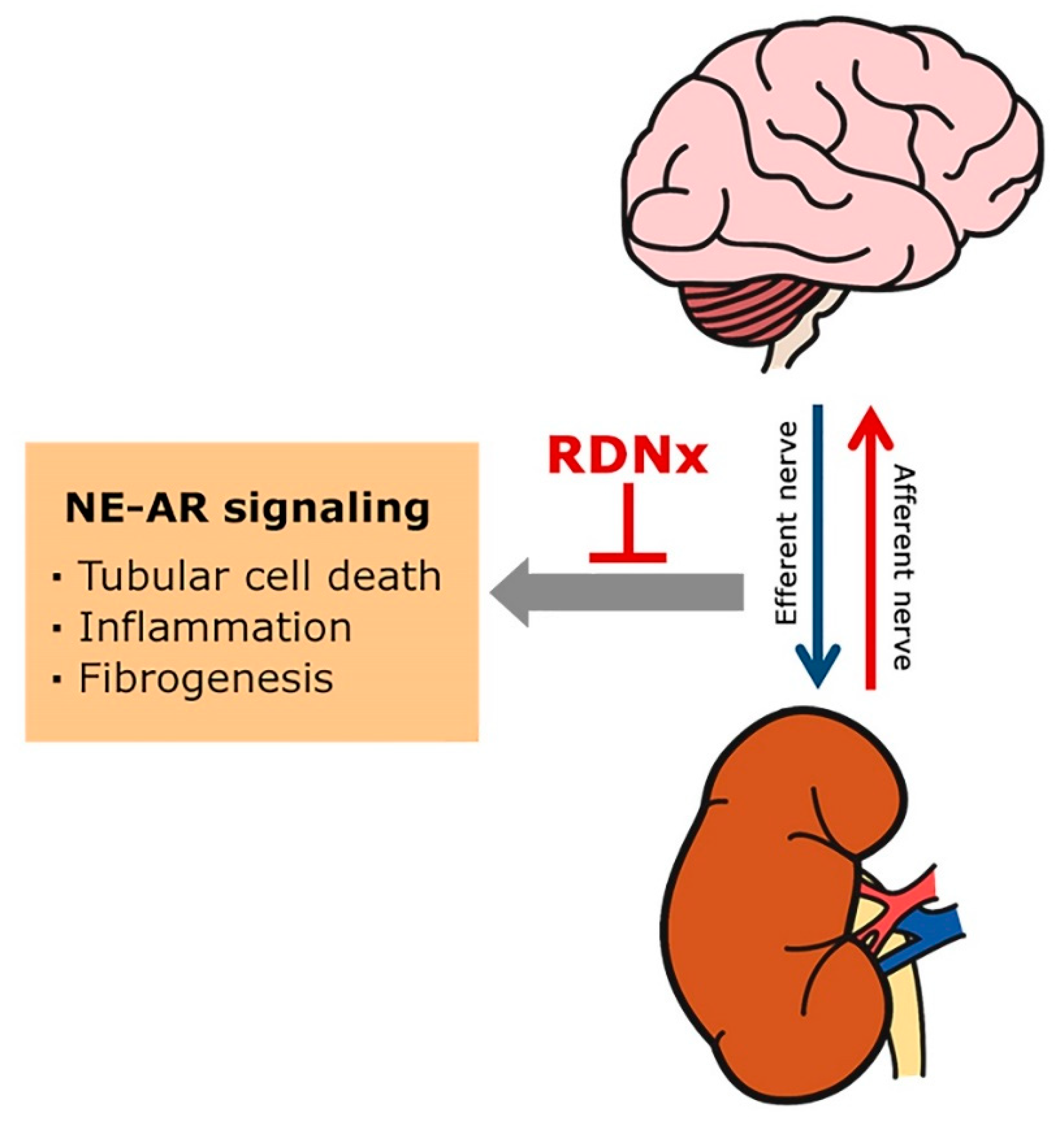
4. Inactivation of NE-AR Signaling in AKI and CKD
5. Mechanisms of NE-AR Signaling in AKI and CKD
6. Role of Sympathetic Nerves in Other Organs
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- He, L.; Wei, Q.; Liu, J.; Yi, M.; Liu, Y.; Liu, H.; Sun, L.; Peng, Y.; Liu, F.; Venkatachalam, M.A.; et al. AKI on CKD: heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 2017, 92, 1071–1083. [Google Scholar] [CrossRef]
- Belayev, L.Y.; Palevsky, P.M. The link between acute kidney injury and chronic kidney disease. Curr. Opin. Nephrol. Hypertens 2014, 23, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.; Yang, C.W. Chronic kidney disease: global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Kaushal, G.P.; Shah, S.V. Challenges and advances in the treatment of AKI. J. Am. Soc. Nephrol. 2014, 25, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, T.; Oka, M.; Maesato, K.; Mano, T.; Ikee, R.; Moriya, H.; Kobayashi, S. Pathological regression by angiotensin II type 1 receptor blockade in patients with mesangial proliferative glomerulonephritis. Hypertens Res. 2008, 31, 387–394. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sata, Y.; Head, G.A.; Denton, K.; May, C.N.; Schlaich, M.P. Role of the Sympathetic Nervous System and Its Modulation in Renal Hypertension. Front. Med. 2018, 5, 82. [Google Scholar] [CrossRef]
- Nishi, E.E.; Bergamaschi, C.T.; Campos, R.R. The crosstalk between the kidney and the central nervous system: the role of renal nerves in blood pressure regulation. Exp. Physiol. 2015, 100, 479–484. [Google Scholar] [CrossRef]
- Johns, E.J.; Kopp, U.C.; DiBona, G.F. Neural control of renal function. Compr. Physiol. 2011, 1, 731–767. [Google Scholar] [CrossRef] [PubMed]
- McCorry, L.K. Physiology of the autonomic nervous system. Am. J. Pharm. Educ. 2007, 71, 78. [Google Scholar] [CrossRef]
- Kanagy, N.L. Alpha(2)-adrenergic receptor signalling in hypertension. Clin. Sci. 2005, 109, 431–437. [Google Scholar] [CrossRef]
- Molinoff, P.B. Alpha- and beta-adrenergic receptor subtypes properties, distribution and regulation. Drugs 1984, 28 (Suppl. 2), 1–15. [Google Scholar] [CrossRef]
- Converse, R.L., Jr.; Jacobsen, T.N.; Toto, R.D.; Jost, C.M.; Cosentino, F.; Fouad-Tarazi, F.; Victor, R.G. Sympathetic overactivity in patients with chronic renal failure. N. Engl. J. Med. 1992, 327, 1912–1918. [Google Scholar] [CrossRef] [PubMed]
- Veelken, R.; Vogel, E.M.; Hilgers, K.; Amann, K.; Hartner, A.; Sass, G.; Neuhuber, W.; Tiegs, G. Autonomic renal denervation ameliorates experimental glomerulonephritis. J. Am. Soc. Nephrol. 2008, 19, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Young, B.E.; Fadel, P.J. Sympathetic Overactivity in Chronic Kidney Disease: Consequences and Mechanisms. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Zoccali, C.; Mallamaci, F.; Tripepi, G.; Parlongo, S.; Cutrupi, S.; Benedetto, F.A.; Cataliotti, A.; Malatino, L.S.; Investigators, C. Norepinephrine and concentric hypertrophy in patients with end-stage renal disease. Hypertension 2002, 40, 41–46. [Google Scholar] [CrossRef]
- Cronin, R.E.; Erickson, A.M.; de Torrente, A.; McDonald, K.M.; Schrier, R.W. Norepinephrine-induced acute renal failure: a reversible ischemic model of acute renal failure. Kidney Int. 1978, 14, 187–190. [Google Scholar] [CrossRef]
- Conger, J.D.; Robinette, J.B.; Hammond, W.S. Differences in vascular reactivity in models of ischemic acute renal failure. Kidney Int. 1991, 39, 1087–1097. [Google Scholar] [CrossRef]
- Bellomo, R.; Wan, L.; May, C. Vasoactive drugs and acute kidney injury. Crit. Care Med. 2008, 36, S179–S186. [Google Scholar] [CrossRef]
- Schlaich, M.P.; Sobotka, P.A.; Krum, H.; Lambert, E.; Esler, M.D. Renal sympathetic-nerve ablation for uncontrolled hypertension. N. Engl. J. Med. 2009, 361, 932–934. [Google Scholar] [CrossRef]
- Krum, H.; Schlaich, M.; Whitbourn, R.; Sobotka, P.A.; Sadowski, J.; Bartus, K.; Kapelak, B.; Walton, A.; Sievert, H.; Thambar, S.; et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009, 373, 1275–1281. [Google Scholar] [CrossRef]
- Kannan, A.; Medina, R.I.; Nagajothi, N.; Balamuthusamy, S. Renal sympathetic nervous system and the effects of denervation on renal arteries. World J. Cardiol. 2014, 6, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Mulder, J.; Hokfelt, T.; Knuepfer, M.M.; Kopp, U.C. Renal sensory and sympathetic nerves reinnervate the kidney in a similar time-dependent fashion after renal denervation in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R675–R682. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.R.; Denton, K.M. Renal Denervation. Hypertension 2018, 72, 528–536. [Google Scholar] [CrossRef] [PubMed]
- DiBona, G.F.; Esler, M. Translational medicine: the antihypertensive effect of renal denervation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R245–R253. [Google Scholar] [CrossRef]
- Kim, J.; Padanilam, B.J. Renal nerves drive interstitial fibrogenesis in obstructive nephropathy. J. Am. Soc. Nephrol. 2013, 24, 229–242. [Google Scholar] [CrossRef]
- Azizi, M.; Schmieder, R.E.; Mahfoud, F.; Weber, M.A.; Daemen, J.; Davies, J.; Basile, J.; Kirtane, A.J.; Wang, Y.; Lobo, M.D.; et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet 2018, 391, 2335–2345. [Google Scholar] [CrossRef]
