The Role of Cell Adhesion Molecule 1 (CADM1) in Cutaneous Malignancies
Abstract
1. Introduction
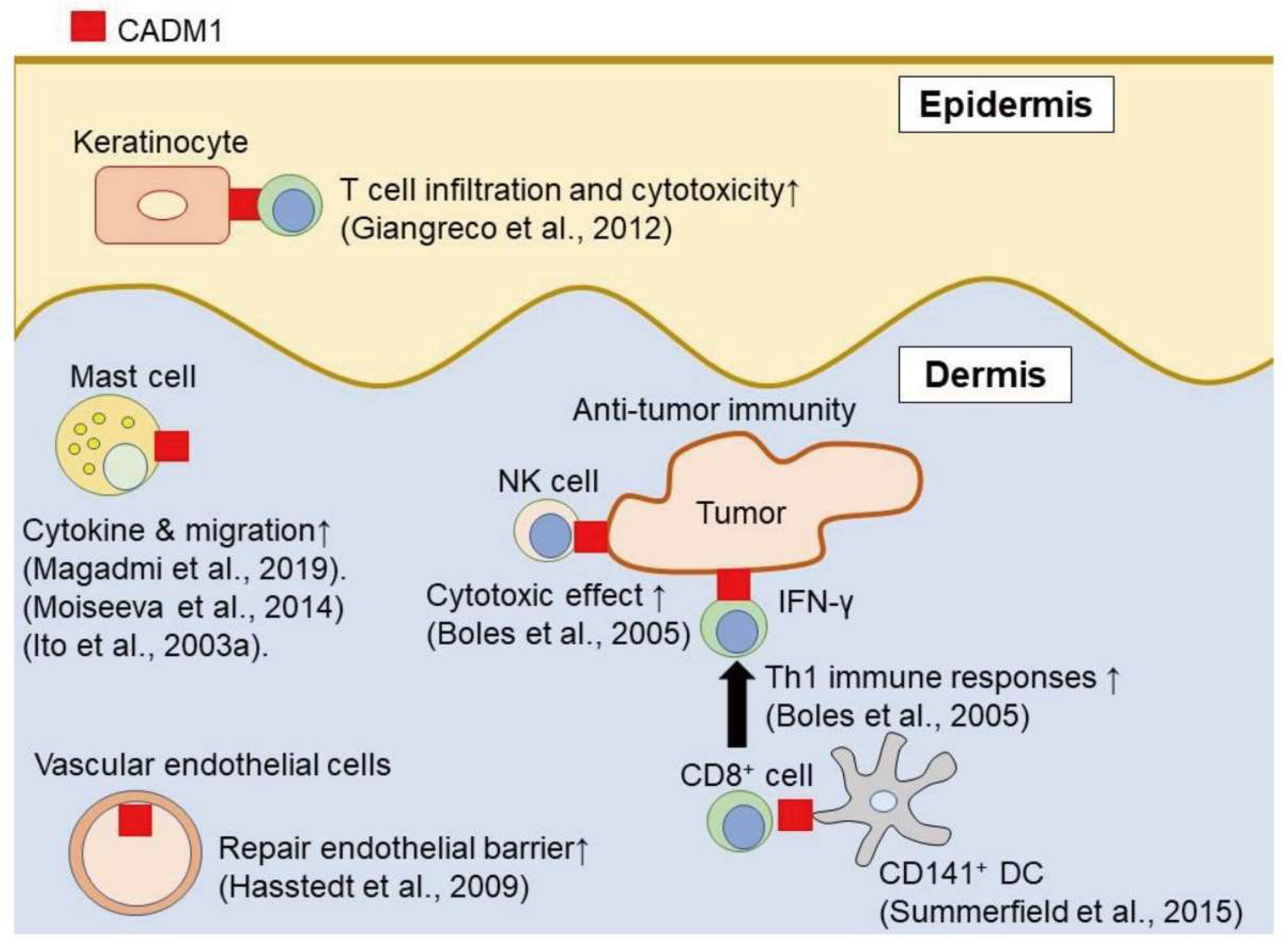
2. The Role of CADM1 in Cell Types
2.1. Keratinocytes
2.2. Mast Cells
2.3. Dendritic Cells
2.4. NK Cells and CD8+ Cells
2.5. Neuron Cells
3. CADM1 and Human Cutaneous Malignancies
3.1. Cutaneous Squamous Cell Carcinoma
3.2. Malignant Melanoma
3.3. Cutaneous Lymphoma
3.3.1. ATLL
3.3.2. Mycosis Fungoides
3.3.3. Sézary Syndrome
4. Merkel Cell Carcinoma
4.1. Advances of CADM1 Research in Non-Dermatological Fields
4.2. CADM1 as a Biomarker
4.3. Epigenetic Modification of CADM1
4.4. The Future Direction of Clinical Application of CADM1-Targeted Therapy
| Cutaneous Malignancies | Possible Therapeutic Options |
|---|---|
| Solid tumor | |
| Melanoma | MicroRNA-214 inhibitor [102] |
| Squamous cell carcinoma | miR-424-5p inhibitor [103] |
| Lymphoma | |
| Adult T cell leukemia/lymphoma | OD-2100 [93], anti-CADM1 antibody [101] |
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Steinberg, M.S. Adhesion in development: An historical overview. Dev. Biol. 1996, 180, 377–388. [Google Scholar] [CrossRef]
- Kuramochi, M.; Fukuhara, H.; Nobukuni, T.; Kanbe, T.; Maruyama, T.; Ghosh, H.P.; Pletcher, M.; Isomura, M.; Onizuka, M.; Kitamura, T.; et al. TSLC1 is a tumor-suppressor gene in human non-small-cell lung cancer. Nat. Genet. 2001, 27, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Okada, M.; Uchino, K.; Wakayama, T.; Koma, Y.; Iseki, S.; Tsubota, N.; Okita, Y.; Kitamura, Y. Expression of the TSLC1 adhesion molecule in pulmonary epithelium and its down-regulation in pulmonary adenocarcinoma other than bronchioloalveolar carcinoma. Lab. Investig. 2003, 83, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Uchino, K.; Ito, A.; Wakayama, T.; Koma, Y.; Okada, T.; Ohbayashi, C.; Iseki, S.; Kitamura, Y.; Tsubota, N.; Okita, Y.; et al. Clinical implication and prognostic significance of the tumor suppressor TSLC1 gene detected in adenocarcinoma of the lung. Cancer 2003, 98, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Nishikata, I.; Shiraga, T.; Akamatsu, E.; Fukami, T.; Hidaka, T.; Kubuki, Y.; Okayama, A.; Hamada, K.; Okabe, H.; et al. Overexpression of a cell adhesion molecule, TSLC1, as a possible molecular marker for acute-type adult T-cell leukemia. Blood 2005, 105, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Moiseeva, E.P.; Straatman, K.R.; Leyland, M.L.; Bradding, P. CADM1 controls actin cytoskeleton assembly and regulates extracellular matrix adhesion in human mast cells. PLoS ONE 2014, 9, e85980. [Google Scholar] [CrossRef]
- Biederer, T.; Sara, Y.; Mozhayeva, M.; Atasoy, D.; Liu, X.; Kavalali, E.T.; Südhof, T.C. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science 2002, 297, 1525–1531. [Google Scholar] [CrossRef]
- Hasstedt, S.J.; Bezemer, I.D.; Callas, P.W.; Vossen, C.Y.; Trotman, W.; Hebbel, R.P.; Demers, C.; Rosendaal, F.R.; Bovill, E.G. Cell adhesion molecule 1: A novel risk factor for venous thrombosis. Blood 2009, 114, 3084–3091. [Google Scholar] [CrossRef]
- Schwartz, R.A.; McDonough, P.H.; Lee, B.W. Toxic epidermal necrolysis: Part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J. Am. Acad. Dermatol. 2013, 69, 203–204. [Google Scholar] [CrossRef]
- Masuda, M.; Maruyama, T.; Ohta, T.; Ito, A.; Hayashi, T.; Tsukasaki, K.; Kamihira, S.; Yamaoka, S.; Hoshino, H.; Yoshida, T.; et al. CADM1 interacts with Tiam1 and promotes invasive phenotype of human T-cell leukemia virus type I-transformed cells and adult T-cell leukemia cells. J. Biol. Chem. 2010, 285, 15511–15522. [Google Scholar] [CrossRef]
- Yageta, M.; Kuramochi, M.; Masuda, M.; Fukami, T.; Fukuhara, H.; Maruyama, T.; Shibuya, M.; Murakami, Y. Direct association of TSLC1 and DAL-1, two distinct tumor suppressor proteins in lung cancer. Cancer Res. 2002, 62, 5129–5133. [Google Scholar] [PubMed]
- Shingai, T.; Ikeda, W.; Kakunaga, S.; Morimoto, K.; Takekuni, K.; Itoh, S.; Satoh, K.; Takeuchi, M.; Imai, T.; Monden, M.; et al. Implications of nectin-like molecule-2/IGSF4/RA175/SgIGSF/TSLC1/SynCAM1 in cell-cell adhesion and transmembrane protein localization in epithelial cells. J. Biol. Chem. 2003, 278, 35421–35427. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Honda, T.; Hanakawa, S.; Nakamizo, S.; Murata, T.; Ueharaguchi-Tanada, Y.; Ono, S.; Amano, W.; Nakajima, S.; Egawa, G.; et al. Resolvin E1 inhibits dendritic cell migration in the skin and attenuates contact hypersensitivity responses. J. Exp. Med. 2015, 212, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Giangreco, A.; Hoste, E.; Takai, Y.; Rosewell, I.; Watt, F.M. Epidermal Cadm1 expression promotes autoimmune alopecia via enhanced T cell adhesion and cytotoxicity. J. Immunol. 2012, 188, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Crivellato, E.; Ribatti, D. The mast cell: An evolutionary perspective. Biol. Rev. Camb. Philos. Soc. 2010, 85, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, A.; Kubo, M.; Honda, T.; Egawa, G.; Nakajima, S.; Tanizaki, H.; Kim, B.; Matsuoka, S.; Watanabe, T.; Nakae, S.; et al. Requirement of interaction between mast cells and skin dendritic cells to establish contact hypersensitivity. PLoS ONE 2011, 6, e25538. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, C.; Ishida, Y.; Kitoh, A.; Otsuka, A.; Kabashima, K. Interaction of peripheral nerves and mast cells, eosinophils, and basophils in the development of pruritus. Exp. Dermatol. 2019, 28, 1405–1411. [Google Scholar] [CrossRef]
- Moiseeva, E.P.; Leyland, M.L.; Bradding, P. CADM1 is expressed as multiple alternatively spliced functional and dysfunctional isoforms in human mast cells. Mol. Immunol. 2013, 53, 345–354. [Google Scholar] [CrossRef]
- Magadmi, R.; Meszaros, J.; Damanhouri, Z.A.; Seward, E.P. Secretion of Mast Cell Inflammatory Mediators Is Enhanced by CADM1-Dependent Adhesion to Sensory Neurons. Front. Cell. Neurosci. 2019, 13, 262. [Google Scholar] [CrossRef]
- Ito, A.; Jippo, T.; Wakayama, T.; Morii, E.; Koma, Y.; Onda, H.; Nojima, H.; Iseki, S.; Kitamura, Y. SgIGSF: A new mast-cell adhesion molecule used for attachment to fibroblasts and transcriptionally regulated by MITF. Blood 2003, 101, 2601–2608. [Google Scholar] [CrossRef]
- Sawada, Y.; Honda, T.; Nakamizo, S.; Otsuka, A.; Ogawa, N.; Kobayashi, Y.; Nakamura, M.; Kabashima, K. Resolvin E1 attenuates murine psoriatic dermatitis. Sci. Rep. 2018, 8, 11873. [Google Scholar] [CrossRef] [PubMed]
- Saito-Sasaki, N.; Sawada, Y.; Mashima, E.; Yamaguchi, T.; Ohmori, S.; Yoshioka, H.; Haruyama, S.; Okada, E.; Nakamura, M. Maresin-1 suppresses imiquimod-induced skin inflammation by regulating IL-23 receptor expression. Sci. Rep. 2018, 8, 5522. [Google Scholar] [CrossRef] [PubMed]
- Summerfield, A.; Meurens, F.; Ricklin, M.E. The immunology of the porcine skin and its value as a model for human skin. Mol. Immunol. 2015, 66, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Jongbloed, S.L.; Kassianos, A.J.; McDonald, K.J.; Clark, G.J.; Ju, X.; Angel, C.E.; Chen, C.J.; Dunbar, P.R.; Wadley, R.B.; Jeet, V.; et al. Human CD141+ (BDCA-3) + dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J. Exp. Med. 2010, 207, 1247–1260. [Google Scholar] [CrossRef]