- Kandzari, D.E.; Bohm, M.; Mahfoud, F.; Townsend, R.R.; Weber, M.A.; Pocock, S.; Tsioufis, K.; Tousoulis, D.; Choi, J.W.; East, C.; et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet 2018, 391, 2346–2355. [Google Scholar] [CrossRef]
- Smithwick, R.H.; Thompson, J.E. Splanchnicectomy for essential hypertension; results in 1,266 cases. J. Am. Med. Assoc. 1953, 152, 1501–1504. [Google Scholar] [CrossRef]
- Azizi, M.; Sapoval, M.; Gosse, P.; Monge, M.; Bobrie, G.; Delsart, P.; Midulla, M.; Mounier-Vehier, C.; Courand, P.Y.; Lantelme, P.; et al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015, 385, 1957–1965. [Google Scholar] [CrossRef]
- Mahfoud, F.; Ukena, C.; Schmieder, R.E.; Cremers, B.; Rump, L.C.; Vonend, O.; Weil, J.; Schmidt, M.; Hoppe, U.C.; Zeller, T.; et al. Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation 2013, 128, 132–140. [Google Scholar] [CrossRef]
- Kim, J.; Padanilam, B.J. Renal denervation prevents long-term sequelae of ischemic renal injury. Kidney Int. 2015, 87, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Mahfoud, F.; Bohm, M.; Schmieder, R.; Narkiewicz, K.; Ewen, S.; Ruilope, L.; Schlaich, M.; Williams, B.; Fahy, M.; Mancia, G. Effects of renal denervation on kidney function and long-term outcomes: 3-year follow-up from the Global SYMPLICITY Registry. Eur. Heart J. 2019, 40, 3474–3482. [Google Scholar] [CrossRef] [PubMed]
- Schlaich, M.P.; Bart, B.; Hering, D.; Walton, A.; Marusic, P.; Mahfoud, F.; Bohm, M.; Lambert, E.A.; Krum, H.; Sobotka, P.A.; et al. Feasibility of catheter-based renal nerve ablation and effects on sympathetic nerve activity and blood pressure in patients with end-stage renal disease. Int. J. Cardiol. 2013, 168, 2214–2220. [Google Scholar] [CrossRef] [PubMed]
- Esler, M.; Jennings, G.; Korner, P.; Willett, I.; Dudley, F.; Hasking, G.; Anderson, W.; Lambert, G. Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension 1988, 11, 3–20. [Google Scholar] [CrossRef]
- Oberhauser, V.; Vonend, O.; Rump, L.C. Neuropeptide Y and ATP interact to control renovascular resistance in the rat. J. Am. Soc. Nephrol. 1999, 10, 1179–1185. [Google Scholar]
- Cotecchia, S. The alpha1-adrenergic receptors: diversity of signaling networks and regulation. J. Recept Signal. Transduct Res. 2010, 30, 410–419. [Google Scholar] [CrossRef]
- Arif, E.; Nihalani, D. Beta2-adrenergic receptor in kidney biology: A current prospective. Nephrology (Carlton) 2019, 24, 497–503. [Google Scholar] [CrossRef]
- Procino, G.; Carmosino, M.; Milano, S.; Dal Monte, M.; Schena, G.; Mastrodonato, M.; Gerbino, A.; Bagnoli, P.; Svelto, M. beta3 adrenergic receptor in the kidney may be a new player in sympathetic regulation of renal function. Kidney Int. 2016, 90, 555–567. [Google Scholar] [CrossRef]
- Jang, H.S.; Kim, J.; Padanilam, B.J. Renal sympathetic nerve activation via alpha2-adrenergic receptors in chronic kidney disease progression. Kidney Res. Clin. Pract. 2019, 38, 6–14. [Google Scholar] [CrossRef]
- Summers, R.J.; Stephenson, J.A.; Kuhar, M.J. Localization of beta adrenoceptor subtypes in rat kidney by light microscopic autoradiography. J. Pharmacol. Exp. Ther. 1985, 232, 561–569. [Google Scholar]
- Summers, R.J.; Kuhar, M.J. Autoradiographic localization of beta-adrenoceptors in rat kidney. Eur. J. Pharmacol. 1983, 91, 305–310. [Google Scholar] [CrossRef]
- Struyker-Boudier, H.A.; Janssen, B.J.; Smits, J.F. Adrenoceptors in the kidney: localization and pharmacology. Clin. Exp. Hypertens A 1987, 9 (Suppl. 1), 135–150. [Google Scholar] [CrossRef]
- Boivin, V.; Jahns, R.; Gambaryan, S.; Ness, W.; Boege, F.; Lohse, M.J. Immunofluorescent imaging of beta 1- and beta 2-adrenergic receptors in rat kidney. Kidney Int. 2001, 59, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Thomson, S.C.; Vallon, V. Alpha 2-adrenoceptors determine the response to nitric oxide inhibition in the rat glomerulus and proximal tubule. J. Am. Soc. Nephrol. 1995, 6, 1482–1490. [Google Scholar] [PubMed]
- Snavely, M.D.; Ziegler, M.G.; Insel, P.A. Subtype-selective down-regulation of rat renal cortical alpha- and beta-adrenergic receptors by catecholamines. Endocrinology 1985, 117, 2182–2189. [Google Scholar] [CrossRef] [PubMed]
- Morla, L.; Edwards, A.; Crambert, G. New insights into sodium transport regulation in the distal nephron: Role of G-protein coupled receptors. World J. Biol. Chem. 2016, 7, 44–63. [Google Scholar] [CrossRef]
- Makris, K.; Spanou, L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin. Biochem. Rev. 2016, 37, 85–98. [Google Scholar]
- Basile, D.P.; Anderson, M.D.; Sutton, T.A. Pathophysiology of acute kidney injury. Compr. Physiol. 2012, 2, 1303–1353. [Google Scholar] [CrossRef]
- Forni, L.G.; Darmon, M.; Ostermann, M.; Oudemans-van Straaten, H.M.; Pettila, V.; Prowle, J.R.; Schetz, M.; Joannidis, M. Renal recovery after acute kidney injury. Intensive Care Med. 2017, 43, 855–866. [Google Scholar] [CrossRef]
- Ishani, A.; Xue, J.L.; Himmelfarb, J.; Eggers, P.W.; Kimmel, P.L.; Molitoris, B.A.; Collins, A.J. Acute kidney injury increases risk of ESRD among elderly. J. Am. Soc. Nephrol. 2009, 20, 223–228. [Google Scholar] [CrossRef]