- Kaur, G.; Trowsdale, J.; Fugger, L. Natural killer cells and their receptors in multiple sclerosis. Brain 2013, 136, 2657–2676. [Google Scholar] [CrossRef]
- Long, E.O.; Kim, H.S.; Liu, D.; Peterson, M.E.; Rajagopalan, S. Controlling natural killer cell responses: Integration of signals for activation and inhibition. Annu. Rev. Immunol. 2013, 31, 227–258. [Google Scholar] [CrossRef]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef]
- Boles, K.S.; Barchet, W.; Diacovo, T.; Cella, M.; Colonna, M. The tumor suppressor TSLC1/NECL-2 triggers NK-cell and CD8+ T-cell responses through the cell-surface receptor CRTAM. Blood 2005, 106, 779–786. [Google Scholar] [CrossRef]
- Takahashi, S.; Ishida, A.; Kubo, A.; Kawasaki, H.; Ochiai, S.; Nakayama, M.; Koseki, H.; Amagai, M.; Okada, T. Homeostatic pruning and activity of epidermal nerves are dysregulated in barrier-impaired skin during chronic itch development. Sci. Rep. 2019, 9, 8625. [Google Scholar] [CrossRef]
- Perner, C.; Flayer, C.H.; Zhu, X.; Aderhold, P.A.; Dewan, Z.N.A.; Voisin, T.; Camire, R.B.; Chow, O.A.; Chiu, I.M.; Sokol, C.L. Substance P Release by Sensory Neurons Triggers Dendritic Cell Migration and Initiates the Type-2 Immune Response to Allergens. Immunity 2020, 53, 1063–1077. [Google Scholar] [CrossRef]
- Mou, K.; Zhang, X.; Mu, X.; Ge, R.; Han, D.; Zhou, Y.; Wang, L. LNMAT1 Promotes Invasion-Metastasis Cascade in Malignant Melanoma by Epigenetically Suppressing CADM1 Expression. Front. Oncol. 2019, 9, 569. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Zhang, J.; Li, Y.; Li, Y.; Shi, G.; Ma, L.; Wei, H. CADM1/TSLC1 inhibits melanoma cell line A375 invasion through the suppression of matrix metalloproteinases. Mol. Med. Rep. 2014, 10, 2621–2626. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hartsough, E.J.; Weiss, M.B.; Heilman, S.A.; Purwin, T.J.; Kugel, C.H., III; Rosenbaum, S.R.; Erkes, D.A.; Tiago, M.; HooKim, K.; Chervoneva, I.; et al. CADM1 is a TWIST1-regulated suppressor of invasion and survival. Cell. Death Dis. 2019, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Ma, L.; You, M.; Li, X.; Wang, S.; Li, H.; Wu, D.; Yang, H.; Li, Z.Y. TSLC1 gene silencing in cutaneous melanoma. Melanoma Res. 2010, 20, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Feng, X.; Wu, X.; Li, Z.; Wang, W.; Tao, Y.; Xia, Y. Tumor suppressor in lung cancer 1 (TSLC1), a novel tumor suppressor gene, is implicated in the regulation of proliferation, invasion, cell cycle, apoptosis, and tumorigenicity in cutaneous squamous cell carcinoma. Tumour Biol. 2013, 34, 3773–3783. [Google Scholar] [CrossRef] [PubMed]
- Chahal, H.S.; Lin, Y.; Ransohoff, K.J.; Hinds, D.A.; Wu, W.; Dai, H.J.; Qureshi, A.A.; Li, W.Q.; Kraft, P.; Tang, J.Y.; et al. Genome-wide association study identifies novel susceptibility loci for cutaneous squamous cell carcinoma. Nat. Commun. 2016, 7, 12048. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Matsushita, M.; Nonaka, D.; Nagata, K.; Kato, M.; Kuwamoto, S.; Murakami, I.; Hayashi, K. Lower expression of CADM1 and higher expression of MAL in Merkel cell carcinomas are associated with Merkel cell polyomavirus infection and better prognosis. Hum. Pathol. 2016, 48, 1–8. [Google Scholar] [CrossRef]
- Dewan, M.Z.; Takamatsu, N.; Hidaka, T.; Hatakeyama, K.; Nakahata, S.; Fujisawa, J.; Katano, H.; Yamamoto, N.; Morishita, K. Critical role for TSLC1 expression in the growth and organ infiltration of adult T-cell leukemia cells in vivo. J. Virol. 2008, 82, 11958–11963. [Google Scholar] [CrossRef]