- Lo, L.J.; Go, A.S.; Chertow, G.M.; McCulloch, C.E.; Fan, D.; Ordonez, J.D.; Hsu, C.Y. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009, 76, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Zager, R.A.; Johnson, A.C.; Becker, K. Acute unilateral ischemic renal injury induces progressive renal inflammation, lipid accumulation, histone modification, and "end-stage" kidney disease. Am. J. Physiol. Renal Physiol. 2011, 301, F1334–F1345. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Bonventre, J.V.; Mehta, R.; Nangaku, M.; Unwin, R.; Rosner, M.H.; Kellum, J.A.; Ronco, C.; Group, A.X.W. Progression after AKI: Understanding Maladaptive Repair Processes to Predict and Identify Therapeutic Treatments. J. Am. Soc. Nephrol. 2016, 27, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Baines, A.D.; DeBold, A.J.; Sonnenberg, H. Natriuretic effect of atrial extract on isolated perfused rat kidney. Can. J. Physiol. Pharmacol. 1983, 61, 1462–1466. [Google Scholar] [CrossRef]
- Iaina, A.; Eliahou, H.E. The sympathetic nervous system in the pathogenesis of acute renal failure. Clin. Exp. Dial. Apheresis 1983, 7, 115–125. [Google Scholar] [CrossRef]
- Bellomo, R.; Giantomasso, D.D. Noradrenaline and the kidney: friends or foes? Crit. Care 2001, 5, 294–298. [Google Scholar] [CrossRef]
- Conger, J.D.; Robinette, J.B.; Guggenheim, S.J. Effect of acetylcholine on the early phase of reversible norepinephrine-induced acute renal failure. Kidney Int. 1981, 19, 399–409. [Google Scholar] [CrossRef]
- Fujii, T.; Kurata, H.; Takaoka, M.; Muraoka, T.; Fujisawa, Y.; Shokoji, T.; Nishiyama, A.; Abe, Y.; Matsumura, Y. The role of renal sympathetic nervous system in the pathogenesis of ischemic acute renal failure. Eur. J. Pharmacol. 2003, 481, 241–248. [Google Scholar] [CrossRef]
- Tanaka, R.; Tsutsui, H.; Ohkita, M.; Takaoka, M.; Yukimura, T.; Matsumura, Y. Sex differences in ischemia/reperfusion-induced acute kidney injury are dependent on the renal sympathetic nervous system. Eur. J. Pharmacol. 2013, 714, 397–404. [Google Scholar] [CrossRef]
- Mutoh, J.; Ohsawa, M.; Hisa, H. Involvement of renal sympathetic nerve activation on the progression of ischemic acute kidney injury in the mouse. J. Pharmacol. Sci. 2014, 125, 415–421. [Google Scholar] [CrossRef]
- Tsutsui, H.; Tanaka, R.; Yamagata, M.; Yukimura, T.; Ohkita, M.; Matsumura, Y. Protective effect of ischemic preconditioning on ischemia/reperfusion-induced acute kidney injury through sympathetic nervous system in rats. Eur. J. Pharmacol. 2013, 718, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Li, A.; Li, J.; Wu, C.; Cui, S.; Zhou, Z.; Liu, Y.; Wilcox, C.S.; Hou, F.F. Reno-Cerebral Reflex Activates the Renin-Angiotensin System, Promoting Oxidative Stress and Renal Damage After Ischemia-Reperfusion Injury. Antioxid Redox Signal. 2017, 27, 415–432. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef] [PubMed]
- Goulding, N.E.; Johns, E.J. Neural regulation of the kidney function in rats with cisplatin induced renal failure. Front. Physiol. 2015, 6, 192. [Google Scholar] [CrossRef]
- Matsushima, H.; Yonemura, K.; Ohishi, K.; Hishida, A. The role of oxygen free radicals in cisplatin-induced acute renal failure in rats. J. Lab. Clin. Med. 1998, 131, 518–526. [Google Scholar] [CrossRef]
- Bagnis, C.; Beaufils, H.; Jacquiaud, C.; Adabra, Y.; Jouanneau, C.; Le Nahour, G.; Jaudon, M.C.; Bourbouze, R.; Jacobs, C.; Deray, G. Erythropoietin enhances recovery after cisplatin-induced acute renal failure in the rat. Nephrol. Dial. Transplant. 2001, 16, 932–938. [Google Scholar] [CrossRef]
- Hye Khan, M.A.; Sattar, M.A.; Abdullah, N.A.; Johns, E.J. Influence of combined hypertension and renal failure on functional alpha(1)-adrenoceptor subtypes in the rat kidney. Br. J. Pharmacol. 2008, 153, 1232–1241. [Google Scholar] [CrossRef]
- Kribben, A.; Edelstein, C.L.; Schrier, R.W. Pathophysiology of acute renal failure. J. Nephrol. 1999, 12 (Suppl. 2), S142–S151. [Google Scholar]
- Yatsu, T.; Aoki, M.; Inagaki, O. Preventive effect of zelandopam, a dopamine D1 receptor agonist, on cisplatin-induced acute renal failure in rats. Eur. J. Pharmacol. 2003, 461, 191–195. [Google Scholar] [CrossRef]
- Hasking, G.J.; Esler, M.D.; Jennings, G.L.; Burton, D.; Johns, J.A.; Korner, P.I. Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation 1986, 73, 615–621. [Google Scholar] [CrossRef]
- Hausberg, M.; Kosch, M.; Harmelink, P.; Barenbrock, M.; Hohage, H.; Kisters, K.; Dietl, K.H.; Rahn, K.H. Sympathetic nerve activity in end-stage renal disease. Circulation 2002, 106, 1974–1979. [Google Scholar] [CrossRef] [PubMed]
- Denker, M.G.; Cohen, D.L. Resistant Hypertension and Renal Nerve Denervation. Methodist Debakey Cardiovasc. J. 2015, 11, 240–244. [Google Scholar] [CrossRef] [PubMed]
- DiBona, G.F. Functionally specific renal sympathetic nerve fibers: role in cardiovascular regulation. Am. J. Hypertens 2001, 14, 163S–170S. [Google Scholar] [CrossRef]
- Taddei, S.; Virdis, A.; Abdel-Haq, B.; Giovannetti, R.; Duranti, P.; Arena, A.M.; Favilla, S.; Salvetti, A. Indirect evidence for vascular uptake of circulating renin in hypertensive patients. Hypertension 1993, 21, 852–860. [Google Scholar] [CrossRef][Green Version]