- Mashima, E.; Sawada, Y.; Yamaguchi, T.; Yoshioka, H.; Ohmori, S.; Haruyama, S.; Yoshioka, M.; Okada, E.; Nakamura, M. A high expression of cell adhesion molecule 1 (CADM1) is an unfavorable prognostic factor in mycosis fungoides. Clin. Immunol. 2018, 193, 121–122. [Google Scholar] [CrossRef]
- Yuki, A.; Ansai, O.; Abe, R. CADM1 expression of mast cells in mycosis fungoides. J. Am. Acad. Dermatol. 2020, 82, e143–e144. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Morizane, S.; Hamada, T.; Miyake, T.; Sugaya, M.; Iwata, H.; Fujii, K.; Haramoto-Shiratsuki, R.; Nakagawa, Y.; Miura, M.; et al. The expression of cell adhesion molecule 1 and its splicing variants in Sézary cells and cell lines from cutaneous T-cell lymphoma. J. Dermatol. 2019, 46, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Corchado-Cobos, R.; García-Sancha, N.; González-Sarmiento, R.; Pérez-Losada, J.; Cañueto, J. Cutaneous Squamous Cell Carcinoma: From Biology to Therapy. Int. J. Mol. Sci. 2020, 21, 2956. [Google Scholar] [CrossRef] [PubMed]
- Burton, K.A.; Ashack, K.A.; Khachemoune, A. Cutaneous Squamous Cell Carcinoma: A Review of High-Risk and Metastatic Disease. Am. J. Clin. Dermatol. 2016, 17, 491–508. [Google Scholar] [CrossRef]
- Claveau, J.; Archambault, J.; Ernst, D.S.; Giacomantonio, C.; Limacher, J.J.; Murray, C.; Parent, F.; Zloty, D. Multidisciplinary management of locally advanced and metastatic cutaneous squamous cell carcinoma. Curr. Oncol. 2020, 27, e399–e407. [Google Scholar] [CrossRef]
- Wu, H.; Lotan, R.; Menter, D.; Lippman, S.M.; Xu, X.C. Expression of E-cadherin is associated with squamous differentiation in squamous cell carcinomas. Anticancer. Res. 2000, 20, 1385–1390. [Google Scholar]
- Jenkins, R.W.; Fisher, D.E. Treatment of Advanced Melanoma in 2020 and Beyond. J. Investig. Dermatol. 2020. [Google Scholar] [CrossRef]
- Simiczyjew, A.; Dratkiewicz, E.; Mazurkiewicz, J.; Ziętek, M.; Matkowski, R.; Nowak, D. The Influence of Tumor Microenvironment on Immune Escape of Melanoma. Int. J. Mol. Sci. 2020, 21, 8359. [Google Scholar] [CrossRef]
- You, Y.; Wang, S.H.; Zhang, J.F.; Zheng, S.Y. TSLC1 expression discriminates cutaneous melanomas from dysplastic nevi. Melanoma Res. 2012, 22, 430–435. [Google Scholar] [CrossRef]
- Munhoz de Paula Alves Coelho, K.; Stall, J.; Fronza Júnior, H.; Blasius, R.; de França, P.H.C. Evaluation of expression of genes CADM1, TWIST1 and CDH1 by immunohistochemestry in melanocytic lesions. Pathol. Res. Pract. 2017, 213, 1067–1071. [Google Scholar] [CrossRef]
- Willemze, R.; Jaffe, E.S.; Burg, G.; Cerroni, L.; Berti, E.; Swerdlow, S.H.; Ralfkiaer, E.; Chimenti, S.; Diaz-Perez, J.L.; Duncan, L.M.; et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005, 105, 3768–3785. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.B.; Fuji, S.; Hermine, O.; Bazarbachi, A.; Ramos, J.C.; Ratner, L.; Horwitz, S.; Fields, P.; Tanase, A.; Bumbea, H.; et al. Revised Adult T-Cell Leukemia-Lymphoma International Consensus Meeting Report. J. Clin. Oncol. 2019, 37, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Hino, R.; Hama, K.; Ohmori, S.; Fueki, H.; Yamada, S.; Fukamachi, S.; Tajiri, M.; Kubo, R.; Yoshioka, M.; et al. Type of skin eruption is an independent prognostic indicator for adult T-cell leukemia/lymphoma. Blood 2011, 117, 3961–3967. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Shimauchi, T.; Yamaguchi, T.; Okura, R.; Hama-Yamamoto, K.; Fueki-Yoshioka, H.; Ohmori, S.; Yamada, S.; Yoshizawa, M.; Hiromasa, K.; et al. Combination of skin-directed therapy and oral etoposide for smoldering adult T-cell leukemia/lymphoma with skin involvement. Leuk. Lymphoma 2013, 54, 520–527. [Google Scholar] [CrossRef]