- Campese, V.M.; Mitra, N.; Sandee, D. Hypertension in renal parenchymal disease: why is it so resistant to treatment? Kidney Int. 2006, 69, 967–973. [Google Scholar] [CrossRef]
- DiBona, G.F.; Kopp, U.C. Neural control of renal function. Physiol. Rev. 1997, 77, 75–197. [Google Scholar] [CrossRef]
- Schlaich, M.P.; Socratous, F.; Hennebry, S.; Eikelis, N.; Lambert, E.A.; Straznicky, N.; Esler, M.D.; Lambert, G.W. Sympathetic activation in chronic renal failure. J. Am. Soc. Nephrol. 2009, 20, 933–939. [Google Scholar] [CrossRef]
- Stegbauer, J.; Vonend, O.; Habbel, S.; Quack, I.; Sellin, L.; Gross, V.; Rump, L.C. Angiotensin II modulates renal sympathetic neurotransmission through nitric oxide in AT2 receptor knockout mice. J. Hypertens 2005, 23, 1691–1698. [Google Scholar] [CrossRef]
- Hall, M.E.; do Carmo, J.M.; da Silva, A.A.; Juncos, L.A.; Wang, Z.; Hall, J.E. Obesity, hypertension, and chronic kidney disease. Int. J. Nephrol. Renov. Dis. 2014, 7, 75–88. [Google Scholar] [CrossRef]
- Schiffl, H.; Lang, S.M. Obesity, acute kidney injury and outcome of critical illness. Int. Urol. Nephrol. 2017, 49, 461–466. [Google Scholar] [CrossRef]
- Richards, R.J.; Thakur, V.; Reisin, E. Obesity-related hypertension: its physiological basis and pharmacological approaches to its treatment. J. Hum. Hypertens 1996, 10 (Suppl. 3), S59–S64. [Google Scholar]
- Wofford, M.R.; Anderson, D.C., Jr.; Brown, C.A.; Jones, D.W.; Miller, M.E.; Hall, J.E. Antihypertensive effect of alpha- and beta-adrenergic blockade in obese and lean hypertensive subjects. Am. J. Hypertens 2001, 14, 694–698. [Google Scholar] [CrossRef]
- Rocchini, A.P.; Mao, H.Z.; Babu, K.; Marker, P.; Rocchini, A.J. Clonidine prevents insulin resistance and hypertension in obese dogs. Hypertension 1999, 33, 548–553. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kassab, S.; Kato, T.; Wilkins, F.C.; Chen, R.; Hall, J.E.; Granger, J.P. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension 1995, 25, 893–897. [Google Scholar] [CrossRef]
- Hoch, H.; Stegbauer, J.; Potthoff, S.A.; Hein, L.; Quack, I.; Rump, L.C.; Vonend, O. Regulation of renal sympathetic neurotransmission by renal alpha(2A)-adrenoceptors is impaired in chronic renal failure. Br. J. Pharmacol. 2011, 163, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Solez, K.; Freshwater, M.F.; Su, C.T. The effect of propranolol on postischemic acute renal failure in the rat. Transplantation 1977, 24, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Solez, K.; Ideura, T.; Silvia, C.B.; Hamilton, B.; Saito, H. Clonidine after renal ischemia to lessen acute renal failure and microvascular damage. Kidney Int. 1980, 18, 309–322. [Google Scholar] [CrossRef]
- Chevalier, R.L.; Finn, W.F. Effects of propranolol on post-ischemic acute renal failure. Nephron 1980, 25, 77–81. [Google Scholar] [CrossRef]
- Amann, K.; Koch, A.; Hofstetter, J.; Gross, M.L.; Haas, C.; Orth, S.R.; Ehmke, H.; Rump, L.C.; Ritz, E. Glomerulosclerosis and progression: effect of subantihypertensive doses of alpha and beta blockers. Kidney Int. 2001, 60, 1309–1323. [Google Scholar] [CrossRef]
- Shimokawa, T.; Tsutsui, H.; Miura, T.; Nishinaka, T.; Terada, T.; Takama, M.; Yoshida, S.; Tanba, T.; Tojo, A.; Yamagata, M.; et al. Renoprotective effect of yohimbine on ischaemia/reperfusion-induced acute kidney injury through alpha2C-adrenoceptors in rats. Eur. J. Pharmacol. 2016, 781, 36–44. [Google Scholar] [CrossRef]
- Salman, I.M.; Ameer, O.Z.; Sattar, M.A.; Abdullah, N.A.; Yam, M.F.; Najim, H.S.; Abdulkarim, M.F.; Abdullah, G.Z.; Kaur, G.; Khan, M.A.; et al. Characterization of renal hemodynamic and structural alterations in rat models of renal impairment: role of renal sympathoexcitation. J. Nephrol. 2011, 24, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Sattar, M.A.; Rathore, H.A.; Abdulla, M.H.; Ud Din Ahmad, F.; Ahmad, A.; Afzal, S.; Abdullah, N.A.; Johns, E.J. Renal denervation restores the baroreflex control of renal sympathetic nerve activity and heart rate in Wistar-Kyoto rats with cisplatin-induced renal failure. Acta Physiol. 2014, 210, 690–700. [Google Scholar] [CrossRef]
- Ogawa, T.; Mimura, Y.; Kaminishi, M. Renal denervation abolishes the protective effects of ischaemic preconditioning on function and haemodynamics in ischaemia-reperfused rat kidneys. Acta Physiol. Scand. 2002, 174, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Hering, D.; Mahfoud, F.; Walton, A.S.; Krum, H.; Lambert, G.W.; Lambert, E.A.; Sobotka, P.A.; Bohm, M.; Cremers, B.; Esler, M.D.; et al. Renal denervation in moderate to severe CKD. J. Am. Soc. Nephrol. 2012, 23, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.; Schmid, A.; Toennes, S.W.; Ditting, T.; Veelken, R.; Uder, M.; Schmieder, R.E. Central pulse pressure predicts BP reduction after renal denervation in patients with treatment-resistant hypertension. EuroIntervention 2015, 11, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, M.H. Renal nerves and CKD: is renal denervation the answer? J. Am. Soc. Nephrol. 2012, 23, 1132–1135. [Google Scholar] [CrossRef]
- Desir, G. Novel insights into the physiology of renalase and its role in hypertension and heart disease. Pediatr. Nephrol. 2012, 27, 719–725. [Google Scholar] [CrossRef]