- Tokura, Y.; Sawada, Y.; Shimauchi, T. Skin manifestations of adult T-cell leukemia/lymphoma: Clinical, cytological and immunological features. J. Dermatol. 2014, 41, 19–25. [Google Scholar] [CrossRef]
- Yamada, Y.; Tomonaga, M.; Fukuda, H.; Hanada, S.; Utsunomiya, A.; Tara, M.; Sano, M.; Ikeda, S.; Takatsuki, K.; Kozuru, M.; et al. A new G-CSF-supported combination chemotherapy, LSG15, for adult T-cell leukaemia-lymphoma: Japan Clinical Oncology Group Study 9303. Br. J. Haematol. 2001, 113, 375–382. [Google Scholar] [CrossRef]
- Tsukasaki, K.; Utsunomiya, A.; Fukuda, H.; Shibata, T.; Fukushima, T.; Takatsuka, Y.; Ikeda, S.; Masuda, M.; Nagoshi, H.; Ueda, R.; et al. VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study JCOG9801. J. Clin. Oncol. 2007, 25, 5458–5464. [Google Scholar] [CrossRef]
- Stanchina, M.; Soong, D.; Zheng-Lin, B.; Watts, J.M.; Taylor, J. Advances in Acute Myeloid Leukemia: Recently Approved Therapies and Drugs in Development. Cancers 2020, 12, 3225. [Google Scholar] [CrossRef]
- Bladé, J.; Dimopoulos, M.; Rosiñol, L.; Rajkumar, S.V.; Kyle, R.A. Smoldering (asymptomatic) multiple myeloma: Current diagnostic criteria, new predictors of outcome, and follow-up recommendations. J. Clin. Oncol. 2010, 28, 690–697. [Google Scholar] [CrossRef]
- Nakahata, S.; Saito, Y.; Marutsuka, K.; Hidaka, T.; Maeda, K.; Hatakeyama, K.; Shiraga, T.; Goto, A.; Takamatsu, N.; Asada, Y.; et al. Clinical significance of CADM1/TSLC1/IgSF4 expression in adult T-cell leukemia/lymphoma. Leukemia 2012, 26, 1238–1246. [Google Scholar] [CrossRef]
- Vermeer, M.H.; Nicolay, J.P.; Scarisbrick, J.J.; Zinzani, P.L. The importance of assessing blood tumour burden in cutaneous T-cell lymphoma. Br. J. Dermatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, P.; Fava, P.; Pileri, A.; Grandi, V.; Sanlorenzo, M.; Panasiti, V.; Guglielmo, A.; Alberti-Violetti, S.; Novelli, M.; Astrua, C.; et al. Phenotypical Markers, Molecular Mutations, and Immune Microenvironment as Targets for New Treatments in Patients with Mycosis Fungoides and/or Sézary Syndrome. J. Investig. Dermatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wieselthier, J.S.; Koh, H.K. Sézary syndrome: Diagnosis, prognosis, and critical review of treatment options. J. Am. Acad. Dermatol. 1990, 22, 381–401. [Google Scholar] [CrossRef]
- Goessling, W.; McKee, P.H.; Mayer, R.J. Merkel cell carcinoma. J. Clin. Oncol. 2002, 20, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Heath, M.; Jaimes, N.; Lemos, B.; Mostaghimi, A.; Wang, L.C.; Peñas, P.F.; Nghiem, P. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: The AEIOU features. J. Am. Acad. Dermatol. 2008, 58, 375–381. [Google Scholar] [CrossRef]
- Babadzhanov, M.; Doudican, N.; Wilken, R.; Stevenson, M.; Pavlick, A.; Carucci, J. Current concepts and approaches to merkel cell carcinoma. Arch. Dermatol. Res. 2020. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Russell, J.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbé, C.; Linette, G.P.; Milella, M.; et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 1374–1385. [Google Scholar] [CrossRef]
- Qian, J.B.; Liu, H.B.; Zhu, Y.; Lu, F.; Yang, Q.C.; Shen, Y. CADM1 mRNA expression and clinicopathological significance in esophageal squamous cell carcinoma tissue. Genet. Mol. Res. 2017, 16. [Google Scholar] [CrossRef]