- Lee, H.T.; Kim, J.Y.; Kim, M.; Wang, P.; Tang, L.; Baroni, S.; D’Agati, V.D.; Desir, G.V. Renalase protects against ischemic AKI. J. Am. Soc. Nephrol 2013, 24, 445–455. [Google Scholar] [CrossRef]
- Johns, E.J.; Abdulla, M.H. Renal nerves in blood pressure regulation. Curr. Opin. Nephrol. Hypertens 2013, 22, 504–510. [Google Scholar] [CrossRef]
- Lee, J.Y.; Walsh, G.M. Systemic and regional haemodynamic effects of renal denervation in spontaneously hypertensive rats. J. Hypertens 1983, 1, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Krueger, A.D.; Lee, J.Y.; Yang, P.C.; Papaioannou, S.E.; Walsh, G.M. Selective vasodilation produced by renal denervation in adult spontaneously hypertensive rats. Hypertension 1986, 8, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Foss, J.D.; Fink, G.D.; Osborn, J.W. Reversal of genetic salt-sensitive hypertension by targeted sympathetic ablation. Hypertension 2013, 61, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Nishida, H.; Nomura, G.; Van Vliet, B.N.; Toshima, H. Hypertension induced by nitric oxide synthesis inhibition is renal nerve dependent. Hypertension 1994, 23, 971–975. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jacob, F.; Clark, L.A.; Guzman, P.A.; Osborn, J.W. Role of renal nerves in development of hypertension in DOCA-salt model in rats: a telemetric approach. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1519–H1529. [Google Scholar] [CrossRef] [PubMed]
- Kandlikar, S.S.; Fink, G.D. Splanchnic sympathetic nerves in the development of mild DOCA-salt hypertension. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1965–H1973. [Google Scholar] [CrossRef]
- Singh, R.R.; McArdle, Z.M.; Iudica, M.; Easton, L.K.; Booth, L.C.; May, C.N.; Parkington, H.C.; Lombardo, P.; Head, G.A.; Lambert, G.; et al. Sustained Decrease in Blood Pressure and Reduced Anatomical and Functional Reinnervation of Renal Nerves in Hypertensive Sheep 30 Months After Catheter-Based Renal Denervation. Hypertension 2019, 73, 718–727. [Google Scholar] [CrossRef]
- Ma, S.; Evans, R.G.; Iguchi, N.; Tare, M.; Parkington, H.C.; Bellomo, R.; May, C.N.; Lankadeva, Y.R. Sepsis-induced acute kidney injury: A disease of the microcirculation. Microcirculation 2019, 26, e12483. [Google Scholar] [CrossRef]
- Tan, F.; Chen, Y.; Yuan, D.; Gong, C.; Li, X.; Zhou, S. Dexmedetomidine protects against acute kidney injury through downregulating inflammatory reactions in endotoxemia rats. Biomed. Rep. 2015, 3, 365–370. [Google Scholar] [CrossRef]
- Si, Y.; Bao, H.; Han, L.; Shi, H.; Zhang, Y.; Xu, L.; Liu, C.; Wang, J.; Yang, X.; Vohra, A.; et al. Dexmedetomidine protects against renal ischemia and reperfusion injury by inhibiting the JAK/STAT signaling activation. J. Transl. Med. 2013, 11, 141. [Google Scholar] [CrossRef]
- Gu, J.; Sun, P.; Zhao, H.; Watts, H.R.; Sanders, R.D.; Terrando, N.; Xia, P.; Maze, M.; Ma, D. Dexmedetomidine provides renoprotection against ischemia-reperfusion injury in mice. Crit. Care 2011, 15, R153. [Google Scholar] [CrossRef]
- Noh, H.; Yu, M.R.; Kim, H.J.; Lee, J.H.; Park, B.W.; Wu, I.H.; Matsumoto, M.; King, G.L. Beta 2-adrenergic receptor agonists are novel regulators of macrophage activation in diabetic renal and cardiovascular complications. Kidney Int. 2017, 92, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Imaizumi, A.; Yanagawa, Y.; Kohsaka, T.; Johns, E.J. beta(2)-Adrenoceptor activation attenuates endotoxin-induced acute renal failure. J. Am. Soc. Nephrol. 2004, 15, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yanagita, M. Immune cells and inflammation in AKI to CKD progression. Am. J. Physiol. Renal. Physiol. 2018, 315, F1501–F1512. [Google Scholar] [CrossRef] [PubMed]
- Ferenbach, D.A.; Bonventre, J.V. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat. Rev. Nephrol. 2015, 11, 264–276. [Google Scholar] [CrossRef]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. Inflammatory processes in renal fibrosis. Nat. Rev. Nephrol. 2014, 10, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Kirabo, A.; Wu, J.; Saleh, M.A.; Zhu, L.; Wang, F.; Takahashi, T.; Loperena, R.; Foss, J.D.; Mernaugh, R.L.; et al. Renal Denervation Prevents Immune Cell Activation and Renal Inflammation in Angiotensin II-Induced Hypertension. Circ. Res. 2015, 117, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Le Clef, N.; Verhulst, A.; D’Haese, P.C.; Vervaet, B.A. Unilateral Renal Ischemia-Reperfusion as a Robust Model for Acute to Chronic Kidney Injury in Mice. PLoS ONE 2016, 11, e0152153. [Google Scholar] [CrossRef]
- Padro, C.J.; Sanders, V.M. Neuroendocrine regulation of inflammation. Semin Immunol. 2014, 26, 357–368. [Google Scholar] [CrossRef]
- Severn, A.; Rapson, N.T.; Hunter, C.A.; Liew, F.Y. Regulation of tumor necrosis factor production by adrenaline and beta-adrenergic agonists. J. Immunol. 1992, 148, 3441–3445. [Google Scholar]
- Spengler, R.N.; Allen, R.M.; Remick, D.G.; Strieter, R.M.; Kunkel, S.L. Stimulation of alpha-adrenergic receptor augments the production of macrophage-derived tumor necrosis factor. J. Immunol. 1990, 145, 1430–1434. [Google Scholar]