- Tsuboi, Y.; Oyama, M.; Kozuka-Hata, H.; Ito, A.; Matsubara, D.; Murakami, Y. CADM1 suppresses c-Src activation by binding with Cbp on membrane lipid rafts and intervenes colon carcinogenesis. Biochem. Biophys. Res. Commun. 2020, 529, 854–860. [Google Scholar] [CrossRef]
- Saito, M.; Goto, A.; Abe, N.; Saito, K.; Maeda, D.; Ohtake, T.; Murakami, Y.; Takenoshita, S. Decreased expression of CADM1 and CADM4 are associated with advanced stage breast cancer. Oncol. Lett. 2018, 15, 2401–2406. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Guo, Z.; Wang, Y.; Yang, Y.; Liu, X. Lost expression of cell adhesion molecule 1 is associated with bladder cancer progression and recurrence and its overexpression inhibited tumor cell malignant behaviors. Oncol. Lett. 2019, 17, 2047–2056. [Google Scholar] [PubMed]
- Si, X.; Xu, F.; Xu, F.; Wei, M.; Ge, Y.; Chenge, S. CADM1 inhibits ovarian cancer cell proliferation and migration by potentially regulating the PI3K/Akt/mTOR pathway. Biomed. Pharmacother. 2020, 123, 109717. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Hagiyama, M.; Takashima, Y.; Yoneshige, A.; Ito, A. Cell adhesion molecule-1 shedding induces apoptosis of renal epithelial cells and exacerbates human nephropathies. Am. J. Physiol. Ren. Physiol. 2018, 314, F388–F398. [Google Scholar] [CrossRef] [PubMed]
- Hagiyama, M.; Kimura, R.; Yoneshige, A.; Inoue, T.; Otani, T.; Ito, A. Cell Adhesion Molecule 1 Contributes to Cell Survival in Crowded Epithelial Monolayers. Int. J. Mol. Sci. 2020, 21, 4123. [Google Scholar] [CrossRef]
- Sun, S.; Liu, W.; Li, Y. CADM1 enhances intestinal barrier function in a rat model of mild inflammatory bowel disease by inhibiting the STAT3 signaling pathway. J. Bioenerg. Biomembr. 2020, 52, 343–354. [Google Scholar] [CrossRef]
- Kobayashi, M.; Shu, S.; Marunaka, K.; Matsunaga, T.; Ikari, A. Weak Ultraviolet B Enhances the Mislocalization of Claudin-1 Mediated by Nitric Oxide and Peroxynitrite Production in Human Keratinocyte-Derived HaCaT Cells. Int. J. Mol. Sci. 2020, 21, 7138. [Google Scholar] [CrossRef]
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J. Cell. Biol. 2002, 156, 1099–1111. [Google Scholar] [CrossRef]
- Bergmann, S.; von Buenau, B.; Vidal, Y.S.S.; Haftek, M.; Wladykowski, E.; Houdek, P.; Lezius, S.; Duplan, H.; Bäsler, K.; Dähnhardt-Pfeiffer, S.; et al. Claudin-1 decrease impacts epidermal barrier function in atopic dermatitis lesions dose-dependently. Sci. Rep. 2020, 10, 2024. [Google Scholar] [CrossRef]
- Rathjen, T.; Yan, X.; Kononenko, N.L.; Ku, M.C.; Song, K.; Ferrarese, L.; Tarallo, V.; Puchkov, D.; Kochlamazashvili, G.; Brachs, S.; et al. Regulation of body weight and energy homeostasis by neuronal cell adhesion molecule 1. Nat. Neurosci. 2017, 20, 1096–1103. [Google Scholar] [CrossRef]
- Yan, X.; Kononenko, N.L.; Brüel, A.; Thomsen, J.S.; Poy, M.N. Neuronal Cell Adhesion Molecule 1 Regulates Leptin Sensitivity and Bone Mass. Calcif. Tissue Int. 2018, 102, 329–336. [Google Scholar] [CrossRef]
- Ito, T.; Nakamura, A.; Tanaka, I.; Tsuboi, Y.; Morikawa, T.; Nakajima, J.; Takai, D.; Fukayama, M.; Sekido, Y.; Niki, T.; et al. CADM1 associates with Hippo pathway core kinases; membranous co-expression of CADM1 and LATS2 in lung tumors predicts good prognosis. Cancer Sci. 2019, 110, 2284–2295. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Sakurai-Yageta, M.; Maruyama, T.; Murakami, Y. Trans-homophilic interaction of CADM1 activates PI3K by forming a complex with MAGuK-family proteins MPP3 and Dlg. PLoS ONE 2014, 9, e110062. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Corum, L.; Meng, Q.; Blenis, J.; Zheng, J.Z.; Shi, X.; Flynn, D.C.; Jiang, B.H. PI3K induced actin filament remodeling through Akt and p70S6K1: Implication of essential role in cell migration. Am. J. Physiol. Cell. Physiol. 2004, 286, C153–C163. [Google Scholar] [CrossRef] [PubMed]