- Spengler, R.N.; Chensue, S.W.; Giacherio, D.A.; Blenk, N.; Kunkel, S.L. Endogenous norepinephrine regulates tumor necrosis factor-alpha production from macrophages in vitro. J. Immunol. 1994, 152, 3024–3031. [Google Scholar] [PubMed]
- Shimokawa, T.; Tsutsui, H.; Miura, T.; Takama, M.; Hayashi, K.; Nishinaka, T.; Terada, T.; Yoneda, K.; Yamagata, M.; Yukimura, T. Post-treatment with JP-1302 protects against renal ischemia/reperfusion-induced acute kidney injury in rats. J. Pharmacol. Sci. 2019, 139, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Zaldivia, M.T.; Rivera, J.; Hering, D.; Marusic, P.; Sata, Y.; Lim, B.; Eikelis, N.; Lee, R.; Lambert, G.W.; Esler, M.D.; et al. Renal Denervation Reduces Monocyte Activation and Monocyte-Platelet Aggregate Formation: An Anti-Inflammatory Effect Relevant for Cardiovascular Risk. Hypertension 2017, 69, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Szelenyi, J.; Kiss, J.P.; Vizi, E.S. Differential involvement of sympathetic nervous system and immune system in the modulation of TNF-alpha production by alpha2- and beta-adrenoceptors in mice. J. Neuroimmunol. 2000, 103, 34–40. [Google Scholar] [CrossRef]
- Flierl, M.A.; Rittirsch, D.; Nadeau, B.A.; Chen, A.J.; Sarma, J.V.; Zetoune, F.S.; McGuire, S.R.; List, R.P.; Day, D.E.; Hoesel, L.M.; et al. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature 2007, 449, 721–725. [Google Scholar] [CrossRef]
- Mount, P.F.; Power, D.A. Nitric oxide in the kidney: functions and regulation of synthesis. Acta Physiol. 2006, 187, 433–446. [Google Scholar] [CrossRef]
- Eppel, G.A.; Denton, K.M.; Malpas, S.C.; Evans, R.G. Nitric oxide in responses of regional kidney perfusion to renal nerve stimulation and renal ischaemia. Pflugers Arch. 2003, 447, 205–213. [Google Scholar] [CrossRef]
- Basile, D.P.; Yoder, M.C. Renal endothelial dysfunction in acute kidney ischemia reperfusion injury. Cardiovasc. Hematol. Disord Drug Targets 2014, 14, 3–14. [Google Scholar] [CrossRef]
- Aiello, S.; Noris, M.; Todeschini, M.; Zappella, S.; Foglieni, C.; Benigni, A.; Corna, D.; Zoja, C.; Cavallotti, D.; Remuzzi, G. Renal and systemic nitric oxide synthesis in rats with renal mass reduction. Kidney Int. 1997, 52, 171–181. [Google Scholar] [CrossRef]
- Baylis, C.; Braith, R.; Santmyire, B.R.; Engels, K. Renal nerves do not mediate vasoconstrictor responses to acute nitric oxide synthesis inhibition in conscious rats. J. Am. Soc. Nephrol. 1997, 8, 887–892. [Google Scholar]
- Bruck, H.; Gossl, M.; Spitthover, R.; Schafers, R.F.; Kohnle, M.; Philipp, T.; Wenzel, R.R. The nitric oxide synthase inhibitor L-NMMA potentiates noradrenaline-induced vasoconstriction: effects of the alpha2-receptor antagonist yohimbine. J. Hypertens 2001, 19, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Tojo, A.; Kobayashi, N.; Kimura, K.; Hirata, Y.; Matsuoka, H.; Yagi, S.; Omata, M. Effects of antihypertensive drugs on nitric oxide synthase activity in rat kidney. Kidney Int. Suppl. 1996, 55, S138–S140. [Google Scholar]
- Wangensteen, R.; O’Valle, F.; Del Moral, R.; Vargas, F.; Osuna, A. Chronic alpha1-adrenergic blockade improves hypertension and renal injury in L-NAME and low-renin L-NAME-DOCA hypertensive rats. Med. Sci. Monit 2002, 8, BR378–BR384. [Google Scholar] [PubMed]
- Erley, C.M.; Rebmann, S.; Strobel, U.; Schmidt, T.; Wehrmann, M.; Osswald, H.; Risler, T. Effects of antihypertensive therapy on blood pressure and renal function in rats with hypertension due to chronic blockade of nitric oxide synthesis. Exp. Nephrol. 1995, 3, 293–299. [Google Scholar] [PubMed]
- Van den Branden, C.; Gabriels, M.; Vamecq, J.; Vanden Houte, K.; Verbeelen, D. Carvedilol protects against glomerulosclerosis in rat remnant kidney without general changes in antioxidant enzyme status. A comparative study of two beta-blocking drugs, carvedilol and propanolol. Nephron 1997, 77, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.V.; Shifow, A.A.; Naidu, M.U.; Ratnakar, K.S. Carvedilol: a beta blocker with antioxidant property protects against gentamicin-induced nephrotoxicity in rats. Life Sci. 2000, 66, 2603–2611. [Google Scholar] [CrossRef]
- Barone, F.C.; Nelson, A.H.; Ohlstein, E.H.; Willette, R.N.; Sealey, J.E.; Laragh, J.H.; Campbell, W.G., Jr.; Feuerstein, G.Z. Chronic carvedilol reduces mortality and renal damage in hypertensive stroke-prone rats. J. Pharmacol. Exp. Ther. 1996, 279, 948–955. [Google Scholar]
- Yang, L.; Besschetnova, T.Y.; Brooks, C.R.; Shah, J.V.; Bonventre, J.V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 2010, 16, 535–543. [Google Scholar] [CrossRef]
- Canaud, G.; Bonventre, J.V. Cell cycle arrest and the evolution of chronic kidney disease from acute kidney injury. Nephrol. Dial. Transplant. 2015, 30, 575–583. [Google Scholar] [CrossRef]
- Agarwal, A.; Dong, Z.; Harris, R.; Murray, P.; Parikh, S.M.; Rosner, M.H.; Kellum, J.A.; Ronco, C.; Acute Dialysis Quality Initiative, X.W.G. Cellular and Molecular Mechanisms of AKI. J. Am. Soc. Nephrol. 2016, 27, 1288–1299. [Google Scholar] [CrossRef]
- Daemen, M.A.; van’t Veer, C.; Denecker, G.; Heemskerk, V.H.; Wolfs, T.G.; Clauss, M.; Vandenabeele, P.; Buurman, W.A. Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J. Clin. Invest. 1999, 104, 541–549. [Google Scholar] [CrossRef]
- Jang, H.S.; Padanilam, B.J. Simultaneous deletion of Bax and Bak is required to prevent apoptosis and interstitial fibrosis in obstructive nephropathy. Am. J. Physiol. Renal. Physiol. 2015, 309, F540–F550. [Google Scholar] [CrossRef] [PubMed]
- Homsi, E.; Janino, P.; de Faria, J.B. Role of caspases on cell death, inflammation, and cell cycle in glycerol-induced acute renal failure. Kidney Int. 2006, 69, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Servais, H.; Ortiz, A.; Devuyst, O.; Denamur, S.; Tulkens, P.M.; Mingeot-Leclercq, M.P. Renal cell apoptosis induced by nephrotoxic drugs: cellular and molecular mechanisms and potential approaches to modulation. Apoptosis 2008, 13, 11–32. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.C.; Yin, S.C.; Chi, C.S.; Hwang, B.; Hsu, S.L. Norepinephrine induces apoptosis in neonatal rat endothelial cells via a ROS-dependent JNK activation pathway. Apoptosis 2006, 11, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Wang, N.; Qian, J.; Bai, L.; Zheng, X.; Hou, G.; Qiu, X.; Yang, B. Renal sympathetic denervation improves myocardial apoptosis in rats with isoproterenol-induced heart failure by downregulation of tumor necrosis factor-alpha and nuclear factor-kappaB. Exp. Ther Med. 2017, 14, 4104–4110. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chi, X.; Wu, S.; Jin, Y.; Yao, H.; Wang, Y.; Xia, Z.; Cai, J. Dexmedetomidine Pretreatment Attenuates Kidney Injury and Oxidative Stress during Orthotopic Autologous Liver Transplantation in Rats. Oxid Med. Cell Longev 2016, 2016, 4675817. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, X.; Hu, X.; Sha, J.; Li, B.; Zhang, H.; Fan, H. Dexmedetomidine Ameliorates Acute Stress-Induced Kidney Injury by Attenuating Oxidative Stress and Apoptosis through Inhibition of the ROS/JNK Signaling Pathway. Oxid Med. Cell Longev 2018, 2018, 4035310. [Google Scholar] [CrossRef]
- Koca, U.; Olguner, C.G.; Ergur, B.U.; Altekin, E.; Tasdogen, A.; Duru, S.; Girgin, P.; Gunduz, K.; Cilaker Micili, S.; Guzeldag, S.; et al. The effects of dexmedetomidine on secondary acute lung and kidney injuries in the rat model of intra-abdominal sepsis. Sci. World J. 2013, 2013, 292687. [Google Scholar] [CrossRef]
- Schiller, A.M.; Pellegrino, P.R.; Zucker, I.H. The renal nerves in chronic heart failure: efferent and afferent mechanisms. Front. Physiol. 2015, 6, 224. [Google Scholar] [CrossRef]
- Park, J. Cardiovascular risk in chronic kidney disease: role of the sympathetic nervous system. Cardiol. Res. Pract 2012, 2012, 319432. [Google Scholar] [CrossRef] [PubMed]
- Kon, V.; Yared, A.; Ichikawa, I. Role of renal sympathetic nerves in mediating hypoperfusion of renal cortical microcirculation in experimental congestive heart failure and acute extracellular fluid volume depletion. J. Clin. Invest. 1985, 76, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Swedberg, K.; Viquerat, C.; Rouleau, J.L.; Roizen, M.; Atherton, B.; Parmley, W.W.; Chatterjee, K. Comparison of myocardial catecholamine balance in chronic congestive heart failure and in angina pectoris without failure. Am. J. Cardiol. 1984, 54, 783–786. [Google Scholar] [CrossRef]
- Bohm, M.; La Rosee, K.; Schwinger, R.H.; Erdmann, E. Evidence for reduction of norepinephrine uptake sites in the failing human heart. J. Am. Coll Cardiol. 1995, 25, 146–153. [Google Scholar] [CrossRef]
- Bohm, M.; Gierschik, P.; Jakobs, K.H.; Pieske, B.; Schnabel, P.; Ungerer, M.; Erdmann, E. Increase of Gi alpha in human hearts with dilated but not ischemic cardiomyopathy. Circulation 1990, 82, 1249–1265. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, D.; Freeman, R.H.; Johnson, R.A.; Simmons, J.C. Effects of renal denervation on postprandial sodium excretion in experimental heart failure. Am. J. Physiol. 1994, 266, R1599–R1604. [Google Scholar] [CrossRef]
- Masaki, H.; Imaizumi, T.; Harasawa, Y.; Takeshita, A. Dynamic arterial baroreflex in rabbits with heart failure induced by rapid pacing. Am. J. Physiol. 1994, 267, H92–H99. [Google Scholar] [CrossRef]
- Clayton, S.C.; Haack, K.K.; Zucker, I.H. Renal denervation modulates angiotensin receptor expression in the renal cortex of rabbits with chronic heart failure. Am. J. Physiol. Renal Physiol. 2011, 300, F31–F39. [Google Scholar] [CrossRef]
- Linz, D.; Wirth, K.; Ukena, C.; Mahfoud, F.; Poss, J.; Linz, B.; Bohm, M.; Neuberger, H.R. Renal denervation suppresses ventricular arrhythmias during acute ventricular ischemia in pigs. Heart Rhythm 2013, 10, 1525–1530. [Google Scholar] [CrossRef]
- Brandt, M.C.; Mahfoud, F.; Reda, S.; Schirmer, S.H.; Erdmann, E.; Bohm, M.; Hoppe, U.C. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J. Am. Coll. Cardiol. 2012, 59, 901–909. [Google Scholar] [CrossRef]
- Brede, M.; Wiesmann, F.; Jahns, R.; Hadamek, K.; Arnolt, C.; Neubauer, S.; Lohse, M.J.; Hein, L. Feedback inhibition of catecholamine release by two different alpha2-adrenoceptor subtypes prevents progression of heart failure. Circulation 2002, 106, 2491–2496. [Google Scholar] [CrossRef] [PubMed]