- Gouëffic, Y.; Guilluy, C.; Guérin, P.; Patra, P.; Pacaud, P.; Loirand, G. Hyaluronan induces vascular smooth muscle cell migration through RHAMM-mediated PI3K-dependent Rac activation. Cardiovasc. Res. 2006, 72, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Lien, E.C.; Dibble, C.C.; Toker, A. PI3K signaling in cancer: Beyond AKT. Curr. Opin. Cell. Biol. 2017, 45, 62–71. [Google Scholar] [CrossRef]
- Nakahata, S.; Syahrul, C.; Nakatake, A.; Sakamoto, K.; Yoshihama, M.; Nishikata, I.; Ukai, Y.; Matsuura, T.; Kameda, T.; Shide, K.; et al. Clinical significance of soluble CADM1 as a novel marker for adult T-cell leukemia/lymphoma. Haematologica 2020. [Google Scholar] [CrossRef]
- Makiyama, J.; Kobayashi, S.; Watanabe, E.; Ishigaki, T.; Kawamata, T.; Nakashima, M.; Yamagishi, M.; Nakano, K.; Tojo, A.; Watanabe, T.; et al. CD4(+) CADM1(+) cell percentage predicts disease progression in HTLV-1 carriers and indolent adult T-cell leukemia/lymphoma. Cancer Sci. 2019, 110, 3746–3753. [Google Scholar] [CrossRef]
- Rong, G.; Zhang, M.; Xia, W.; Li, D.; Miao, J.; Wang, H. Plasma CADM1 promoter hypermethylation and D-dimer as novel metastasis predictors of cervical cancer. J. Obstet. Gynaecol. Res. 2019, 45, 1251–1259. [Google Scholar] [CrossRef]
- Hagiyama, M.; Nakatani, Y.; Takashima, Y.; Kato, T.; Inoue, T.; Kimura, R.; Otani, T.; Sato, Y.; Mori, H.; Arima, S.; et al. Urinary Cell Adhesion Molecule 1 Is a Novel Biomarker That Links Tubulointerstitial Damage to Glomerular Filtration Rates in Chronic Kidney Disease. Front. Cell. Dev. Biol. 2019, 7, 111. [Google Scholar] [CrossRef]
- Crick, F. Central dogma of molecular biology. Nature 1970, 227, 561–563. [Google Scholar] [CrossRef]
- Sawada, Y.; Gallo, R. Role of epigenetics in the regulation of immune functions of the skin. J. Investig. Dermatol. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Heard, E. Advances in epigenetics link genetics to the environment and disease. Nature 2019, 571, 489–499. [Google Scholar] [CrossRef]
- Watanabe, T.; Yamashita, S.; Ureshino, H.; Kamachi, K.; Kurahashi, Y.; Fukuda-Kurahashi, Y.; Yoshida, N.; Hattori, N.; Nakamura, H.; Sato, A.; et al. Targeting aberrant DNA hypermethylation as a driver of ATL leukemogenesis by using the new oral demethylating agent OR-2100. Blood 2020, 136, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Yanatatsaneejit, P.; Chalertpet, K.; Sukbhattee, J.; Nuchcharoen, I.; Phumcharoen, P.; Mutirangura, A. Promoter methylation of tumor suppressor genes induced by human papillomavirus in cervical cancer. Oncol. Lett. 2020, 20, 955–961. [Google Scholar] [CrossRef]
- Holubekova, V.; Mersakova, S.; Grendar, M.; Snahnicanova, Z.; Kudela, E.; Kalman, M.; Lasabova, Z.; Danko, J.; Zubor, P. The Role of CADM1 and MAL Promoter Methylation in Inflammation and Cervical Intraepithelial Neoplasia. Genet. Test. Mol. Biomark. 2020, 24, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Meršaková, S.; Holubeková, V.; Grendár, M.; Višňovský, J.; Ňachajová, M.; Kalman, M.; Kúdela, E.; Žúbor, P.; Bielik, T.; Lasabová, Z.; et al. Methylation of CADM1 and MAL together with HPV status in cytological cervical specimens serves an important role in the progression of cervical intraepithelial neoplasia. Oncol. Lett. 2018, 16, 7166–7174. [Google Scholar] [CrossRef]
- Fiano, V.; Trevisan, M.; Fasanelli, F.; Grasso, C.; Marabese, F.; da Graça Bicalho, M.; de Carvalho, N.S.; Maestri, C.A.; Merletti, F.; Sacerdote, C.; et al. Methylation in host and viral genes as marker of aggressiveness in cervical lesions: Analysis in 543 unscreened women. Gynecol. Oncol. 2018, 151, 319–326. [Google Scholar] [CrossRef]
- Hassan, Z.K.; Hafez, M.M.; Kamel, M.M.; Zekri, A.R. Human Papillomavirus Genotypes and Methylation of CADM1, PAX1, MAL and ADCYAP1 Genes in Epithelial Ovarian Cancer Patients. Asian Pac. J. Cancer Prev. 2017, 18, 169–176. [Google Scholar]
- Ribeiro, I.P.; Caramelo, F.; Esteves, L.; Oliveira, C.; Marques, F.; Barroso, L.; Melo, J.B.; Carreira, I.M. Genomic and epigenetic signatures associated with survival rate in oral squamous cell carcinoma patients. J. Cancer 2018, 9, 1885–1895. [Google Scholar] [CrossRef]
- Vermeulen, M.A.; van Deurzen, C.H.M.; Doebar, S.C.; de Leng, W.W.J.; Martens, J.W.M.; van Diest, P.J.; Moelans, C.B. Promoter hypermethylation in ductal carcinoma in situ of the male breast. Endocr. Relat. Cancer 2019, 26, 575–584. [Google Scholar] [CrossRef]
- Chilmi, S.; Nakahata, S.; Fauzi, Y.R.; Ichikawa, T.; Tani, C.; Suwanruengsri, M.; Yamaguchi, R.; Matsuura, T.; Kurosawa, G.; Morishita, K. Development of anti-human CADM1 monoclonal antibodies as a potential therapy for adult T-cell leukemia/lymphoma. Int. J. Hematol. 2020, 112, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Li, W.W.; Wen, C.J.; Diao, Y.L.; Zhao, T.L. MicroRNA-214 promotes the EMT process in melanoma by downregulating CADM1 expression. Mol. Med. Rep. 2020, 22, 3795–3803. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Hu, W.; Zhang, Y.; Sang, J.; Li, H.; Ma, T.; Bo, Y.; Bai, T.; Guo, H.; et al. MiR-424-5p Promotes Proliferation, Migration and Invasion of Laryngeal Squamous Cell Carcinoma. Onco Targets Ther. 2019, 12, 10441–10453. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Nong, S.; Gong, J.; Zhang, X.; Tang, H.; Zhou, T.; Li, W. MiR-194 promotes hepatocellular carcinoma through negative regulation of CADM1. Int. J. Clin. Exp. Pathol. 2020, 13, 1518–1528. [Google Scholar]
| Cutaneous Malignancies | The Role of CADM1 in the Risk of Each Malignancies |
|---|---|
| Solid tumor | |
| Melanoma | Favorable [31,32,33,34]. |
| Squamous cell carcinoma | Favorable [35,36] |
| Merkel cell carcinoma | Unfavorable [37]. |
| Lymphoma | |
| Adult T cell leukemia/lymphoma | Unfavorable [10,38]. |
| Mycosis fungoides | Unfavorable [39,40] |
| Sezary syndrome | Unfavorable [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawada, Y.; Mashima, E.; Saito-Sasaki, N.; Nakamura, M. The Role of Cell Adhesion Molecule 1 (CADM1) in Cutaneous Malignancies. Int. J. Mol. Sci. 2020, 21, 9732. https://doi.org/10.3390/ijms21249732
Sawada Y, Mashima E, Saito-Sasaki N, Nakamura M. The Role of Cell Adhesion Molecule 1 (CADM1) in Cutaneous Malignancies. International Journal of Molecular Sciences. 2020; 21(24):9732. https://doi.org/10.3390/ijms21249732
Chicago/Turabian StyleSawada, Yu, Emi Mashima, Natsuko Saito-Sasaki, and Motonobu Nakamura. 2020. "The Role of Cell Adhesion Molecule 1 (CADM1) in Cutaneous Malignancies" International Journal of Molecular Sciences 21, no. 24: 9732. https://doi.org/10.3390/ijms21249732
APA StyleSawada, Y., Mashima, E., Saito-Sasaki, N., & Nakamura, M. (2020). The Role of Cell Adhesion Molecule 1 (CADM1) in Cutaneous Malignancies. International Journal of Molecular Sciences, 21(24), 9732. https://doi.org/10.3390/ijms21249732