- Brede, M.; Philipp, M.; Knaus, A.; Muthig, V.; Hein, L. alpha2-adrenergic receptor subtypes - novel functions uncovered in gene-targeted mouse models. Biol. Cell 2004, 96, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Sugiura, T.; Hayashi, K.; Ohkita, M.; Takaoka, M.; Yukimura, T.; Matsumura, Y. Moxonidine prevents ischemia/reperfusion-induced renal injury in rats. Eur. J. Pharmacol. 2009, 603, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Gilsbach, R.; Schneider, J.; Lother, A.; Schickinger, S.; Leemhuis, J.; Hein, L. Sympathetic alpha(2)-adrenoceptors prevent cardiac hypertrophy and fibrosis in mice at baseline but not after chronic pressure overload. Cardiovasc. Res. 2010, 86, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.K.; Chan, M.H.; Tai, M.H.; Lam, C.W. Hepatorenal syndrome. Clin. Biochem. Rev. 2007, 28, 11–17. [Google Scholar]
- Moreau, R.; Lebrec, D. Acute renal failure in patients with cirrhosis: perspectives in the age of MELD. Hepatology 2003, 37, 233–243. [Google Scholar] [CrossRef]
- Stadlbauer, V.; Wright, G.A.; Banaji, M.; Mukhopadhya, A.; Mookerjee, R.P.; Moore, K.; Jalan, R. Relationship between activation of the sympathetic nervous system and renal blood flow autoregulation in cirrhosis. Gastroenterology 2008, 134, 111–119. [Google Scholar] [CrossRef]
- Bendtsen, F.; Christensen, N.J.; Sorensen, T.I.; Henriksen, J.H. Effect of oral propranolol administration on azygos, renal and hepatic uptake and output of catecholamines in cirrhosis. Hepatology 1991, 14, 237–243. [Google Scholar] [CrossRef]
- Henriksen, J.H.; Ring-Larsen, H. Hepatorenal disorders: role of the sympathetic nervous system. Semin Liver Dis. 1994, 14, 35–43. [Google Scholar] [CrossRef]
- Arroyo, V.; Guevara, M.; Gines, P. Hepatorenal syndrome in cirrhosis: pathogenesis and treatment. Gastroenterology 2002, 122, 1658–1676. [Google Scholar] [CrossRef]
- Angeli, P.; Volpin, R.; Gerunda, G.; Craighero, R.; Roner, P.; Merenda, R.; Amodio, P.; Sticca, A.; Caregaro, L.; Maffei-Faccioli, A.; et al. Reversal of type 1 hepatorenal syndrome with the administration of midodrine and octreotide. Hepatology 1999, 29, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Roulot, D.; Braillon, A.; Gaudin, C.; Ozier, Y.; Girod, C.; Lebrec, D. Mechanisms of a clonidine-induced decrease in portal pressure in normal and cirrhotic conscious rats. Hepatology 1989, 10, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Serste, T.; Melot, C.; Francoz, C.; Durand, F.; Rautou, P.E.; Valla, D.; Moreau, R.; Lebrec, D. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology 2010, 52, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Krag, A.; Wiest, R.; Albillos, A.; Gluud, L.L. The window hypothesis: haemodynamic and non-haemodynamic effects of beta-blockers improve survival of patients with cirrhosis during a window in the disease. Gut 2012, 61, 967–969. [Google Scholar] [CrossRef] [PubMed]
- Simoes, E.S.A.C.; Miranda, A.S.; Rocha, N.P.; Teixeira, A.L. Renin angiotensin system in liver diseases: Friend or foe? World J. Gastroenterol. 2017, 23, 3396–3406. [Google Scholar] [CrossRef] [PubMed]
- Grace, J.A.; Herath, C.B.; Mak, K.Y.; Burrell, L.M.; Angus, P.W. Update on new aspects of the renin-angiotensin system in liver disease: clinical implications and new therapeutic options. Clin. Sci. 2012, 123, 225–239. [Google Scholar] [CrossRef]
- Ahmadian, E.; Pennefather, P.S.; Eftekhari, A.; Heidari, R.; Eghbal, M.A. Role of renin-angiotensin system in liver diseases: an outline on the potential therapeutic points of intervention. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 1279–1288. [Google Scholar] [CrossRef]
- Ott, C.; Mahfoud, F.; Schmid, A.; Toennes, S.W.; Ewen, S.; Ditting, T.; Veelken, R.; Ukena, C.; Uder, M.; Bohm, M.; et al. Renal denervation preserves renal function in patients with chronic kidney disease and resistant hypertension. J. Hypertens 2015, 33, 1261–1266. [Google Scholar] [CrossRef]
- Hering, D.; Marusic, P.; Duval, J.; Sata, Y.; Head, G.A.; Denton, K.M.; Burrows, S.; Walton, A.S.; Esler, M.D.; Schlaich, M.P. Effect of renal denervation on kidney function in patients with chronic kidney disease. Int. J. Cardiol. 2017, 232, 93–97. [Google Scholar] [CrossRef]
| Subtype | Localization in Kidney | Action |
|---|---|---|
| α1-AR | Arterioles |
|
| α2-AR | Proximal tubules |
|
| β-AR | All nephron segments |
|
| Organ | Consequences |
|---|---|
| Heart |
|
| Liver |
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, M.R.; Jang, H.-S.; Kim, J.; Padanilam, B.J. Renal Sympathetic Nerve-Derived Signaling in Acute and Chronic Kidney Diseases. Int. J. Mol. Sci. 2020, 21, 1647. https://doi.org/10.3390/ijms21051647
Noh MR, Jang H-S, Kim J, Padanilam BJ. Renal Sympathetic Nerve-Derived Signaling in Acute and Chronic Kidney Diseases. International Journal of Molecular Sciences. 2020; 21(5):1647. https://doi.org/10.3390/ijms21051647
Chicago/Turabian StyleNoh, Mi Ra, Hee-Seong Jang, Jinu Kim, and Babu J. Padanilam. 2020. "Renal Sympathetic Nerve-Derived Signaling in Acute and Chronic Kidney Diseases" International Journal of Molecular Sciences 21, no. 5: 1647. https://doi.org/10.3390/ijms21051647
APA StyleNoh, M. R., Jang, H.-S., Kim, J., & Padanilam, B. J. (2020). Renal Sympathetic Nerve-Derived Signaling in Acute and Chronic Kidney Diseases. International Journal of Molecular Sciences, 21(5), 1647. https://doi.org/10.3390/ijms21051647







